Abstract
Parkinsonism caused by MPTP exposure was first identified in intravenous drug users. The neurotoxicant has since been used extensively in nonhuman primates to induce an experimental model of Parkinson’s disease. We examined the intraoperative physiology and the efficacy of subthalamic nucleus deep brain stimulation (DBS) in 1 of only 4 known living humans with MPTP-induced parkinsonism. The physiologic recordings were consistent with recordings from MPTP-treated primates and humans with Parkinson’s disease, thus further validating the MPTP model for the study of the neurophysiology of the nigrostriatal dopaminergic deficit in Parkinson’s disease. Furthermore, DBS offered significant clinical improvement in this patient similar to that seen in idiopathic Parkinson’s disease. This unique case has important implications for translational research that employs the MPTP-primate model for symptomatic therapy in Parkinson’s disease.
Keywords: Parkinson’s disease, toxin, parkinsonism, Parkinsonian, deep brain stimulation, subthalamic nucleus, physiology
Parkinsonism caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) exposure was first identified in intravenous drug users in 1983.5 Although exposure was time-limited, long-term follow up has shown that the parkinsonian syndrome is permanent and in some cases progresses. Moreover, positron emission tomographic imaging has demonstrated a loss of fluorodopa uptake similar to that seen in Parkinson’s disease (PD).13 The discovery that exposure to a relatively simple molecule induced so many of the motor features of PD has stimulated new directions for research, ranging from epidemiologic studies on the cause, to in vitro tools for the study of mechanisms of neurodegeneration. However, one of the most important applications of this discovery was the development of an animal model for PD.
The MPTP-induced parkinsonian primate model has been particularly useful for investigating pharmacologic therapies as well as side-effects of treatment, such as dyskinesias.3,4 The primate model has also been used for the study of cell replacement therapies, including both fetal cells and embryonic stem cells. It has also proved invaluable in elucidating much of our current knowledge of basal ganglia circuitry and the therapeutic value of manipulating its output through deep brain stimulation (DBS) as a treatment for the idiopathic disease.14 However, the direct testing of this model for expected electrophysiology changes in basal ganglia firing patterns caused by MPTP in humans has been lacking.
We now report the benefit of DBS in one of the original individuals with MPTP-induced parkinsonism. This is the first time DBS has been used to treat MPTP-induced parkinsonism in a human, and it has provided a unique opportunity to compare subthalamic nucleus (STN) physiology in human MPTP-induced parkinsonism with that seen in idiopathic PD and in non-human primates with MPTP-induced parkinsonism. Our results have important implications for validity of this widely used model of basal ganglia dysfunction due to a failing nigrostriatal system.
Case History
In 1982, when 24 years old, this man developed tremor and parkinsonism over a three day period after intravenous self-administration of what he thought to a form of “synthetic heroin.” This drug was eventually found to be composed of a chemical mixture containing MPTP, generated as an inadvertent by-product of illicit narcotic synthesis.5 His motor function improved dramatically with levodopa therapy. However, over time, he developed disabling wearing-off, freezing, dyskinesias, and severe tremor.
His medical history was otherwise notable for polysubstance abuse (including alcohol, heroin, marijuana, and cocaine), hepatitis C, and a long history of antisocial behavior that resulted in incarceration at state detention centers beginning in his teenage years. He developed auditory hallucinations about 1 year after beginning treatment with levodopa which were controlled with atypical antipsychotic medications.
Prior to surgery, he estimated that he spent about half the day with significant tremor involving upper extremities that interfered with eating and spent about 25% of the day in the “off state.” He experienced falls about once daily because of freezing of gait. Moreover, he experienced hallucinations and had assaulted a number of individuals in his facility likely secondary to paranoia. The preoperative medications were carbidopa/levodopa 10/100 seven daily, clozapine 175 mg daily, pramipexole 0.5 mg three times daily, benztropine 2 mg twice daily, tolterodine 2 mg twice daily, quitiapine 50 mg twice daily, and trazodone 200 mg daily.
Methods
The patient underwent careful assessments 1 month preoperatively and at 8, 15, and 40 months postoperatively using the Unified Parkinson’s disease Rating Scale, Part III (UPDRS III)12 and the stand-walk-sit test.9 On October 28, 2003 date, he underwent placement of bilateral STN DBS (Medtronic model 3387 lead) using single unit microelectrode recording to confirm location. Neuronal firing patterns were analyzed according to methods described elsewhere in detail.10 Electrode location was determined by postoperative MRI, performed in accordance with guidelines issued by the device manufacturer.
For all postoperative evaluations, the evaluator was blinded as to the medication and stimulator state. The practically defined “off-medication” state consisted of the patient refraining from dopaminergic medication for 12 hours prior to evaluation. The practically defined “on-medication” state was defined as the state of the patient 1 hour after taking the usual first dopaminergic doses of the day. Both stimulators were turned off for 1 hour prior to the evaluation of the off-stimulator state. The “on-stimulator” state was defined as both stimulators being on for at least 1 hour. Simulator settings were as follows: left side contact 1 negative/case positive, 2 V, pulse width 60 µs and 185 Hz; right side contact 1 negative/case positive, 2 V, pulse width 60 µs and 185 Hz. These settings remained unchanged throughout the 2 1/2 years of follow-up. Neuropsychological evaluations were performed in the on-medication state 1 month preoperatively and in the on-medication/on-stimulator state 10 months postoperatively.
Results
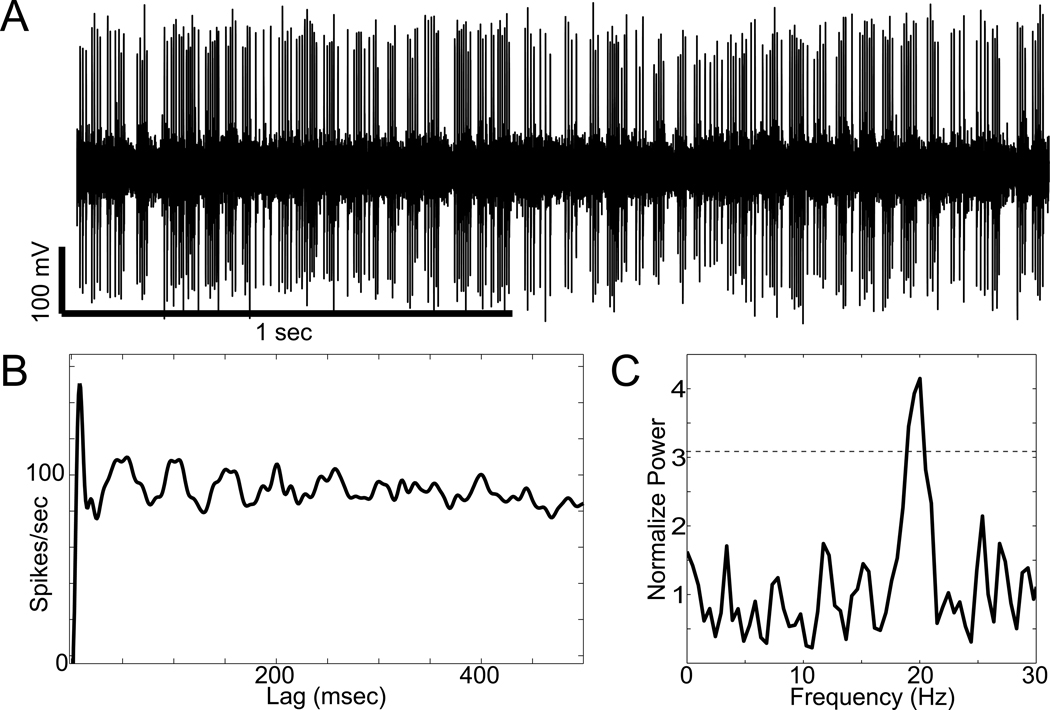
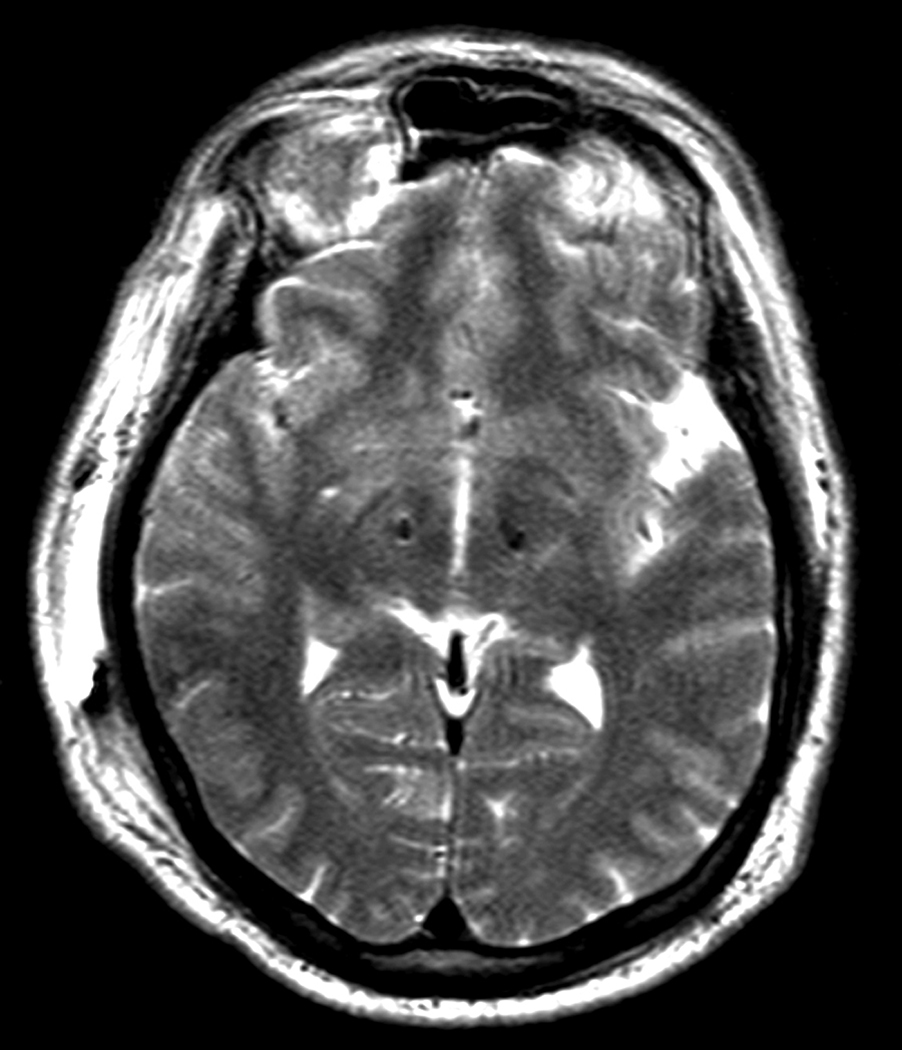
Intra-operatively, many STN neuronal action potentials were recorded, but only five were amenable to formal analysis based on the presence of a well isolated single unit recorded continuously for at least 20 seconds. These cells (Figure 1) demonstrated a mean firing frequency of 76 +/− 27 Hz (SD) and oscillatory firing patterns (significant spectral peaks at ~4 Hz and/or ~20 Hz in 4/5 cells) similar to that seen in idiopathic PD and in parkinsonian non-human primates.1 All five neurons had mean firing frequencies greater than 50 spikes/sec, which is above the 99% confidence interval for normative STN firing rates based on published data from non-human primates.1 Published data also indicate that oscillatory firing patterns are rare in the normal primate STN (found in 3.8% of cells1). Therefore, it is very unlikely that 4 out of five cells picked at random from a population of normal STN neurons would have oscillatory activity (P<0.00001; Chi-square test). Post-operative MRI demonstrated that the leads were appropriately located with the active contacts at left lateral 10.5, AP-3.4, vert −3.8 and right lateral 12.1, AP - 0.7, vert −2.8, with respect to the mid-commissural point (Figure 2).
Figure 1.
Characteristic oscillatory activity recorded from an STN neuron. A, Segment of the raw signal recorded from a well-isolated neuron. B, Autocorrelogram for the same spike-train illustrating rhythmic peaks in firing probability ~50 milliseconds apart. C, Frequency spectrum for the spike-train (normalized to the mean of 100 spectra from shuffled interspike intervals). Horizontal dotted line, threshold for significance (P<0.01 adjusted for multiple comparisons, based on the mean and SD in normalized spectrum between 300–500 Hz).
Figure 2.
Postoperative axial T2 weighted MRI in the plane of the dorsal STN (4 mm inferior to the intercommissural line). The leads are seen as signal voids, lateral to the anterior border of the red nucleus.
The patient tolerated the surgical procedure without incident. When evaluated after activation of the DBS, the patient reported a substantial reduction in the daily percentage of “off” time from 25% preoperatively to 0% per day postoperatively; this benefit persisted throughout the 40 months of observation. In addition, activation of the DBS allowed reduction in medications: levodopa was reduced from 700 mg to 400 mg daily; clozapine was reduced from 175 mg to 75 mg daily; while pramipexole (0.5 mg 3 times daily) and benztropine (2 mg twice daily), and quetiapine (50 mg twice daily) were discontinued altogether. At the 40 month visit, he continued to report stable motor benefit and denied any recent hallucinations.
Formal postoperative evaluations also showed improvement (Table 1). When evaluated 40 months after surgery, there was a 72% reduction in the UPDRS III score (lower scores reflect milder parkinsonsism) comparing the practically defined off-medication score preoperatively with the postoperative off-medication/on-stimulation score. There was also a 55% reduction in the on-medication score, comparing the preoperative on-medication score with the on-medication/on-stimulation score at 40 months. There were similar improvements in the stand-walk-sit test, in which shorter times reflect better mobility. For example, at 40 months, there was a 32% reduction in the timed stand-walk-sit test comparing the preoperative off-medication time with the off-medication/on-stimulation time. The on-medication/on-stimulation time was 29% shorter than the preoperative on-medication state (Table 1). At 40 months, no changes in stimulation parameters had been made since the initial months after placement.
Table 1.
UPDRS and Stand-walk-sit test
| Unified Parkinson’s disease rating scale, Part 3 | |||||
|---|---|---|---|---|---|
| Off Meds | On Meds | Off Meds/ Off Stim |
Off Meds/ On Stim |
On Meds/On Stim |
|
| Pre Op | 53 | 20 | |||
| 8 Months Post | 31 | 19 | 12 | ||
| 15 Months Post | 39 | 20 | 15 | ||
| 40 Months Post | 30 | 15 | 9 | ||
| Stand-walk-sit test (Seconds) | |||||
| Off Meds | On Meds | Off Meds/ Off Stim |
Off Meds/ On Stim |
On Meds/ On Stim |
|
| Pre Op | 22.3 | 20.5 | |||
| 8 Months Post | 20 | 17 | 17 | ||
| 15 Months Post | 17 | 15 | 15 | ||
| 40 Months Post | 18 | 15 | 15 | ||
Surgery and STN DBS had little effect on the patient's neuropsychological status. Preoperative testing in the on-medication state showed a Wechsler Adult Intelligence Scale III verbal scale IQ of 77, performance scale IQ of 74, and full scale IQ of 74. Difficulties were noted on verbal articulation, fine motor dexterity, 3-step motor sequencing, visual-spatial organization, delayed recall of complex abstract visual stimuli, abstract problem solving, recognizing higher-order concepts and intruding extra words on list learning. Post-operatively, with the stimulators on and on-medication, the Wechsler Adult verbal scale IQ was 71, the performance scale IQ was 80, and the full scale IQ was 73.
Discussion
This report documents the first example of using STN DBS to treat MPTP-induced parkinsonism in a human. Furthermore the procedure provided robust clinical benefit. It also represents the first time that STN neuronal action potentials have been recorded in a human with what is presumably a relatively pure nigrostriatal lesion, i.e., a lesion induced by MPTP. Single cell recording of STN neurons showed high spontaneous discharge rates and oscillatory firing patterns, consistent with published physiological studies of idiopathic parkinsonism6 and MPTP-induced parkinsonism in the nonhuman primate.1 Thus this study is the final step of cross-validation of the neurophysiologic changes in basal ganglia circuitry between MPTP-induced parkinsonism in non-human primates, that seen in humans with PD, and a human with MPTP induced parkinsonism.
The STN is a key structure in the "indirect pathway." one of the two major intrinsic basal ganglia pathways connecting the striatum to the internal globus pallidus. The indirect pathway is thought to suppress the activation of unwanted motor programs. In PD, dopamine denervation of the striatal cells that originate the indirect pathway (D2 receptor positive cells) is thought to increase their neuronal discharge. This in turn would be expected to suppress spontaneous firing in the external pallidum and increase spontaneous firing in the STN and GPi. In the patient with MPTP-induced parkinsonism reported here, STN physiology demonstrated high firing rates and oscillatory activity highly analogous to that seen in the idiopathic disease, lending support to the classical model of firing rate abnormalities in the indirect pathway in the parkinsonian state.
That STN DBS produced favorable functional consequents in this patient appears to highly likely, particularly in view of the fact that his parkinsonism had been disabling for nearly 20 years. Indeed, comparing either UPDRS III scores or stand-walk-sit times preoperatively with off-medication/off-stimulation values shows a surprisingly robust improvement postoperatively. A number of factors may explain this improvement including 1) a carry over benefit of DBS lasting longer than the 1 hour during which the stimulator is off before testing in the off stimulation state8, 2) reduction in the dose of clozapine and elimination of quetiapine (both dopamine receptor blocking antipsychotics), 3) a micro-lesion effect on the STN, or 4) an unusually poor performance at his baseline visit. Regardless of which factor(s) explain this effect, stimulation clearly provided additional benefit as his UPDRS scores and stand-walk-sit times were lower in the on-stimulation/off-medication state compared to the off-stimulation/off-medication state. Furthermore, after DBS, it was possible to make substantial reductions in dopaminergic medications, which in turn may have provided further benefits by limiting side-effects of therapy. Although the pattern of neuropsychological deficits are consistent with the cognitive deficits in PD and MPTP induced parkinsonism11, this subject’s deficits are more global and more severe, likely reflecting the baseline abnormalities in this individual with a history of antisocial behavior and substance abuse.
How well MPTP-induced parkinsonism models idiopathic PD continues to be a topic of debate.2 Major criticisms relate to the lack of wide-spread synuclein-based neuropathology similar to that seen in PD, and the fact that, in animals at least, it does not induce a clearly progressive disorder. However, these and other similar arguments can be reconciled with those supporting its use by clearly defining what is being modeled. If the objective is to provide insight regarding protein misfolding, or investigating therapeutic approaches to disease modification, then the MPTP model may not be an appropriate choice for an animal model (indeed, an experimental model meeting these specifications represents one of the most urgent needs in the field of PD research). On the other hand, if the goal is to model the abnormalities in basal ganglia circuitry that follow degeneration of the dopaminergic nigrostriatal system, then this model has proven to be extraordinarily predictive. When viewed in this light, our case report further substantiates the close approximation of MPTP-induced parkinsonism to the parkinsonism and changes in basal ganglia physiology seen in the idiopathic disease because. For example, STN physiology demonstrated high firing rates and oscillatory activity highly analogous to that seen in the idiopathic disease. Furthermore, STN DBS produced a striking clinical improvement just as seen in the MPTP-induced non-human primate models, and patients with idiopathic PD. This is precisely what was predicted in the original report by Bergman and colleagues in 1989, when they used the MPTP-non-human primate model to demonstrate over-activity in the STN, and then found that a lesion of the nucleus reduced parkinsonism.
In closing, it is worth noting that this report represents yet another irony in the MPTP story7 as it completes of a series of events coming full circle. While the outbreak of MPTP-induced parkinsonism in the early 1980’s represented a tragedy for a group of young drug abusers, the discovery of the biologic effects of this neurotoxicant lead to the development of a stable animal model of PD. This model has made it possible to understand the role of the STN in parkinsonism, a discovery that was central to the development of STN DBS. Over the past decade, STN DBS has improved the lives of many PD patients, and now, with this report, has benefited one of the original patients in whom the effects of MPTP were discovered.
Supplementary Material
Acknowledgement
No funding sources supported this work.
We thank Danny Herrick for help in preparing the manuscript.
Footnotes
Portions of this work were presented in abstract and poster form at the International Congress of Parkinson’s disease and Movement Disorders in New Orleans, Louisanna 2005
References
- 1.Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- 2.Bove J, Prou D, Perier C, Przedborski S. Toxin-induced models of Parkinson's disease. NeuroRx. 2005;2:484–494. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenner P. The contribution of the MPTP-treated primate model to the development of new treatment strategies for Parkinson's disease. Parkinsonism Relat Disord. 2003;9:131–137. doi: 10.1016/s1353-8020(02)00115-3. [DOI] [PubMed] [Google Scholar]
- 4.Jenner P. The MPTP-treated primate as a model of motor complications in PD: primate model of motor complications. Neurology. 2003;61:S4–S11. doi: 10.1212/wnl.61.6_suppl_3.s4. [DOI] [PubMed] [Google Scholar]
- 5.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 6.Levy R, Hutchison WD, Lozano AM, Dostrovsky JO. High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J Neurosci. 2000;20:7766–7775. doi: 10.1523/JNEUROSCI.20-20-07766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewin R. Trail of ironies to Parkinson's disease. Science. 1984;224:1083–1085. doi: 10.1126/science.6426059. [DOI] [PubMed] [Google Scholar]
- 8.Nutt JG, Rufener SL, Carter JH, Anderson VC, Pahwa R, Hammerstad JP, et al. Interactions between deep brain stimulation and levodopa in Parkinson's disease. Neurology. 2001;57:1835–1842. doi: 10.1212/wnl.57.10.1835. [DOI] [PubMed] [Google Scholar]
- 9.O'Sullivan JD, Said CM, Dillon LC, Hoffman M, Hughes AJ. Gait analysis in patients with Parkinson's disease and motor fluctuations: influence of levodopa and comparison with other measures of motor function. Mov Disord. 1998;13:900–906. doi: 10.1002/mds.870130607. [DOI] [PubMed] [Google Scholar]
- 10.Starr PA, Christine CW, Theodosopoulos PV, Lindsey N, Byrd D, Mosley A, et al. Implantation of deep brain stimulators into the subthalamic nucleus: technical approach and magnetic resonance imaging-verified lead locations. J Neurosurg. 2002;97:370–387. doi: 10.3171/jns.2002.97.2.0370. [DOI] [PubMed] [Google Scholar]
- 11.Stern Y, Langston JW. Intellectual changes in patients with MPTP-induced parkinsonism. Neurology. 1985;35:1506–1509. doi: 10.1212/wnl.35.10.1506. [DOI] [PubMed] [Google Scholar]
- 12.van Hilten JJ, van der Zwan AD, Zwinderman AH, Roos RA. Rating impairment and disability in Parkinson's disease: evaluation of the Unified Parkinson's Disease Rating Scale. Mov Disord. 1994;9:84–88. doi: 10.1002/mds.870090113. [DOI] [PubMed] [Google Scholar]
- 13.Vingerhoets FJ, Snow BJ, Tetrud JW, Langston JW, Schulzer M, Calne DB. Positron emission tomographic evidence for progression of human MPTP-induced dopaminergic lesions. Ann Neurol. 1994;36:765–770. doi: 10.1002/ana.410360513. [DOI] [PubMed] [Google Scholar]
- 14.Wichmann T, DeLong MR. Pathophysiology of Parkinson's disease: the MPTP primate model of the human disorder. Ann N Y Acad Sci. 2003;991:199–213. doi: 10.1111/j.1749-6632.2003.tb07477.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.