Abstract
Synaptic plasticity in the ventral tegmental area (VTA) is modulated by drugs of abuse and stress and is hypothesized to contribute to specific aspects of addiction. Both excitatory and inhibitory synapses on dopamine neurons in the VTA are capable of undergoing long-term changes in synaptic strength. While the strengthening or weakening of excitatory synapses in the VTA has been widely examined, the role of inhibitory synaptic plasticity in brain reward circuitry is less established. Here, we investigated the effects of drugs of abuse, as well as acute stress, on long-term potentiation of GABAergic synapses onto VTA dopamine neurons (LTPGABA). Morphine (10 mg/kg i.p.) reduced the ability of inhibitory synapses in midbrain slices to express LTPGABA both at 2 and 24 hours after drug exposure but not after 5 days. Cocaine (15 mg/kg i.p.) impaired LTPGABA 24 hours after exposure, but not at 2 hours. Nicotine (0.5 mg/kg i.p.) impaired LTPGABA 2 hours after exposure, but not after 24 hours. Furthermore, LTPGABA was completely blocked 24 hours following brief exposure to a stressful stimulus, a forced swim task. Our data suggest that drugs of abuse and stress trigger a common modification to inhibitory plasticity, synergizing with their collective effect at excitatory synapses. Together, the net effect of addictive substances or stress is expected to increase excitability of VTA dopamine neurons, potentially contributing to the early stages of addiction.
Keywords: addiction, GABA, synaptic plasticity, patch clamp
Introduction
The ability of addictive drugs to usurp brain reward circuits relevant to natural rewards and establish addictive behaviors is hypothesized to be due in part to changes in synaptic plasticity of the mesolimbic dopamine system triggered by drugs of abuse. Initial studies seeking to identify common adaptations in the brain produced by drugs of abuse ascertained that these substances all increased dopamine release probability of ventral tegmental area (VTA) dopamine neurons that project to the nucleus accumbens (Wise, 1996; Koob et al., 1998; Nestler, 2001). Other investigators found that in vivo administration of addictive drugs is sufficient to induce a long-lasting change in synaptic transmission of excitatory inputs onto dopamine neurons in the VTA (Ungless et al., 2001; Saal et al., 2003; Faleiro et al., 2004; Argilli et al., 2008; Chen et al., 2008; Engblom et al., 2008; Mameli et al., 2009). Long-term potentiation (LTP) of excitatory synapses is a mechanism that underlies the ability of neural circuits to form memories, in addition to many other brain functions (Malenka & Bear, 2004). Thus, addictive drugs may co-opt brain reward circuitry to ‘overlearn’ the value of a drug to the organism (Kauer & Malenka, 2007; Niehaus et al., 2009). Since the net output of a neuron depends upon the balance between excitatory and inhibitory inputs onto the cell, some studies have considered whether inhibitory synaptic transmission is modified by addictive drugs. Cocaine, morphine and ethanol have all been found to affect inhibitory synaptic plasticity in the VTA (Melis et al., 2002; Liu et al., 2005; Nugent et al., 2007). Long-lasting potentiation of GABAergic synapses onto dopamine neurons in the VTA (LTPGABA) is blocked by in vivo administration of morphine (Nugent et al., 2007); however, it is unknown whether other drugs of abuse, many of which potentiate excitatory synapses onto VTA dopamine neurons (Saal et al., 2003), can analogously alter LTPGABA.
In addition to addictive drugs, acute stress can also affect synaptic plasticity. In the hippocampus, acute stress impairs LTP and simultaneously facilitates LTD (Shors et al., 1989; Kim et al., 1996; Xu et al., 1997). Acute stress, as well as various drugs of abuse, potentiates excitatory synaptic strength at dopamine neurons in the VTA (Saal et al., 2003; Dong et al., 2004) suggesting that stress and drugs of abuse converge on a common pathway that modifies dopamine neuron output and thereby reward circuitry. Stress can increase dopamine release (Inglis & Moghaddam, 1999) and can facilitate behaviors such as drug-seeking and drug-taking that may develop into addiction, perhaps by augmenting the rewarding effects of drug administration (Piazza & Le Moal, 1998; Miczek et al., 2008). Here we examine whether in vivo administration of addictive drugs or exposure to acute stress regulate inhibitory inputs onto dopamine neurons in the VTA. Our findings suggest that drugs of abuse and stress may utilize common mechanisms to modulate synaptic plasticity of inhibitory synapses.
Methods
Animals and in vivo manipulations
All procedures were carried out in accordance with the guidelines of the National Institutes of Health for animal care and use, and were approved by the Brown University Institutional Animal Care and Use Committee. Sprague-Dawley rats were maintained on a 12-hour light/dark cycle and provided food and water ad libitum. For drug administration, rats were given a single injection i.p. at the same time of day of either saline (volume matched with experimental injections), morphine (10 mg/kg), cocaine (15 mg/kg) or nicotine (0.5 mg/kg), housed singly in a new cage for 2 hours and then returned to the home cage. For morphine, cocaine, nicotine and stress experiments, only one brain slice per rat was used for each experiment so that reported n numbers represent the number of animals. Acute stress was administered by a modified Porsolt forced swim task (Saal et al., 2003), wherein rats were placed in a 2 L plastic beaker containing approximately 1 L of cold water (6 °C) for 5 min. Following the swim task, animals were dried off, wrapped in a dry cloth for 5 min, placed singly in a warmed cage that was heated underneath by a heating pad for 2 hours and then returned to the home cage. RU486 (40 mg/ml in DMSO) or DMSO (as vehicle) were administered i.p. 30 min prior to the cold water swim.
Preparation of brain slices
Preparation of slices was performed as previously described (Jones et al., 2000; Nugent et al., 2007). Sprague-Dawley rats (15–21 days old) were deeply anesthetized using isoflurane and quickly decapitated. Unless stated otherwise, brain slices were prepared 24 hours after drug administration or stress exposure. The brain was rapidly removed into ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): 126 NaCl, 21.4 NaHCO3, 2.5 KCl, 1.2 NaH2PO4, 2.4 CaCl2, 1.2 MgSO4, 11.1 glucose and 0.4 ascorbic acid, saturated with 95% O2/5% CO2 (pH 7.4). Horizontal midbrain slices containing the VTA were cut at a thickness of 250 µm using a vibratome (Leica Microsystems), stored for at least one hour at 34°C, and transferred to a recording chamber where the slice was submerged in warmed ACSF.
Electrophysiology
Midbrain slices were continuously perfused with ACSF (no ascorbic acid) at 28–32°C at 1.5–2 ml/min containing: 6,7-dinitroquinoxaline-2,3-dione (DNQX; 10µM), strychnine (1µM), and 1, 3-dipropyl-8-cyclopentylxanthine (DPCPX; 1µM) to block AMPA-, glycine-, and A1 adenosine receptors, respectively. A1 receptors were blocked to remove endogenous levels of adenosine-mediated inhibition of GABA release (Floran et al., 2002). Patch pipettes were filled with (in mM): 125 KCl, 2.8 NaCl, 2 MgCl2, 2 ATP-Na+, 0.3 GTP-Li+, 0.6 EGTA, and 10 HEPES. Whole-cell patch-clamp recordings were made from neurons visually identified in the VTA. Dopamine neurons were identified by the presence of a large Ih-current (>60 pA) during a voltage step from −50 mV to −100 mV. A recent study showed that expression of Ih alone is not sufficient to identify DA cells unequivocally (Margolis et al., 2006), but see (Chen et al., 2008). Therefore in each set of our experiments, a subset of the neurons recorded from and reported here are possibly non-dopaminergic neurons. GABAA receptor-mediated IPSCs were stimulated at 0.1 Hz (100 µsec) using a bipolar stainless steel stimulating electrode placed 200–500 µm rostral to the recording site in the VTA. Cells were voltage-clamped at −70mV and the cell input resistance and series resistance were monitored throughout the experiment and were discarded if these values changed by more than 10% during the experiment. IPSCs were amplified using an AxoClamp 2B amplifier (Axon Instruments) and Brownlee Precision model 210 postamplifier, low-pass filtered at 3 kHz and digitally sampled to computer at 30 kHz using an analog-to-digital interface (National Instruments). Custom-designed software for use with LabVIEW (National Instruments) was used to measure the peak IPSC amplitudes.
Materials
3-isobutyl-1-methylxanthine (IBMX; 100 µm) was used to inhibit phosphodiesterase-mediated degradation of cGMP and applied via perfused ACSF for at least 10 minutes prior to induction of LTPGABA by SNAP or BAY 41-2272. Brain slices from control animals (vehicle-injected) were interleaved with brain slices from experimental animals (morphine, cocaine or nicotine injected). Cocaine was provided by Brown University Animal Care Facility. SNAP (S-nitroso-N-acetylpenicillamine) was obtained from Cayman Chemical. IBMX and DPCPX were obtained from Tocris Bioscience. Morphine, nicotine, BAY 41-2272, RU 486, DNQX, strychnine and all other chemicals were obtained from Sigma-Aldrich.
Analysis
Data are presented as means ± s.e.m. Data from Figures 1–3, 5 and 7 were analyzed with a two-factor analysis of variance (ANOVA), using the experimental condition (saline vs. drug or stress vs. RU486) as the between-subjects factor and time (in minutes) as the within-subjects factor. Data from Figures 4 and 6 were analyzed by one-way ANOVA to assess drug effect (SNAP or BAY 41-2272) on IPSC amplitude over time course of experiment (in minutes). Main effects and interactions from ANOVAs were determined with a significance level of p < 0.05. Magnitude of LTP was calculated by averaging 30 consecutive IPSCs 5 min prior to drug application and 20–25 min after drug application.
Figure 1. LTPGABA is impaired by morphine 2 hours following injection, but recovers after 5 days.
A, Diagram of injection protocol. B, Averaged experiments show that SNAP potentiates IPSCs in slices prepared from saline-treated animals 2 hours after injection (n = 7), but is impaired in morphine-treated animals (n = 9). We analyzed the effect of morphine on LTPGABA using two way ANOVA with the drug treatment (saline vs. morphine) as the between subjects factor and time (in minutes) as the within subjects factor. This analysis revealed significant effects of drug treatment (F1,39 = 99.2, p < 0.001), time (F1,39 = 7.00, p < 0.001) and an interaction between these two factors (F1,39 = 0.11, p < 0.001). C, Averaged experiments show that SNAP potentiation of IPSCs is not significantly different in slices prepared from saline (n = 6) or morphine-treated animals (n = 8) 5 days after a single injection of morphine. Statistical analysis revealed a significant effect of time (F1,39 = 5.29, p < 0.001), but no significant effects of drug treatment (F1,39 = 0.63, p > 0.05) or interaction between the two factors (F1,39 = 0.37, p > 0.05).
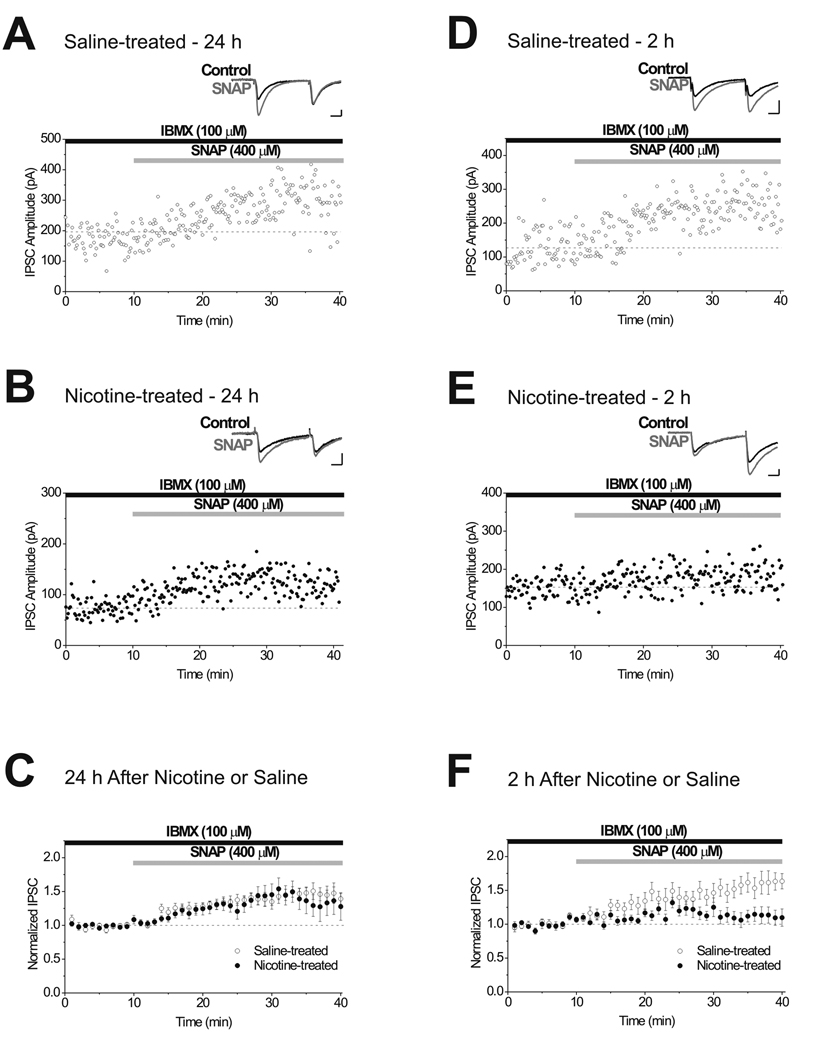
Figure 3. A single in vivo administration of nicotine reduces LTPGABA 2 hours after exposure, but not at 24 hours.
A, Single experiment illustrating LTPGABA induced by bath application of SNAP onto a brain slice prepared 24 hours after saline-injection. Insets, Representative IPSCs evoked before (Control) and 15 min after (SNAP) drug application. B, Single experiment illustrating that LTPGABA is unaffected in a slice prepared 24 hours after nicotine-injection. C, At 24 hours after exposure, averaged experiments show no significant difference in SNAP-induced potentiation of IPSCs from saline (n = 11) or nicotine-treated animals (n = 13). Statistical analysis revealed a significant effect of time (F1,39 = 8.44, p < 0.001), but no significant effects of drug treatment (F1,39 = 3.55, p > 0.05) or interaction between the two factors (F1,39 = 0.36, p > 0.05). D, Single experiment illustrating LTPGABA induced by bath application of SNAP onto a brain slice prepared 2 hours after saline-injection. E, Single experiment illustrating that LTPGABA is impaired 2 hours after nicotine-injection. F, At 2 hours after exposure, averaged experiments show SNAP potentiation of IPSCs in slices from nicotine-treated animals (n = 7) is reduced compared to those from saline-treated animals (n = 7). There were significant effects of drug treatment (F1,39 = 102.0, p < 0.001), time (F1,39 = 5.1, p < 0.001) and an interaction between these two factors (F1,39 = 1.99, p < 0.001). Scale bars: 10 ms, 50 pA.
Figure 5. Morphine-induced blockade of LTPGABA occurs independently of the stress pathway.
A, Diagram of injection protocol. B, Single experiment illustrating the inability of SNAP to induce LTPGABA in a slice from an animal injected with RU486 30 min prior to morphine injection. Inset, Representative IPSCs evoked before (Control) and 15 min after (SNAP) drug application. C, Averaged experiments show the inability of SNAP to potentiate IPSCs in slices from morphine-treated animals previously injected with RU486 (n = 8). One-way ANOVA revealed no significant effect of SNAP over time (p > 0.05). Scale bars: 10 ms, 50 pA.
Figure 7. Activation of sGC overcomes morphine-induced blockade of LTPGABA 24 hours after drug exposure.
A, Single experiment illustrating LTPGABA induced by application of 10 µM BAY 41-2272 onto a brain slice from a saline-treated animal. Insets, Representative IPSCs evoked before (Control) and 15 min after (BAY 41-2272) drug application. B, Single experiment illustrating the ability of BAY 41-2272 to induce LTPGABA in a slice from an animal injected with morphine. C, Averaged experiments show BAY 41-2272 potentiation of IPSCs is not significantly different in slices from saline (n = 12) or morphine-treated animals (n = 13). The statistical analysis revealed a significant effect of time (F1,39 = 5.07, p < 0.001), but no significant effects of drug treatment (F1,39 = 0.04, p > 0.05) or interaction between the two factors (F1,39 = 0.39, p > 0.05). Scale bars: 10 ms, 50 pA.
Figure 4. Acute stress blocks LTPGABA via glucocorticoid receptors.
A, Diagram of experimental protocol for injection and acute stress. B, Single experiment illustrating the inability of SNAP to induce LTPGABA in a slice prepared 24 hours after the animal was exposed to stress. Insets, Representative IPSCs evoked before (Control) and 15 min after (SNAP) drug application. C, Single experiment illustrating the recovery of SNAP-induced LTPGABA in a slice from an animal treated with RU486 prior to stress. D, Averaged experiments show that SNAP potentiation of IPSCs is abolished in slices from animals exposed to acute stress (n = 8), whereas SNAP potentiates IPSCs in animals pretreated with RU486 (n = 8). Statistical analysis revealed significant effects of drug treatment (F1,39 = 119.0, p < 0.001), time (F1,39 = 2.37, p < 0.001) and an interaction between these two factors (F1,39 = 2.38, p < 0.001). Scale bars: 10 ms, 50 pA.
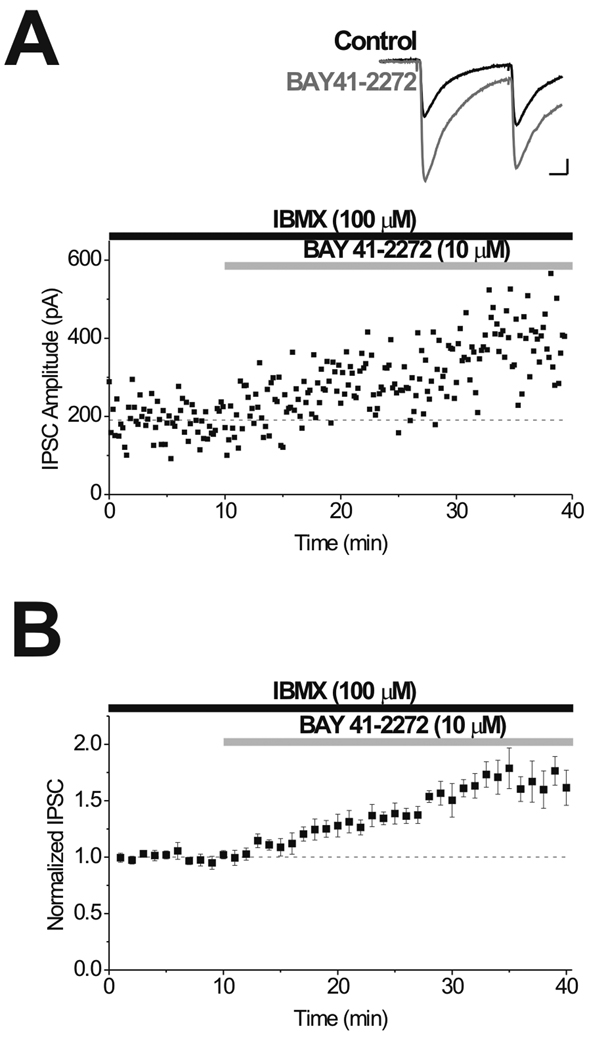
Figure 6. Activation of sGC by BAY 41-2272 induces LTPGABA in slices from drug-naive animals.
A, Single experiment illustrating potentiation of IPSCs by application of 10 µM BAY 41-2272 to induce LTPGABA. Inset, Representative IPSCs evoked before (Control) and 15 min after (BAY 41-2272) drug application. B, Averaged experiments showing induction of LTPGABA by BAY 41-2272 (n = 8). One-way ANOVA revealed a significant effect of SNAP over time (p < 0.001). Scale bars: 10 ms, 50 pA.
Results
LTPGABA, a potentiation of inhibitory GABAergic synapses onto dopamine neurons in the VTA, requires NO as a retrograde messenger to activate presynaptic soluble guanylate cyclase (sGC). Cyclic GMP (cGMP) produced by sGC then activates cGMP-dependent protein kinase (PKG), resulting in the observed persistent enhancement of GABA release (Nugent et al., 2007; Nugent et al., 2009). LTPGABA can be elicited by synaptic stimulation or alternatively by direct bath application of an NO donor (Nugent et al., 2007; Nugent et al., 2009). The maintenance of LTPGABA does not require the persistent presence of NO, as an NO scavenger fails to reverse the potentiation (Nugent et al., 2009). In agreement with our previous studies, bath-applied SNAP, an NO donor, induced LTPGABA in midbrain slices prepared from saline-treated animals (Supplementary Fig. S1); however, in brain slices prepared from animals 24 hours after a single in vivo morphine exposure, SNAP was unable to potentiate GABA IPSCs and induce LTPGABA (Supplementary Fig. S1).
Time course of morphine blockade of LTPGABA
How soon after morphine exposure is LTPGABA impaired and how long does this blockade persist? A more detailed understanding of the process by which morphine alters LTPGABA could provide valuable information about the effects of morphine on the VTA and the brain reward circuit. We previously found that acute, in vitro application of μ-opioids had no effect on SNAP-induced LTPGABA whereas 24 hours after in vivo exposure to morphine LTPGABA was completely blocked (Nugent et al., 2007). We therefore next tested the effect of a single in vivo injection of morphine at an early time point, preparing brain slices 2 hours following drug or saline exposure. SNAP potentiated GABA IPSCs in slices from saline-treated animals; however, the SNAP-induced potentiation was already significantly reduced in slices from morphine-treated animals even 2 hours after morphine (Fig. 1B). We next asked whether LTPGABA remained impaired 5 days following a single morphine injection. Animals were injected once with either morphine or saline and brain slices were prepared 5 days following the single injection. In both groups, SNAP potentiated GABA IPSCs (Fig. 1C) indicating that although the LTPGABA blockade by morphine was observed at 2 or 24 hours after morphine, the blockade persisted for less than 5 days. The temporal window during which LTPGABA is altered by morphine exposure appears to take place between 2 hours and 5 days following drug administration.
Cocaine and nicotine impair LTPGABA at different times
A single administration of several diverse addictive drugs increases the strength of excitatory synapses on VTA dopamine neurons, increasing the AMPA/NMDA ratio (Saal et al., 2003). Is morphine unique in obstructing LTPGABA or do other addictive drugs similarly alter inhibitory synaptic plasticity in the VTA? Based on their differing mechanisms of action from that of morphine, we chose to examine the effects of cocaine, a psychostimulant that enhances brain dopamine levels by blocking reuptake, and nicotine, which acts on nicotinic acetylcholine receptors (nAChRs) to increase dopamine neuron excitability. In vivo administration of cocaine impaired (but did not prevent) the expression of LTPGABA in brain slices prepared 24 hours after cocaine injection (Fig. 2A–C). Since LTPGABA was not completely blocked 24 hours after cocaine exposure, we tested whether cocaine might have a greater impact on LTPGABA at 2 hours after administration. However, cocaine did not significantly impair the expression of LTPGABA 2 hours after exposure as compared to saline-injected controls (Fig. 2D–F). In contrast to the significant attenuation observed 24 hours after cocaine, the induction and persistence of LTPGABA were unaffected by in vivo exposure to nicotine 24 hours after administration (Fig. 3A–C). To determine whether there was a time-dependent effect of nicotine, we tested the effect of nicotine on LTPGABA at 2 hours after administration. Surprisingly, two hours after exposure, LTPGABA was impaired in nicotine-treated animals as compared to saline-treated controls (Fig 3D–F). Taken together, the reduction in inhibitory plasticity induced by morphine, cocaine and nicotine, drugs with different mechanisms of action, demonstrates that drugs of abuse that potentiate excitatory synapses can also inhibit plasticity at inhibitory synapses. However, drugs of abuse do not necessarily impair LTPGABA at the same time point after a single administration.
Figure 2. A single in vivo administration of cocaine reduces LTPGABA 24 hours after exposure, but not at 2 hours.
A, Single experiment illustrating LTPGABA in a brain slice prepared 24 hours after saline-treatment. Insets, Representative IPSCs evoked before (Control) and 15 min after (SNAP) drug application. In this and all subsequent figures, ten IPSCs were averaged for illustration. B, Single experiment illustrating impaired ability of SNAP to induce LTPGABA in a slice from an animal injected with cocaine 24 hours earlier. C, At 24 hours after exposure, averaged experiments show SNAP potentiation of IPSCs in slices from cocaine-treated animals (n = 10) is reduced compared to those from saline-treated animals (n = 10). Two-way ANOVA analysis revealed significant effects of drug treatment (F1,39 = 99.2, p < 0.001), time (F1,39 = 10.2, p < 0.001) and an interaction between these two factors (F1,39 = 1.85, p < 0.001). D, Single experiment illustrating LTPGABA in a brain slice prepared 2 hours after saline-treatment. E, Single experiment illustrating LTPGABA in a slice from an animal injected with cocaine 2 hours earlier. F, At 2 hours after exposure, averaged experiments show no significant difference in SNAP-induced potentiation of IPSCs in slices from cocaine-treated animals (n = 9) compared to saline-treated animals (n = 7). There were significant effects of drug treatment (F1,39 = 24.8, p < 0.001) and time (F1,39 = 3.70, p < 0.001), but not a significant interaction between these two factors (F1,39 = 0.50, p > 0.05). Scale bars: 10 ms, 50 pA.
Stress blocks LTPGABA similarly to drugs of abuse
Like addictive drugs, acute stress also potently potentiates glutamatergic synapses onto VTA dopamine neurons (Saal et al., 2003). At the behavioral level, reinstatement of drug-taking can be triggered by a stressor, by a single episode of drug administration, or by environmental cues associated with prior drug taking or stress (Piazza & Le Moal, 1998; Shaham et al., 2000), suggesting a possible link between stress/drug effects on VTA synapses and stress/drug effects on relapse. Since morphine, cocaine and stress all potentiate excitatory synapses, we next investigated the effect of an acute stress on plasticity of inhibitory synapses on VTA dopamine neurons. Indeed, a modified Porsolt forced swim task administered 24 hours prior to brain slice preparation completely blocked the ability of SNAP to induce LTPGABA (Fig. 4B,D). As previously demonstrated by Saal and colleagues, the potentiation of excitatory synapses by stress depends on glucocorticoid receptor activation and was completely blocked by pretreatment with the glucocorticoid antagonist RU486 (Saal et al., 2003). Similarly, we found that RU486 administered 30 minutes prior to the swim task entirely prevented the effect of stress on LTPGABA, as SNAP effectively potentiated GABAergic IPSCs and induced LTPGABA (Fig. 4C,D). These data indicate that the stress-induced blockade of inhibitory synaptic plasticity occurs through the glucocorticoid receptor pathway, and that both brief stress and a single exposure to drugs of abuse can impair inhibitory synaptic plasticity at VTA dopamine neurons.
Morphine blockade of LTPGABA is independent of the stress pathway
Do morphine and acute stress block LTPGABA via a common mechanism? To determine whether morphine might be acting through an acute stress pathway, RU486 was administered 30 minutes prior to morphine injection. In brain slices prepared 24 hours later, bath-application of SNAP failed to potentiate GABAergic IPSCs (Fig. 5). This result shows that the glucocorticoid receptor antagonist cannot rescue impairment of inhibitory plasticity, suggesting that the morphine blockade of LTPGABA is likely independent of the effects of stress.
Soluble guanylate cyclase activator overcomes morphine blockade of LTPGABA
We next examined in more detail the locus of morphine’s action on GABAergic nerve terminals. In vivo morphine exposure appears to prevent LTPGABA by disrupting the signaling cascade between NO generation and cGMP generation (Nugent et al., 2007). Normally, NO binds to the heme moiety of soluble guanylate cyclase leading to activation and production of cGMP. To determine whether in vivo exposure to morphine might be affecting the guanylate cyclase, we used a sGC activator, BAY 41-2272, which activates sGC independently of NO (Stasch et al., 2001). First, BAY 41-2272 was bath-applied to directly stimulate sGC in VTA brain slices in order to test whether chemical activation of guanylate cyclase by this drug could produce LTPGABA as expected. BAY 41-2272 increased the amplitude of GABAergic IPSCs (Fig. 6) in naïve brain slices through an increase in cGMP levels. Thus, BAY 41-2272 mimicked synaptic- or SNAP-induced LTPGABA, and confirmed the requirement of cGMP in the signaling cascade.
Morphine most likely interferes with LTPGABA downstream of NO generation and upstream of cGMP production, as an NO donor is unable to potentiate synapses in slices from morphine treated animals whereas a cGMP analog induces LTPGABA (Nugent et al., 2007). If morphine prevents NO from activating sGC, BAY 41-2272 should potentiate GABAergic transmission by directly activating sGC. In agreement with this idea, BAY 41-2272 not only potentiated GABA IPSCs from saline-treated animals (Fig. 7A,C), but also potentiated GABA IPSCs from morphine-treated animals (Fig. 7B,C). The ability of BAY 41-2272 to induce LTPGABA in slices from morphine-treated animals indicates that sGC is still present at significant levels in VTA inhibitory synapses and is capable of generating cGMP that can overcome the upstream block of the signaling cascade and trigger LTPGABA.
Presynaptic protein RIM1α is not necessary for LTPGABA
The expression of LTPGABA occurs via a sustained increase in GABA release after initial activation of the NO-cGMP-PKG pathway. Since RIM1α has been linked to other forms of synaptic plasticity, we considered the possibility that the active zone protein RIM1α is necessary for LTPGABA by observing LTPGABA in RIM1α-deficient mice. RIM1α knockout mice have decreased neurotransmitter release at GABAergic synapses in the hippocampus during paired stimulation (Schoch et al., 2002) and mossy fibre LTP, a presynaptic form of LTP, is abolished in RIM1α−/− mice (Castillo et al., 2002). Furthermore, LTP at GABAergic synapses in the cerebellum requires RIM1α for the sustained increase in GABA release (Lachamp 2009). However, at GABAergic synapses on VTA dopamine cells, SNAP induced LTPGABA in both RIM1α-deficient mice (137.4 ± 10.3 % of baseline, n = 8) and wild-type littermates (138.5 ± 5.7 % of baseline, n = 5) indicating that unlike other presynaptic forms of plasticity RIM1α is not necessary for expression of LTPGABA. Two-way ANOVA revealed significant effects of time (F1,39 = 7.94, p < 0.001) and genotype (F1,39 = 11.59, p < 0.001), but no significant interaction between the two factors (F1,39 = 0.78, p > 0.05).
Discussion
Our data demonstrate that morphine, cocaine and nicotine all cause a loss of LTP at inhibitory synapses onto dopamine neurons in the VTA (Fig. 8). Different drugs of abuse were previously shown to enhance excitatory synaptic strength onto VTA dopamine neurons (White et al., 1995; Zhang et al., 1997; Ungless et al., 2001; Saal et al., 2003; Faleiro et al., 2004; Borgland et al., 2004). Our results suggest that drug-induced changes in inhibitory synaptic plasticity in the VTA also represent a significant neuroadaptation in the brain reward circuit. Remarkably, acute stress blocked LTPGABA in a manner similar to that caused by morphine, cocaine and nicotine (Fig. 8), again in parallel with previous results at excitatory synapses (Saal et al., 2003). The potentiation of excitatory synapses along with the loss of LTPGABA at GABAergic synapses will act in concert to increase dopamine cell excitability. Taken together, these observations suggest that the effects of drugs of abuse and stress on both excitatory and inhibitory synapses in the VTA are intertwined, resulting in adaptations in reward circuitry that could contribute not only to reward learning but perhaps to drug seeking, use and relapse.
Figure 8. Proposed model of LTPGABA signaling cascade.
Drugs of abuse and stress alter presynaptic GABA release onto VTA dopamine neurons. In vivo injection of morphine and nicotine impairs LTPGABA at 2 hours after drug administration, while cocaine does not. LTPGABA is impaired at 24 hours following morphine and cocaine injection, but not after nicotine. LTPGABA appears to be normal 5 days after morphine exposure. Acute stress potently blocked LTPGABA at the 24 hour time point. The relevant glucocorticoid receptors may be located in the presynaptic neuron as drawn, but may also be located in the postsynaptic dopamine neuron.
Time course of drug induced block of LTPGABA
Previously we found that SNAP-induced LTPGABA was blocked 24 hours after in vivo administration of morphine, but was completely unaffected by an acute in vitro application (Nugent et al., 2007). How quickly after in vivo treatment with drugs of abuse is LTPGABA blocked? As early as 2 hours after morphine injection, the magnitude of LTPGABA was reduced compared to saline controls. Five days after a single morphine injection, the magnitude of LTPGABA was no longer significantly different from saline injected controls, indicating that morphine initiates a synaptic change that is evident within 2 hours, persists for 24 hours, but is reversed within 5 days. Interestingly, LTPGABA was not impaired 2 hours after cocaine exposure, suggesting that the effect of cocaine is time-dependent and requires more than 2 hours to impact the expression of LTPGABA. Even after 24 hours, cocaine did not entirely block LTPGABA, although we found a significant attenuation. This raises the possibility that psychostimulants may not affect LTPGABA as much as morphine or nicotine, or alternatively that the block of LTPGABA by cocaine peaks either at a time between 2 and 24 hours, or later than 24 hours. Cocaine has been reported to decrease GABAergic input onto VTA dopamine neurons following repeated cocaine exposure, by Liu and colleagues, and a decrease in maximal IPSC amplitude was not detected until 5 days of cocaine treatment, although the authors determined that the cocaine-induced reduction was postsynaptic (Liu et al., 2005). Thus, cocaine reduces GABAergic transmission in the VTA by at least two distinct mechanisms.
In vivo administration of nicotine impaired LTPGABA 2 hours after administration, but did not result in persistent blockade of LTPGABA when examined 24 hours after initial exposure. The distribution of pharmacologically distinct nicotinic acetylcholine receptors (nAChRs) in the VTA might partially explain the time dependent effect of nicotine on GABAergic plasticity. Glutamatergic synapses onto VTA dopamine neurons express α7-containing nAChRs that are resistant to desensitization (Pidoplichko et al., 1997; Wooltorton et al., 2003) whereas non α7-containing nAChRs present on GABAergic nerve terminals rapidly desensitize in the presence of nicotine (Mansvelder et al., 2002). The observed potentiation of glutamatergic synapses 24 hours following in vivo exposure to nicotine may involve α7-containing nAChRs, while LTPGABA might be affected by the desensitizing non α7-containing nAChRs present at GABAergic synapses.
Acute stress blocks LTPGABA
We demonstrated that acute stress potently blocks LTPGABA at synapses on VTA dopamine neurons. Importantly, a glucocorticoid receptor antagonist prevented the blockade of LTPGABA by acute stress, but had no effect on morphine-induced blockade of LTPGABA. These results indicate that the effect of morphine on GABAergic plasticity was not simply due to stress associated with drug administration and that while drugs of abuse and acute stress produce a similar effect on LTPGABA, the mechanisms leading to the reduction in GABAergic plasticity are distinct. As observed at excitatory synapses (Saal et al., 2003; Dong et al., 2004), drugs of abuse and stress act in a similar manner to converge in a reduction of GABAergic transmission. Reduced plasticity of GABAergic synapses impairs the ability to persistently increase GABA release and is permissive for increased excitation of a dopamine neuron by excitatory synapses or other neuromodulators. During drug withdrawal, a coordinated increase in excitatory synaptic strength and impairment of inhibitory synaptic plasticity might contribute to stress-induced relapse as a result of increased dopamine neuron firing rate and dopamine release in VTA projection areas. Infusion of GABA agonists into the VTA or infusion of D1/D2 receptor antagonists into the dorsal prefrontal cortex both prevent footshock-induced reinstatement of cocaine-seeking behavior (McFarland et al., 2004) suggesting that dopamine released by afferents from the VTA projecting to the prefrontal cortex is necessary for stress-induced relapse. McFarland and colleagues hypothesized that footshock stress activates the central extended amygdala, which subsequently activates motor circuitry via dopaminergic input to the prefrontal cortex. The increased dopamine concentration in the prefrontal cortex initiates reinstatement via glutamatergic projections to the nucleus accumbens core (Yap & Miczek, 2008). One effect of stressful stimuli is to increase the release of corticotropin-releasing factor (CRF) in the brain, which triggers glutamate release in the VTA and in turn dopamine release (Wang et al., 2005). In cocaine-experienced animals, footshock stress causes release of CRF in the VTA, triggering local glutamate release and subsequent dopamine release to reinstate cocaine-seeking behavior (Wise & Morales, 2010). Activation of the CRF receptor, CRF-R1, increases the firing rate of VTA dopamine neurons (Korotkova et al., 2006; Wanat et al., 2008) leading to an increase in dopamine release in brain regions that are innervated by the VTA (Inglis & Moghaddam, 1999; McFarland et al., 2004). The increase in dopamine neuron firing frequency occurs via an enhancement of the hyperpolarization activated cation current, Ih (Wanat et al., 2008); however, a loss of potentiation of GABAergic synapses on dopamine neurons would be expected to further facilitate an increase in firing rate. Stress or CRF infusions can reinstate drug-seeking in animals that previously had extinguished this behavior, while CRF antagonists have some efficacy in reducing drug seeking behavior following stress (Sarnyai et al., 2001). Although CRF does not appear to be involved in drug-primed reinstatement (Sarnyai et al., 2001), the neuronal adaptations induced by exposure to drugs of abuse may increase the probability of relapse triggered by environmental stress.
Mechanism of morphine-induced block of LTPGABA
Soluble guanylate cyclase is a critical component of the LTPGABA signaling cascade and a possible target of the events initiated by exposure to drugs of abuse. When sGC was inhibited by ODQ, neither synaptic stimulation nor exogenous NO donors were able to induce LTPGABA; however, a cGMP analog induced LTPGABA by circumventing sGC generation of cGMP (Nugent et al., 2007). Morphine-induced blockade of LTPGABA appears to occur at the level of sGC activation because an NO donor is unable to initiate LTPGABA whereas the sGC activator BAY 41-2272 produced LTPGABA. Since BAY 41-2272 requires the reduced prosthetic heme group of sGC (Stasch et al., 2001), our results show that in vivo treatment of morphine does not prevent LTPGABA by oxidation or removal of the heme group in sGC, as BAY 41-2272 would be ineffective if the heme group was oxidized or absent. We conclude that sGC is still capable of producing cGMP in morphine-treated animals, but through an incompletely understood mechanism, sGC is not sufficiently activated by NO to generate levels of cGMP necessary to potentiate GABAergic transmission. We do not yet know whether morphine has a direct or indirect effect on sGC to diminish the capacity of NO to activate the cyclase. The block of LTPGABA was observed within 2 hours after morphine injection, and this time course limits the possible mechanisms of morphine’s action. Post-translational modification of sGC or an associated protein appears most likely. It will be interesting to determine whether nicotine, cocaine, and stress also act on sGC to block the potentiation of LTPGABA. If all three drugs and stress interfere with sGC function, this might suggest that they all act on a common intermediate.
The NO-cGMP-sGC-PKG signaling pathway, while more extensively studied in the cardiovascular system, provides important functions in the nervous system, including effects on synaptic plasticity (Kleppisch & Feil, 2009). NO and cGMP are known modulators of neurotransmitter release in the hippocampus, cerebellum, cortex and striatum (Prast and Philippu, 2001) although the molecular mechanisms are varied and not completely understood. Presynaptic neurotransmitter release could be regulated by cGMP through effects on vesicle docking, fusion and recycling (Kleppisch and Feil, 2009). Recent data indicate that PKG is required for induction of LTPGABA, but not for its maintenance (Nugent et al., 2009). Based on its involvement in other forms of presynaptically-maintained plasticity, the possible role of RIM1α in LTPGABA was investigated. In the cerebellum, a form of LTP expressed by GABAergic interneurons via a presynaptic cAMP/PKA signaling pathway was abolished in RIM1α knockout mice (Lachamp et al., 2009). However, in the VTA we found that RIM1α is not necessary for induction or maintenance of LTPGABA. Although activation of PKA and PKG both potentiate GABAergic IPSCs (Melis et al., 2002; Nugent et al., 2009), our results indicate the kinases do not converge upon RIM1α as a common cellular mechanism to increase GABA transmission. Further investigation is needed to determine the downstream molecular targets of NO-sGC-cGMP signaling in VTA dopamine neurons and potential role of these signaling components in the brain reward circuit.
Comparison of the temporal effects of drugs on excitatory and inhibitory synapses
At excitatory synapses, in vivo cocaine or amphetamine enhanced the AMPA/NMDA ratio in brain slices prepared 2 hours after exposure and this increase was of similar magnitude to that observed 24 hours after cocaine (Faleiro et al., 2004; Argilli et al., 2008). Nicotine and morphine both enhanced the AMPA/NMDA ratio at 24 hours, and other time points have not yet been examined. It is intriguing that psychostimulants, morphine and nicotine all potentiate glutamatergic synapses 24 hours later (Saal et al., 2003), while the effects of nicotine and cocaine on inhibitory synapses appear strongly dependent on the temporal window examined. An intriguing question that remains unresolved is whether repeated drug administration would prolong the impairment of LTPGABA. At excitatory synapses onto VTA dopamine neurons, daily cocaine administration over a period of 7 days still caused potentiation, but the magnitude of the increase was similar to that of a single injection of cocaine (Borgland et al., 2004). Furthermore, the increased AMPA/NMDA ratio induced by repeated cocaine administration persisted for at least 5 days following the final cocaine injection, but not 10 days, (a time course seen after a single cocaine administration (Ungless et al., 2001), suggesting that repeated injections enhanced neither the extent nor the duration of the potentiation (Borgland et al., 2004). A more recent study found that even an escalating dose of cocaine over 14 days does not further elevate the AMPA/NMDA ratio (Chen et al., 2008). These findings suggest that a single injection of cocaine saturates plasticity at excitatory synapses and repeated drug administration has no additive effect on AMPA/NMDA ratio (Chen et al., 2008; Mameli et al., 2009). If our results parallel those found at excitatory synapses then LTPGABA may remain impaired following chronic drug administration. Alternatively, it is possible that the transient impairment of LTPGABA may be unaffected by repeated drug administration, perhaps due to a compensatory mechanism involved in restoring inhibitory synaptic plasticity following a single exposure to morphine (Fig. 1). At excitatory synapses in the VTA, induction of a form of LTD mediated by type 1 metabotropic glutamate receptors reverses cocaine-evoked elevation of the AMPA/NMDA ratio (Bellone & Luscher, 2006; Mameli et al., 2009). A parallel to this endogenous reversal of cocaine-induced potentiation might exist at inhibitory synapses to restore normal LTPGABA function.
It is appealing to speculate that addictive drugs might begin to seize control of reward circuits by simultaneously enhancing excitation and reducing inhibition in the local VTA circuit. Perhaps the robust neuroadaptations that occur in response to drugs of abuse and stress are related to the combined ability to alter both excitatory and inhibitory synapses. It is striking that even a single exposure to addictive drugs, or to a stressful stimulus, is sufficient to catapult the local VTA circuit into a different state that persists for several days. The effects must involve the majority of fast synapses in the VTA, since in the randomly sampled dopamine neurons in our studies and those of others, striking synaptic alterations are observed. One puzzling issue is how different drugs (and stressful stimuli) with entirely non-overlapping mechanisms of action converge to both potentiate excitatory synapses and inhibit LTPGABA over a period of hours after administration. This observation suggests that drugs or stress may act on a common substrate that in turn influences synaptic strength beginning within hours after the stimulus, and lasting as long as several days (Nestler, 2005). Several such intermediates have been suggested. As mentioned above, peptides such as CRF and orexins influence synaptic function in the VTA and interact with behavioral stress and with drugs (Bonci & Borgland, 2009). The involvement of transcription factors and growth factors (e.g. bFGF, BDNF, Δ FosB, and CREB) has also been proposed in recent years, and these potent regulators of cell physiology and structure might reasonably play the role of intermediates (Forget et al., 2006; Russo et al., 2009). Our present findings strengthen the argument that a common mediator may exist and provide clues to the time course of activation of such a mediator, at least in the VTA. Moreover, if LTPGABA is controlled by multiple addictive drugs and stress, members of the LTPGABA signaling cascade may represent valuable targets for therapeutic intervention to treat drug addiction.
Supplementary Material
Figure S1. A single in vivo administration of morphine impairs GABAergic synaptic plasticity onto VTA dopamine neurons 24 hours later. A, Single experiment illustrating LTPGABA induced by application of 400 µM SNAP onto a brain slice prepared from a saline-treated animal. Insets, Representative GABAA receptor-mediated IPSCs evoked before (Control) and 15 min after (SNAP) drug application. B, Single experiment illustrating inability of SNAP to induce LTPGABA in slice prepared from an animal injected with morphine 24 hours earlier. C, Averaged experiments show that SNAP potentiates IPSCs in slices from saline-treated animals (n = 10), but is ineffective in slices from morphine-treated animals (n = 12). Analysis using two-way ANOVA revealed significant effects of drug treatment (F1,39 = 118.8, p < 0.001), time (F1,39 = 6.18, p < 0.001) and an interaction between these two factors (F1,39 = 1.85, p < 0.01). Scale bars: 10 ms, 50 pA.
Acknowledgements
This work was supported by NIH grants from the National Institute on Drug Abuse to JAK (DA011289) and JLN (DA024527). We thank laboratory members for helpful discussion and suggestions.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- cGMP
cyclic GMP
- IPSC
inhibitory postsynaptic current
- LTP
long-term potentiation
- nAChR
nicotinic acetylcholine receptor
- NO
nitric oxide
- PKG
cGMP-dependent protein kinase
- sGC
soluble guanylate cyclase
- SNAP
S-nitroso-N-acetylpenicillamine
- VTA
ventral tegmental area
References
- Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J. Neurosci. 2008;28:9092–9100. doi: 10.1523/JNEUROSCI.1001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- Bonci A, Borgland S. Role of orexin/hypocretin and CRF in the formation of drug-dependent synaptic plasticity in the mesolimbic system. Neuropharmacology. 2009;56 Suppl 1:107–111. doi: 10.1016/j.neuropharm.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J. Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC. RIM1alpha is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Chou JK, Bonci A. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien WL, Liang KC, Teng CM, Kuo SC, Lee FY, Fu WM. Enhancement of learning behaviour by a potent nitric oxide-guanylate cyclase activator YC-1. Eur. J. Neurosci. 2005;21:1679–1688. doi: 10.1111/j.1460-9568.2005.03993.x. [DOI] [PubMed] [Google Scholar]
- Dong Y, Saal D, Thomas M, Faust R, Bonci A, Robinson T, Malenka RC. Cocaine-induced potentiation of synaptic strength in dopamine neurons: behavioral correlates in GluRA(−/−) mice. Proc. Natl Acad. Sci. USA. 2004;101:14282–14287. doi: 10.1073/pnas.0401553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engblom D, Bilbao A, Sanchis-Segura C, Dahan L, Perreau-Lenz S, Balland B, Parkitna JR, Lujan R, Halbout B, Mameli M, Parlato R, Sprengel R, Luscher C, Schutz G, Spanagel R. Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron. 2008;59:497–508. doi: 10.1016/j.neuron.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Faleiro LJ, Jones S, Kauer JA. Rapid synaptic plasticity of glutamatergic synapses on dopamine neurons in the ventral tegmental area in response to acute amphetamine injection. Neuropsychopharmacology. 2004;29:2115–2125. doi: 10.1038/sj.npp.1300495. [DOI] [PubMed] [Google Scholar]
- Floran B, Barajas C, Floran L, Erlij D, Aceves J. Adenosine A1 receptors control dopamine D1-dependent [(3)H]GABA release in slices of substantia nigra pars reticulata and motor behavior in the rat. Neuroscience. 2002;115:743–751. doi: 10.1016/s0306-4522(02)00479-7. [DOI] [PubMed] [Google Scholar]
- Forget C, Stewart J, Trudeau LE. Impact of basic FGF expression in astrocytes on dopamine neuron synaptic function and development. Eur. J. Neurosci. 2006;23:608–616. doi: 10.1111/j.1460-9568.2006.04570.x. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Moghaddam B. Dopaminergic innervation of the amygdala is highly responsive to stress. J. Neurochem. 1999;72:1088–1094. doi: 10.1046/j.1471-4159.1999.0721088.x. [DOI] [PubMed] [Google Scholar]
- Jones S, Kornblum JL, Kauer JA. Amphetamine blocks long-term synaptic depression in the ventral tegmental area. J. Neurosci. 2000;20:5575–5580. doi: 10.1523/JNEUROSCI.20-15-05575.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nature reviews. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Foy MR, Thompson RF. Behavioral stress modifies hippocampal plasticity through N-methyl-D-aspartate receptor activation. Proc. Natl Acad. Sci. USA. 1996;93:4750–4753. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleppisch T, Feil R. cGMP signalling in the mammalian brain: role in synaptic plasticity and behaviour. Handb. Exp. Pharmacol. 2009:549–579. doi: 10.1007/978-3-540-68964-5_24. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur. J. Neurosci. 2006;23:2677–2685. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- Lachamp PM, Liu Y, Liu SJ. Glutamatergic modulation of cerebellar interneuron activity is mediated by an enhancement of GABA release and requires protein kinase A/RIM1alpha signaling. J Neurosci. 2009;29:381–392. doi: 10.1523/JNEUROSCI.2354-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Gallegos RA, Henriksen SJ, van der Kooy D. Opiate state controls bi-directional reward signaling via GABAA receptors in the ventral tegmental area. Nat. Neurosci. 2004;7:160–169. doi: 10.1038/nn1182. [DOI] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Luscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat. Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J. Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J. Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Camarini R, Ungless MA, Bonci A. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J. Neurosci. 2002;22:2074–2082. doi: 10.1523/JNEUROSCI.22-06-02074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE., 3rd Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol. Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat. Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Niehaus JL, Cruz-Bermudez ND, Kauer JA. Plasticity of addiction: a mesolimbic dopamine short-circuit? Am. J. Addict. 2009;18:259–271. doi: 10.1080/10550490902925946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent FS, Niehaus JL, Kauer JA. PKG and PKA signaling in LTP at GABAergic synapses. Neuropsychopharmacology. 2009;34:1829–1842. doi: 10.1038/npp.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;446:1086–1090. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol. Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog. Neurobiol. 2001;64:51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology. 2009;56 Suppl 1:73–82. doi: 10.1016/j.neuropharm.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol. Rev. 2001;53:209–243. [PubMed] [Google Scholar]
- Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, Schmitz F, Malenka RC, Sudhof TC. RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002;415:321–326. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res. Brain Res. Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Seib TB, Levine S, Thompson RF. Inescapable versus escapable shock modulates long-term potentiation in the rat hippocampus. Science. 1989;244:224–226. doi: 10.1126/science.2704997. [DOI] [PubMed] [Google Scholar]
- Stasch JP, Becker EM, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, Gerzer R, Minuth T, Perzborn E, Pleiss U, Schroder H, Schroeder W, Stahl E, Steinke W, Straub A, Schramm M. NO-independent regulatory site on soluble guanylate cyclase. Nature. 2001;410:212–215. doi: 10.1038/35065611. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J. Physiol. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FJ, Hu XT, Zhang XF, Wolf ME. Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. J. Pharmacol. Exp. Ther. 1995;273:445–454. [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr. Opin. Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Wise RA, Morales M. A ventral tegmental CRF-glutamate-dopamine interaction in addiction. Brain Res. 2010;1314:38–43. doi: 10.1016/j.brainres.2009.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23:3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Anwyl R, Rowan MJ. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387:497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- Yap JJ, Miczek KA. Stress and Rodent Models of Drug Addiction: Role of VTA-Accumbens-PFC-Amygdala Circuit. Drug Discov. Today Dis. Models. 2008;5:259–270. doi: 10.1016/j.ddmod.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ, Wolf ME. Increased responsiveness of ventral tegmental area dopamine neurons to glutamate after repeated administration of cocaine or amphetamine is transient and selectively involves AMPA receptors. J. Pharmacol. Exp. Ther. 1997;281:699–706. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. A single in vivo administration of morphine impairs GABAergic synaptic plasticity onto VTA dopamine neurons 24 hours later. A, Single experiment illustrating LTPGABA induced by application of 400 µM SNAP onto a brain slice prepared from a saline-treated animal. Insets, Representative GABAA receptor-mediated IPSCs evoked before (Control) and 15 min after (SNAP) drug application. B, Single experiment illustrating inability of SNAP to induce LTPGABA in slice prepared from an animal injected with morphine 24 hours earlier. C, Averaged experiments show that SNAP potentiates IPSCs in slices from saline-treated animals (n = 10), but is ineffective in slices from morphine-treated animals (n = 12). Analysis using two-way ANOVA revealed significant effects of drug treatment (F1,39 = 118.8, p < 0.001), time (F1,39 = 6.18, p < 0.001) and an interaction between these two factors (F1,39 = 1.85, p < 0.01). Scale bars: 10 ms, 50 pA.