Abstract
Purpose
Polymorphisms in factor H (fH), an inhibitor of the alternative pathway (AP) of complement activation, are associated with increased risk for age-related macular degeneration (AMD). The authors investigated the therapeutic use of a novel recombinant form of fH, CR2-fH, which is targeted to sites of complement activation, in mouse choroidal neovascularization (CNV). CR2-fH consists of the N terminus of mouse fH, which contains the AP-inhibitory domain, linked to a complement receptor 2 (CR2) targeting fragment that binds complement activation products.
Methods
Laser-induced CNV was analyzed in factor-B–deficient mice or in mice treated with CR2-fH, soluble CR2 (targeting domain), or PBS. CNV progression was analyzed by molecular, histologic, and electrophysiological readouts.
Results
Intravenously administered CR2-fH reduced CNV size, preserved retina function, and abrogated the injury-associated expression of C3 and VEGF mRNA. CR2 and PBS treatment was without effect. In therapeutically relevant paradigms involving delayed treatment after injury, CR2-fH was effective in reducing CNV and provided approximately 60% of the amount of protection of that seen in factor B–deficient mice that lacked functional AP. After intravenous injection, CR2-fH localized to sites of C3 deposition in RPE-choroid.
Conclusions
Specific inhibition of the AP reduces angiogenesis in mouse CNV. Of note, intravenous injection of C3d-targeted CR2-fH is protective even though endogenous fH is present in serum at a higher relative concentration, and serum fH contains native C3d and cell surface binding domains that target it to cell surfaces. The most common AMD-associated variant of fH resides within a native cell-binding region of fH (Tyr402His). These data may open new avenues for AMD treatment strategies.
Age-related macular degeneration (AMD) occurs in two forms, dry and wet, and is the leading cause of blindness in Americans 60 years of age and older. Dry AMD, the more prevalent form, leads to slow photoreceptor degeneration in the macula. Wet AMD accounts for most cases with severe vision loss because it is associated with neovascularization, leakage of new vessels, acute hemorrhage, and rapid photoreceptor degeneration. Studies on the pathogenesis of AMD indicate that inflammation is a fundamental component of the disease process and that the alternative pathway (AP) of complement plays a critical role in driving the inflammatory response. Genetic evidence has identified variations in the complement inhibitory protein factor H (fH)1–5 and in the complement activation-proteins factor B (fB), C2, and C36,7 as major risk factors for AMD. The most prevalent polymorphism in fH, a mutation at position 402 (Y402H), is associated with a 48% risk for AMD by 95 years of age for homozygotes compared with a 22% risk for noncarriers.8 In addition, several noncoding regions in fH have been identified that contribute to disease susceptibility.5
The alternative pathway is 1 of 3 complement activation pathways (the others are the classical and lectin pathways) that share a common terminal pathway. Although the AP can be activated spontaneously on foreign surfaces, it also serves an important function as an amplification loop for the other two pathways through formation of the AP C3 convertase, an enzymatic complex that cleaves C3 into C3a and C3b. The C3b fragment can initiate the formation of additional AP C3 convertase after the binding and cleavage of factor B. Factor H is a fluid-phase inhibitor of the AP present in human and rodent sera at concentrations of approximately 500 μg/mL. It associates with C3b and interferes with the formation and stability of the AP C3 convertase, and it functions as a cofactor for factor I that mediates cleavage and inactivation of C3b. The protein is composed of 20 repetitive units of 60 amino acids called short consensus repeats (SCRs), which make up functional domains in fH. Factor H has binding sites for C3b/C3d (SCR1–4, SCR12–14, SCR19–20), heparin (SCR7, SCR13, SCR19–20), sialic acid and glycosaminoglycans (SCR19–20, SCR7), and C-reactive protein (SCR7). The C3b binding site in SCR1–4 is essential for its factor I cofactor activity, and the C3b/C3d/polyanion-binding site in SCR19–20 is important for surface binding and cell surface regulation (for a review, see Ref. 9). Y402H in fH lies at the center of a major binding site in SCR7.10 It has been suggested that this mutation may alter the interaction of fH with target surface polyanions, particularly glycosaminoglycan heparin-sulfate,10,11 and may reduce its binding affinity to surface-attached C-reactive protein,12 although recent results argue this to be an experimental artifact.13 Mutations in the noncoding regions of fH associated with AMD may modulate expression levels, as may other risk factors correlated with fH mutations.
There remains a significant need for novel therapeutic approaches to treat AMD. Here, we characterize a novel, targeted inhibitor of the AP in a mouse model of choroidal neovascularization (CNV). The inhibitor, CR2-fH, comprises a targeting domain (SCR1–4 fragment of complement receptor 2 [CR2]) and a complement inhibitory domain (SCR1–5 fragment of fH). The CR2 targeting moiety binds iC3b, C3dg, and C3d, cell-bound cleavage fragments of C3 present at sites of complement activation.14 The same CR2 targeting domain has been used previously to target Crry, a rodent inhibitor of all complement activation pathways,15 and we have recently shown that target binding and complement inhibitory activity of CR2-fH is dependent on CR2-C3d–mediated interactions.16
Materials and Methods
Animals
CFB−/− mice on a C57BL/6 background were generously provided by V. Michael Holers (University of Colorado Health Science Center, Denver, CO). C57BL/6 mice were generated from breeding pairs (Harlan Laboratories, Indianapolis, IN). Animals were housed under a 12-hour light/12-hour dark cycle with access to food and water ad libitum.
For CNV lesions, 3-month-old mice were anesthetized (20 mg/kg xylazine, 80 mg/kg ketamine), and pupils were dilated (2.5% phenylephrine HCl and 1% atropine sulfate). Argon laser photocoagulation (532 nm, 100 μm spot size, 0.1 second duration, 100 mW) was used to generate four laser spots in each eye surrounding the optic nerve with the use of a handheld coverslip as a contact lens. Bubble formation at the laser spot indicated the rupture of Bruch membrane.17
For tail-vein injections, the vein was vasodilated by heat, a 25-gauge needle was inserted, and a 50 μL volume was injected (250 μg CR2-fH in PBS, molar equivalent of CR2 in PBS [113 μg], or PBS only). Dosing and treatment schedules are outlined in Results. All experiments were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the University Animal Care and Use Committee.
Plasmid Construction, Expression, and Purification of CR2-fH and CR2 Control
Two expression plasmids were prepared, one for the generation of the CR2 control protein and one to produce CR2-fH. Both contained the sequence encoding the four N-terminal SCRs of mouse CR2 (residues 1–257 of mature protein; RefSeq accession number NM_007758; www.ncbi.nlm.nih.gov/locuslink/refseq/provided in the public domain by the National Center for Biotechnology, Bethesda, MD); in the CR2-fH vector, mouse CR2 is linked to the sequence encoding the 5 N-terminal SCR of mouse fH (residues 1–303 of mature protein; RefSeq NM 009888). Both constructs were prepared with a (G4S)2 linker, and all procedures were carried out using standard PCR methods. The expression plasmid was the previously described PBM vector with a CD5 signal peptide sequence.18 Final plasmid constructs were transfected into Chinese hamster ovary cells with transfection reagent (FuGene HD; Roche Applied Science, Indianapolis, IN) according to the manufacturer instructions, and protein expression was quantified by ELISA and dot blot analysis. For protein purification, Chinese hamster ovary cells expressing CR2 or CR2-fH were cultured in flasks (375 cm2), and culture supernatant was centrifuged at 2000g for 20 minutes, then filtered through a 0.22-μm filter. CR2 and CR2-fH were purified from the filtered supernatant by anti–mouse CR2 (mAb 7G6) affinity chromatography, as described previously.18 Purified CR2 and CR2-fH gave a single band of appropriate molecular mass by SDS-PAGE. Detailed in vitro characterization of the fusion protein demonstrating AP specific activity is presented elsewhere.16
Assessment of CNV Lesions
Relative CNV size was determined in flatmount preparations of RPE-choroid stained with isolectin B (which binds to terminal μ-D-galactose residues on endothelial cells and selectively labels the murine vasculature; see Fig. 1A–C).17 In brief, eyes were collected and immersion-fixed in 4% paraformaldehyde (PFA) for 2 hours at 4°C, after which the anterior chamber, lens, and retina were removed. Eyecups were incubated in blocking solution (3% bovine serum albumin, 10% normal goat serum, and 0.4% Triton-X in Tris-buffered saline) for 1 hour. Isolectin B (1:100 of 1 mg/mL solution; Sigma-Aldrich, St. Louis, MO) was applied to eyecups overnight at 4°C in blocking solution. After extensive washing, eyecups were flattened using four relaxing cuts, cover-slipped with mounting medium (Fluoromount; Southern Biotechnology Associates, Inc., Birmingham, AL), and examined by confocal microscopy (TCS SP2 AOBS; Leica, Bannockburn, IL). Fluorescence measurements, taken from 2 μm sections using confocal microscopy (40 × oil lens), were used for size determination. A Z-stack of images through the entire depth of the CNV lesion was obtained at the same laser intensity setting for all experiments. For each slice, overall fluorescence was determined to obtain pixel intensity against depth (Fig. 1D), from which the peak intensity and the area under the curve (indirect volume measurement) were calculated (Fig. 1E).17 Data are expressed as mean ± SEM per eye. Individual CNV lesions were also photographed under a microscope (Zeiss, Thornwood, NY) equipped for fluorescence and digital microscopy (Spot camera; Diagnostic Instruments, Sterling Heights, MI).
Figure 1.
Documentation of CNV development. CNV was induced using laser photocoagulation of Bruch membrane. (A–C) Isolectin-B4 staining, which highlights newly formed murine vessels, is used to document the size increase of the CNV lesion over time. Confocal microscopy, capturing Z-stacks of images through the entire depth of each CNV lesion, was performed using identical laser intensity settings for all experiments. (D, E) Intensity profiles were plotted and analyzed for lesion size (peak fluorescence and area under the curve; integrated fluorescence). (F) Effects of the lesion (or the treatments) on retina function were determined by electroretinography, revealing a decrease in ERG amplitudes for both a- and b-waves (photoreceptor and bipolar cell response, respectively) 6 days after the induction of CNV. (G) The increase in the size of the lesion as determined by confocal microscopy correlated with a decrease in the ERG amplitude (lesions were normalized to maximum size, as determined after 2 weeks; ERG b-waves were expressed as a percentage of baseline amplitudes). Scale bar, 50 μm.
Immunohistochemistry
Immunohistochemistry was performed as previously described with minor modifications.19 Complement deposition is documented using C3d-FITC (1:100; DakoCytomation, Peterborough, UK; see, for example, Ref. 16). For localization of CR2-fH, the CR2 portion of the fusion protein was localized with a mAb against CR2 (1:100; hybridoma clone 7G6, generously provided by V. Michael Holers) and visualized by using anti–rat Alexa 488 (1:400; Invitrogen, Carlsbad, CA). No primary antibody condition was used as negative control. This antibody also recognizes mouse CR1, an alternatively spliced protein transcribed from the same gene as CR2.20 Localization was performed either in flatmounts of RPE-choroid lightly fixed in 2% PFA or fresh-frozen sections postfixed in ice-cold acetone. Each staining was performed on more than three eyes per condition. Staining was examined by fluorescence microscopy (Zeiss) equipped with a digital black-and-white camera using a fixed exposure time per experimental condition (e.g., time series for C3d or CR2 staining after CR2-fH or PBS injection and negative control). C3d images were false-colored (Photoshop; Adobe Systems, San Jose, CA). Quantification of CR2 fluorescence was performed with ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html) to measure a grayscale value within the range of 0 to 256.
Electroretinography
ERG recordings and data analyses were performed as previously detailed21,22 (EPIC-2000 system; LKC Technologies, Inc., Gaithersburg, MD). Stimuli to determine overall retinal responsiveness consisted of 10 μs single flashes at a fixed intensity (2.48 cd · s/m2) under scotopic conditions (Fig. 1F). Measurements were performed before (baseline ERG) and after the CNV lesion period. Peak a-wave amplitude was measured from baseline to the initial negative-going voltage, whereas peak b-wave amplitude was measured from the trough of the a-wave to the peak of the positive b-wave. ERG responses were found to decline with increasing size of the CNV lesion (Fig. 1G), affecting a- and b-waves equally.
Quantitative RT-PCR
RPE-choroid fractions were isolated from control and CNV eyes and were stored at −80°C until use. Quantitative RT-PCR analyses were performed as described in detail previously.23 Primers used were: β-actin forward 5′-AGCTGAGAGGGAAATCGTGC-3′ and reverse 5′-ACCAGACAGCACTGTGTTG-3′; C3 forward 5′-GGAAACGGTGGAGAAAGC-3′ and reverse 5′-CTCTTGACAGGAATGCCATCGG-3′; VEGF forward 5′-CAGGCTGCTGTAACGATGAA-3′ and reverse 5′-GCATTCACATCTGCTGTGCT-3′. Real-time PCR analyses were performed in triplicate in a sequence detection system (GeneAmp 5700; Applied Bio-systems, Foster City, CA) using standard cycling conditions. Quantitative values were obtained by the cycle number.
Statistical Analysis
For data consisting of multiple groups, one-way ANOVA followed by Fisher post hoc test (P < 0.05) was used; single comparisons were made with t-test analysis (P < 0.05).
Results
Effect of Alternative Pathway Inhibition and Deficiency on CNV Development
Activation of the AP and an associated inflammatory response are involved in the development of CNV in mice and humans. We investigated the use of CR2-fH, a novel targeted inhibitor specific for the AP, in a mouse model of CNV. The model involves the induction of lesions by laser photocoagulation of Bruch membrane, which produces characteristics typical of human CNV with vessels penetrating the RPE-Bruch membrane to invade the retina (Fig. 1A–C; for examples, see Refs. 17, 24, 25).
The development of CNV and retinal function after laser photocoagulation were assessed in mice treated with intravenous injections of CR2-fH or vehicle (CR2 or PBS). Animals received tail-vein injections of CR2-fH (250 μg), CR2 (133 μg, molar equivalent), or PBS immediately after laser photocoagulation and on days 2 and 4. On day 6, mice were analyzed electrophysiologically and killed. Factor-B–deficient mice (CFB−/−) that lack a functional AP served as a positive control. CR2-fH significantly reduced the size of the CNV lesion as measured histologically by isolectin B4 staining of RPE-choroid flatmounts (Fig. 2). Intravenous injections of CR2-fH significantly reduced CNV (approximately 40%) compared with PBS injections (P < 0.01; PBS, 19 eyes; CR2-fH, 11 eyes), and there was no difference in CNV between PBS- and CR2-treated mice (P = 0.1; CR2, 9 eyes). CNV development was also significantly reduced in CFB−/− mice compared with wild-type (wt) mice (approximately 65%; P < 0.001; Fig. 2).
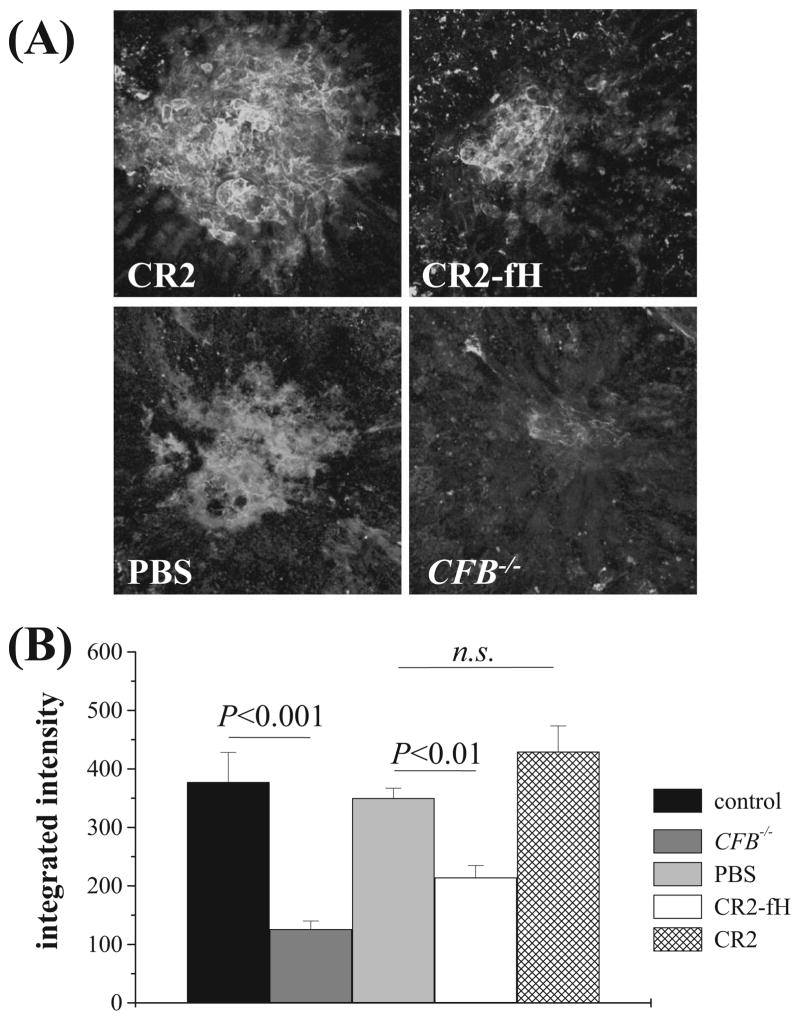
Figure 2.
CNV in CR2-fH–treated and fB-deficient mice. CNV development was evaluated 6 days after laser photocoagulation using isolectin-B4 staining. (A) Representative images, (B) with corresponding quantification of data (lesion size, integrated pixel intensity). (A) CFB−/− mice (n = 18) or wt mice (n = 12) treated with intravenous injections of PBS (n = 19), CR2 (133 μg; n = 9), or CR2-fH (250 μg; n = 11) on days 0, 2, and 4 after laser photocoagulation. Isolectin-B4 staining intensity is reduced in CR2-fH–treated and CFB−/− animals compared with PBS and CR2-treated controls. (B) Comparison of the size of the lesions suggests that the absence of the alternative pathway (CFB−/−) reduced CNV size by approximately 65%, whereas AP inhibition with CR2-fH reduced CNV size by 40% compared with PBS or CR2 controls. n.s., not significant.
ERG recordings were assessed to follow the extent of the lesion as described by Caicedo et al. 26 (Fig. 1F, ERG traces). Reduction in ERG amplitudes is most likely a compound effect of damage to the retina because of the overlying CNV lesion26 and a reduction of retinal oxygen supply27,28 resulting from the impaired RPE-choroid integrity and is correlated with the extent of the lesion (Fig. 1G). Two parameters of the ERG response were analyzed: a-wave amplitudes, which are a direct reflection of the photoreceptor currents generated by the absorption of light; and b-wave amplitudes, which are a mass potential generated by the bipolar cells that sum photoreceptor output (for a review, see Ref. 29). The four lesions caused a reduction in ERG amplitudes to approximately 60% of the baseline values in wt animals for a- and b-wave amplitudes (Fig. 3A). Eliminating or blocking AP activation blunted but did not prevent this decrease in ERG amplitudes. Photoreceptor function was significantly preserved in CFB−/− mice (Fig. 3A; CFB−/− vs. wt after CNV; P < 0.01), whereas b-wave amplitudes did not reach significance. Intravenous injections of CR2-fH similarly protected retina function. In PBS-injected animals, a- and b-wave amplitudes were reduced to almost 55% of the baseline values (Fig. 3B). Both parameters were significantly preserved after CR2-fH treatment compared with the PBS-injected controls (Fig. 3B; a-wave, P < 0.01; b-wave, P < 0.02).
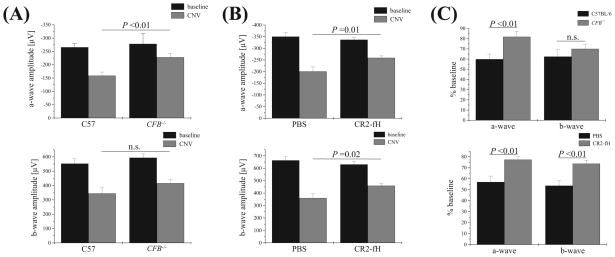
Figure 3.
CNV and retina function in CR2-fH–treated and fB-deficient mice. ERG recordings were performed before laser photocoagulation (baseline) and at the end of the experiment (after 6 days of CNV) using single-flash ERG recordings at a single light intensity of 2.48 photopic cd · s/m2. (See Figure 1 for ERG traces from wt, C57BL/6 animals.) ERG responses were analyzed from (A) CFB+/+ and CFB−/− mice and (B) wt mice treated with either PBS or CR2-fH. (A) A-wave and b-wave amplitudes at baseline were identical for CFB+/+ and CFB −/− mice. However, after CNV induction, a genotype-dependent difference was identified. CFB+/+ mice exhibited a significant loss of ERG amplitudes after CNV, whereas ERG amplitudes were relatively preserved in CFB−/− mice. (B) Reductions in ERG a- and b-wave amplitudes were significantly lower in mice treated intravenously with CR2-fH (250 μg on days 0, 2, and 4 after laser injury) compared with PBS-treated mice. (C) Summary of ERG data showing ERG a-waves as percentage of baseline amplitudes for the CFB+/+ and CFB−/− and the PBS and CR2-fH comparisons.
Effect of AP Inhibition and Deficiency on CNV-Associated Upregulation of C3 and VEGF
To gain further insight into how CR2-fH may modulate angiogenesis, we examined the local expression of VEGF and C3. The angiogenic factor VEGF plays an important role in the development of CNV in mice and humans,17,30 and recent data indicate that AP-dependent inflammation plays a role in the upregulated expression of VEGF in the RPE-choroid.31 Given that the combined data in the literature have shown that CNV growth is associated with changes in mRNA and protein for VEGF and complement components,17,30–33 we investigated mRNA changes only. Quantitative RT-PCR revealed that VEGF and C3 mRNA were significantly upregulated in RPE-choroid samples from CNV mice at 1, 3, and 6 days after injury, with peak levels seen at day 3 (Fig. 4A). Thus, the RPE-choroid can be a source of C3 in this model, raising the possibility that locally produced complement may contribute to VEGF release and CNV. CR2-fH treatment resulted in approximately 50% reduction in VEGF and C3 mRNA levels from that seen in control-treated mice (P = 0.008 and P = 0.002, respectively; Fig. 4B). In CFB−/− mice, VEGF and C3 mRNA levels were reduced by approximately 95% and 90%, respectively, compared with wt mice (P = 0.001 and P = 0.03, respectively; Fig. 4B). These data indicate that local VEGF and C3 expression by the RPE-choroid after laser injury is largely AP dependent. There does appear to be a correlation between VEGF mRNA expression and CNV because the level of VEGF mRNA expression in CR2-fH–treated mice was significantly higher than in fB-deficient mice, which correlated with the greater level of reduction of CNV in the CFB−/− mice.
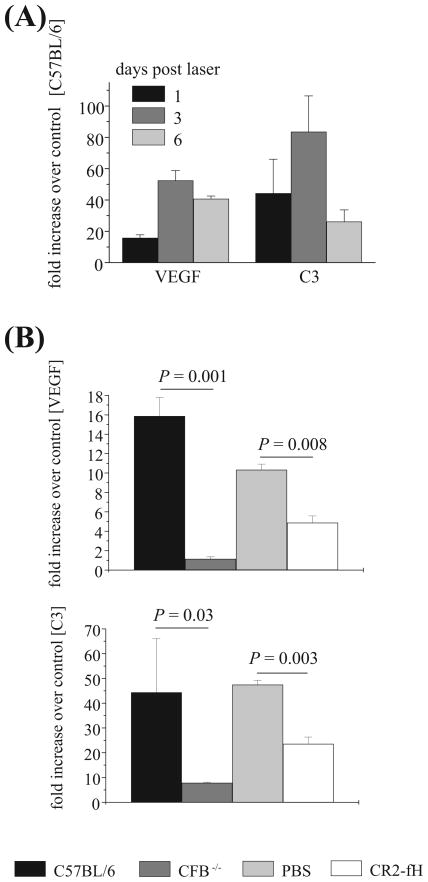
Figure 4.
Analysis of VEGF and C3 mRNA expression in CNV. (A) Quantitative RT-PCR was performed on RPE-choroid samples removed 1, 3, and 6 days after laser photocoagulation. Quantitative values were obtained by cycle number (Ct value), determining the difference between the mean experimental (VEGF or C3) and control (β-actin) ΔCt values. VEGF and C3 mRNA expression was significantly increased as early as 1 day after laser injury and reached a peak by 3 days. (B) Fold difference for QRT-PCR on RPE-choroid samples removed 1 day after laser photocoagulation from wt and CFB−/−, and wt mice treated with PBS or CR2-fH. VEGF and C3 mRNA expression was significantly decreased when AP pathway activation was eliminated (CFB−/− ) or inhibited (CR2-fH). Eyes from four animals were evaluated per group.
Retinal Localization of C3d and CR2-fH
To correlate CR2-fH treatment with the pathogenesis of disease, we analyzed the localization of CR2-fH and the complement activation product C3d, a covalently attached breakdown product of C3 and a target ligand for CR2-fH. C3d was deposited at the edge of the CNV lesion as early as 12 hours after laser damage, with decreased levels 6 days later (Fig. 5A). C3d deposition at the edge of the lesion was also apparent in RPE-choroid flatmounts (Fig. 5B). To investigate the bioavailability of CR2-fH at the site of injury, CR2-fH (250 μg) was administered intravenously on day 3 after laser injury, and eyes were harvested 24 hours later. Anti–CR2 immunofluorescence was used to probe for CR2-fH in CNV lesions (Fig. 6A). Some immunopositive staining was localized at the edge of the CNV lesion in the PBS-treated animals. This may in part be due to presence of certain immune cells that normally express CR2; alternatively, CR1-positive cells could also be detected by this antibody.20 No nonspecific staining was observed when the primary antibody was eliminated. However, CR2 staining was significantly higher in CR2-fH–treated mice (Fig. 6B; P < 0.001), and C3d deposition and CR2-fH binding showed similar patterns of staining, with both proteins localized to the lesion edge, indicating CR2-fH targeting. Thus, intravenously administered CR2-fH gains access to the RPE after laser injury, where it can bind its target ligand, C3d.
Figure 5.
C3d deposition in the CNV lesion. (A) Comparison of C3d deposition by immunohistochemistry using a C3d antibody directly conjugated to FITC (left), and C3d staining overlaid with the differential interference contrast image of mouse retinas (right) frozen sections. Photographs of sections were taken through a CNV lesion, which can be identified by the damage in the pigmented RPE-choroid. Significant C3d deposition was seen by 12 hours after laser; it remained elevated through day 3 and appeared to decline by day 6. (B) Flatmount analysis of the RPE-choroid showed the anti-C3d labeling to be located preferentially at the edge of the lesion. IS/OS (photoreceptor), inner segment/outer segment; ONL, outer nuclear layer, OPL, outer plexiform layer.
Figure 6.
Localization of CR2-fH to the CNV lesion. Anti–CR2 immunofluorescent staining for CR2-fH. Animals were injected with a therapeutic dose of CR2-fH (250 μg) on day 3 after laser photocoagulation, and eyes were collected 24 hours later. (A) Wholemount immunofluorescence microscopy revealed the presence of CR2-positive material in the CR2-fH–injected eyes, which was significantly reduced in the PBS-injected eyes; no staining was observed when the primary antibody was omitted (negative control). (B) Semiquantitative analysis of the amount of immunofluorescence confirmed the localization of CR2-fH to the site of the lesion.
Therapeutic Analysis of CR2-fH
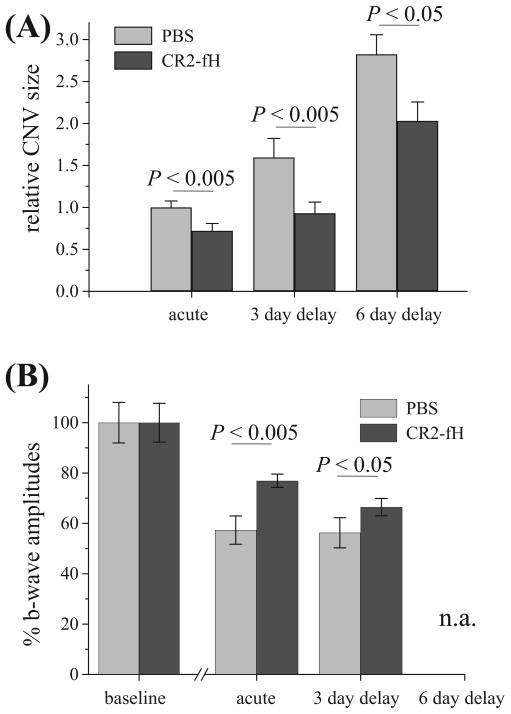
AMD is a slowly progressing disease. We investigated the use of CR2-fH in more therapeutically relevant paradigms involving delayed CR2-fH treatment after injury. Additional treatment protocols consisted of administering CR2-fH on days 3, 5, and 7 after laser injury or on days 6, 8, and 10 after injury. In both cases, histologic and electrophysiological analyses were performed 2 days after final CR2-fH treatment (day 9 or 12). Because b-wave amplitudes have been reported to be more prone to deterioration in CNV,26 only b-waves are reported here, though similar results were obtained analyzing either ERG component. As was acute treatment, delayed CR2-fH treatment was therapeutically effective (Fig. 7). Delaying initiation of CR2-fH treatment until day 3 after photocoagulation, which represents the peak expression of C3, significantly reduced the amount of CNV (P < 0.002) and preserved retinal function (P = 0.05; Fig. 7). CR2-fH was also protective when treatment was initiated on day 6 after photocoagulation, which was beyond the peak of C3 mRNA expression and C3 deposition (P = 0.02; Fig. 7). Thus, CNV progression can be reduced in animals subsequent to peak levels of complement activation and VEGF expression in the lesion.
Figure 7.
Effect of delayed CR2-fH treatment on CNV progression. Three temporal paradigms of CR2-fH treatment were compared for efficacy in reducing CNV size. All animals were exposed to laser photocoagulation on day 0. For acute treatment, the first injection of CR2-fH was administered at the time of laser injury (with analysis on day 6; see Fig. 2); for the delayed treatments, the first treatment with CR2-fH was administered either on day 3 (analysis on day 9) or on day 6 (analysis on day 12) after laser injury. Data are plotted based on the day of the start of treatment (acute, 3-day delay, and 6-day delay). Compared are (A) indirect size measurements and (B) ERG b-wave amplitudes. CNV size measurements are normalized to the maximum size obtained with PBS treatment using the acute treatment paradigm. b-Wave ERG amplitudes are normalized to the baseline values before laser treatment. CNV sizes and the decrease in ERG b-wave amplitudes are significantly reduced in acutely treated animals and in animals treated with a 3- or 6-day delay (ERGs are not available for the 6-day group because of equipment failure). Eight animals per treatment group were evaluated (*P < 0.005; #P < 0.05).
Discussion
An association of AMD with complement was originally indicated by the deposition of various complement proteins in the drusen of patients with AMD.30 More recently, a close association between AMD and the alternative pathway of complement has been shown by the discovery that polymorphisms of the human fH gene are a risk factor for all forms of AMD.1–4 It has been shown subsequently that polymorphisms of genes encoding the alternative pathway proteins fB and C3,6,7 as well as the classical pathway protein C2,6 are also associated with AMD. Studies in animal models of CNV, a major pathologic occurrence with wet AMD, also support an important role for complement in AMD.
In mouse CNV, a role for the terminal complement pathway and the cytolytic membrane attack complex (MAC) is indicated by combined data showing that CNV is reduced in C5-deficient mice and in mice treated with MAC inhibitors,32,34 though the complement activation products C3a and C5a have also been implicated.17 Deficiency or inhibition of classical and lectin pathway proteins has no impact on murine CNV,31 but inhibition of the alternative pathway by fB siRNA appears to significantly reduce CNV.31 However, the data from studies showing that fB siRNA protects against CNV must be interpreted with caution, based on the recent report that siRNA can suppress angiogenesis and CNV through TLR3 activation independent of the knockdown target gene.35 In the present study, we confirm a key role for the alternative pathway in murine CNV by demonstrating that fB deficiency is associated with a significant reduction in the size of CNV after laser injury, preserved retinal function, and decreased VEGF mRNA expression. Furthermore, we demonstrate the importance of the alternative pathway in a clinically relevant therapeutic paradigm using CR2-fH, a novel complement inhibitor specific for the alternative pathway that targeted to sites of C3 deposition in the RPE-choroid after intravenous injection. Of therapeutic relevance, CR2-fH slowed CNV progression and reduced retina function loss when administered days after laser injury and during the rapid growth of the CNV lesion.
Although defects in complement regulation are associated with AMD, available evidence indicates that additional insults are required for the development of wet AMD, and here laser photocoagulation is used to induce injury that leads to a time-dependent increase in complement activation, VEGF production, and CNV. Of note, fH deficiency results in retinal abnormalities and vision dysfunction in aged mice, and while these studies suggest that there is no requirement for an additional trigger for complement-dependent retinal disease, fH-deficient mice do not develop vasculature changes.36 It should also be noted that fH-deficient mice contain very low levels of circulating C3 because of uncontrolled activation of complement in the fluid phase,37 whereas the polymorphisms in the human fH gene associated with AMD occur within a region of fH that affects cell binding and cell-surface complement regulation but not fluid-phase regulation. Together, these results indicate that complement activation is involved in murine photoreceptor health (see also Ref. 38) but suggest that other factors, such as mechanical lesions, are required for CNV. The situation is similar for VEGF: increased expression of VEGF in the RPE is not in itself sufficient for CNV development, but when additional insults to the integrity of RPE-Bruch membrane are provided, CNV is rapidly induced.39 Although it is possible that these results reflect differences between murine and human CNV, they are more likely an indication that multiple insults are required for the development of wet AMD. The human data corroborate this statement because approximately 30% of carriers of the Y402H AMD susceptibility mutation do not develop AMD.4 Furthermore, no single variant of CFH alone has been shown to account for disease in humans, but other unidentified factors are necessary for disease (for a review, see Ref. 8). Together, these findings argue that variants in CFH or the lack of CFH (CFH−/− ) in a normal/healthy environment do not produce neovascularization and that although complement activation is strongly associated with AMD, it is not alone responsible.
Factor H is a soluble complement inhibitor that can bind to deposited C3 and certain membrane structures and provide cells with protection from complement. Indeed, fH mutations associated with AMD are within fH “targeting” domains distinct from its SCR1–4 complement inhibitory-domain. An interesting finding here is that CR2-fH delivered by intravenous injection is able to provide protection from CNV, whereas endogenous fH, present at much higher concentrations (300–600 μg/mL), is not. It is unclear why the fH complement inhibitory-domain targeted by means of CR2 is able to provide such effective protection from CNV compared with endogenous fH, but it may be related to different binding affinities of CR2 compared with native fH at the cell surface, or it may be that CR2-mediated binding orients the complement inhibitory SCR1–5 domain of fH in a more favorable position for interaction with C3 and C3 convertases (for further discussion, see Ref. 16). Recently, CR2-fH was also shown to be protective in a model of intestine ischemia reperfusion injury,16 and a similar targeting approach has been shown to enhance the efficacy of soluble Crry, an inhibitor of all complement pathways, by more than 10-fold.15
The similar pattern of C3d deposition and CR2-fH binding at the edge of the lesion suggests that complement activation occurs on injured RPE cells at the edge of the lesion, enlarging the area of damage over time, and allowing for choroidal vessels to penetrate. The origin of the complement proteins deposited at the site of CNV is unclear. A likely source is the circulation, but our findings that C3 mRNA is expressed in the RPE-choroid and that expression is upregulated after laser treatment, support the concept that locally-produced complement proteins may play a role in injury. This hypothesis is also supported by findings that fB and fH mRNA can be isolated from RPE-choroid in human and mouse CNV.30,31 Irrespective of the origin of the complement proteins, they mark the site of the lesion to which therapeutic inhibitors must be targeted. Intravenous injections of CR2-fH inhibited CNV, suggesting that CR2-fH accesses the site of CNV by way of the impaired blood-retina barrier. In mouse CNV, the barrier function of RPE-Bruch membrane is compromised by laser damage; human wet AMD exhibits a compromised blood-retina barrier, with the formation of new vessels with high substance permeability.
In summary, we show that a targeted inhibitor specific for the AP of complement significantly reduces CNV and the physiological consequences of CNV on retina function. In the CNV model, AP dependence was shown in a therapeutic setting and, importantly, the therapeutic effect of CR2-fH was achieved with intravenous injection. CR2-mediated targeting of the fH complement inhibitory-domain may open new avenues for the development of treatment strategies for wet AMD.
Acknowledgments
The authors thank Michael Holers, Carl Atkinson, and Anand Swaroop for helpful discussions; Luanna Bartholomew for critical review; Helga Sandoval and Luis Fernandez de Castro for training in the use of the argon laser; and Efrain Martinez for producing CR2-fH.
Supported in part by National Institutes of Health Grants EY013520 (BR), EY017465 (BR), HL86576 (ST), and Vision Core Grant EY014793; Foundation Fighting Blindness; and an unrestricted grant to Medical University of South Carolina from Research to Prevent Blindness. Animal studies were conducted in a facility constructed with support from the National Institutes of Health (Grant C06 RR015455).
Footnotes
Disclosure: B. Rohrer, Taligen Therapeutics Inc. (C), P; Q. Long, None; B. Coughlin, None; R.B. Wilson, None; Y. Huang, None; F. Qiao, None; P.H. Tang, None; K. Kunchithapautham, None; G.S. Gilkeson, Taligen Therapeutics Inc. (C), P; S. Tomlinson, Taligen Therapeutics Inc. (C), P
References
- 1.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 3.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 4.Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li M, Atmaca-Sonmez P, Othman M, et al. CFH haplotypes without the Y402H coding variant show strong association with susceptibility to age-related macular degeneration. Nat Genet. 2006;38:1049–1054. doi: 10.1038/ng1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gold B, Merriam JE, Zernant J, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38:458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yates JR, Sepp T, Matharu BK, et al. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357:553–561. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- 8.Despriet DD, Klaver CC, Witteman JC, et al. Complement factor H polymorphism, complement activators, and risk of age-related macular degeneration. JAMA. 2006;296:301–309. doi: 10.1001/jama.296.3.301. [DOI] [PubMed] [Google Scholar]
- 9.de Cordoba SR, de Jorge EG. Translational mini-review series on complement factor H: genetics and disease associations of human complement factor H. Clin Exp Immunol. 2008;151:1–13. doi: 10.1111/j.1365-2249.2007.03552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prosser BE, Johnson S, Roversi P, et al. Structural basis for complement factor H linked age-related macular degeneration. J Exp Med. 2007;204:2277–2283. doi: 10.1084/jem.20071069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark SJ, Higman VA, Mulloy B, et al. His-384 allotypic variant of factor H associated with age-related macular degeneration has different heparin binding properties from the non-disease-associated form. J Biol Chem. 2006;281:24713–24720. doi: 10.1074/jbc.M605083200. [DOI] [PubMed] [Google Scholar]
- 12.Ormsby RJ, Ranganathan S, Tong JC, et al. Functional and structural implications of the complement factor H Y402H polymorphism associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:1763–1770. doi: 10.1167/iovs.07-1297. [DOI] [PubMed] [Google Scholar]
- 13.Hakobyan S, Harris CL, van den Berg CW, et al. Complement factor H binds to denatured rather than to native pentameric C-reactive protein. J Biol Chem. 2008;283:30451–30460. doi: 10.1074/jbc.M803648200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atkinson C, Qiao F, Song H, Gilkeson GS, Tomlinson S. Low-dose targeted complement inhibition protects against renal disease and other manifestations of autoimmune disease in MRL/lpr mice. J Immunol. 2008;180:1231–1238. doi: 10.4049/jimmunol.180.2.1231. [DOI] [PubMed] [Google Scholar]
- 15.Atkinson C, Song H, Lu B, et al. Targeted complement inhibition by C3d recognition ameliorates tissue injury without apparent increase in susceptibility to infection. J Clin Invest. 2005;115:2444–2453. doi: 10.1172/JCI25208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y, Qiao F, Atkinson C, Holers VM, Tomlinson S. A novel targeted inhibitor of the alternative pathway of complement and its therapeutic application in ischemia/reperfusion injury. J Immunol. 2008;181:8068–8076. doi: 10.4049/jimmunol.181.11.8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nozaki M, Raisler BJ, Sakurai E, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A. 2006;103:2328–2333. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song H, He C, Knaak C, Guthridge JM, Holers VM, Tomlinson S. Complement receptor 2-mediated targeting of complement inhibitors to sites of complement activation. J Clin Invest. 2003;111:1875–1885. doi: 10.1172/JCI17348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rohrer B, Korenbrot JI, LaVail MM, Reichardt LF, Xu B. Role of neurotrophin receptor TrkB in the maturation of rod photoreceptors and establishment of synaptic transmission to the inner retina. J Neurosci. 1999;19:8919–8930. doi: 10.1523/JNEUROSCI.19-20-08919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molina H, Kinoshita T, Inoue K, Carel JC, Holers VM. A molecular and immunochemical characterization of mouse CR2: evidence for a single gene model of mouse complement receptors 1 and 2. J Immunol. 1990;145:2974–2983. [PubMed] [Google Scholar]
- 21.Gresh J, Goletz PW, Crouch RK, Rohrer B. Structure-function analysis of rods and cones in juvenile, adult, and aged C57bl/6 and Balb/c mice. Vis Neurosci. 2003;20:211–220. doi: 10.1017/s0952523803202108. [DOI] [PubMed] [Google Scholar]
- 22.Richards A, Emondi AA, Rohrer B. Long-term ERG analysis in the partially light-damaged mouse retina reveals regressive and compensatory changes. Vis Neurosci. 2006;23:91–97. doi: 10.1017/S0952523806231080. [DOI] [PubMed] [Google Scholar]
- 23.Lohr HR, Kuntchithapautham K, Sharma AK, Rohrer B. Multiple, parallel cellular suicide mechanisms participate in photoreceptor cell death. Exp Eye Res. 2006;83:380–389. doi: 10.1016/j.exer.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Espinosa-Heidmann DG, Suner I, Hernandez EP, Frazier WD, Csaky KG, Cousins SW. Age as an independent risk factor for severity of experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2002;43:1567–1573. [PubMed] [Google Scholar]
- 25.Bora PS, Hu Z, Tezel TH, et al. Immunotherapy for choroidal neovascularization in a laser-induced mouse model simulating exudative (wet) macular degeneration. Proc Natl Acad Sci U S A. 2003;100:2679–2684. doi: 10.1073/pnas.0438014100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caicedo A, Espinosa-Heidmann DG, Hamasaki D, Pina Y, Cousins SW. Photoreceptor synapses degenerate early in experimental choroidal neovascularization. J Comp Neurol. 2005;483:263–277. doi: 10.1002/cne.20413. [DOI] [PubMed] [Google Scholar]
- 27.Johnson MA, Marcus S, Elman MJ, McPhee TJ. Neovascularization in central retinal vein occlusion: electroretinographic findings. Arch Ophthalmol. 1988;106:348–352. doi: 10.1001/archopht.1988.01060130374025. [DOI] [PubMed] [Google Scholar]
- 28.Sabates R, Hirose T, McMeel JW. Electroretinography in the prognosis and classification of central retinal vein occlusion. Arch Ophthalmol. 1983;101:232–235. doi: 10.1001/archopht.1983.01040010234010. [DOI] [PubMed] [Google Scholar]
- 29.Pugh EN, Jr, Falsini B, Lyubarsky AL. The Origin of the Major Rod-and Cone-Driven Components of the Rodent Electroretinogram and the Effect of Age and Light-Rearing History on the Magnitude of These Components. New York: Plenum Press; 1998. pp. 93–128. [Google Scholar]
- 30.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 31.Bora NS, Kaliappan S, Jha P, et al. Complement activation via alternative pathway is critical in the development of laser-induced choroidal neovascularization: role of factor B and factor H. J Immunol. 2006;177:1872–1878. doi: 10.4049/jimmunol.177.3.1872. [DOI] [PubMed] [Google Scholar]
- 32.Bora NS, Kaliappan S, Jha P, et al. CD59, a complement regulatory protein, controls choroidal neovascularization in a mouse model of wet-type age-related macular degeneration. J Immunol. 2007;178:1783–1790. doi: 10.4049/jimmunol.178.3.1783. [DOI] [PubMed] [Google Scholar]
- 33.Hu W, Criswell MH, Fong SL, et al. Differences in the temporal expression of regulatory growth factors during choroidal neovascular development. Exp Eye Res. 2009;88:79–91. doi: 10.1016/j.exer.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Bora PS, Sohn JH, Cruz JM, et al. Role of complement and complement membrane attack complex in laser-induced choroidal neovascularization. J Immunol. 2005;174:491–497. doi: 10.4049/jimmunol.174.1.491. [DOI] [PubMed] [Google Scholar]
- 35.Kleinman ME, Yamada K, Takeda A, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coffey PJ, Gias C, McDermott CJ, et al. Complement factor H deficiency in aged mice causes retinal abnormalities and visual dysfunction. Proc Natl Acad Sci U S A. 2007;104:16651–16656. doi: 10.1073/pnas.0705079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose KL, Paixao-Cavalcante D, Fish J, et al. Factor I is required for the development of membranoproliferative glomerulonephritis in factor H-deficient mice. J Clin Invest. 2008;118:608–618. doi: 10.1172/JCI32525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rohrer B, Guo Y, Kunchithapautham K, Gilkeson GS. Eliminating complement factor D reduces photoreceptor susceptibility to light-induced damage. Invest Ophthalmol Vis Sci. 2007;48:5282–5289. doi: 10.1167/iovs.07-0282. [DOI] [PubMed] [Google Scholar]
- 39.Oshima Y, Oshima S, Nambu H, et al. Increased expression of VEGF in retinal pigmented epithelial cells is not sufficient to cause choroidal neovascularization. J Cell Physiol. 2004;201:393–400. doi: 10.1002/jcp.20110. [DOI] [PubMed] [Google Scholar]