Abstract
Neuroendocrine mechanisms underlying the progression of sleep-wake gonadotropin-releasing hormone (GnRH) pulse secretion across puberty have remained enigmatic. This article reviews the changes of sleep-wake luteinizing hormone (LH) (and by inference GnRH) pulse secretion across puberty in normal girls, primarily focusing on available human data. Herein I suggest that primary control of GnRH pulse frequency changes across puberty, with sex steroid feedback exerting minimal control during childhood but primary control during adulthood. I offer a working model regarding how such a transfer of GnRH pulse frequency control may partly account for the prominent day-night differences of GnRH pulse frequency characteristic of puberty. I then describe how this model may be relevant to the genesis of abnormal GnRH secretion in peripubertal girls with hyperandrogenemia.
Keywords: Puberty, gonadotropin releasing hormone, luteinising hormone
The importance of GnRH pulse frequency modulation in women
Gonadotropin-releasing hormone (GnRH) is a decapeptide hormone secreted in pulses from a functionally-integrated network of neurons—the GnRH pulse generator—located in the mediobasal hypothalamus. GnRH is the primary if not exclusive feedforward signal to pituitary gonadotropes, stimulating the synthesis and secretion of both luteinising hormone (LH) and follicle-stimulating hormone (FSH). Nonetheless, LH and FSH levels change differentially throughout ovulatory cycles, with FSH predominance in the early follicular phase and LH predominance in the late follicular phase. Sequential FSH and LH predominance is important for appropriate follicular maturation, sex steroid production, and subsequent ovulation.
At least two mechanisms govern differential gonadotropin (LH and FSH) secretion throughout ovulatory cycles. First, both oestradiol and inhibins selectively inhibit FSH release from gonadotropes during the mid-follicular, late follicular, and luteal phases (1, 2); this contributes to the transition from FSH- to LH-predominance across the follicular phase. Second, different patterns of pulsatile GnRH release differentially affect gonadotropin synthesis and secretion (3). Specifically, high GnRH pulse frequencies favor LH synthesis and secretion, while low GnRH pulse frequencies favor FSH synthesis and secretion. For example, in studies of ovariectomized, GnRH-deficient monkeys, decreasing the frequency of exogenous GnRH “pulses” from one pulse per hour to one pulse every 3 hours results in a 65% increase in plasma FSH, despite a 50% decrease in LH concentrations (4). Similar phenomena have been described in rats (3), sheep (5), and humans (6, 7).
The rapid half-life of GnRH makes peripheral measurements an unreliable reflection of pulsatile secretion, and the hypophyseal portal system is inaccessible in humans. However, patterns of LH pulsatility accurately mirror GnRH secretion in animal studies (8, 9). Therefore, in human studies, GnRH pulse frequency is inferred from LH pulse frequency. Some data suggests that a once hourly GnRH pulse frequency represents an inherent characteristic of the GnRH pulse generator in adult women. For example, a once hourly LH (and by inference GnRH) pulse frequency is observed in women in the (near) absence of sex steroid feedback (e.g., surgical or natural menopause) (10, 11). In addition, LH pulses do not appear to exceed this frequency during any phase of the menstrual cycle (10, 12). Furthermore, the isolated human mediobasal hypothalamus secretes GnRH pulses every 60–100 minutes (13), in contrast to the marked changes of GnRH pulse frequency observed across the menstrual cycle. Therefore, day-to-day changes of GnRH pulse frequency in women may chiefly reflect the imposition or removal of sex steroid negative feedback.
Progesterone appears to be the most important effector of GnRH pulse frequency reduction in women. For example, LH pulse frequency decreases coincident with progesterone increases in the luteal phase (12); and administration of progesterone to women during the follicular phase reduces LH pulse frequency (14). Also, progesterone plus oestradiol—but not oestradiol alone—slows LH pulse frequency in postmenopausal women (11). Importantly, low GnRH pulse frequency during the luteal phase appears to be physiologically relevant for maintenance of long-term cyclicity. For example, in monkeys, administration of hourly GnRH pulses during the luteal phase increased follicular phase length and decreased luteal progesterone production in the subsequent 3–5 cycles (15). Low GnRH pulse frequencies during the luteal phase may be important to increase FSH synthesis for later release during the luteal-follicular transition (16). With these things in mind, an ability of sex steroids (namely progesterone) to slow GnRH pulse frequency is likely an important facet of the adult menstrual cycle.
GnRH and gonadotropin changes across normal puberty
Puberty represents the transition from childhood to biological adulthood. It involves a series of highly complex and incompletely understood processes that result in the attainment of secondary sexual characteristics and reproductive competence. Normal puberty is initiated by the central nervous system (CNS) and hypothalamus, mediated by pulsatile GnRH secretion. There are many unknowns regarding puberty, including triggers for central pubertal initiation and the mechanisms underlying the evolution of sleep-wake GnRH secretion across puberty. Although I primarily discuss human data in this review, insights into GnRH pulse generator control during both childhood and puberty are often derived from animal studies; and a number of excellent reviews describing these important data are available (17–19).
Although pulsatile GnRH drives gonadotropins during mid-fetal life (the “mini-puberty of infancy”), gonadotropins decrease by late infancy or early childhood, and the hypothalamic-pituitary-gonadal axis becomes quiescent (the “juvenile pause”) (20). Studies during childhood using highly sensitive assays reveal low LH and FSH concentrations, a high FSH-to-LH ratio, and low LH pulse amplitude and frequency (21–25). Minor nocturnal increases of LH pulse amplitude occur during childhood, but gonadotropin levels are insufficient for significant gonadal stimulation. Near the close of the first decade, before the appearance of any clinical signs of puberty, amplification of nocturnal LH (GnRH) secretion heralds the reemergence of GnRH pulse generator activity and the beginning of puberty.
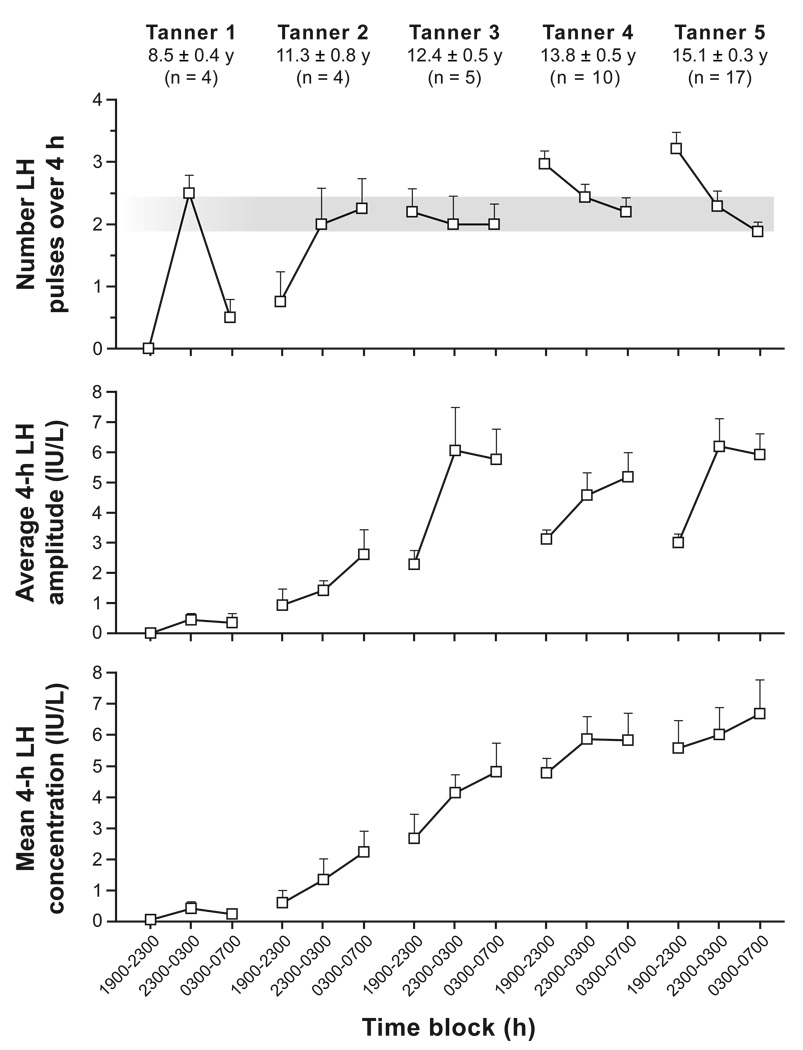
Most studies describing sleep-associated changes of LH (GnRH) secretion during puberty were performed in boys. A majority of these studies suggest that LH pulse frequency increases at night during early puberty (22, 26–32). Importantly, nocturnal increases of LH pulse frequency were evident in early pubertal subjects in many (22, 27, 29, 31), but not all (33), studies using highly sensitive assays (i.e., reported LH sensitivity ≤0.04 IU/liter). Similarly, most (22, 26–31), but not all (32, 33), studies reveal higher nighttime LH pulse amplitude compared to daytime. Although there are fewer data in pubertal girls, available studies reveal similar findings. For instance, a study by Cemeroglu and colleagues demonstrated a 2-fold nocturnal increase in LH pulse frequency in pre-, early, and mid-pubertal girls (34). (These girls also exhibited an overnight doubling of LH pulse amplitude—a change that did not achieve statistical significance.) Apter et al. observed nocturnal increases of both LH pulse frequency and amplitude in clinically prepubertal girls, with sleep-associated increases of LH amplitude persisting throughout puberty (23). A recent study by our group revealed overnight increases of LH pulse frequency and amplitude in pre- and early pubertal girls (i.e., Tanner breast stages 1 and 2) (Figure 1) (35). Notably, the aforementioned amplification of GnRH/LH pulsatility is specifically related to sleep: it generally begins within an hour of sleep onset, and it follows sleep reversal (23, 36). Overnight increases of LH pulsatility are followed by increased testosterone concentrations in boys (24, 37, 38), and by morning increases of oestradiol (39–41) and progesterone (42) in girls.
Figure 1. Late evening and overnight LH secretory characteristics in non-obese peripubertal girls stratified by Tanner breast stage.
Data are presented as mean ± SEM. Late evening (1900–2300 hours) LH pulse frequency serves as a surrogate for daytime (waking) frequency. Subjects generally slept from 2300 to 0700 hours. The gray bar encompasses mean overnight LH pulse frequencies in Tanner 2–5 girls. (Adapted with permission from J Clin Endocrinol Metab 2009; 94: 56–66 (35); Copyright 2009, The Endocrine Society.)
Many studies also suggest that both LH pulse frequency and amplitude generally increase across pubertal maturation (20, 22, 25, 27–30, 43). In girls, for example, LH pulse frequency and amplitude increase 4- and 9-fold, respectively, across puberty (23). Of interest, the sleep-wake patterns of LH secretion change markedly across puberty as well. The aforementioned study by our group (Figure 1) suggests that waking LH pulse frequency increases across puberty in girls, while nighttime frequency does not change substantially (35). Thus, during early puberty, sleep-associated LH pulse frequency increases compared to daytime frequency; but by late puberty, sleep-associated LH pulse frequency decreases compared to daytime frequency. A similar phenomenon is suggested by the aforesaid study by Apter and colleagues, although less detail was provided (23). Sleep-related slowing of LH (GnRH) pulse frequency persists into adulthood, being most prominent during the early follicular phase (44), but also observed in the late follicular phase (45). Nocturnal slowing of LH pulse frequency in women during the early follicular phase is also specifically related to sleep (e.g., it follows sleep reversal) (44).
Importance of sleep-wake differences of pulsatile GnRH secretion across puberty
The physiological relevance of sleep-wake GnRH pulse frequency changes and the pubertal evolution thereof are unknown. In children with hypogonadotropic hypogonadism, pubertal development may be achieved by administering GnRH at an unvarying frequency. However, many of these regimens utilize pulsatile GnRH only at night until midpuberty. Also, escalating doses of exogenous GnRH (per pulse) may be required for “normal” progress of pubertal development (46).
It seems plausible that daytime slowing of GnRH pulse frequency during early puberty is important for enhancement of FSH synthesis, follicular development, and eventual ovulation—analogous to the importance of luteal GnRH pulse frequency slowing in adults. In particular, follicular development requires FSH, which is enhanced by slow GnRH pulses. FSH stimulation of ovarian follicles is also important for oestradiol secretion, which will eventually provide the positive feedback at the pituitary required for an ovulatory LH surge. On the other hand, there must be enough releasable LH for sex steroid production and an eventual LH surge; and LH synthesis is enhanced by high-frequency GnRH pulses. Therefore, alternating episodes of low and high GnRH pulse frequency may support appropriate production of both FSH and LH, respectively, prior to the development of the cyclic GnRH pulse frequency changes characteristic of normal women.
The physiological relevance of decreased nocturnal GnRH pulse frequency in adult women is also uncertain, but it is interesting that urinary FSH concentrations appear to correlate with sleep duration in women (47); and that FSH secretion predominates when nocturnal slowing is most prominent (i.e., the early follicular phase). Hall and colleagues have suggested that disruption of day-night GnRH pulse frequency changes may disrupt LH and FSH secretion, which may then contribute to menstrual disturbances (as may be seen in night shift workers) (44).
Mechanisms underlying the evolution of sleep-wake GnRH pulse secretion across puberty in girls
The mechanisms accounting for the hypothalamic-pituitary-gonadal axis quiescence during childhood, and its reawakening at puberty, are unclear. Environmental and genetic elements play prominent roles in the onset of puberty, and a number of specific factors such as the kisspeptin-GPR54 system and leptin appear to be fundamental to this process (48). Importantly, though, the GnRH pulse generator represents the final common pathway for the central control of puberty. Historically, there have been two primary models regarding the control of GnRH secretion during childhood and at puberty: “central” and “gonadostat” hypotheses. These models are not mutually exclusive; indeed, both are thought to be operative in humans, although the central mechanism appears to be dominant during childhood (20).
By the central hypothesis, the reduction of gonadotropins during childhood reflects a CNS maturation that strongly inhibits the GnRH pulse generator (a neurobiological “brake”) (17, 20). This view is supported by the finding of low gonadotropins during mid-childhood in girls with gonadal dysgenesis (17). Also, whereas ovariectomy increases GnRH and LH pulsatility in early and midpubertal monkeys, it fails to do so in prepubertal monkeys (17). At puberty, removal of the neurobiological brake—along with stimulatory factors such as kisspeptin—leads to increased GnRH (and gonadotropin) pulse secretion. In this paradigm, the early pubertal increase of gonadal steroids largely follows that of gonadotropins in a feedforward fashion. As an extension of this model, sleep-associated increases of GnRH pulsatility in early puberty may reflect release from CNS inhibition and/or CNS augmentation during sleep.
The gonadostat hypothesis holds that low gonadotropin concentrations during childhood reflect exquisite sensitivity of the GnRH pulse generator-gonadotrope unit to the negative feedback actions of sex steroids (17, 49); and that the increase of gonadotropin secretion across puberty reflects decreasing GnRH pulse generator sensitivity to negative feedback. This model has largely fallen out of favor (19, 20), although some evidence supports the relevance of this hypothesis. For example, the amount of oestradiol required to suppress gonadotropins in prepubertal children is lower than amounts required in adults (50); and the prepubertal hypothalamic-pituitary unit is estimated to be 6–15 times more sensitive than that of adults (49). Similar observations have been described in rats, sheep, and monkeys (51–53).
Borrowing from the gonadostat concept, our group has suggested that a pubertal decrease of GnRH pulse generator sensitivity to negative feedback may be related to rising testosterone concentrations (35, 40, 41). In support of this idea, hyperandrogenemia appears to mediate relative GnRH pulse generator insensitivity to sex steroid negative feedback in polycystic ovary syndrome (PCOS) (54); and androgens augment GnRH neuron firing rate in adult female mice (55). We have also proposed that sleep-wake changes of GnRH pulse frequency during early puberty—reduced daytime GnRH pulse frequency specifically—may be related to rapid negative feedback effects of morning increases of progesterone (42, 56). Evidence for this hypothesis includes the absence of sleep-wake LH pulse frequency changes in girls with gonadal dysgenesis (in contrast to age-matched controls) (34). This view is also in line with the notion that day-to-day changes of post-pubertal GnRH pulse frequency primarily reflect the imposition or removal of sex steroid negative feedback. Thus, daytime slowing of GnRH pulse frequency during early puberty may be akin to luteal GnRH pulse frequency slowing in adults, albeit on a shorter time scale. This idea may also be consistent with a study of pubertal girls in which oestradiol infusion (to raise oestradiol levels 2.5-fold) reduced 14-hour (2200-1200 h) LH pulse amplitude and frequency by 24% and 21%, respectively, although the latter did not achieve statistical significance (57). Other than some preliminary data from our group, we know of no published data in pubertal girls regarding acute LH pulse frequency changes after administration of progesterone—the primary determinant of LH pulse frequency slowing in adult women.
Overall, though, it seems that sleep-wake changes of GnRH pulse frequency during puberty cannot be adequately explained using only the gonadostat concept. The very close temporal association between altered LH pulsatility and sleep is very strong evidence that higher CNS inputs play a major role in the day-night changes of GnRH pulse generator activity during puberty. Similarly, while early morning increases of sex steroid concentrations could rapidly decrease daytime GnRH pulse frequency during early puberty, it is unclear how such interactions would relate to sleep-wake differences in later puberty, when daytime LH pulse frequency exceeds nighttime LH frequency.
My research group is currently investigating the possibility that sleep-wake changes of GnRH pulse frequency reflect differential regulation of sleep-associated and waking GnRH pulse frequency by sex steroids. As mentioned above, girls exhibit remarkably similar nocturnal LH pulse frequency across puberty (approximately one pulse every two hours), while LH pulse frequency while awake gradually increases across puberty (from no detectable pulses in Tanner 1 girls to 3.2 ± 0.3 pulses/4 hours in Tanner 5 girls) (Figure 1). These data suggest that, once neuroendocrine puberty starts, sleep-activated CNS inputs impose a given GnRH pulse frequency regardless of pubertal stage, at least in the absence of luteal progesterone concentrations. Thus, nocturnal LH pulse frequency increases in early pubertal girls, but decreases in late pubertal girls. Furthermore, preliminary data from our group—thus far presented in abstract form only (58)—suggests that progesterone administration to early to mid-pubertal girls markedly suppresses late evening (waking) LH pulse frequency without affecting nighttime (sleep-associated) LH pulse frequency. The concept that different mechanisms control waking and sleep-associated GnRH pulse frequency is also supported by two studies by Loucks and colleagues, who reported that dietary restriction slows daytime—but not nighttime—LH pulse frequency in adult women during the late follicular phase (59, 60).
If correct, these concepts may also provide insight into abnormal developmental patterns of LH secretion in girls with hyperandrogenemia. Prior to offering a working model regarding sleep-wake GnRH pulse frequency during puberty in girls with and without hyperandrogenemia, I will briefly review GnRH/LH secretion in the setting of hyperandrogenemia, with PCOS serving as a prototype.
Altered neuroendocrine function in PCOS and adolescent hyperandrogenemia
PCOS is a common reproductive disorder marked by hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphology (61). The hyperandrogenemia of PCOS is predominantly of ovarian origin, but the pathogenesis of PCOS is complex and poorly understood. Hyperinsulinemia and abnormal steroidogenesis appear to be key players in the pathogenesis of PCOS. Likewise, abnormal neuroendocrine function appears to be an important pathophysiological factor in this disorder (56). For example, women with PCOS demonstrate increased mean LH and LH pulse amplitude; and the ovarian hyperandrogenemia of PCOS is LH-dependent (16).
In contrast to patterns observed in normal cycles, episodic slowing of GnRH pulse frequency does not regularly occur in PCOS (56). Persistently rapid GnRH pulse frequency likely plays a major role in the primary manifestations of PCOS, since it augments LH secretion and contributes to relative FSH deficiency (16). Excessive LH drive enhances ovarian hyperandrogenemia and can hinder follicular development. Relative FSH deficiency also impairs follicular development; and it can enhance hyperandrogenemia via relative reductions of granulosa cell aromatase activity.
The cause of persistently rapid day-to-day GnRH pulse frequency in PCOS is not clear. Infrequent ovulation—with corresponding low progesterone levels—contributes in adults, but this does not explain why women with PCOS often never established regular periods during puberty, nor does it explain rapid GnRH pulses seen in girls with hyperandrogenemia before menarche (i.e., before ovulation has occurred) (62). Also, small increases of progesterone observed in occasional anovulatory cycles appear to be sufficient for normal GnRH pulse frequency slowing in cycling women (63). Importantly, the GnRH pulse generator is relatively resistant to the feedback effects of progesterone in adults with PCOS (64, 65). For example, seven days of exogenous progesterone and oestradiol (to achieve luteal concentrations) suppressed LH pulse frequency by 60% in cycling controls, but by only 25% in women with PCOS (65).
Hyperandrogenemia during adolescence can represent a precursor of adult PCOS, and the clinical manifestations of PCOS often begin during or shortly after puberty (66, 67). Available data suggest that gonadotropin secretion is abnormal in adolescent girls with hyperandrogenemia as well (62, 68–72). For example, Apter et al., found that late pubertal adolescents with hyperandrogenism (age 15.1 ± 0.6 y; 3.1 ± 0.5 y postmenarche) had increased LH pulse frequency during both daytime and nighttime, and higher mean 24-hour LH:FSH ratios (62). Relative overnight slowing of LH pulse frequency was apparent, and regression analysis of sleep-wake differences vs. age suggested that the transition from primarily sleep-related LH secretion to predominance of daytime LH secretion occurred 2 years early in adolescents with hyperandrogenism (62). Zumoff and colleagues reported abnormal LH pulsatility in 4 of 5 adolescents with PCOS (age 15.2 ± 0.6 y; 3.7 ± 0.7 y postmenarche); and these adolescents demonstrated increases of LH pulsatility upon awakening, contrasting with sleep-associated increases characteristic of normal adolescent controls (68). Similarly, Yoo et al., published that daytime and nighttime LH pulse frequency was increased in late pubertal girls meeting criteria for PCOS (age 15.1 ± 0.4 y; 3.5 ± 0.5 y postmenarche) (72). Our group has also reported that, as a group, adolescents with hyperandrogenemia demonstrate relative GnRH pulse generator resistance to sex steroid negative feedback (73).
Overall, these data suggest that LH pulse frequency is high during the day and night in late later pubertal girls with hyperandrogenemia. Unfortunately, there are no detailed data regarding the (presumably early pubertal) genesis of abnormal gonadotropin secretion in such girls. The underlying cause(s) of these abnormalities is (are) also unknown, but hyperandrogenemia during pubertal development may play a prominent role. For example, women with congenital adrenal hyperplasia (CAH) demonstrate elevated LH levels (74). Prenatally androgenized monkeys have elevated LH concentrations as adults (75); prenatally androgenized lambs demonstrate increased LH pulse frequency and resistance of the GnRH pulse generator to the feedback actions of progesterone as adults (76); and prenatally androgenized rats demonstrate increased activity of the GnRH pulse generator during adulthood, possibly related to reduced progesterone receptors in the hypothalamus (77). And as mentioned above, androgen receptor blockade restores GnRH pulse generator sensitivity to progesterone negative feedback in women with PCOS (54).
Our group has proposed that abnormally elevated androgens may reduce GnRH pulse generator sensitivity beyond that observed in normal adolescents, in part accounting for the development of LH secretory abnormalities (42, 73). For example, if sex steroid negative feedback plays a role in directing sleep-wake changes (i.e., daytime slowing) of GnRH pulse frequency during early puberty, then hyperandrogenemia may be associated with relatively rapid GnRH pulse frequency during both daytime and nighttime, contributing to increased LH drive and relative FSH deficiency. This hypothesis is difficult to test directly, though, in part because the manifestations of hyperandrogenemia (e.g., hirsutism) do not usually develop until later puberty. However, we and others have published data suggesting that obesity is commonly associated with hyperandrogenemia in peripubertal girls, including pre- and early pubertal girls (42, 78, 79). Thus, the study of early pubertal girls with obesity may provide insight into the effects of hyperandrogenemia on GnRH secretion throughout puberty.
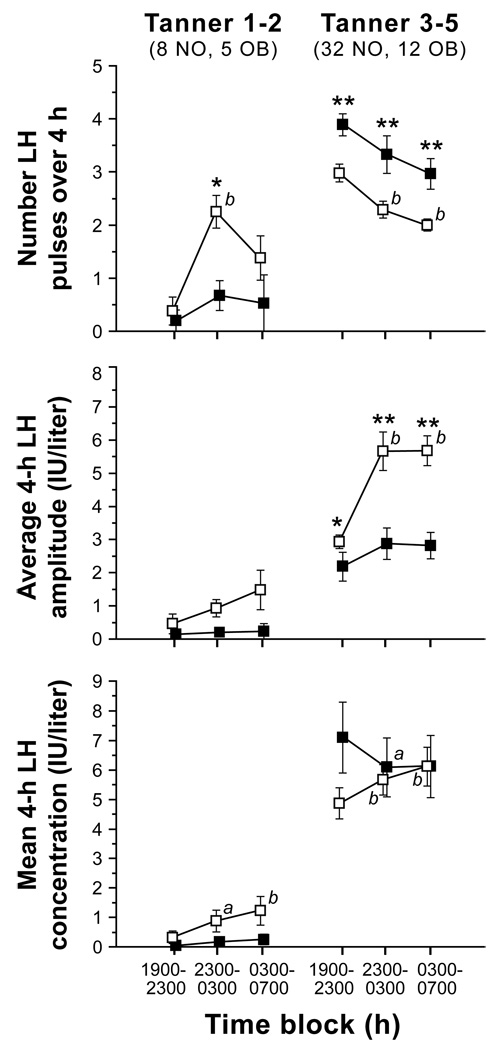
Our group has evaluated late evening and overnight LH pulse frequency in a cohort of peripubertal girls with obesity. In contrast to normal pre- and early pubertal girls (Tanner breast stages 1–2), similarly-staged girls with obesity demonstrated low LH pulse frequency with no nocturnal increase (Figure 2) (35). (Another study also revealed a blunted sleep-related rise of LH in early pubertal girls with excessive weight (80).) However, late evening and overnight LH pulse frequency was abnormally elevated in Tanner 3–5 girls with obesity (35). We hypothesized that testosterone-induced resistance to progesterone negative feedback leads to increased GnRH pulse frequency in girls with obesity, at least once the increased GnRH neuronal activity of puberty begins. That is, early breast development (i.e., Tanner breast stage 2) in obese girls may possibly reflect increased peripheral aromatization of adrenal androgens to estrogens rather than GnRH/gonadotropin-dependent ovarian function. Thus, the Tanner 2 obese girls in these studies (35, 80) may not have started neuroendocrine puberty. Also, not all girls with obesity have hyperandrogenemia (81), and the early pubertal obese girls in these particular studies did not have demonstrable hyperandrogenemia (35, 80).
Figure 2. Late evening and overnight LH secretory characteristics in non-obese (open squares) and obese (solid circles) peripubertal girls stratified by Tanner breast stage grouping.
Data are presented as mean ± SEM. Numbers per group are shown beneath Tanner stage labels (NO = non-obese; OB = obese). a = P < 0.05 vs. baseline (1900–2300 h), b = P < 0.01 vs. baseline (1900–2300 h). * = P < 0.05 vs. non-obese in the same time block, ** = P < 0.01 vs. non-obese in the same time block. (Adapted with permission from J Clin Endocrinol Metab 2009; 94: 56–66 (35); Copyright 2009, The Endocrine Society.)
Working model
Below I describe a working hypothesis regarding mechanisms controlling GnRH pulse frequency in pubertal girls with and without hyperandrogenemia (Figure 3). Notions foundational to this model include the following: puberty involves a transfer of primary GnRH pulse frequency control from the CNS (childhood) to the ovaries via sex steroid negative feedback (adulthood); and hyperandrogenemia during puberty can interfere with progesterone negative feedback of the GnRH pulse generator.
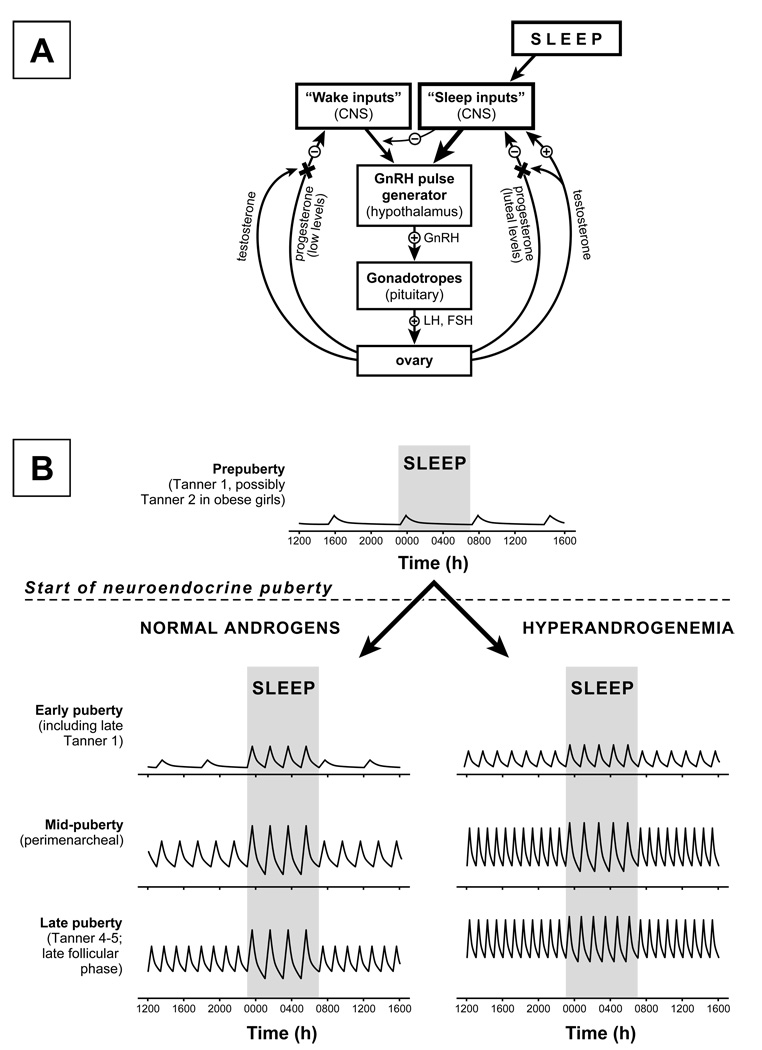
Figure 3. Working model.
Model of working hypothesis regarding control of GnRH pulse frequency after the onset of neuroendocrine puberty (i.e., once GnRH neuronal activity increases at the onset of puberty) (panel A); and predicted day-night LH pulse frequency changes in normal and hyperandrogenemic peripubertal girls (panel B). By this model, the principal controller of pubertal GnRH pulse frequency depends on sleep status. “Wake inputs” are highly sensitive to negative feedback by progesterone. However, sensitivity to negative feedback decreases across normal puberty (related to interference by rising testosterone concentrations), allowing waking pulse frequency to gradually increase. In contrast, “sleep inputs” are insensitive to low concentrations of sex steroids and supersede “wake inputs.” High concentrations of progesterone may decrease, and hyperandrogenemia may increase, the frequency imposed by sleep inputs; however, in the absence of both conditions, the GnRH pulse frequency imposed by sleep inputs remains constant across pubertal development. When neuroendocrine puberty begins in the setting of hyperandrogenemia, waking GnRH pulse frequency is immediately elevated due to androgen-induced resistance to progesterone negative feedback. Thus, such girls do not pass through a stage of low GnRH pulse frequency while awake and high GnRH pulse frequency while asleep. The LH secretory patterns shown for girls with normal androgen concentrations and later pubertal girls with hyperandrogenemia (panel B) are consistent with available data (e.g., references 35 and 62). To my knowledge, no data are available regarding LH secretory patterns in hyperandrogenemic girls shortly after neuroendocrine puberty.
GnRH pulse generator activity increases at the start of puberty, a process that involves removal of the neurobiological brake, stimulatory factors such as the kisspeptin-GPR54 system, permissive factors such as leptin, etc. Once GnRH pulse generator activity increases (i.e., at the beginning of neuroendocrine puberty), the control of GnRH pulse frequency depends primarily on sleep status. During waking hours, GnRH pulse frequency is governed by neuronal inputs (“wake inputs”) that are highly sensitive to sex steroid negative feedback. Specifically, waking GnRH pulse frequency is determined by both (a) the prevailing progesterone and oestradiol milieu (plasma levels) and (b) the sensitivity of “wake inputs” to the suppressive effects of progesterone. However, during sleep, different neuronal inputs (“sleep inputs”) take over control of the GnRH pulse generator. These inputs are not influenced by pubertal concentrations of progesterone (e.g., concentrations < 1 ng/ml); supersede wake inputs; and, during normal puberty, impose a pulse frequency of approximately one pulse every two hours. Waking GnRH pulse frequency is initially low because of exquisite sensitivity of the “wake inputs” to negative feedback by progesterone. But as normal puberty progresses, increases of testosterone reduce “wake input” sensitivity to progesterone feedback, allowing waking GnRH pulse frequency to gradually increase and eventually exceed sleep-associated frequency. Since “sleep inputs” are not affected by pubertal levels of progesterone, sleep-associated GnRH pulse frequency remains relatively fixed, regardless of the preceding (waking) frequency. Upon waking, control of the GnRH pulse generator is shifted from “sleep inputs” to “wake inputs” (i.e., sex steroid negative feedback).
In contrast to this normal developmental sequence, once GnRH pulse generator activity increases in girls with hyperandrogenemia, waking GnRH pulse frequency is elevated due to androgen-induced resistance to progesterone negative feedback. Thus, these girls do not pass through a stage of low GnRH pulse frequency while awake and high GnRH pulse frequency while asleep—a stage that is important for appropriate FSH/LH secretion, follicular development, and eventual ovulation. Relatively rapid GnRH pulses during both day and night also increase LH release, driving ovarian androgen synthesis and promoting progression toward PCOS.
Of note, sleep inputs would not be completely insensitive to sex steroids. For example, sleep-associated LH pulse frequency can be suppressed by 7 days of oestradiol and progesterone administration (approximating luteal serum concentrations) (82). It is also possible that hyperandrogenemia directly increases sleep-associated GnRH pulse frequency, since hyperandrogenic girls demonstrate elevated nighttime LH pulse frequency during later puberty (35, 62, 71, 72).
Conclusion
Mechanisms underlying the developmental maturation of sleep-wake GnRH secretion across puberty, and how these patterns may be adversely influenced by hyperandrogenemia, remain unknown. I have offered a working model that represents a revision of previous models from our group. This model may provide insight into the apparent transition of power over the GnRH pulse generator across puberty—from the CNS during childhood to the gonads (via sex steroid negative feedback) in adults. It may also help explain observed patterns of GnRH secretion across puberty in girls with and without hyperandrogenemia. These concepts clearly require further study, and many of the basic tenets of this working model can be tested in humans.
Acknowledgements
This work was/is supported by K23 HD044742; R01 HD058671; the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement U54 HD 28934; and General Clinical Research Center Grant M01 RR00847. Special thanks to Dr. John C. Marshall for excellent early-career mentorship and continued collaboration; and to Drs. Susan K. Blank, Kristin D. Helm, Kathleen A. Prendergast, Sandhya Chhabra, Christine A. Eagleson, Karen L. Knudsen, and Jessicah SP Collins, who are or have been integral members of our research group.
Bibliography
- 1.Marshall JC, Case GD, Valk TW, Corley KP, Sauder SE, Kelch RP. Selective inhibition of follicle-stimulating hormone secretion by estradiol. Mechanism for modulation of gonadotropin responses to low dose pulses of gonadotropin-releasing hormone. J Clin Invest. 1983;71(2):248–257. doi: 10.1172/JCI110765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregory SJ, Kaiser UB. Regulation of gonadotropins by inhibin and activin. Semin Reprod Med. 2004;22(3):253–267. doi: 10.1055/s-2004-831901. [DOI] [PubMed] [Google Scholar]
- 3.Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC. Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol. 2004;33(3):559–584. doi: 10.1677/jme.1.01600. [DOI] [PubMed] [Google Scholar]
- 4.Wildt L, Hausler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981;109(2):376–385. doi: 10.1210/endo-109-2-376. [DOI] [PubMed] [Google Scholar]
- 5.Clarke IJ, Cummins JT, Findlay JK, Burman KJ, Doughton BW. Effects on plasma luteinizing hormone and follicle-stimulating hormone of varying the frequency and amplitude of gonadotropin-releasing hormone pulses in ovariectomized ewes with hypothalamo-pituitary disconnection. Neuroendocrinology. 1984;39(3):214–221. doi: 10.1159/000123982. [DOI] [PubMed] [Google Scholar]
- 6.Gross KM, Matsumoto AM, Bremner WJ. Differential control of luteinizing hormone and follicle-stimulating hormone secretion by luteinizing hormone-releasing hormone pulse frequency in man. J Clin Endocrinol Metab. 1987;64(4):675–680. doi: 10.1210/jcem-64-4-675. [DOI] [PubMed] [Google Scholar]
- 7.Spratt DI, Finkelstein JS, Butler JP, Badger TM, Crowley WF., Jr Effects of increasing the frequency of low doses of gonadotropin-releasing hormone (GnRH) on gonadotropin secretion in GnRH-deficient men. J Clin Endocrinol Metab. 1987;64(6):1179–1186. doi: 10.1210/jcem-64-6-1179. [DOI] [PubMed] [Google Scholar]
- 8.Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111(5):1737–1739. doi: 10.1210/endo-111-5-1737. [DOI] [PubMed] [Google Scholar]
- 9.Levine JE, Pau KY, Ramirez VD, Jackson GL. Simultaneous measurement of luteinizing hormone-releasing hormone and luteinizing hormone release in unanesthetized, ovariectomized sheep. Endocrinology. 1982;111(5):1449–1455. doi: 10.1210/endo-111-5-1449. [DOI] [PubMed] [Google Scholar]
- 10.Rossmanith WG, Liu CH, Laughlin GA, Mortola JF, Suh BY, Yen SS. Relative changes in LH pulsatility during the menstrual cycle: using data from hypogonadal women as a reference point. Clin Endocrinol (Oxf) 1990;32(5):647–660. doi: 10.1111/j.1365-2265.1990.tb00909.x. [DOI] [PubMed] [Google Scholar]
- 11.Gill S, Lavoie HB, Bo-Abbas Y, Hall JE. Negative feedback effects of gonadal steroids are preserved with aging in postmenopausal women. J Clin Endocrinol Metab. 2002;87(5):2297–2302. doi: 10.1210/jcem.87.5.8510. [DOI] [PubMed] [Google Scholar]
- 12.Filicori M, Santoro N, Merriam GR, Crowley WF., Jr Characterization of the physiological pattern of episodic gonadotropin secretion throughout the human menstrual cycle. J Clin Endocrinol Metab. 1986;62(6):1136–1144. doi: 10.1210/jcem-62-6-1136. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen DD, Gambacciani M, Swartz W, Tueros VS, Yen SS. Pulsatile gonadotropin-releasing hormone release from the human mediobasal hypothalamus in vitro: opiate receptor-mediated suppression. Neuroendocrinology. 1989;49(2):150–156. doi: 10.1159/000125107. [DOI] [PubMed] [Google Scholar]
- 14.Soules MR, Steiner RA, Clifton DK, Cohen NL, Aksel S, Bremner WJ. Progesterone modulation of pulsatile luteinizing hormone secretion in normal women. J Clin Endocrinol Metab. 1984;58(2):378–383. doi: 10.1210/jcem-58-2-378. [DOI] [PubMed] [Google Scholar]
- 15.Lam NY, Ferin M. Is the decrease in the hypophysiotropic signal frequency normally observed during the luteal phase important for menstrual cyclicity in the primate? Endocrinology. 1987;120(5):2044–2049. doi: 10.1210/endo-120-5-2044. [DOI] [PubMed] [Google Scholar]
- 16.Marshall JC, Eagleson CA. Neuroendocrine aspects of polycystic ovary syndrome. Endocrinol Metab Clin North Am. 1999;28(2):295–324. doi: 10.1016/s0889-8529(05)70071-2. [DOI] [PubMed] [Google Scholar]
- 17.Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22(1):111–151. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- 18.Ojeda SR, Roth C, Mungenast A, Heger S, Mastronardi C, Parent AS, Lomniczi A, Jung H. Neuroendocrine mechanisms controlling female puberty: new approaches, new concepts. Int J Androl. 2006;29(1):256–263. doi: 10.1111/j.1365-2605.2005.00619.x. discussion 86–90. [DOI] [PubMed] [Google Scholar]
- 19.Plant TM. Hypothalamic control of the pituitary-gonadal axis in higher primates: key advances over the last two decades. J Neuroendocrinol. 2008;20(6):719–726. doi: 10.1111/j.1365-2826.2008.01708.x. [DOI] [PubMed] [Google Scholar]
- 20.Grumbach MM. The neuroendocrinology of human puberty revisited. Horm Res. 2002;57 Suppl 2:2–14. doi: 10.1159/000058094. [DOI] [PubMed] [Google Scholar]
- 21.Wu FC, Butler GE, Kelnar CJ, Stirling HF, Huhtaniemi I. Patterns of pulsatile luteinizing hormone and follicle-stimulating hormone secretion in prepubertal (midchildhood) boys and girls and patients with idiopathic hypogonadotropic hypogonadism (Kallmann's syndrome): a study using an ultrasensitive time-resolved immunofluorometric assay. J Clin Endocrinol Metab. 1991;72(6):1229–1237. doi: 10.1210/jcem-72-6-1229. [DOI] [PubMed] [Google Scholar]
- 22.Dunkel L, Alfthan H, Stenman UH, Selstam G, Rosberg S, Albertsson-Wikland K. Developmental changes in 24-hour profiles of luteinizing hormone and follicle-stimulating hormone from prepuberty to midstages of puberty in boys. J Clin Endocrinol Metab. 1992;74(4):890–897. doi: 10.1210/jcem.74.4.1548356. [DOI] [PubMed] [Google Scholar]
- 23.Apter D, Butzow TL, Laughlin GA, Yen SS. Gonadotropin-releasing hormone pulse generator activity during pubertal transition in girls: pulsatile and diurnal patterns of circulating gonadotropins. J Clin Endocrinol Metab. 1993;76(4):940–949. doi: 10.1210/jcem.76.4.8473410. [DOI] [PubMed] [Google Scholar]
- 24.Goji K, Tanikaze S. Spontaneous gonadotropin and testosterone concentration profiles in prepubertal and pubertal boys: temporal relationship between luteinizing hormone and testosterone. Pediatr Res. 1993;34(2):229–236. doi: 10.1203/00006450-199308000-00026. [DOI] [PubMed] [Google Scholar]
- 25.Wu FC, Butler GE, Kelnar CJ, Huhtaniemi I, Veldhuis JD. Ontogeny of pulsatile gonadotropin releasing hormone secretion from midchildhood, through puberty, to adulthood in the human male: a study using deconvolution analysis and an ultrasensitive immunofluorometric assay. J Clin Endocrinol Metab. 1996;81(5):1798–1805. doi: 10.1210/jcem.81.5.8626838. [DOI] [PubMed] [Google Scholar]
- 26.Hale PM, Khoury S, Foster CM, Beitins IZ, Hopwood NJ, Marshall JC, Kelch RP. Increased luteinizing hormone pulse frequency during sleep in early to midpubertal boys: effects of testosterone infusion. J Clin Endocrinol Metab. 1988;66(4):785–791. doi: 10.1210/jcem-66-4-785. [DOI] [PubMed] [Google Scholar]
- 27.Wennink JM, Delemarre-van de Waal HA, van Kessel H, Mulder GH, Foster JP, Schoemaker J. Luteinizing hormone secretion patterns in boys at the onset of puberty measured using a highly sensitive immunoradiometric assay. J Clin Endocrinol Metab. 1988;67(5):924–928. doi: 10.1210/jcem-67-5-924. [DOI] [PubMed] [Google Scholar]
- 28.Wu FC, Borrow SM, Nicol K, Elton R, Hunter WM. Ontogeny of pulsatile gonadotrophin secretion and pituitary responsiveness in male puberty in man: a mixed longitudinal and cross-sectional study. J Endocrinol. 1989;123(2):347–359. doi: 10.1677/joe.0.1230347. [DOI] [PubMed] [Google Scholar]
- 29.Dunkel L, Alfthan H, Stenman UH, Tapanainen P, Perheentupa J. Pulsatile secretion of LH and FSH in prepubertal and early pubertal boys revealed by ultrasensitive time-resolved immunofluorometric assays. Pediatr Res. 1990;27(3):215–219. doi: 10.1203/00006450-199003000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Oerter KE, Uriarte MM, Rose SR, Barnes KM, Cutler GB., Jr Gonadotropin secretory dynamics during puberty in normal girls and boys. J Clin Endocrinol Metab. 1990;71(5):1251–1258. doi: 10.1210/jcem-71-5-1251. [DOI] [PubMed] [Google Scholar]
- 31.Dunkel L, Alfthan H, Stenman UH, Perheentupa J. Gonadal control of pulsatile secretion of luteinizing hormone and follicle-stimulating hormone in prepubertal boys evaluated by ultrasensitive time-resolved immunofluorometric assays. J Clin Endocrinol Metab. 1990;70(1):107–114. doi: 10.1210/jcem-70-1-107. [DOI] [PubMed] [Google Scholar]
- 32.Kletter GB, Padmanabhan V, Beitins IZ, Marshall JC, Kelch RP, Foster CM. Acute effects of estradiol infusion and naloxone on luteinizing hormone secretion in pubertal boys. J Clin Endocrinol Metab. 1997;82(12):4010–4014. doi: 10.1210/jcem.82.12.4458. [DOI] [PubMed] [Google Scholar]
- 33.Clark PA, Iranmanesh A, Veldhuis JD, Rogol AD. Comparison of pulsatile luteinizing hormone secretion between prepubertal children and young adults: evidence for a mass/amplitude-dependent difference without gender or day/night contrasts. J Clin Endocrinol Metab. 1997;82(9):2950–2955. doi: 10.1210/jcem.82.9.4262. [DOI] [PubMed] [Google Scholar]
- 34.Cemeroglu AP, Foster CM, Warner R, Kletter GB, Marshall JC, Kelch RP. Comparison of the neuroendocrine control of pubertal maturation in girls and boys with spontaneous puberty and in hypogonadal girls. J Clin Endocrinol Metab. 1996;81(12):4352–4357. doi: 10.1210/jcem.81.12.8954041. [DOI] [PubMed] [Google Scholar]
- 35.McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, Marshall JC. Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: evidence for altered regulation in obese peripubertal girls. J Clin Endocrinol Metab. 2009;94(1):56–66. doi: 10.1210/jc.2008-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyar R, Finkelstein J, Roffwarg H, Kapen S, Weitzman E, Hellman L. Synchronization of augmented luteinizing hormone secretion with sleep during puberty. N Engl J Med. 1972;287(12):582–586. doi: 10.1056/NEJM197209212871203. [DOI] [PubMed] [Google Scholar]
- 37.Boyar RM, Rosenfeld RS, Kapen S, Finkelstein JW, Roffwarg HP, Weitzman ED, Hellman L. Human puberty. Simultaneous augmented secretion of luteinizing hormone and testosterone during sleep. J Clin Invest. 1974;54(3):609–618. doi: 10.1172/JCI107798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albertsson-Wikland K, Rosberg S, Lannering B, Dunkel L, Selstam G, Norjavaara E. Twenty-four-hour profiles of luteinizing hormone, follicle-stimulating hormone, testosterone, and estradiol levels: a semilongitudinal study throughout puberty in healthy boys. J Clin Endocrinol Metab. 1997;82(2):541–549. doi: 10.1210/jcem.82.2.3778. [DOI] [PubMed] [Google Scholar]
- 39.Goji K. Twenty-four-hour concentration profiles of gonadotropin and estradiol (E2) in prepubertal and early pubertal girls: the diurnal rise of E2 is opposite the nocturnal rise of gonadotropin. J Clin Endocrinol Metab. 1993;77(6):1629–1635. doi: 10.1210/jcem.77.6.8263151. [DOI] [PubMed] [Google Scholar]
- 40.Ankarberg C, Norjavaara E. Diurnal rhythm of testosterone secretion before and throughout puberty in healthy girls: correlation with 17beta-estradiol and dehydroepiandrosterone sulfate. J Clin Endocrinol Metab. 1999;84(3):975–984. doi: 10.1210/jcem.84.3.5524. [DOI] [PubMed] [Google Scholar]
- 41.Mitamura R, Yano K, Suzuki N, Ito Y, Makita Y, Okuno A. Diurnal rhythms of luteinizing hormone, follicle-stimulating hormone, testosterone, and estradiol secretion before the onset of female puberty in short children. J Clin Endocrinol Metab. 2000;85(3):1074–1080. doi: 10.1210/jcem.85.3.6445. [DOI] [PubMed] [Google Scholar]
- 42.McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, Helm KD, Yoo R, Chang RJ, Foster CM, Caprio S, Marshall JC. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab. 2007;92(2):430–436. doi: 10.1210/jc.2006-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu FC, Butler GE, Kelnar CJ, Sellar RE. Patterns of pulsatile luteinizing hormone secretion before and during the onset of puberty in boys: a study using an immunoradiometric assay. J Clin Endocrinol Metab. 1990;70(3):629–637. doi: 10.1210/jcem-70-3-629. [DOI] [PubMed] [Google Scholar]
- 44.Hall JE, Sullivan JP. Richardson GS. Brief wake episodes modulate sleep-inhibited luteinizing hormone secretion in the early follicular phase. J Clin Endocrinol Metab. 2005;90(4):2050–2055. doi: 10.1210/jc.2004-2033. [DOI] [PubMed] [Google Scholar]
- 45.McCartney CR, Blank SK, Marshall JC. Progesterone acutely increases LH pulse amplitude but does not acutely influence nocturnal LH pulse frequency slowing during the late follicular phase in women. Am J Physiol Endocrinol Metab. 2007;292(3):E900–E906. doi: 10.1152/ajpendo.00371.2006. [DOI] [PubMed] [Google Scholar]
- 46.Stanhope R, Brook CG, Pringle PJ, Adams J, Jacobs HS. Induction of puberty by pulsatile gonadotropin releasing hormone. Lancet. 1987;2(8558):552–555. doi: 10.1016/s0140-6736(87)92932-1. [DOI] [PubMed] [Google Scholar]
- 47.Touzet S, Rabilloud M, Boehringer H, Barranco E, Ecochard R. Relationship between sleep and secretion of gonadotropin and ovarian hormones in women with normal cycles. Fertil Steril. 2002;77(4):738–744. doi: 10.1016/s0015-0282(01)03254-x. [DOI] [PubMed] [Google Scholar]
- 48.DiVall SA, Radovick S. Endocrinology of female puberty. Current opinion in endocrinology, diabetes, and obesity. 2009;16(1):1–4. doi: 10.1097/med.0b013e3283207937. [DOI] [PubMed] [Google Scholar]
- 49.Grumbach MM, Styne DM. Puberty: ontogeny, neuroendocrinology, physiology, and disorders. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Williams Textbook of Endocrinology. 10th ed. Philadelphia: Saunders; 2003. pp. 1115–1239. [Google Scholar]
- 50.Kelch RP, Kaplan SL, Grumbach MM. Suppression of urinary and plasma follicle-stimulating hormone by exogenous estrogens in prepubertal and pubertal children. J Clin Invest. 1973;52(5):1122–1128. doi: 10.1172/JCI107278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steele RE, Weisz J. Changes in sensitivity of the estradiol-LH feedback system with puberty in the female rat. Endocrinology. 1974;95(2):513–520. doi: 10.1210/endo-95-2-513. [DOI] [PubMed] [Google Scholar]
- 52.Foster DL, Ryan KD. Endocrine mechanisms governing transition into adulthood: a marked decrease in inhibitory feedback action of estradiol on tonic secretion of luteinizing hormone in the lamb during puberty. Endocrinology. 1979;105(4):896–904. doi: 10.1210/endo-105-4-896. [DOI] [PubMed] [Google Scholar]
- 53.Rapisarda JJ, Bergman KS, Steiner RA, Foster DL. Response to estradiol inhibition of tonic luteinizing hormone secretion decreases during the final stage of puberty in the rhesus monkey. Endocrinology. 1983;112(4):1172–1179. doi: 10.1210/endo-112-4-1172. [DOI] [PubMed] [Google Scholar]
- 54.Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, Marshall JC. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85(11):4047–4052. doi: 10.1210/jcem.85.11.6992. [DOI] [PubMed] [Google Scholar]
- 55.Pielecka J, Quaynor SD, Moenter SM. Androgens increase gonadotropin-releasing hormone neuron firing activity in females and interfere with progesterone negative feedback. Endocrinology. 2006;147(3):1474–1479. doi: 10.1210/en.2005-1029. [DOI] [PubMed] [Google Scholar]
- 56.Blank SK, McCartney CR, Marshall JC. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update. 2006;12(4):351–361. doi: 10.1093/humupd/dml017. [DOI] [PubMed] [Google Scholar]
- 57.Cemeroglu AP, Kletter GB, Guo W, Brown MB, Kelch RP, Marshall JC, Padmanabhan V, Foster CM. In pubertal girls, naloxone fails to reverse the suppression of luteinizing hormone secretion by estradiol. J Clin Endocrinol Metab. 1998;83(10):3501–3506. doi: 10.1210/jcem.83.10.5207. [DOI] [PubMed] [Google Scholar]
- 58.McCartney CR, Blank SK, Helm KD, Marshall JC. Evidence That Sex Steroid Feedback Milieu Influences Daytime More Than Nighttime GnRH Frequency; 90th Meeting of the Endocrine Society; San Francisco, CA. 2008. [Google Scholar]
- 59.Loucks AB, Heath EM. Dietary restriction reduces luteinizing hormone (LH) pulse frequency during waking hours and increases LH pulse amplitude during sleep in young menstruating women. J Clin Endocrinol Metab. 1994;78(4):910–915. doi: 10.1210/jcem.78.4.8157720. [DOI] [PubMed] [Google Scholar]
- 60.Loucks AB, Verdun M, Heath EM. Low energy availability, not stress of exercise, alters LH pulsatility in exercising women. J Appl Physiol. 1998;84(1):37–46. doi: 10.1152/jappl.1998.84.1.37. [DOI] [PubMed] [Google Scholar]
- 61.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352(12):1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 62.Apter D, Butzow T, Laughlin GA, Yen SS. Accelerated 24-hour luteinizing hormone pulsatile activity in adolescent girls with ovarian hyperandrogenism: relevance to the developmental phase of polycystic ovarian syndrome. J Clin Endocrinol Metab. 1994;79(1):119–125. doi: 10.1210/jcem.79.1.8027216. [DOI] [PubMed] [Google Scholar]
- 63.Clayton RN, Royston JP, Chapman J, Wilson M, Obhrai M, Sawers RS, Lynch SS. Is changing hypothalamic activity important for control of ovulation? Br Med J (Clin Res Ed) 1987;295(6589):7–12. doi: 10.1136/bmj.295.6589.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Daniels TL, Berga SL. Resistance of gonadotropin releasing hormone drive to sex steroid-induced suppression in hyperandrogenic anovulation. J Clin Endocrinol Metab. 1997;82(12):4179–4183. doi: 10.1210/jcem.82.12.4402. [DOI] [PubMed] [Google Scholar]
- 65.Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC. Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 1998;83(2):582–590. doi: 10.1210/jcem.83.2.4604. [DOI] [PubMed] [Google Scholar]
- 66.Shayya R, Chang RJ. Reproductive endocrinology of adolescent polycystic ovary syndrome. Bjog. 2010;117(2):150–155. doi: 10.1111/j.1471-0528.2009.02421.x. [DOI] [PubMed] [Google Scholar]
- 67.Franks S. Adult polycystic ovary syndrome begins in childhood. Best practice & research. 2002;16(2):263–272. doi: 10.1053/beem.2002.0203. [DOI] [PubMed] [Google Scholar]
- 68.Zumoff B, Freeman R, Coupey S, Saenger P, Markowitz M, Kream J. A chronobiologic abnormality in luteinizing hormone secretion in teenage girls with the polycystic-ovary syndrome. N Engl J Med. 1983;309(20):1206–1209. doi: 10.1056/NEJM198311173092002. [DOI] [PubMed] [Google Scholar]
- 69.Venturoli S, Porcu E, Fabbri R, Magrini O, Gammi L, Paradisi R, Flamigni C. Longitudinal evaluation of the different gonadotropin pulsatile patterns in anovulatory cycles of young girls. J Clin Endocrinol Metab. 1992;74(4):836–841. doi: 10.1210/jcem.74.4.1548348. [DOI] [PubMed] [Google Scholar]
- 70.Porcu E, Venturoli S, Longhi M, Fabbri R, Paradisi R, Flamigni C. Chronobiologic evolution of luteinizing hormone secretion in adolescence: developmental patterns and speculations on the onset of the polycystic ovary syndrome. Fertil Steril. 1997;67(5):842–848. doi: 10.1016/s0015-0282(97)81395-7. [DOI] [PubMed] [Google Scholar]
- 71.Garcia-Rudaz MC, Ropelato MG, Escobar ME, Veldhuis JD, Barontini M. Augmented frequency and mass of LH discharged per burst are accompanied by marked disorderliness of LH secretion in adolescents with polycystic ovary syndrome. Eur J Endocrinol. 1998;139(6):621–630. doi: 10.1530/eje.0.1390621. [DOI] [PubMed] [Google Scholar]
- 72.Yoo RY, Dewan A, Basu R, Newfield R, Gottschalk M, Chang RJ. Increased luteinizing hormone pulse frequency in obese oligomenorrheic girls with no evidence of hyperandrogenism. Fertil Steril. 2006;85(4):1049–1056. doi: 10.1016/j.fertnstert.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 73.Blank SK, McCartney CR, Chhabra S, Helm KD, Eagleson CA, Chang RJ, Marshall JC. Modulation of GnRH pulse generator sensitivity to progesterone inhibition in hyperandrogenic adolescent girls - implications for regulation of pubertal maturation. J Clin Endocrinol Metab. 2009;94:2360–2366. doi: 10.1210/jc.2008-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barnes RB, Rosenfield RL, Ehrmann DA, Cara JF, Cuttler L, Levitsky LL, Rosenthal IM. Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: evidence for perinatal masculinization of neuroendocrine function in women. J Clin Endocrinol Metab. 1994;79(5):1328–1333. doi: 10.1210/jcem.79.5.7962325. [DOI] [PubMed] [Google Scholar]
- 75.Dumesic DA, Abbott DH, Eisner JR, Goy RW. Prenatal exposure of female rhesus monkeys to testosterone propionate increases serum luteinizing hormone levels in adulthood. Fertil Steril. 1997;67(1):155–163. doi: 10.1016/s0015-0282(97)81873-0. [DOI] [PubMed] [Google Scholar]
- 76.Robinson JE, Forsdike RA, Taylor JA. In utero exposure of female lambs to testosterone reduces the sensitivity of the gonadotropin-releasing hormone neuronal network to inhibition by progesterone. Endocrinology. 1999;140(12):5797–5805. doi: 10.1210/endo.140.12.7205. [DOI] [PubMed] [Google Scholar]
- 77.Foecking EM, Szabo M, Schwartz NB, Levine JE. Neuroendocrine consequences of prenatal androgen exposure in the female rat: absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biol Reprod. 2005;72(6):1475–1483. doi: 10.1095/biolreprod.105.039800. [DOI] [PubMed] [Google Scholar]
- 78.Reinehr T, de Sousa G, Roth CL, Andler W. Androgens before and after weight loss in obese children. J Clin Endocrinol Metab. 2005;90(10):5588–5595. doi: 10.1210/jc.2005-0438. [DOI] [PubMed] [Google Scholar]
- 79.McCartney CR, Prendergast KA, Chhabra S, Eagleson CA, Yoo R, Chang RJ, Foster CM, Marshall JC. The Association of Obesity and Hyperandrogenemia during the Pubertal Transition in Girls: Obesity as a Potential Factor in the Genesis of Postpubertal Hyperandrogenism. J Clin Endocrinol Metab. 2006;91(5):1714–1722. doi: 10.1210/jc.2005-1852. [DOI] [PubMed] [Google Scholar]
- 80.Bordini B, Littlejohn E, Rosenfield RL. Blunted sleep-related luteinizing hormone rise in healthy premenarcheal pubertal girls with elevated body mass index. J Clin Endocrinol Metab. 2009;94(4):1168–1175. doi: 10.1210/jc.2008-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Knudsen KL, Blank SK, Burt Solorzano C, Patrie JT, Chang RJ, Caprio C, Marshall JC, McCartney CR. Variable hyperandrogenemia in obese peripubertal girls: correlates and potential etiological determinants. Obesity. 2010 doi: 10.1038/oby.2010.58. advance online publication (Epub ahead of print) March 25, 2010, as doi:10.1038/oby.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blank SK, McCartney CR, Chhabra S, Helm KD, Eagleson CA, Chang RJ, Marshall JC. Modulation of gonadotropin-releasing hormone pulse generator sensitivity to progesterone inhibition in hyperandrogenic adolescent girls--implications for regulation of pubertal maturation. J Clin Endocrinol Metab. 2009;94(7):2360–2366. doi: 10.1210/jc.2008-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]