Abstract
Overexpression of the endothelin A (ẸTA) receptor has been found in a number of human cancer cell lines. Activation of the ETA receptor by endothelin-1 (ET-1) promotes cell proliferation and survival in these tumors, whereas activation of the endothelin B (ETB) receptor results in an opposing effect. Therefore, blockade of ETA may have antitumor effects, while sparing ETB-mediated effects such as induction of apoptosis and clearance of ET-1. ZD4054 is an orally bioavailable, specific ETA antagonist currently being investigated in prostate cancer. In receptor-binding studies, ZD4054 only bound to ETA with no binding detected towards ETB. Prostate cancer cell lines are known to produce ET-1 and there is a relative increase in expression of ETA to ETB in these cancers. There is also an association of greater ETA expression in higher grade versus lower grade tumors, suggesting that ETA may be involved in the malignant transformation process. Since ET-1 may also mediate nociceptive effects and osteoblastic effects, there is much interest in clinically assessing ZD4054 in prostate cancer.
Keywords: Prostate cancer, endothelin receptor antagonist, osteoblast, bone metastasis
1. Introduction
Prostate cancer is the most common solid malignancy in men. It is estimated to be diagnosed in approximately 186,320 men, resulting in 28,660 deaths, in the United States in 2008 alone 1. While androgen-deprivation therapy is the mainstay of treatment in those with advanced disease, eventually all patients will develop castrate-resistant prostate cancer (CRPC), Mitoxantrone with prednisone was approved by the Food and Drug Administration (FDA) in 1996 for men with metastatic CRPC, based on an improvement in pain and quality of life (QOL) parameters, with no difference in overall survival observed 2, 3. Docetaxel and prednisone are currently the standard of care for patients with metastatic CRPC, and was FDA approved based on a statistically significant improvement in median survival of 2 – 2.5 months over mitoxantrone chemotherapy 4. While many may not consider this a giant leap forward, it does represent a new standard in this disease, against which future drugs will be judged. As a result, the approval of docetaxel-based chemotherapy has changed the current drug development strategies in metastatic CRPC to focus on either the pre-chemotherapy (pre-docetaxel) population, front-line chemotherapy, assessing newer agents in combination with docetaxel, or the post-docetaxel population in which no standard exists. Depending on which population is being targeted, an appropriate benchmark for drug activity success will need to be chosen based on the drug’s mechanism of action, goals of therapy, as well as acceptance by regulatory bodies.
Metastatic prostate cancer is associated with significant morbidity, as it results in the development of osteoblastic bony metastases 5. This can be accompanied by pain, increased fracture risk, and a general decrease in QOL 3. In addition, hematological complications and neurologic compromise can occur, adding to the morbidity of this disease. Management of osseous tumor involvement is necessary. Androgen-deprivation therapy (ADT) is a temporary measure, but will accelerate osteoporosis, potentially increasing skeletal events 6, 7. Bisphosphonates, which inhibit osteoclast activity, have been shown to decrease pain and musculoskeletal events in men with osseous metastasis8.
While there is great need for newer therapies in CRPC, the road ahead is not well marked. Although the gold standard for drug development is showing a survival improvement, limitations include difficulties showing survival differences early in the disease course as well as in the pre-taxane state, due to the heterogeneity of disease as well as competing mortality in this older population 9. In addition, the traditional reliance on changes in objective disease response is problematic in a disease manifesting primarily with bony metastasis10. As a result, we have become overly dependent on changes in the prostate specific antigen (PSA), which we are beginning to learn may not always correlate with clinical benefit or survival 11, 12.
Recent advances in the understanding of prostate cancer biology and its progression to bone metastasis have led to the development of drugs that target specific molecular alterations in the prostate tumor cell, host environment and bone. Targeting specific pathways to inhibit cancer growth, proliferation, and metastasis may be less toxic and better tolerated than conventional cytotoxic chemotherapies. The endothelin axis and its receptor-signaling pathway, is one such target that may be particularly relevant in prostate cancer, and drugs that antagonize this pathway, specifically ZD4054 is the subject of this review.
2. Endothelin and its role in prostate cancer
2.1 Endothelin Biology
The endothelins (ETs) are a family of three 21 amino acid peptides: ET-1, -2, and -3. Widely expressed in mammalian cells, they exert autocrine effects through cell-surface receptors to influence normal cellular processes involving maintenance of vasomotor tone, cellular proliferation, tissue repair and development. A number of disease states, such as hypertension (systemic and pulmonary), congestive heart failure, asthma, post-ischemic renal failure, and cerebral vasospasm, as well as neoplasia, are contributed, in part, by the dysregulation of these receptors and signaling pathways 13–19.
2.2 Pathophysiology of endothelin
2.2.1 The endothelin axis
The endothelin axis initiates with large pro-peptides (preproendothelins) in tissues, and undergo a two-step enzymatic cleavage to ETs. The ETs are expressed differentially depending on the tissue type. ET-1, which is not organ specific, is expressed predominantly in endothelial, epithelial, and smooth muscle cells. ET-2 is primarily found in the kidney and intestine. Lastly, ET-3 is mainly seen in the brain, intestine, lung and kidney 14, 19. The ETs transmit their actions through two G-protein membrane receptors, ETA and ETB. ETA has a ten-fold affinity for ET-1 and ET-2 over ET-3, and is the major effector of the ET axis. ETB has equal affinity for all three ETs 13–15, 18, 19. The differential expression and activation of ETA and ETB in tissues determines the eventual effect that the endothelin axis exerts on the cells 13, 14, 19, 20.
2.2.2 Endothelin signal cascade in carcinogenesis
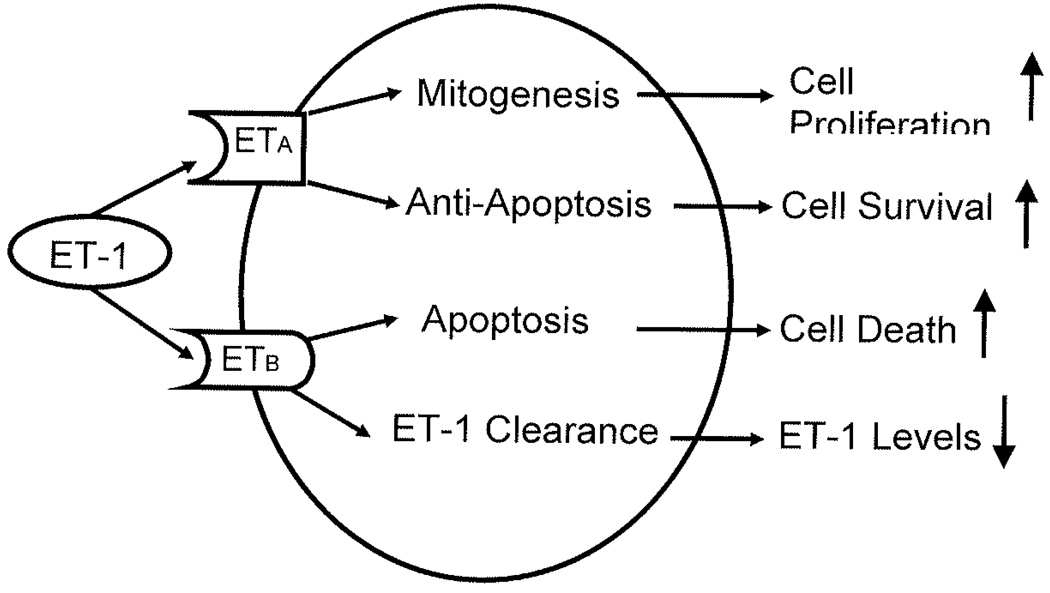
The ET-1/ETA signaling cascade is the main endothelin interaction implicated in carcinogenesis. ET-1 activates ETA via an intracellular G-protein, triggering several cellular signaling pathways (see Figure 1). This cascade leads to the activation of phospholipase C (PLC) and protein kinase C (PKC) resulting in increased levels of intracellular calcium stores14, 15, 21. In addition, activation of the RAS/MAPK pathway occurs through phosphorylation of epidermal growth factor receptor (EGFR) tyrosine kinase 22, 23. Together, the activation of MAPK and PKC pathways, combined with increased intracellular calcium level, leads to the induction of protooncogenes, such as c-MYC, c-FOS, and c-JUN and hence stimulates cellular growth and mitogenesis 23, 24. Finally, ET-1 has a negative regulatory effect on apoptosis. These effects are dependant upon activation of phosphatidylinositol-3-kinase (PI3-K) and Akt protein kinase pathways. Pretreatment of cells with a selective ETA receptor antagonist has been shown to reverse the anti-apoptotic effects of ET-1, implicating the ET-1/ETA interaction as a survival mechanism in cancer progression 25, 26.
Figure 1.
Shown is the endothelin axis and cascade. Activation of endothelin receptor A (ETA) by enodothelin-1 (ET-1) leads to increased cell proliferation and survival. Activation of endothelin receptor B (ETB) by ET-1 facilitates cell apoptosis and results in decreased levels endothelin-1 (ET-1).
2.3 Role of endothelin and the ETA receptor in cancer
2.3.1 Expression in cancer cells
ETs and their receptors ETA/B are expressed in several cancer cell lines and tumor types13 including cancers of the prostate 27, ovary 28, cervix 29, 30, breast31, melanocytes32, kidney, lung, colon, central nervous system, and Kaposi’s sarcoma 13. ETA receptors are present in high density throughout the prostate gland, and seminal fluid contains the highest concentrations of ET-1, with concentrations near 500 times that of plasma 27. Increased ETA expression correlates significantly with increased tumor stage and aggressiveness 33, 34. Conversely, ETB appears to be down-regulated in the presence of cancer. Though ETB binding sites are found on benign prostatic tissue, they are much reduced or absent in metastatic prostate cancer20, 35. ETB counter-regulates ET-1/ETA activity through a variety of mechanisms: 1) it increases production of nitrous oxide, thus counteracting ETA ET -induced calcium-dependant vasoconstriction; 2) provokes apoptotic pathways, balancing the cell growth and survival pathways of ET; 3) it directly incites clearance of ET-1; and 4) it may inhibit secretion of ET-1 20, 34, 35.
The balance of ETA and ETB activation in tumor cells appears to be important in the progression of most cancers 21, especially prostate cancer36, contributing to increased tumor cell survival and growth.
2.3.2 Cell growth and survival
There is accumulating evidence to suggest activation of ETA by ET-1 has a role in regulating growth and proliferation of tumors 17. ET-1 induces DNA synthesis and cell proliferation in numerous cells, including osteoblast, fibroblast, prostatic smooth muscle and epithelium and prostate cancers 27, 36, 37. In vitro, the activity of ET-1 in prostate cancer is demonstrated by the induced proliferation of all prostate cancer cell lines by exogenous ET-127. In preclinical studies, selective ETA receptor antagonist, ZD4054, specifically inhibited ETA mediated anti-apoptotic effects on human smooth muscle cells.38, 39
The binding of ET-1 to ETA and ETB causes distinct and opposing effects on cell growth and survival (see Figure 1), In most cells, activation of ETA promotes cell growth 17, while activation of ETB induces apoptosis 40. Therefore selective targeting of ETA may be useful in the treatment of prostate cancer
2.3.3 Angiogenesis in the malignant process
ET-1 and ETA have been linked to neovascularization in both tumor and the surrounding environment36. Activation of ETA by ET-1 modulates the production of vascular endothelin growth factor (VEGF), which can promote angiogenesis, in part by inducing hypoxia-inducible factor-1, promoting endothelial cell proliferation and enhancing vascular permeability 17, 41–44. VEGF is overexpressed in many tumors, including prostate cancer. In vivo, the combination of ET-1 and VEGF produce significantly more angiogenesis than either alone 45, 46.
2.3.4 Spread and development of bone metastasis
The activation of ETA by ET-1 upregulates tumor proteases (matrix metalloproteinases) and urokinase-type plasminogen activator 47. These mechanisms of invasion and migration are inhibited when ET-1 is blocked by ETA-selective receptor antagonists 47–49.
Additionally, activation of ETA by ET-1 leads to proliferation of osteoblasts, bone remodeling and release of growth factors that stimulate survival and growth of metastatic50–52tumor cells within osseous metastasis 53, 54. ET-1 has also been shown to stimulate mitogenesis in the osteoblast and, at the same time, decrease osteoclastic bone resorption and motility53. Studies have demonstrated ET-1 stimulation increased alkaline phosphatases activity, suggesting an osteoblastic response 27. Prostate cancer cell lines co-cultured with bone slices had increased levels of ET-1 and inhibited osteoclastic bone resorption. This effect was negated by the addition of a specific anti-ET-1 antibody 37, 53, 55. In pre-clinical studies, selective endothelin A receptor antagonist, ZD4054, was shown to block ETA- mediated activation of p44/42 mitogen-activated protein kinase in murine osteoblast cells and inhibit ET-1 induced proliferation of human immature pre-osteoblast cells.56
2.3.5 Role in the nociceptive response
Human subjects injected with ET-1 experience intense dose-dependant pain; this pain can be reduced on administration of an ETA receptor antagonists 57. High levels of ET-1 are found in dorsal root ganglion and ETA receptors can be found on small to medium sized root ganglion neurons and their axons 58. Through a recently defined intrinsic feedback mechanism, ET-1 is able to trigger pain by stimulating the ETA receptors located in the root ganglion neurons. Conversely, ET-1 activation of ETB produces an anti-nociceptive or analgesic effect by the release of β- endorphin 59. Selective inhibition of ETA markedly reduces the ET-1 stimulated pain response while preserving the favorable anti-nociceptive or analgesic effects of ETB activation 60.
In summary, numerous studies implicate the ET-1/ETA interaction as a pivotal player in cancer cell signaling pathways, growth, proliferation, avoidance of apoptosis, invasion, angiogenesis. metastasis (particularly to bone) and the propagation of pain. All of which are inhibited by the use of selective ETA receptor antagonists, while retaining beneficial ETB receptor mediated effects such as apoptosis and clearance of ET-1 and antinociceptive or analgesic activity.
3. Endothelin antagonists in cancer therapy
3.1 Endothetin antagonists
There are several endothelin antagonists which have been actively studied for cancer treatment in clinical trials. These drugs selectively target ETA over ETB, but in varying degrees.
YM598 (Astellas Pharma Inc.) is a highly selective ETA antagonist that is 816-fold more selective for the ETA receptor than ETB (Ki = 0.697 and 569nM (n=6) for ETA and ETB respectively)61. YM598 has been combined with mitoxantrone and prednisone in a randomized, double-blind, placebo-controlled phase II trial to assess benefits in cancer related pain in men with metastatic CRPC. However, this trial was terminated early due to lack of efficacy.
Atrasentan (ABT-627; Xinlay: Abbott Laboratories). It is an orally bioavailable, selective ETA receptor inhibitor (Ki= .069 and 138.6 nM (n=3) for ETA and ETB. IC50 = 0.11 nM and 98.2nM (n=5) for ETA and ETB) - more than 2000 times more selective for ETA over ETB62. In phase I studies it was found to be have good tolerability and safety in a diverse population.
Several phase II trials have been conducted evaluating Atrasentan in men with CRPC, The first assessed pain relief and changes in bone markers in men with metastatic CRPC requiring opioids. The second assessed time to clinical progression in asymptomatic men with metastatic CRPC. Though both trials showed positive trends, their primary endpoints were not statistically significant 63, 64. A third study combined Atrasentan with zolendronic acid in men with metastatic prostate cancer. This trial too failed to demonstrate its primary endpoint of bone marker improvement 65.
In a phase III, multinational, double-blind, randomized, placebo-controlled trial of 809 men with metastatic CRPC, despite encouraging trends, its primary endpoint of time to disease progression did not reach statistical significance 66.
A current phase III trial combing Atrasentan and Taxotere in patients with advanced CRPC is ongoing and we await its results upon completion.
While each of these studies had numerous challenges and failed to reach their primary end points, they contribute largely to the accumulating and consistent data of endothelin antagonist research and offer information for future trial designs.
4. ZD4054
4.1 Pharmacology
4.1.1 Chemical name, structure and properties
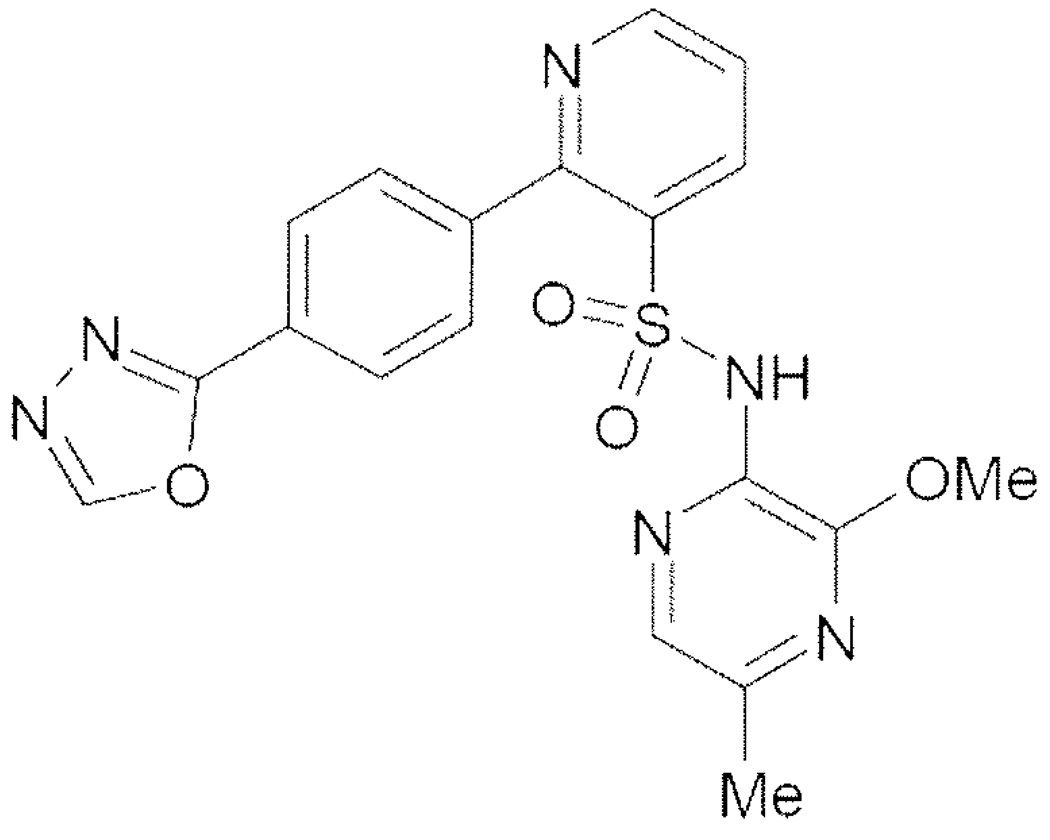
ZD4054, chemically designated as N-(3-Methoxy-5-niethylpyrazine-2-yl)-2-(4-[1,3,4-oxadiazol-2-yl]phenyl)pyridine-3-sulfonamide is a crystalline solid with two measurable pKa values at 1.46 and 5.66 (see Figure 2). It is soluble in distilled water (0.12mg/ml at 25 Celsius). The molecular weight is 424.4. 67.
Figure 2.
Chemical structure of ZD4054
4.1.2 Receptor specificity
Of the orally available endothelin receptor antagonists, ZD4054 most potently and selectively binds ETA over ETB. In multi-receptor binding screens the mean Ki values measured were 13nM and mean pIC50 values were 21 nM. In contrast, ZD4054 had no measurable affinity for cloned human ETB. In the same multi-receptor binding screens ZD4054 was inactive at ETB at a concentration of >10µM.54
4.1.3 Distribution and metabolism
In studies of [14 C]-ZD4054 in healthy volunteers reported by Clarkson-Jones, et al show radioactivity in whole blood was generally less than in plasma and mean plasma protein binding level was 73%. This suggests a limited association of drug-related material with blood cells and is unlikely to be affected by co-medication with other drugs, at least as consequence of protein binding displacement interactions68.
Concentrations of ZD4054 were similar to total radioactivity until 12 hours post dose, after which concentrations of radioactivity were slightly higher than zd4054, indicating the presence of circulating metabolites. P6 (hydroxide) was the only detectable metabolite, accounting 4% of plasma radioactivity and no other metabolites were detected after 24 hours68.
There were similar metabolite profiles for urine and feces samples. The main metabolites in urine and feces were P3 (despyrazine), P4 (hydroxylated pyrazine), P6( hydroxide) and P3, P4. respectively. Overall recoveries of radioactivity were high, ranging from 81–99% (mean 93.4%). Excretion via the urine was rapid and extensive: recoveries ranged from 71–94% of the dose, with mean renal clearance of ZD4054 calculated at 1.1 liter/hour. Renal clearance contributes significantly to the overall clearance of ZD405468.
Preclinical results indicate that ZD4054 is metabolized via the cytochrome P450 system using isozyme 3A4 (Cyp3A4). A study was designed to evaluate the effect of a potent CYP3A4 inducer and CYP3A4 inhibitor on the pharmacokinetics and metabolism of ZD405469. This trial showed that ZD4054 15mg given to healthy volunteers predosed with rifampicin 600mg reduced the Cmax and the AUC of ZD4054 by 29 and 68%, respectively. Rifampicin also reduced the t ½ from 8.2 to 2.7 hours, while the t max did not seem to be affected. This suggests that CYP3A4-inducing drugs may reduce the efficacy of ZD4054. Conversely, in volunteers receiving ZD4054 10mg predosed with CYP3A4 inhibitor itraconazole increased the C max and AUC of ZD4054 by 29 and 27%, respectively. This is a small increase of ZD4054 exposure and effect that is unlikely to significantly alter the safety profile69.
4.2 Clinical development of ZD4054
4.2.1 Pharmacokinetics
Pharmacokinetics of ZD4054 has been studied in both healthy volunteers and patients68, 70. Single dosing of 15mg ZD4054 was rapidly absorbed with peak plasma concentrations (Cmax) observed in 1–3 hours after dosing and exposure increased with dose68, 70. Plasma concentrations declined in a monophasic manner and the mean half-life was between 9–12 hours70.
In multiple dose kinetics studies there was minimal accumulation of ZD4054 after 4 or 5 daily consecutive doses. AUC 0–24 values on day 15 were generally in accordance with predicted values from the single dose data70.
In studies comparing between ethnic groups of Caucasian and Japanese patients there were no marked differences in the Cmax and AUC0–24 in either single or multiple dosing schedules. In Japanese patients who received ZD4054 15mg (single or multiple dose), some patients achieved higher exposure than Caucasian counterparts, but these differences disappeared when the data were normalized for the body weights of the patients70.
4.2.3 Clinical Safety
Safety and tolerability profiles remain consistent throughout a number of clinical trials69–71. In a phase IIa, open-label, multi-center dose-escalation study the starting dose was 10mg and doses were escalated to 15mg and 22.5mg71. The therapy was well tolerated. Dose limiting toxicities were encountered at 22.5mg with dyspnea, peripheral edema, headache and intraventricular hemorrhage. At 15mg, no dose limiting toxicities were seen. Secondary to vasodilation and fluid retention, the most frequent adverse events at 15mg were headache, peripheral edema, fatigue, nasal congestion and nausea. Mild weight gain (0.7kg) and decrease in hemoglobin of 0.8mg/dl (from hemodilution) were also observed. These events were reversible upon drug cessation. This observed tolerability profile is consistent with effects rising from specific ETA antagonism 71.
4.2.4 Efficacy of ZD4054: phase II prostate cancer study data
ZD4054 was further studied in a multi-center phase II, randomized, double blind, placebo-controlled trial. A total of 312 asymptomatic or mildly symptomatic CRPC patients with bone metastasis were randomized into one of three treatment arms: 15mg ZD4054 once daily; 10mg ZD4054 once daily or a placebo tablet. The primary end point of the study was progression free survival (PFS) and a secondary endpoint was overall survival (OS). While the PFS data did not show a statistically significant difference between ZD4054 and placebo treatment arms, preliminary survival data suggested an improvement in OS. Patients who received ZD4054 10mg once daily experienced a 45% reduction in the risk of death compared with placebo (HR 0.55; 80% CI 0.41, 0.73), translating into an improved median OS of 24.5 months with ZD4054 10mg once-daily compared with 17.3 months in the placebo arm.
Patients who received ZD4054 15mg once-daily experienced a 35% reduction in the risk of death (HR 0.65; 80 CI 0.49, 0.86), again translating into an improved median OS of 23.5 months with ZD4054 15mg once daily compared with 17.3 months in the placebo arm 72, 73.
4.2.5 Efficacy of ZD4054: phase III prostate cancer study data
Currently, there are 3 phase III trials conducted as part of a program known as Endothelin A Use (ETHUSE). All of the trials will use a once daily dose of ZD4054 at 10mg. The first is a large phase III study of the safety and efficacy of ZD4054 vs. placebo in patients with CRPC and bone metastases. The primary outcomes focus on overall survival. The second trial tests the efficacy of ZD4054 vs. placebo in men with CRPC without evidence of metastases, but have rising PSA values. The primary outcomes are overall survival and progression free survival. Lastly, the third of the phase III trials combines ZD4054 with docetaxel in men with non-metastatic CRPC. The primary outcome in this study is overall survival.
Conclusion
For many patients with CRPC chemotherapy may not be a viable or desirable treatment option. Therefore, targeting the endothelin axis offers a new and promising approach in the treatment of CRPC. There is considerable preclinical data suggesting that the activation of the ETA receptor by ET-1 promotes prostate cancer growth, bone metastasis and pain. Drugs such ZD4054 have been shown to specifically block ETA with high affinity while sparing the ETB receptor54. Phase II clinical trials demonstrate its safety and tolerability in patients with CRPC, with suggestions that ZD4054 may be beneficial in patients with asymptomatic or mildly symptomatic metastatic CRPC by improving overall survival72. These results offer the impetus for further trials. Phase III studies with ZD4054 are planned in CRPC, administered either as monotherapy or in combination with chemotherapy, with overall survival as the primary endpoint.
Expert opinion
Metastases to bone are the principal cause of morbidity and mortality in prostate cancer. This is an ongoing challenge not only for the clinical management of prostate cancer, but also prostate cancer drug development, as current imaging modalities are not capable of assessing response in osseous metastasis with certainty 74. As a result, we have necessitated that differences in survival be the prerequisite for prostate cancer drug approval. While reducing mortality from prostate cancer is the stated goal, the reduction of morbidity from prostate cancer should be considered of equal importance. The scientific rationale for use of endothelin receptor antagonists in prostate cancer is unique in that it may address both of these issues simultaneously. The finding of over-expressed ETA receptors on prostate cancers with elevated circulating ET-1 levels in men with prostate cancer strongly suggests that the endothelin axis is important in prostate cancer proliferation and survival. Likewise, the finding that ET-1 activity induces osteoblastic and osteoclastic responses in bone strongly suggest that there is a synergistic feedback loop between the prostate cancer cells and osteoblasts/osteoclasts, which facilitates the propagation of the tumor in bone. Early clinical trials with the ETA antagonist atrasentan 63 did suggest improvements in time to progression in patients with prostate cancer and osseous metastasis 66, as well as improvements in bone pain 41 supporting the importance of the endothelin axis in prostate cancer. Unfortunately, these trials failed to show a statistically significant improvement in time to progression (as defined in the protocol) resulting in the FDA’s recommendation not to approve atrasentan. ZD4054 is a specific ETA receptor antagonist currently being investigated in prostate cancer. Learning from the protocol failures seen with atrasentan, the randomized, placebo-controlled study of ZD4054 was powered to show a progression free survival improvement, but was more careful in defining progression with a composite endpoint. This included clinically meaningful endpoints such as clinical progression, pain requiring opioids, progressive soft tissue metastasis, and death, but excluded clinically controversial endpoints such as PSA or bone scan worsening. Although no statistical difference in progression free survival was observed, an improvement in overall survival was seen with ZD4054 versus placebo. We expect that improvements in PFS lead to improvements in overall survival and have, as a prostate research community, been encouraging our regulating bodies to accept PFS as an appropriate surrogate (such as in breast cancer) to facilitate drug development, especially as we are treat patients earlier in their disease course (pre-docetaxel). How does one account for the improvement in overall survival without a difference observed in PFS with ZD4054? One possible conclusion is that the study was underpowered to show an overall survival difference, and thus this observed difference could be due to chance alone. Another is that the benefit in terms of improving PFS may be more appreciable in patients without established osseous metastasis, and that in the presence of established osseous metastasis, the benefit (including survival) of ZD4054 might be due to its palliative effects. In general, we need to take into account several factors: 1) the expected action for the drug on the cancer (cytostatic or cytotoxic), 2) the goals of therapy (curative, prolongation of life, palliative), and 3) the disease state being assessed (e.g. rising PSA only, pre-docetaxel, in combination with docetaxel, or taxane-refractory), when designing clinical trial endpoints. Moving forward, the most clinically meaningful endpoint might be improvements in time to radiographic progression (rising PSA state), survival (metastatic, pre-docetaxel state), or pain improvement (post-docetaxel state).
Further investigation of ZD4054 in prostate cancer is warranted, and Phase III trials are already planned in patients with non-metastatic CRPC with rising PSA values, metastatic (asymptomatic) CRPC, and in metastatic CRPC in combination with docetaxel, assessing either differences in PFS and OS or OS alone. We must keep in mind that the ultimate objective is to reduce the mortality and morbidity from prostate cancer. What this implies is that although survival improvement is important, it represents only one half of the goal.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007 Jan–Feb;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Kantoff PW, Halabi S, Conaway M, Picus J, Kirshner L, Hars V, et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the cancer and leukemia group B 9182 study. J Clin Oncol. 1999 Aug;17(8):2506–2513. doi: 10.1200/JCO.1999.17.8.2506. [DOI] [PubMed] [Google Scholar]
- 3.Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, Moore MJ, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996 Jun;14(6):1756–1764. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 4.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004 Oct 7;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 5.Saitoh H, Hida M, Shimbo T, Nakamura K, Yamagata J, Satoh T. Metastatic patterns of prostatic cancer. Correlation between sites and number of organs involved. Cancer. 1984 Dec 15;54(12):3078–3084. doi: 10.1002/1097-0142(19841215)54:12<3078::aid-cncr2820541245>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 6.Daniell HW, Dunn SR, Ferguson DW, Lomas G, Niazi Z, Stratte PT. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol. 2000 Jan;163(1):181–186. [PubMed] [Google Scholar]
- 7.Stoch SA, Parker RA, Chen L, Bubley G, Ko YJ, Vincelette A, et al. Bone loss in men with prostate cancer treated with gonadotropin-releasing hormone agonists. J Clin Endocrinol Metab. 2001 Jun;86(6):2787–2791. doi: 10.1210/jcem.86.6.7558. [DOI] [PubMed] [Google Scholar]
- 8.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002 Oct 2;94(19):1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 9.Scher HI, Heller G. Clinical states in prostate cancer: toward a dynamic model of disease progression. Urology. 2000 Mar;55(3):323–327. doi: 10.1016/s0090-4295(99)00471-9. [DOI] [PubMed] [Google Scholar]
- 10.Morris MJ, Scher HI. Clinical approaches to osseous metastases in prostate cancer. Oncologist. 2003;8(2):161–173. doi: 10.1634/theoncologist.8-2-161. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong AJ, Garrett-Mayer E, Ou Yang YC, Carducci MA, Tannock I, de Wit R, et al. Prostate-specific antigen and pain surrogacy analysis in metastatic hormone-refractory prostate cancer. J Clin Oncol. 2007 Sep 1;25(25):3965–3970. doi: 10.1200/JCO.2007.11.4769. [DOI] [PubMed] [Google Scholar]
- 12.Prentice R. Surrogate end points in clinical trials: definition and operational criteria. Statistical Medicine. 1989;8:431–440. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 13.Rubanyi GM, Polokoff MA. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev. 1994 Sep;46(3):325–415. [PubMed] [Google Scholar]
- 14.Levin ER. Endothelins. N Engl J Med. 1995 Aug 10;333(6):356–363. doi: 10.1056/NEJM199508103330607. [DOI] [PubMed] [Google Scholar]
- 15.Bagnato A, Catt KJ. Endothelins as Autocrine Regulators of Tumor Cell Growth. Trends in Endocrinology and Metabolism. 1998;9(9):378–383. doi: 10.1016/s1043-2760(98)00094-0. [DOI] [PubMed] [Google Scholar]
- 16.Goldie RG. Endothelins in health and disease: an overview. Clin Exp Pharmacol Physiol. 1999 Feb;26(2):145–148. doi: 10.1046/j.1440-1681.1999.03014.x. [DOI] [PubMed] [Google Scholar]
- 17.Nelson J, Bagnato A, Battistini B, Nisen P. The endothelin axis: emerging role in cancer. Nat Rev Cancer. 2003 Feb;3(2):110–116. doi: 10.1038/nrc990. [DOI] [PubMed] [Google Scholar]
- 18.Bagnato A, Natali PG. Endothelin receptors as novel targets in tumor therapy. J Transl Med. 2004 May 27;2(1):16. doi: 10.1186/1479-5876-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lalich M, McNeel DG, Wilding G, Liu G. Endothelin receptor antagonists in cancer therapy. Cancer Invest. 2007 Dec;25(8):785–794. doi: 10.1080/07357900701522588. [DOI] [PubMed] [Google Scholar]
- 20.Nelson JB, Chan-Tack K, Hedican SP, Magnuson SR, Opgenorth TJ, Bova GS, et al. Endothelin-1 production and decreased endothelin B receptor expression in advanced prostate cancer. Cancer Res. 1996 Feb 15;56(4):663–668. [PubMed] [Google Scholar]
- 21.Nelson JB, Nabulsi AA, Vogelzang NJ, Breul J, Zonnenberg BA, Daliani DD, et al. Suppression of prostate cancer induced bone remodeling by the endothelin receptor A antagonist atrasentan. J Urol. 2003 Mar;169(3):1143–1149. doi: 10.1097/01.ju.0000042162.08938.27. [DOI] [PubMed] [Google Scholar]
- 22.Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996 Feb 8;379(6565):557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 23.Vacca F, Bagnato A, Catt KJ, Tecce R. Transactivation of the epidermal growth factor receptor in endothelin-1-induced mitogenic signaling in human ovarian carcinoma cells. Cancer Res. 2000 Sep 15;60(18):5310–5317. [PubMed] [Google Scholar]
- 24.Bagnato A, Tecce R, Di Castro V, Catt KJ. Activation of mitogenic signaling by endothelin 1 in ovarian carcinoma cells. Cancer Res. 1997 Apr 1;57(7):1306–1311. [PubMed] [Google Scholar]
- 25.Del Bufalo D, Di Castro V, Biroccio A, Varmi M, Salani D, Rosano L, et al. Endothelin-1 protects ovarian carcinoma cells against paclitaxel-induced apoptosis: requirement for Akt activation. Mol Pharmacol. 2002 Mar;61(3):524–532. doi: 10.1124/mol.61.3.524. [DOI] [PubMed] [Google Scholar]
- 26.Rosano L, Spinella F, Salani D, Di Castro V, Venuti A, Nicotra MR, et al. Therapeutic targeting of the endothelin a receptor in human ovarian carcinoma. Cancer Res. 2003 May 15;63(10):2447–2453. [PubMed] [Google Scholar]
- 27.Nelson JB, Hedican SP, George DJ, Reddi AH, Piantadosi S, Eisenberger MA, et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat Med. 1995 Sep;1(9):944–949. doi: 10.1038/nm0995-944. [DOI] [PubMed] [Google Scholar]
- 28.Bagnato A, Tecce R, Moretti C, Di Castro V, Spergel D, Catt KJ. Autocrine actions of endothelin-1 as a growth factor in human ovarian carcinoma cells. Clin Cancer Res. 1995 Sep;1(9):1059–1066. [PubMed] [Google Scholar]
- 29.Drimal J, Drimal J, Jr, Drimal D. Enhanced endothelin ET(B) receptor down-regulation in human tumor cells. Eur J Pharmacol. 2000 May 12;396(1):19–22. doi: 10.1016/s0014-2999(00)00198-9. [DOI] [PubMed] [Google Scholar]
- 30.Bagnato A, Cirilli A, Salani D, Simeone P, Muller A, Nicotra MR, et al. Growth inhibition of cervix carcinoma cells in vivo by endothelin A receptor blockade. Cancer Res. 2002 Nov 15;62(22):6381–6384. [PubMed] [Google Scholar]
- 31.Grimshaw MJ, Naylor S, Balkwill FR. Endothelin-2 is a hypoxia-induced autocrine survival factor for breast tumor cells. Mol Cancer Ther. 2002 Dec;1(14):1273–1281. [PubMed] [Google Scholar]
- 32.Lahav R, Heffner G, Patterson PH. An endothelin receptor B antagonist inhibits growth and induces cell death in human melanoma cells in vitro and in vivo. Proc Natl Acad Sci U S A. 1999 Sep 28;96(20):1469–1500. doi: 10.1073/pnas.96.20.11496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gohji K, Kitazawa S, Tamada H, Katsuoka Y, Nakajima M. Expression of endothelin receptor a associated with prostate cancer progression. J Urol. 2001 Mar;165(3):1033–1036. [PubMed] [Google Scholar]
- 34.Godara G, Cannon GW, Cannon GM, Jr, Bies RR, Nelson JB, Pflug BR. Role of endothelin axis in progression to aggressive phenotype of prostate adenocarcinoma. Prostate. 2005 Sep 15;65(1):27–34. doi: 10.1002/pros.20252. [DOI] [PubMed] [Google Scholar]
- 35.Nelson JB, Lee WH, Nguyen SH, Jarrard DF, Brooks JD, Magnuson SR, et al. Methylation of the 5' CpG island of the endothelin B receptor gene is common in human prostate cancer. Cancer Res. 1997 Jan 1;57(1):35–37. [PubMed] [Google Scholar]
- 36.Kopetz ES, Nelson JB, Carducci MA. Endothelin-1 as a target for therapeutic intervention in prostate cancer. Invest New Drugs. 2002 May;20(2):173–182. doi: 10.1023/a:1015630513908. [DOI] [PubMed] [Google Scholar]
- 37.Chiao JW, Moonga BS, Yang YM, Kancherla R, Mittelman A, Wu-Wong JR, et al. Endothelin-1 from prostate cancer cells is enhanced by bone contact which blocks osteoclastic bone resorption. Br J Cancer. 2000 Aug;83(3):360–365. doi: 10.1054/bjoc.2000.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curtis N, Howard Z, Brooks N, Curwen J. ZD4054 specifically inhibits endothelin A receptor-mediated anti-apoptotic effects, but not endothelin B receptor-medicated pro-apoptotic effects. European Journal of Cancer Supplement. 2004 [Google Scholar]
- 39.Dreicer R, Curtis N, Morris CD, Wilson D, Hughes A, Le Maulf F, et al. ZD4054 specifically inhibits endothelin A receptor-mediated effects but not endothelin B receptor-mediated effects; ASCO Multidisciplinary Prostate Cancer Symposium; 2005. 2005. [Google Scholar]
- 40.Okazawa M, Shiraki T, Ninomiya H, Kobayashi S, Masaki T. Endothelin-induced apoptosis of A375 human melanoma cells. J Biol Chem. 1998 May 15;273(20):12584–12592. doi: 10.1074/jbc.273.20.12584. [DOI] [PubMed] [Google Scholar]
- 41.Lassiter LK, Carducci MA. Endothelin receptor antagonists in the treatment of prostate cancer. Semin Oncol. 2003 Oct 30;30(5):678–688. doi: 10.1016/s0093-7754(03)00353-1. [DOI] [PubMed] [Google Scholar]
- 42.Bagnato A, Salani D, Di Castro V, Wu-Wong JR, Tecce R, Nicotra MR, et al. Expression of endothelin 1 and endothelin A receptor in ovarian carcinoma: evidence for an autocrine role in tumor growth. Cancer Res. 1999 Feb 1;59(3):720–727. [PubMed] [Google Scholar]
- 43.Salani D, Taraboletti G, Rosano L, Di Castro V, Borsotti P, Giavazzi R, et al. Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Am J Pathol. 2000 Nov;157(5):1703–1711. doi: 10.1016/S0002-9440(10)64807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spinella F, Rosano L, Di Castro V, Natali PG, Bagnato A. Endothelin-1 induces vascular endothelial growth factor by increasing hypoxia-inducible factor-1 alpha in ovarian carcinoma cells. J Biol Chem. 2002 Aug 2;277(31):27850–27855. doi: 10.1074/jbc.M202421200. [DOI] [PubMed] [Google Scholar]
- 45.Salani D, Di Castro V, Nicotra MR, Rosano L, Tecce R, Venuti A, et al. Role of endothelin-1 in neovascularization of ovarian carcinoma. Am J Pathol. 2000 Nov;157(5):1537–1547. doi: 10.1016/S0002-9440(10)64791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strohmeyer D, Rossing C, Bauerfeind A, Kaufmann O, Schlechte H, Bartsch G, et al. Vascular endothelial growth factor and its correlation with angiogenesis and p53 expression in prostate cancer. Prostate. 2000 Nov 1;45(3):216–224. doi: 10.1002/1097-0045(20001101)45:3<216::aid-pros3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 47.Rosano L, Salani D, Di Castro V, Spinella F, Natali PG, Bagnato A. Endothelin-1 promotes proteolytic activity of ovarian carcinoma. Clin Sci (Lond) 2002 Aug;103 Suppl 48:306S–309S. doi: 10.1042/CS103S306S. [DOI] [PubMed] [Google Scholar]
- 48.Rosano L, Varmi M, Salani D, Di Castro V, Spinella F, Natali PG, et al. Endothelin-1 induces tumor proteinase activation and invasiveness of ovarian carcinoma cells. Cancer Res. 2001 Nov 15;61(22):8340–8346. [PubMed] [Google Scholar]
- 49.Spinella F, Rosano L, Di Castro V, Nicotra MR, Natali PG, Bagnato A. Endothelin-1 decreases gap junctional intercellular communication by inducing phosphorylation of connexin 43 in human ovarian carcinoma cells. J Biol Chem. 2003 Oct 17;278(42):41294–41301. doi: 10.1074/jbc.M304785200. [DOI] [PubMed] [Google Scholar]
- 50.Carducci MA, Nelson JB, Bowling MK, Rogers T, Eisenberger MA, Sinibaldi V, et al. Atrasentan, an endothelin-receptor antagonist for refractory adenocarcinomas: safety and pharmacokinetics. J Clin Oncol. 2002 Apr 15;20(8):2171–2180. doi: 10.1200/JCO.2002.08.028. [DOI] [PubMed] [Google Scholar]
- 51.Zonnenberg BA, Groenewegen G, Janus TJ, Leahy TW, Humerickhouse RA, Isaacson JD, et al. Phase I dose-escalation study of the safety and pharmacokinetics of atrasentan: an endothelin receptor antagonist for refractory prostate cancer. Clin Cancer Res. 2003 Aug 1;9(8):2965–2972. [PubMed] [Google Scholar]
- 52.Ryan CW, Vogelzang NJ, Vokes EE, Kindler HL, Undevia SD, Humerickhouse R, et al. Dose-ranging study of the safety and pharmacokinetics of atrasentan in patients with refractory malignancies. Clin Cancer Res. 2004 Jul 1;10(13):4406–4411. doi: 10.1158/1078-0432.CCR-04-0083. [DOI] [PubMed] [Google Scholar]
- 53.Nelson JB, Nguyen SH, Wu-Wong JR, Opgenorth TJ, Dixon DB, Chung LW, et al. New bone formation in an osteoblastic tumor model is increased by endothelin-1 overexpression and decreased by endothelin A receptor blockade. Urology. 1999 May;53(5):1063–1069. doi: 10.1016/s0090-4295(98)00658-x. [DOI] [PubMed] [Google Scholar]
- 54.Morris CD, Rose A, Curwen J, Hughes AM, Wilson DJ, Webb DJ. Specific inhibition of the endothelin A receptor with ZD4054: clinical and pre-clinical evidence. Br J Cancer. 2005 Jun 20;92(12):2148–2152. doi: 10.1038/sj.bjc.6602676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Logothetis CJ, Lin SH. Osteoblasts in prostate cancer metastasis to bone. Nat Rev Cancer. 2005 Jan;5(1):21–28. doi: 10.1038/nrc1528. [DOI] [PubMed] [Google Scholar]
- 56.Curtis N, Anderson E, Brooks J, Curwen J. ZD4054 blocks ET-1-stimulated phosphorylation of p44/42 mitogen-activated protein kinase and proliferation of osteoblast cells. Proceedings American Association of Cancer Research. 2005 2005. [Google Scholar]
- 57.Dahlof B, Gustafsson D, Hedner T, Jern S, Hansson L. Regional haemodynamic effects of endothelin-1 in rat and man: unexpected adverse reaction. J Hypertens. 1990 Sep;8(9):811–817. doi: 10.1097/00004872-199009000-00004. [DOI] [PubMed] [Google Scholar]
- 58.Pomonis JD, Rogers SD, Peters CM, Ghilardi JR, Mantyh PW. Expression and localization of endothelin receptors: implications for the involvement of peripheral glia in nociception. J Neurosci. 2001 Feb 1;21(3):999–1006. doi: 10.1523/JNEUROSCI.21-03-00999.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khodorova A, Navarro B, Jouaville LS, Murphy JE, Rice FL, Mazurkiewicz JE, et al. Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injury. Nat Med. 2003 Aug;9(8):1055–1061. doi: 10.1038/nm885. [DOI] [PubMed] [Google Scholar]
- 60.Yuyama H, Koakutsu A, Fujiyasu N, Tanahashi M, Fujimori A, Sato S, et al. Effects of selective endothelin ET(A) receptor antagonists on endothelin-1-induced potentiation of cancer pain. Eur J Pharmacol. 2004 May 25;492(2–3):177–182. doi: 10.1016/j.ejphar.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 61.Yuyama H, Sanagi M, Koakutsu A, Mori M, Fujimori A, Harada H, et al. Pharmacological characterization of YM598, an orally active and highly potent selective endothelin ET(A) receptor antagonist. Eur J Pharmacol. 2003 Sep 30;478(1):61–71. doi: 10.1016/j.ejphar.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 62.Opgenorth TJ, Adler AL, Calzadilla SV, Chiou WJ, Dayton BD, Dixon DB, et al. Pharmacological characterization of A-127722: an orally active and highly potent ETA-selective receptor antagonist. J Pharmacol Exp Ther. 1996 Feb;276(2):473–481. [PubMed] [Google Scholar]
- 63.Carducci MA, Padley RJ, Breul J, Vogelzang NJ, Zonnenberg BA, Daliani DD, et al. Effect of endothelin-A receptor blockade with atrasentan on tumor progression in men with hormone-refractory prostate cancer: a randomized, phase II, placebo-controlled trial. J Clin Oncol. 2003 Feb 15;21(4):679–689. doi: 10.1200/JCO.2003.04.176. [DOI] [PubMed] [Google Scholar]
- 64.Nelson JB. Endothelin inhibition: novel therapy for prostate cancer. J Urol. 2003 Dec;170(6 Pt 2):S65–S67. doi: 10.1097/01.ju.0000096372.07687.86. discussion S7-8. [DOI] [PubMed] [Google Scholar]
- 65.Michaelson MD, Kaufman DS, Kantoff P, Oh WK, Smith MR. Randomized phase II study of atrasentan alone or in combination with zoledronic acid in men with metastatic prostate cancer. Cancer. 2006 Aug 1;107(3):530–535. doi: 10.1002/cncr.22043. [DOI] [PubMed] [Google Scholar]
- 66.Carducci MA, Saad F, Abrahamsson PA, Dearnaley DP, Schulman CC, North SA, et al. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer. 2007 Nov 1;110(9):1959–1966. doi: 10.1002/cncr.22996. [DOI] [PubMed] [Google Scholar]
- 67.AstraZeneca ZD4054 Investigator's Brochure Edition # 9. 2007 [Google Scholar]
- 68.Clarkson-Jones J, Kenyon A, Kemp J, Lenz e, Oliver S, Sandall D, et al. Metabolism of [14 C]-ZD4054 in healthy volunteers; European Conference on Clinical Oncology; 2007. Poster; 2007 Poster. [Google Scholar]
- 69.Swaisland H, Oliver S, Morris T, Jones H, Tootell A, Kudraj C, et al. Clinical drug interactions with ZD4054 in healthy, male volunteers; European Multidisciplinary Meeting on Urological Cancers; 2007. Poster; 2007 Poster. [Google Scholar]
- 70.Ranson M, Usami M, Maruoka M, Yamaguchi A, Cowan R, Logue J, et al. The pharmacokinetics and tolerability profile of once-daily oral ZD4054 in Japanese and Caucasian patients with hormone-resistant prostate cancer; European Conference on Clinical Oncology; 2007. Poster; 2007 Poster. [DOI] [PubMed] [Google Scholar]
- 71.Liu G, Dreicer R, Hou J, Chen Y, Wilding G. Tolerability profile of ZD4054 is consistent with the effects of endothelin A receptor-specific antagonism. Journal of Clinical Oncology. 2005;23(16s):4628. [Google Scholar]
- 72.James ND, Borre M, Zonnenberg BA, Beuzeboc P, Morris T, Phung D, et al. ZD4054, a potent, specific endothelin A receptor antagonist, improves overall survival in pain-free or mildly symptomatic patients with hormone-resistant prostate cancer (MRPC) and bone metastases; European Conference of Clinical Oncologists; 2007; Barcelona, Spain. 2007. abstract # 3LB; abstract # 3LB. [Google Scholar]
- 73.Dawson N, Phung D, Morris M, Borre B, Zonnenberg P, Beuzeboc N, et al. Impact of the specific endothelin A receptor antagonist ZD4054 onoverall survival and bone metastasis in patients with hormone-resistant prostate cancer: Results of a phase II trial; 2008 Genitourinary Cancers Symposium; 2008; San Fransisco, California: 2008. [Google Scholar]
- 74.Scher HI, Morris MJ, Kelly WK, Schwartz LH, Heller G. Prostate cancer clinical trial end points: “RECIST”ing a step backwards. Clin Cancer Res. 2005 Jul 15;11(14):5223–5232. doi: 10.1158/1078-0432.CCR-05-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]