Abstract
Communication between the SR (sarcoplasmic reticulum, SR) and mitochondria is important for cell survival and apoptosis. The SR supplies Ca2+ directly to mitochondria via inositol 1,4,5-trisphosphate receptors (IP3Rs) at close contacts between the two organelles referred to as mitochondrion-associated ER membrane (MAM). Although it has been demonstrated that CaR (calcium sensing receptor) activation is involved in intracellular calcium overload during hypoxia/reoxygenation (H/Re), the role of CaR activation in the cardiomyocyte apoptotic pathway remains unclear. We postulated that CaR activation plays a role in the regulation of SR-mitochondrial inter-organelle Ca2+ signaling, causing apoptosis during H/Re. To investigate the above hypothesis, cultured cardiomyocytes were subjected to H/Re. We examined the distribution of IP3Rs in cardiomyocytes via immunofluorescence and Western blotting and found that type 3 IP3Rs were located in the SR. [Ca2+]i, [Ca2+]m and [Ca2+]SR were determined using Fluo-4, x-rhod-1 and Fluo 5N, respectively, and the mitochondrial membrane potential was detected with JC-1 during reoxygenation using laser confocal microscopy. We found that activation of CaR reduced [Ca2+]SR, increased [Ca2+]i and [Ca2+]m and decreased the mitochondrial membrane potential during reoxygenation. We found that the activation of CaR caused the cleavage of BAP31, thus generating the pro-apoptotic p20 fragment, which induced the release of cytochrome c from mitochondria and the translocation of bak/bax to mitochondria. Taken together, these results reveal that CaR activation causes Ca2+ release from the SR into the mitochondria through IP3Rs and induces cardiomyocyte apoptosis during hypoxia/reoxygenation.
Background
The mitochondrion is a fundamental organelle that is intimately involved in many aspects of cellular physiology, such as energy production, free radical production, regulation of cytosolic Ca2+ signaling pathways and apoptosis [1,2]. The mitochondrion also acts as a spatial Ca2+ buffer that reduces cytosolic Ca2+ overload and regulates Ca2+-dependent signaling in the cytosol. Mitochondrial Ca2+ is taken up from the cytosol via a low-affinity Ca2+ uniporter at mitochondrial membranes [3]. However, the intracellular Ca2+ concentration ([Ca2+]i) is not high enough to initiate the uniporter under physiological conditions. Therefore, it has been postulated that activation of the inositol 1,4,5-trisphosphate receptors (IP3Rs) signaling pathway could release Ca2+ from the sarcoplasmic reticulum (SR) to increase the microdomain Ca2+ concentration ([Ca2+]) at focal contacts, known as mitochondria-associated ER membranes (MAM), between the SR and mitochondria, and then activate the uniporter. Recent studies have suggested that IP3Rs are highly compartmentalized at MAMs, providing direct mitochondrial Ca2+ signaling. Cardiomyocytes contain an abundance of mitochondria, many of which are in close apposition to SR Ca2+ release sites [4].
The SR is a multifunctional organelle that controls protein translation and Ca2+ homeostasis. Under SR stress (e.g., SR Ca2+ depletion), SR chaperone proteins such as Grp78 and Grp94 are up-regulated [5]. Prolonged SR stress will initiate apoptotic signals in the SR, including bax/bak-translocation to the SR to activate the release of Ca2+ from the SR, cleavage and activation of procaspase 12 and BAP31, and Ire 1-mediated activation of apoptosis signal-regulating kinase 1 (ASK1)/c-Jun N-terminal kinase (JNK) [6].
The calcium-sensing receptor (CaR) is a member of the family of G protein-coupled receptors (GPCRs). One of the effects of CaR signal transduction is the activation of phospholipase C, which leads to the generation of the secondary messengers diacylglycerol (DAG) and inositol 1,4,5 trisphosphate (IP3). IP3 then mobilizes Ca2+ from intracellular stores via the activation of specific IP3 receptors [7]. Wang et al. and Tfelt-Hansen et al. reported that CaR was functionally expressed in rat cardiac tissue and rat neonatal ventricular cardiomyocytes, respectively [8,9]. Later, Berra-Romani et al. showed that cardiac microvascular endothelial cells express a functional CaR [10]. Our group has demonstrated that CaR is involved in apoptosis in isolated adult rat hearts and in rat neonatal cardiomyocytes during ischemia/reperfusion [11]. Although it is known that CaR elevates the intracellular calcium concentration and then induces apoptosis, the in-depth mechanisms are still not known. The aim of this study was to investigate whether [Ca2+]SR would change with CaR activation in response to hypoxia/reoxygenation in cardiomyocytes. We specifically focused on the relationship between SR Ca2+ depletion, mitochondrial Ca2+ uptake and cardiomyocyte apoptosis during hypoxia/reoxygenation (H/Re).
Materials and methods
Isolation of neonatal rat cardiomyocytes and H/Re experiments
Primary cultures of neonatal rat cardiomyocytes were performed as previously described [12]. Newborn Wistar rats (1-3 days) were used for this study. The rats were handled in accordance with the Guide for the Care and Use of Laboratory Animals published by the China National Institutes of Health. Briefly, hearts from male Wistar rats (1-3 days old) were minced and dissociated with 0.25% trypsin. Dispersed cells were seeded at 2 × 105 cells/cm2 in 60-mm culture dishes with Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and then cultured in a 5% CO2 incubator at 37°C. Hypoxic conditions were produced using D-Hanks solution (mM: 5.37 KCl, 0.44 KH2PO4, 136.89 NaCl, 4.166 NaHCO3, 0.338 Na2HPO4, 5 D-glucose, pH 7.3-7.4 at 37°C) saturated with 95% N2 and 5% CO2. The pH was adjusted to 6.8 with lactate to mimic ischemic conditions. The dishes were put into a hypoxic incubator that was equilibrated with 1% O2/5%CO2/94%N2. After hypoxic treatment, the culture medium was rapidly replaced with fresh DMEM with 10% FBS (10% FBS/DMEM) to initiate reoxygenation [13].
Experimental protocols
At 72 h post-culturing with 10% FBS/DMEM, the cells were randomly divided into six groups: (1) control group: cells were continuously cultured for 9 h with 10% FBS-DMEM; (2) H/Re: cells were placed in hypoxic culture medium for 3 h and then reoxygenated for 6 h by replacing hypoxic culture medium with fresh DMEM containing 10% FBS; (3) CaCl2 + NiCl2 + CdCl2-H/Re (Ca + Ni + Cd-H/Re): neonatal rat cardiomyocytes were treated with CaCl2 (2.2 mM), NiCl2 (1 mM) and CdCl2 (200 μM) for 30 min in hypoxic medium and then reoxygenated for 6 h by replacing hypoxic culture medium with fresh DMEM containing 10% FBS (CaCl2 is an activator of CaR, NiCl2 is an inhibitor of the Na+-Ca2+ exchanger, CdCl2 is a inhibitor of the L-type calcium channel; these drugs do not affect cardiomyocyte viability); (4) NPS-2390 + CaCl2 + NiCl2 + CdCl2-H/Re (NPS-2390 + Ca + Ni + Cd-H/Re): neonatal rat cardiomyocytes were treated with NPS-2390 (10 μM) for 40 min, and the following steps were the same as for group 3 (NPS-2390 is an allosteric antagonist of the group 1 metabotropic glutamate receptors); (5) 2-APB + CaCl2 + NiCl2 + CdCl2-H/Re (2-APB + Ca + Ni + Cd-H/Re): neonatal rat cardiomyocytes were treated with 2-APB (3 μM) for 40 min, and then other steps were the same as in group 3 (2-APB or 2- aminoethoxydiphenyl borate is a membrane permeable IP3R inhibitor); (6) Ruthenium red + CaCl2 + NiCl2 + CdCl2-H/Re (Ru + Ca + Ni + Cd-H/Re): neonatal rat cardiomyocytes were treated with Ruthenium red (10 μM) for 40 min, and then underwent the same steps as in group 3 (Ruthenium red is an inhibitor of mitochondrial calcium uniporter ).
Immunocytochemistry
Cardiomyocytes were fixed in 10% formaldehyde in phosphate-buffered saline (PBS) for 10 min, permeabilized with 0.1% Triton X-100, washed three times in PBS and blocked in PBS containing 5% bovine serum albumin, 5% horse serum and 0.05% Triton X-100 for 1 h at room temperature (RT). Specific subtype anti-IP3R rabbit polyclonal antibodies were incubated overnight at 4°C at 1:200 or 1:100 (Santa Cruz). FITC-conjugated anti-rabbit IgG was used as a secondary antibody. As indicated, some cells were stained with 4-6- diamidino-2-phenylindole (25 μg/ml) (DAPI, Roche) for 1 h. The results of immunocytochemical staining were read and recorded with a laser confocal scanning microscope (Olympus, LSM, Japan).
3-(4,5-dimethyl thiazol-2yl)-2,5-diphenyltetrazolium bromide(MTT) assay
In the current study, cardiomyocytes were planted in 96-well plates. The MTT assay was performed as described previously [10]. Briefly, MTT (Sigma) was added into the cell cultures at a final concentration of 0.5 mg/mL and the mixture was incubated for 4 h at 37°C. Subsequently, the culture medium was removed and DMSO was added to each well to dissolve the resulting formazan crystals. The absorbance was measured at a wavelength of 570 nm using a microplate reader (Bio-Tek Instruments Inc., Richmond, Va). Background absorbance of medium in the absence of cells was subtracted [14]. Percent viability was defined as the relative absorbance of treated versus untreated control cells.
Hoechst staining
Apoptotic cells were identified by the distinctive condensed or fragmented nuclear structure in cells stained with the chromatin dye Hoechst 33342 (Sigma). Cells were fixed with 4% paraformaldehyde for 10 min at room temperature and were washed twice with phosphate buffer solution (PBS). Cells were then incubated with 5 μg/mL Hoechst 33342 for 15 min. Next, the cells were washed three times and photographed using fluorescence microscope (Leica DFC500 System; Leica Microsystems, Bannockburn, Ill). At least 500 nuclei from randomly selected fields in each group were analyzed for each experiment, and the percentage of apoptotic cells was calculated as the ratio of the number of apoptotic cells versus the total cells counted.
Neonatal rat cardiomyocytes loaded with Fluo-4 AM, Fluo-5N AM and X-rhod-1 AM and cell permeabilization
[Ca2+]i was determined as previously described [15]. Briefly, cells were seeded on the culture slides. After experimentation, cells were loaded with fluo-4 AM in 1% working solution at 37°C for 1 h, washed three times with Ca2+-free PBS to remove extracellular fluo-4 AM, and diluted to the required concentration. The reagents were added in Ca2+-free solution (145 mM NaCl, 5 mM KCl, 1.0 mM EGTA, 1 mM MgCl2, 10 mM HEPES-Na, 5.6 mM glucose, pH 7.4). Fluorescence measurement of Ca2+ was performed using a laser confocal scanning microscope (Olympus, LSM, Japan) at an excitation wavelength of 485 nm for [Ca2+]i and an emission wavelength of 530 nm for [Ca2+]i, using the equation [Ca2+]i = Kd[(F -Fmin)/(Fmax - F)], where Kd is the dissociation constant (345 nM for fluo-4), F is the fluorescence at intermediate Ca2+ levels (corrected from background fluorescence), Fmin is the fluorescence intensity of the indicator in the absence of Ca2+and is obtained by adding a solution of 10 mM EGTA for 15 min, and Fmax is the fluorescence of the Ca2+-saturated indicator and is obtained by adding a solution of 25 μM digitonin in 2.2 nM CaCl2 for 15 min. Final values for [Ca2+]i are expressed in nanomoles.
To determine [Ca2+]SR, cardiomyocytes were treated with Fluo-5N acetoxymethylester (10 μM) for 2 h and deesterified for 1.5 h. For intact myocytes, the superfusate contained (in mM) 140 NaCl, 4 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose (pH 7.4, 23°C). For permeabilization, myocytes were exposed to solution (in mM: 0.1 EGTA, 10 HEPES, 120 K-aspartate, 1 free MgCl2, 5 ATP, 10 reduced glutathione, and 5 phosphocreatine; pH 7.4) and then permeabilized using saponin (50 μg/ml) for 20 seconds. Excitation was set at 488 nm and emission was measured at 530 nm at room temperature [15]. Images of fluorescence reflecting [Ca2+]i and [Ca2+]SR were recorded using a laser confocal scanning microscope (Olympus, LSM, Japan). There were more than 10 cells to be analyzed in each view and quantified using the analysis software for the microscope.
Recent study showed that the mitochondrial Ca2+ concentration ([Ca2+]m) consistently increases during reoxygenation [12]. Therefore, [Ca2+]m was measured at 60 min post-reoxygenation. [Ca2+]m was determined according to the manufacturer's instructions (Molecular Probes). In brief, the cultured cardiomyocytes (1 × 106 cells/sample) were initially washed with HEPES buffer containing (in mM) 130 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 10 HEPES, 11 glucose, and 0.2 CaCl2 at pH 7.4 and then stained with 5 μmol/L X-rhod-1 AM for 30 min at room temperature. To avoid deesterification of intracellular X-rhod-1 AM in the cytosolic compartment, which would interfere with the detection of [Ca2+]m, the cardiomyocytes were rinsed and incubated with 100 μM MnCl2-HEPES for an additional 20 min to quench the cytosolic Ca2+ signal [16]. Fluorescence measurement was determined using a fluorescence plate reader (CytoFluor II; PerSeptive Biosystems; Framingham, MA) at an excitation wavelength of 580 nm and an emission wavelength of 645 nm for [Ca2+]m. To validate the measurement of [Ca2+]m, the cultured cardiomyocytes were transferred into a slide chamber after X-rhod-1 AM staining and were placed on the stage of a fluorescence microscope (×50 objective; Olympus). The images from the slides were captured using a digital camera connected to Image-Pro Plus software (Media Cybernetics; Silver Spring, MD). There were more than 10 cells to be analyzed in each view.
Measurement of mitochondrial membrane potential
Mitochondrial membrane potential (△ψm) was measured with a unique cationic dye of 5,5',6,6'-tetrachloro 1,1'3,3'-tetraethylbenzimidazolcarbocyanine iodide (JC-1), as previously described [12]. Briefly, cells were seeded on culture slides and treated according to experimental protocols. Previous data demonstrated that [Ca2+]m might continuously increase during the process of reoxygenation and result in mitochondrial △ψm collapse [12], so we detected △ψm at 1 h after reoxygenation. At the end of the above-described treatments, cells were stained with JC-1 (1 μg/ml) at 37°C for 15 min and then rinsed three times with PBS. Observations were immediately made using a laser confocal scanning microscope. In live cells, the mitochondria appear red due to the aggregation of accumulated JC-1, which has absorption/emission maxima of 585/590 nm (red). In apoptotic and dead cells, the dye remains in its monomeric form, which has absorption/emission maxima of 510/530 nm (green). More than 100 areas were selected from each image. The average intensity of red and green fluorescence was determined. The ratio of JC-1 aggregate (red) to monomer (green) intensity was calculated. A decrease in this ratio was interpreted as a decrease in the △ψm, whereas an increase in this ratio was interpreted as a gain in the △ψm.
Identification of bax/bak translocation to the mitochondria and assay for cytochrome c release from mitochondria
Western blotting of cellular fractions was used to quantify changes in cytochrome c, bax and bak distribution within cells, as previously described [17]. Briefly, 1 × 107 rat cardiomyocytes were homogenized in ice-cold Tris-sucrose buffer (in mM: 350 sucrose, 10 Tris-HCl, 1 ethylenediaminetetraacetic acid, 0.5 dithiothreitol, and 0.1 phenylmethanesulfonylfluoride; pH 7.5). After 10 min of incubation, cardiomyocyte homogenates were initially centrifuged at 1000 × g for 5 min at 4°C, and the supernatant was further centrifuged at 40,000 × g for another 30 min at 4°C. The supernatant was saved as the cytosolic fraction. The precipitate was re-suspended in the above buffer (containing 0.5% v/v Nonidet P-40) and saved as the mitochondrial fraction. The mitochondrial fractions were blotted with a primary rat anti-bax, bak and cytochrome c monoclonal antibody (Santa Cruz Inc.). The volume of specific bands was measured using a Bio-Rad Chemi EQ densitometer and Bio-Rad QuantityOne software (Bio-Rad laboratories, Hercules, USA).
Western blotting
Western blot analyses were performed as previously described [18]. In brief, the protein concentration of samples was first determined using the Bio-Rad DC protein assay kit (Bio-Rad Laboratories, Hercules, CA). A total of 20 μg of protein was electrophoresed on a 12% SDS-polyacrylamide gel and transferred to nitrocellulose membranes (Amersham International, Amersham, UK). The membranes were blocked with 10% skim milk in TBST buffer (10 mM Tris, pH 7.6, 150 mM NaCl, and 0.1% Tween 20) for 1 h at room temperature and then incubated with a rabbit anti-BAP31 polyclonal antibody (1:500 dilution, sc-48766, Santa Cruz Biotechnology) overnight at 4°C. HRP-conjugated anti-rabbit IgG (1:3000 dilution, Bio-Rad Laboratories) was used as a secondary antibody. Specific bands were visualized with a chemiluminescent substrate (ECL kit, Amersham International).
Statistical analyses
Significance was evaluated using student's t-test, and p < 0.05 was considered statistically significant. Data are expressed as mean ± standard error of the mean (S.E.M.) and are representative of at least three independent experiments. [Ca2+]i data were obtained from 2-3 experiments, and 10-12 images were analyzed in each group.
Results
Asymmetric subcellular distribution of IP3R subtypes in cardiomyocytes
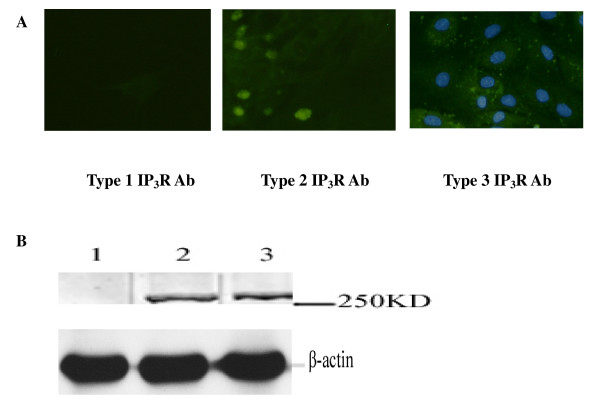
Western blot results showed that type 2 and 3 IP3Rs were expressed in cardiomyocytes, while type 1 IP3R expression was undetectable (Fig. 1A). Similar to the results of the Western blot analysis, type 3 IP3R was distributed in the cytoplasm and intense perinuclear and intranuclear staining was evident for type 2 IP3R in immunofluorescence study, while type 1 IP3R was undetectable.
Figure 1.
Subcellular IP3Rs localization. (A) Immunocytochemical staining of cardiomyocyte with specific antibodies for type 1, type 2 and type 3 IP3Rs. (B) Western blot analysis of cardiomyocyte lysates using antibodies specific for IP3R, type 1, type 2 and type 3, respectively. DAPI and FITC to co-stain nuclei and type 3 IP3 receptors and show the spatial relation between the two structures.
Activation of CaR induces cardiomyocyte apoptosis by H/Re
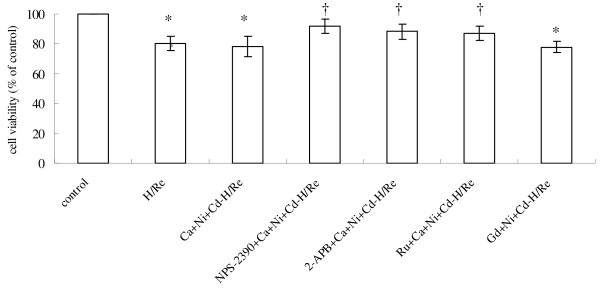
To confirm the role of CaR in cardiomyocyte apoptosis evoked by H/Re, we examined whether activation of CaR induced apoptosis in cultured cardiomyocytes of neonatal rats under our experimental conditions. We used two CaR agonists, CaCl2 and GdCl3, to demonstrate the role of CaR in the induction of apoptosis during H/Re. When cardiomyocytes were exposed to the activation of CaR by H/Re, cell viability was shown to be reduced to 80.2 ± 4.8% (H/Re), 78.3 ± 6.8% (Ca + Ni + Cd-H/Re) and 77.6 ± 5.1% (Gd + Ni + Cd-H/Re), respectively, compared with that of control cells using the MTT assay. Cell viability in NPS-2390 + Ca + Ni + Cd-H/Re (91.7 ± 4.6%), NPS-2390 is an allosteric antagonist of group 1 metabotropic glutamate receptors. 2-APB + Ca + Ni + Cd-H/Re (88.3 ± 5.2%, 2-APB is a selective inhibitor) and Ru + Ca + Ni + Cd-H/Re (87.6 ± 5.6%, Ruthenium red is an inhibitor of mitochondrial calcium uniporter) groups was more than that of the H/Re, Ca + Ni + Cd-H/Re and Gd + Ni + Cd-H/Re groups (Fig. 2).
Figure 2.
Viability of cardiomyocytes was examined using the MTT assay. The cell viability of the control was adjusted to 100%. The data presented are expressed as the mean ± SEM. *p < 0.05 vs Control group; †p < 0.05 vs Ca + Ni + Cd-H/Re .The experiment was repeated three times with similar results.
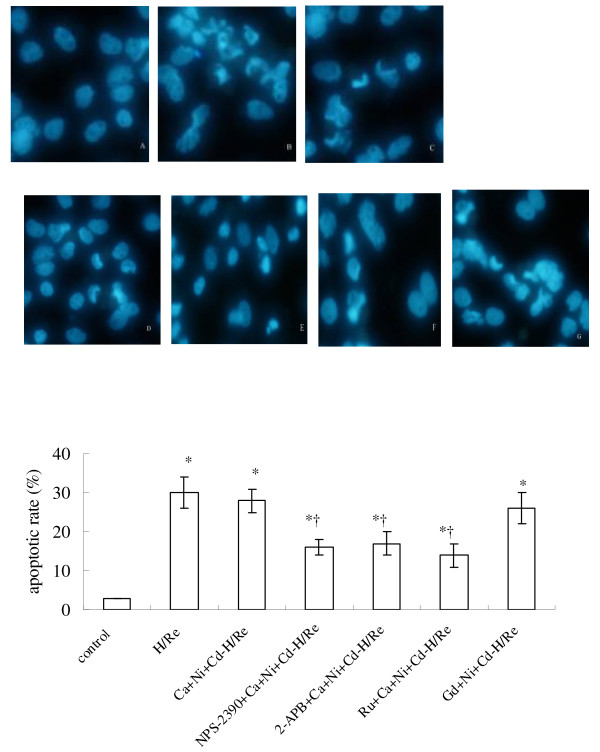
To further determine whether the cell death induced by H/Re and activation of CaR was mediated by apoptosis, the nuclear morphology was analyzed using the Hoechst staining assay. The apoptotic cells exhibited typical fragmented nuclei and condensed chromatin on staining with Hoechst 33342 (Fig. 3). The percentage of apoptotic cells relative to the total number of cells was increased to H/Re (33 ± 6%), Ca + Ni + Cd-H/Re (31 ± 5%) and Gd + Ni + Cd-H/Re (34 ± 3%) compared with the NPS-2390 + Ca + Ni + Cd-H/Re (20 ± 4%), 2-APB + Ca + Ni + Cd-H/Re (18 ± 4%) and Ru + Ca + Ni + Cd-H/Re (23 ± 5%) groups. Therefore, these data show that the activation of CaR is involved in H/Re - induced cardiomyocyte apoptosis.
Figure 3.
Hoechst-stained nuclei of apoptotic myocytes were analyzed morphologically and were expressed as the percentage of total nuclei. (magnification × 400). A: control group. B: H/Re group. C: Ca + Ni + Cd-H/Re group. D: NPS-2390 + Ca + Ni + Cd-H/Re. E: 2-APB + Ca + Ni + Cd-H/Re. F: Ru + Ca + Ni + Cd-H/Re group. G: Gd + Ca + Ni + Cd-H/Re The cardiomyocytes were placed in hypoxic culture medium for 3 h and then reoxygenated for 6 h by replacing hypoxic culture medium with fresh DMEM containing 10% FBS, and were treated with different inhibitors, respectively. The data presented are expressed as the mean ± SEM. *p < 0.05 vs Control group; †p < 0.05 vs Ca + Ni + Cd-H/Re.
CaR-mediated Ca2+ release in cardiomyocytes during hypoxia/reoxygenation
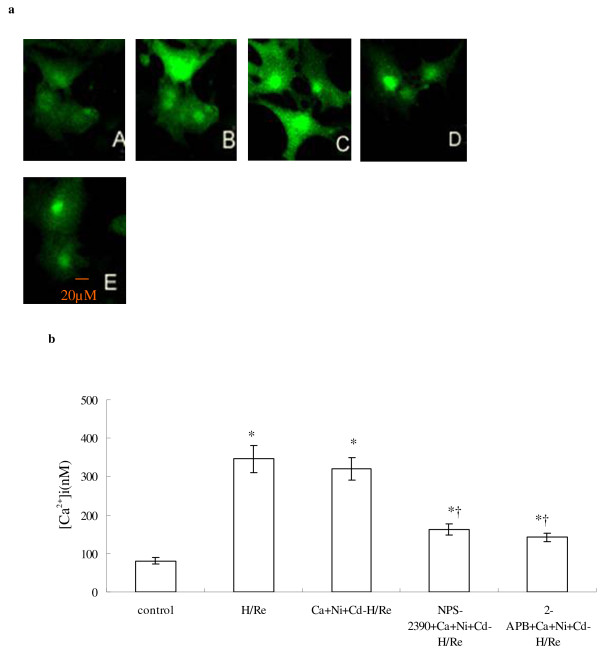
According to previous reports, the increase of [Ca2+]i in cardiomyocytes occurs in the early phase of reoxygenation, concomitant with the burst of calcium overload [19]. In our study, we quantified [Ca2+]i during the first hour after reoxygenation. [Ca2+]i was measured by fluo-4 AM staining (sensitive Ca2+ probe). The calcium concentration of the H/Re (346 ± 35 nM) and Ca + Ni + Cd-H/Re (321 ± 29 nM) groups was significantly increased compared to the control (81 ± 9 nM), NPS-2390 + Ca + Ni + Cd-H/Re (163 ± 15 nM) and 2-APB + Ca + Ni + Cd-H/Re (142 ± 11 nM) groups (Fig.4). The CaCl2 -induced increase in intracellular calcium was significantly attenuated by NPS-2390, which was shown previously to modulate the effects of Ca2+ in other CaR-expressing cells [16]. In our study, we also found similar results in neonatal cardiomyocytes. Likewise, the CaCl2-induced increase in [Ca2+]i was also significantly reduced by 2-APB compared to the Ca + Ni + Cd-H/Re group (Fig. 4).These results suggest that CaCl2 may activate CaR that then induces Ca2+ release through a PLC-mediated/IP3-dependent process.
Figure 4.
The measurement of [Ca2+] after hypoxia/reoxygenation by laser confocal microscopy. (a) A: Control group. B: H/Re group. C: Ca + Ni + Cd-H/Re group. D: NPS-2390 + Ca + Ni + Cd-H/Re. E:2-APB + Ca + Ni + Cd-H/Re -H/Re. (b) Values represent the group mean ± SEM of at least four independent experiments. *p < 0.05 vs Control group; †p < 0.05 vs Ca + Ni + Cd-H/Re.
Activation of CaR depletes [Ca2+]SR during H/Re
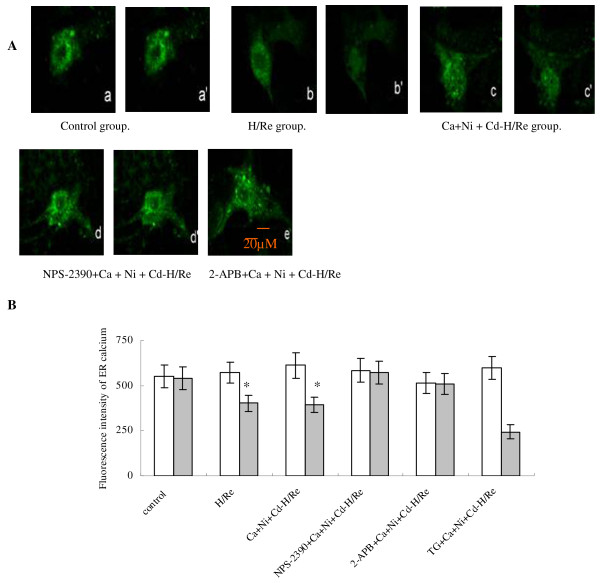
We have demonstrated that CaCl2-activated CaR induces the increase of [Ca2+]i, but the origin of intracellular calcium remains unclear. We examined [Ca2+]SR by Fluo-5N staining. Fluo-5N is a low-affinity Ca2+ indicator (Kd = 400 μmol/L) that is only bright where [Ca2+] is very high, such as in the SR [15]. Rat neonatal cardiomyocytes were loaded with Fluo-5N and permeabilized with saponin. Irregularly distributed bright spots were seen in cardiomyocytes. The Fluo-5N signal was stable at the beginning of reperfusion (Fig. 5). At 60 min after reperfusion, the Fluo-5N signal was detected in the SR. We found that the fluorescence intensity in the SR in the Ca + Ni + Cd-H/Re (376 ± 44) and H/Re (399 ± 42) groups was significantly decreased compared to the control (648 ± 62), NPS-2390 + Ca + Ni + Cd-H/Re (562 ± 64) and 2-APB + Ca + Ni + Cd-H/Re (532 ± 51) groups. Luo et al. have previously demonstrated that 3 μM 2-APB inhibited IP3Rs and prevented PE-induced enhancement of Ca2+ sparks in neonatal cardiomyocytes [20]. Our study also suggests that 3 μM 2-APB may decrease [Ca2+]i through the inhibition of Ca2+ release from the SR via IP3R. Thus, 2-APB treatment could maintain the fluorescence intensity in the SR of cardiomyocytes during reperfusion. These results suggested that the activation of CaR by CaCl2 or H/Re induced SR release of Ca2+.
Figure 5.
CaR activation induced Ca2+ release from the ER during H/Re. (A) a images represent the beginning of reperfusion (0 min). a' images represent 60 min after reperfusion. (B) Values represent the group mean ± SEM of at least four independent experiments. *p < 0.05 vs Control group; †p < 0.05 vs Ca + Ni + Cd-H/Re . White bar represents reoxygenation 0 min; grey bar represents reoxygenation 60 min.
Activation of CaR increases [Ca2+]m and reduces the mitochondrial membrane potential
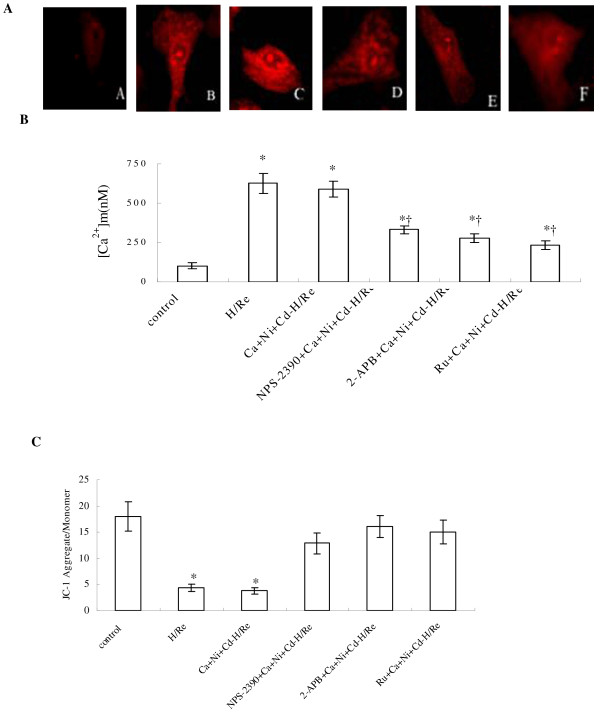
Although CaCl2-activated CaR significantly reduced [Ca2+]SR, the role of type 3 IP3Rs at the MAM in mediating Ca2+ uptake to mitochondria is less clear. To address this question, [Ca2+]m was measured at 60 minutes post-reoxygenation by X-rhod-1 AM staining. The [Ca2+]m was markedly low in the control group (108 ± 11 nM, Fig. 6.A). The [Ca2+]m was significantly greater in the H/Re (626 ± 65 nM) and Ca + Ni + Cd-H/Re (589 ± 52 nM) groups than in the NPS-2390 + Ca + Ni + Cd-H/Re (331 ± 27 nM), 2-APB + Ca + Ni + Cd-H/Re (277 ± 29 nM), or Ru + Ca + Ni + Cd-H/Re (233 ± 26 nM)groups.
Figure 6.
The measurement of [Ca2+]m after 1 h of reoxygenation by laser confocal microscopy. A: control group. B: H/Re group. C: Ca + Ni + Cd-H/Re group. D: NPS-2390 + Ca + Ni + Cd-H/Re E: 2-APB + Ca + Ni + Cd-H/Re -H/Re. F: Ru + Ca + Ni + Cd-H/Re group. (B) Value represents the group mean ± SEM of at least four independent experiments. *p < 0.05 vs Control group; †p < 0.05 vs Ca + Ni + Cd-H/Re . (C) Effect of hypoxia/reoxygenation and CaR activation on △ψm in neonatal rat cardiomyocytes Summarized data for the relative changes of JC-1 fluorescence. Data are mean ± SEM. †p < 0.05 vs sham control group *p < 0.05 vs Ca + Ni + Cd-H/Re group.
The mitochondrial membrane potential was detected with JC-1 staining (Fig. 6C). The ratio of JC-1 aggregates (red) to monomer (green) intensity was reduced in the H/Re (4.4 ± 0.7) and Ca + Ni + Cd-H/Re (3.8 ± 0.6) groups compared with the control (18.1 ± 3.2), NPS-2390 + Ca + Ni + Cd-H/Re (12.9 ± 2.7), 2-APB + Ca + Ni + Cd-H/Re (16.4 ± 2.1) and Ru + Ca + Ni + Cd-H/Re (15.5 ± 2.4) groups.
[Ca2+]SR depletion induced by CaR activation causes apoptosis via a mitochondria-mediated pathway
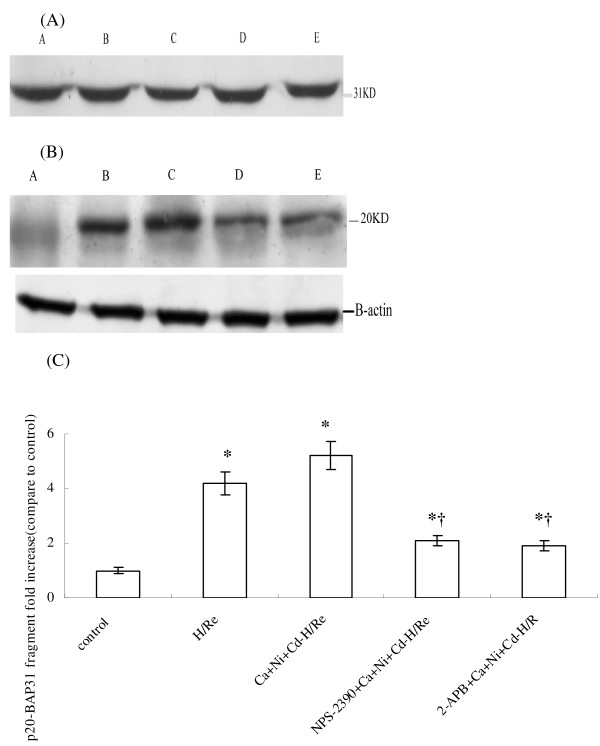
BAP31, an integral membrane protein of the SR, is a caspase-8 substrate [21]. It is cleaved into a p20 fragment following CaCl2 treatment during H/Re (Fig.7). The p20 fragment expression was higher in the H/Re (4.57 ± 0.42) and Ca + Ni + Cd-H/Re (5.28 ± 0.59) groups than in the NPS-2390-+Ca + Ni + Cd-H/Re (2.16 ± 0.27) and 2-APB + Ca + Ni + Cd-H/Re (1.94 ± 0.21) groups.
Figure 7.
The intact (A) and p20 (B) of BAP31 expression during H/Re. A: sham control group. B: H/Re group. C: Ca + Ni + Cd-H/Re group. D: NPS-2390 + Ca + Ni + Cd-H/Re. E: 2-APB + Ca + Ni + Cd-H/Re. The fold change values were mean ± SEM n = 3-4.*p < 0.05 vs control group †p < 0.05 vs H/Re (C)
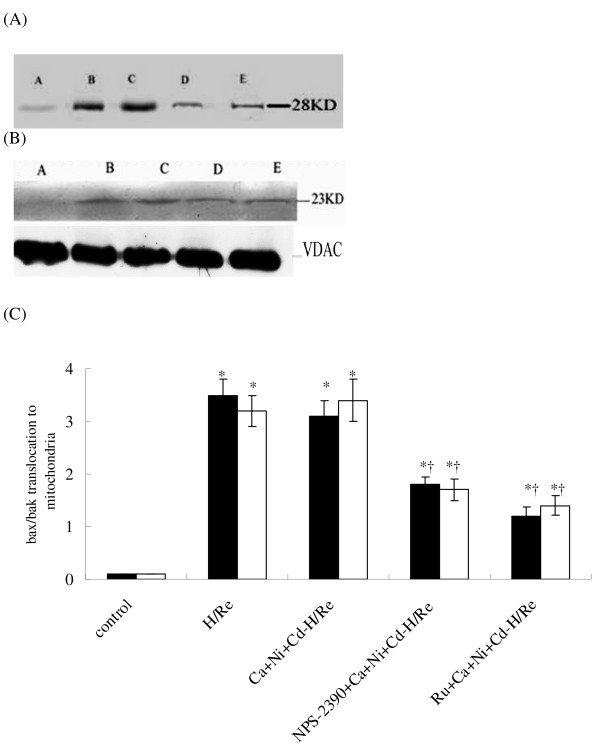
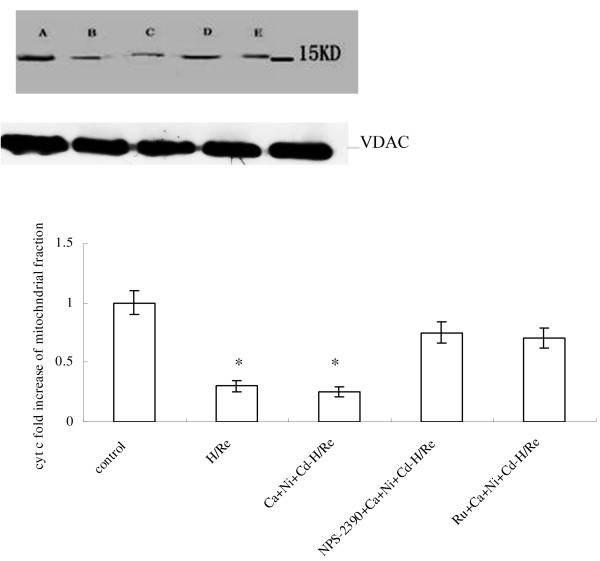
The p20-BAP31 protein has been shown to direct pro-apoptotic signals between the SR and the mitochondria, resulting in the insertion of bax and bak into the outer mitochondria membrane, homo-oligomerization and release of cyt c from the mitochondria [22]. Our results suggest that bax and bak translocation to the mitochondria was significantly increased in the H/Re (3.52 ± 0.31, 3.22 ± 0.28) and Ca + Ni + Cd-H/Re (3.16 ± 0.33, 3.44 ± 0.41) groups compared with the NPS-2390 + Ca + Ni + Cd-H/Re (1.86 ± 0.15, 1.77 ± 0.22) and Ru + Ca + Ni + Cd-H/Re (1.29 ± 0.17, 1.4 ± 0.18) groups (Fig. 8). Next, mitochondrial release of cytochrome c was analyzed to prove the role of the mitochondrial apoptotic pathway. It was found that cytochrome c from mitochondria in the H/Re (0.3 ± 0.05) and Ca + Ni + Cd-H/Re (0.25 ± 0.04) groups was significantly decreased compared with the control (1.0 ± 0.1), NPS-2390- + Ca + Ni + Cd-H/Re (0.75 ± 0.09) and Ru + Ca + Ni + Cd-H/Re (0.69 ± 0.08) groups (Fig. 9).
Figure 8.
Bax (A) and bak (B) translocation to the mitochondrial fractions in rat cardiomyocytes after H/Re. A: control group, B: H/Re group, C: Ca + Ni + Cd-H/Re group, D: NPS-2390 + Ca + Ni + Cd-H/Re group and E: Ru + Ca + Ni + Cd-H/Re group. The fold-change values are mean ± SEM, n = 3-4, *p < 0.05 vs. control group †p < 0.05 vs. H/Re (C). Black bar represented the fold change of bax; white bar represented the fold change of bak.
Figure 9.
The release of cytochrome-C from mitochondrial fractions. A: control group. B: H/Re group. C: Ca + Ni + Cd-H/Re group. D: NPS-2390 + Ca + Ni + Cd-H/Re group. E: Ru + Ca + Ni + Cd-H/Re group. The fold change of cyt c values are mean ± SEM n = 3-4. *p < 0.05 vs control group †p < 0.05 vs H/Re.
Discussion
This study was designed to address the potential involvement of the sarcoplasmic reticulum and mitochondria in regulating cardiomyocyte Ca2+ signaling through MAM subjected to CaR activation and H/Re. The main findings of this study are as follows: (i) Activation of CaR induced the release of Ca2+ from the SR and, simultaneously, the increase of Ca2+ uptake into the mitochondria through MAM during H/Re. (ii) The CaR activation increased the expression of the p20-BAP31 fragment, the translocation of bax/bak from the cytoplasm to the mitochondria and the release of cytochrome c from the mitochondria during H/Re.
The membrane receptor CaR couples to the enzyme PLC, which liberates IP3 from phosphatidylinositol 4,5-bisphosphate (PIP2). The major function of IP3 is to induce endogenous Ca2+ release through IP3Rs [23]. Ca2+ is the primary agonist of CaRs. The EC50 for Ca2+ activation of the CaR is 3-4 mM [24]. CaCl2 was chosen as an agonist to activate CaR, and was shown to increase the expression of CaR (Additional file 1). NPS-2390 was chosen as an antagonist of CaR. In previous study, NPS-2390 is an allosteric antagonist of the group 1 metabotropic glutamate receptors. Group 1 metabotropic glutamate receptors are seven transmembrane domain G protein coupled receptors that activate the Gaq class of G-proteins and stimulate Phospholipase C, resulting in phosphoinositide(PI) hydrolysis and the formation of inositol triphosphate and diacylglycerol.
IP3Rs are ligand-gated Ca2+ channels that function to release intracellular Ca2+ (predominantly from the sarcoplasmic reticulum) in response to IP3 [5]. During reoxygenation, CaR activation caused a significant decrease in the [Ca2+]SR, which could be reversed by either the CaR inhibitor NPS-2390 or the IP3Rs inhibitor 2-APB. Furthermore, the type 3 isoform of the IP3R localized to the SR membranes. Taken together, these results suggest that activation of CaR is involved in the release of Ca2+ from the SR through the IP3R during H/Re.
Rizzuto et al. have provided a structural basis for this hypothesis by showing that mitochondria and ER form an interconnected network in living cells with a restricted number of close contacts [25]. It has been reported that IP3Rs play an important role in establishing macromolecular complexes on the surface of the SR membranes and in modulating the linkage between the SR and mitochondrial membranes. Mitochondria respond rapidly to physiological increases in [Ca2+]e, and stimulation with Gq-coupled receptor agonists, which induce IP3 production and the subsequent release of Ca2+ from ER, causes a rapid rise in [Ca2+]m [26]. This effect has been detected in many cells types: HeLa cells, fibroblasts, endothelial and epithelial cells, cardiac and skeletal muscle cells, neurons and pancreatic β cells [27,28]. CaR, as a Gq-coupled receptor, could be involved in promoting Ca2+ release from ER and then in induced the [Ca2+]m rise. Our results suggest that [Ca2+]m was elevated and mitochondrial membrane potential collapsed in the Ca + Ni + Cd-H/Re group, whereas [Ca2+]m and mitochondrial membrane potentials were maintained in the 2-APB + Ca + Ni + Cd-H/Re group. The rapid mitochondrial Ca2+ uptake is related to the low affinity of the Ca2+ transport system. Therefore, Ruthenium red, an inhibitor of the mitochondrial calcium transporter, was used in our experiment. The results reveal that [Ca2+]m and mitochondrial potentials were maintained in the Ru + Ca + Ni + Cd-H/Re group. These results suggest that both the SR and the mitochondria orchestrate the regulation of Ca2+ signaling between these two organelles.
Although a role for the SR in the mitochondrial redistribution of Ca2+ has been implicated in many models of apoptosis, a primary role for IP3 generation and the activation of IP3Rs in this process has been examined in only a few instances. Caspase-8 cleavage of BAP31 at the SR leads to the generation of a p20 fragment, which directs pro-apoptotic signals between the SR and mitochondria, resulting in early discharge of Ca2+ from the SR and its concomitant uptake into the mitochondria. Early and critical events in apoptosis occur in mitochondria and in the ER, and the release of elements acting as caspase cofactors, such as cytochrome c (from mitochondria) and Ca2+ (from the ER), into the cytosol are requisites for cell death in many cases [29]. The mitochondrial pathway of apoptosis is regulated by members of the Bcl-2 protein family, subdivided into two groups: anti-apoptotic (Bcl-2) and pro-apoptotic (Bax, Bak). The link between Bcl-2 (localized in several intracellular membranes including those of mitochondria and the ER) and Ca2+ homeostasis has been established by showing that Bcl-2 reduces the steady state Ca2+ levels in the ER, thereby dampening the apoptotic signal [30,31]. Jiang et al. showed that CaR was involved in neonatal cardiomyocyte apoptosis in ischemia/reperfusion injury. They suggested that [Ca2+]i was increased, inhibiting the expression of Bcl-2 and elevating the expression of the pro-apoptotic protein caspase-3 in cytoplasm [32]. However, the Ca2+-dependent model of apoptosis was subsequently supported by a series of observations with the pro-apoptotic Bcl-2 family members Bax and Bak. Cells deriving from knockout mice lacking Bax and Bak that are very resistant to apoptotic death have a dramatic reduction in the [Ca2+] within the ER and a drastic reduction in the transfer of Ca2+ from the ER to mitochondria [33].This change prompts mitochondrial fission and cytochrome c release into the cytosol. Green et al. demonstrated that [Ca2+]SR depletion caused bax- and bak-mediated permeability of the outer mitochondrial membrane, thereby releasing pro-apoptotic factors and particularly cytochrome c [34]. Our present data show that CaR activation induced the cleavage of BAP31 with the formation of the pro-apoptotic p20 fragment, causing bax and bak translocation to the mitochondria and cytochrome c release from the mitochondria during H/Re.
In conclusion, our results constitute the first report that CaR plays an important role in the SR-mitochondrial inter-organelle Ca2+ signaling through the IP3Rs, which are also involved in apoptosis during H/Re.
Abbreviations
IP3Rs: inositol 1,4,5-trisphosphate receptors; MAM: mitochondrion-associated ER membrane; H/Re: hypoxia/reoxygenation; CaR: calcium sensing receptor; GPCR: G protein-coupled receptors; PIP2: phosphatidylinositol 4,5-bisphosphate; MTT: 3-(4,5-dimethyl thiazol-2yl)-2,5-diphenyltetrazolium bromide; JC-1: 5,5',6,6'-tetrachloro 1,1'3,3'-tetraethylbenzimidazolcarbocyanine iodide
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
WZ and CX drafted the manuscript, FL and ZT participated in the design of the study and did most of the experiments, YZ conceived of the study, HL, HR, HZ, CL and GH participated in its design and coordination, YT, BY and RW revised the paper and gave some suggestions. All authors read and approved the final manuscript.
Supplementary Material
CaR inducing apoptosis via the sarcoplasmic reticulum-mitochondrion crosstalk in hypoxia/reoxygenation.
Contributor Information
Fang-hao Lu, Email: lufanghao1973@yahoo.com.cn.
Zhiliang Tian, Email: zhangwh116@hotmail.com.
Wei-hua Zhang, Email: zhangwh116@hotmail.com.
Ya-jun Zhao, Email: zhangwh116@hotmail.com.
Hu-lun Li, Email: zhangwh116@hotmail.com.
Huan Ren, Email: zhangwh116@hotmail.com.
Hui-shuang Zheng, Email: zhangwh116@hotmail.com.
Chong Liu, Email: zhangwh116@hotmail.com.
Guang-xia Hu, Email: zhangwh116@hotmail.com.
Ye Tian, Email: zhangwh116@hotmail.com.
Bao-feng Yang, Email: zhangwh116@hotmail.com.
Rui Wang, Email: zhangwh116@hotmail.com.
Chang-qing Xu, Email: xucq45@126.com.
Acknowledgements
This study was supported by grants from the National Basic Research Program of China (973 program No. 2007CB512000), the National Natural Science Foundation of China (No. 30700288, 30770878, 30871012), the Harbin Medical University fund for younger scientists (No. 060015), from Harbin Medical University fund for graduated Students (HCXB2009015) and from Hei Longjiang Province fund for graduated Students (YJSCX209-223HLJ).
References
- Duchen MR. Role of mitochondria in health and disease. Diabetes. 2004;53(Suppl 1):S96–100. doi: 10.2337/diabetes.53.2007.S96. [DOI] [PubMed] [Google Scholar]
- Duchen MR. Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol Aspects Med. 2004;25:365–451. doi: 10.1016/j.mam.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Belmonte S, Morad M. Shear fluid-induced Ca2+ release and the role of mitochondria in rat cardiomyocytes. Ann NY Acad Sci. 2008;1123:58–63. doi: 10.1196/annals.1420.007. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survive. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Alzayady KJ, Wojicikiewicz RJ. The role of Ca2+ in triggering inositol 1,4,5-trisphophate receptor ubiquitination. Biochem J. 2005;392:601–606. doi: 10.1042/BJ20050949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Brown EM, Macleod RJ. Extracellular calcium sensity and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- Wang R, Xu C, Zhao W, Zhang J, Cao K, Yang B, Wu L. Calcium and polyamine regulated calcium-sensing receptors in cardiac tissues. Eur J Biochem. 2003;270:2680–2688. doi: 10.1046/j.1432-1033.2003.03645.x. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen J, Hansen JL, Smajilovic S, Terwilliger EF, Haunso S, Sheikh SP. Calcium receptor is functionally expressed in rat neonatal ventricular cardiomyocytes. Am J Physiol Heart Circ Physiol. 2006;290:H1165–71. doi: 10.1152/ajpheart.00821.2005. [DOI] [PubMed] [Google Scholar]
- Berra Romani R, Raqeeb A, Laforenza U, Scaffino MF, Moccia F, Avelino-Cruz JE, Oldani A, Coltrini D, Milesi V, Taglietti V, Tanzi F. Cardiac microvascular endothelial cells express a functional Ca2+-sensing receptor. J Vasc Res. 2009;46:73–82. doi: 10.1159/000140677. [DOI] [PubMed] [Google Scholar]
- Zhang WH, Fu SB, Lu FH, Wu B, Gong DM, Pan ZW, Lv YJ, Zhao YJ, Li QF, Wang R, Yang BF, Xu CQ. Involvement of calcium-sensing receptor in ischemia/reperfusion-induced apoptosis in rat cardiomyocytes. Biochem Biophys Res Commun. 2006;347:872–881. doi: 10.1016/j.bbrc.2006.06.176. [DOI] [PubMed] [Google Scholar]
- Sun HY, Wang NP, Kerendi F, Halkos M, Kin H, Guyton RA, Vinten-Johansen J, Zhao ZQ. Hypoxic postconditioning reduces cardiomyocyte loss by inhibiting ROS generation and intracellular Ca2+ overload. Am J Physiol Heart Circ Physiol. 2005;288:H1900–H1908. doi: 10.1152/ajpheart.01244.2003. [DOI] [PubMed] [Google Scholar]
- Ladilov Y, Schäfer C, Held A, Schäfer M, Noll T, Piper HM. Mechanism of Ca(2+) overload in endothelial cells exposed to simulated ischemia. Cardiovas Res. 2000;47:394–403. doi: 10.1016/S0008-6363(00)00108-5. [DOI] [PubMed] [Google Scholar]
- Xu J, Lü XW, Huang Y, Zhu PL, Li J. Synergism of simvastatin with losartan prevents angiotensinII-induced cardiomyocyte apoptosis in vitro. J Pharm Pharmacol. 2009;61:503–510. doi: 10.1211/jpp/61.04.0013. [DOI] [PubMed] [Google Scholar]
- Kubalova Z, Györke I, Terentyeva R, Viatchenko-Karpinski S, Terentyev D, Williams SC, Györke S. Modulation of cytosolic and intra-sarcoplasmic reticulum calcium waves by calsequestrin in rat cardiac myocytes. J Physiol. 2004;561:515–524. doi: 10.1113/jphysiol.2004.073940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan P, Mentzer RM Jr, Lasley RD. Annexin V staining during reperfusion detects cardiomyocytes with unique properties. Am J Physiol Heart Circ Physiol. 2001;281:H1931–H1937. doi: 10.1152/ajpheart.2001.281.5.H1931. [DOI] [PubMed] [Google Scholar]
- Sun HY, Wang NP, Halkos ME, Kerendi F, Kin H, Wang RX, Guyton RA, Zhao ZQ. Involvement of Na+/H+ exchanger in hypoxia/re-oxygenation-induced neonatal rat cardiomyocyte apoptosis. Eur J Pharmacol. 2004;486:121–131. doi: 10.1016/j.ejphar.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Tao J, Xu H, Yang C, Liu CN, Li S. Effect of urocortin on L-type calcium currents in adult rat ventricular myocytes. J Pharmacol Res. 2004;50:471–476. doi: 10.1016/j.phrs.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Di Lisa F, Blank PS, Colonna R, Gambassi G, Silverman HS, Stern MD, Hansford RG. Mitochondrial membrane potential in sigle living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. J Physiol. 1995;486:1–13. doi: 10.1113/jphysiol.1995.sp020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Yang D, Lan X, Li K, Li X, Chen J, Zhang Y, Xiao RP, Han Q, Cheng H. Nuclear Ca2+ sparks and waves mediated by inositol 1,4,5-trisphosphate receptors in neonatal rat cardiomyocytes. Cell Calcium. 2008;43:165–174. doi: 10.1016/j.ceca.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson RL, Boehning D, Snyder SH. Inositol 1,4,5-trisphosphate receptors as signal intergrators. Annu Rev Biochem. 2004;73:437–456. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- Kockskämper J, Zima AV, Roderick HL, Pieske B, Blatter LA, Bootman MD. Emerging roles of inositol 1,4,5- triphosphate signaling in cardiac myocytes. J Mol Cell Cardiol. 2008;45:128–147. doi: 10.1016/j.yjmcc.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Chen TH, Pratt S, Shoback D. Amino acids in the second and third intracellular loops of the parathyroid Ca2+-sensing receptor mediate efficient coupling to phospholipase C. J Biol Chem. 2000;275:19955–19963. doi: 10.1074/jbc.M909613199. [DOI] [PubMed] [Google Scholar]
- Handlogten ME, Shiraishi N, Awata H, Huang C, Miller RT. Extracellular Ca2+-sensing receptor is a promiscuous divalent cation sensing that responds to lead. Am J Physiol Renal Physiol. 2000;279:F1083–F1091. doi: 10.1152/ajprenal.2000.279.6.F1083. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+; molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- Roderick HL, Bootman MD. Bi-directional signaling from InsP3 receptor: regulation by calcium and accessory factors. Biochem Soc Trans. 2003;31:950–953. doi: 10.1042/BST0310950. [DOI] [PubMed] [Google Scholar]
- Szado T, Vanderheyden V, Parys JB, De Smedt H, Rietdorf K, Kotelevets L, Chastre E, Khan F, Landegren U, Söderberg O, Bootman MD, Roderick HL. Phosphorylation of inositol 1,4,5-triphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc Natl Acad Sci USA. 2008;105:2427–32. doi: 10.1073/pnas.0711324105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaen L, De Smedt H, Parys JB, Casteels R. Co-actvation of inositol triphosphate-induced Ca2+ release by cytosolic Ca2+ is loading-dependent. J Biol Chem. 1994;269:7238–7242. [PubMed] [Google Scholar]
- Bootman MD, Missiaen L, Parys JB, De Smedt H, Casteels R. Control of inositol triphosphate-induced Ca2+ release by cytosolic Ca2+ Biochem J. 1995;306:445–451. doi: 10.1042/bj3060445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie L, Bootman MD, Laine M, Berridge MJ, Thuring J, Holmes A, Li WH, Lipp P. The role of inositol 1,4,5- triphosphate receptors in Ca2+ signaling and the generation arrhythmias in rat atrial myocytes. J Physiol. 2002;87:457–409. doi: 10.1113/jphysiol.2001.013411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett JK, White C, Cheung KH, Mak DO. Inositol triphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang CM, Han LP, Li HZ, Qu YB, Zhang ZR, Wang R, Xu CQ, Li WM. Calcium sensing receptor induces in cultured neonatal rat ventricular cardiomyocyte apoptosis during simulated ischemia/reperfusion. Cell Biol Int. 2008;38:792–800. doi: 10.1016/j.cellbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Sugimoto s, Iwashiro K, Monti F. The risk of myocardial stunning is decreased concentration-dependent by KATP channel activation with nicorandil before high K+ cardioplegia. Int J Cardiol. 1995;48:11–25. doi: 10.1016/0167-5273(94)02213-3. [DOI] [PubMed] [Google Scholar]
- Green DR. Apoptotic pathways: ten minutes to dead. Cell. 1999;121:671–674. doi: 10.1016/j.cell.2005.05.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CaR inducing apoptosis via the sarcoplasmic reticulum-mitochondrion crosstalk in hypoxia/reoxygenation.