Abstract
Objective
Bullying is the act of intentionally and repeatedly causing harm to someone who has difficulty defending him or herself, and is a relatively wide-spread school-age phenomenon. Being the victim of bullying is associated with a broad spectrum of emotional problems; however, not all children who are bullied go on to develop such problems.
Method
We tested the hypothesis that the relationship between bullying victimization and emotional problems was moderated by variation in the serotonin transporter (5-HTT) gene in 2,232 British children comprising the Environmental Risk (E-Risk) study cohort.
Results
Our data supported the hypothesis that children's bullying victimization leads to their developing emotional problems, and that genetic variation in the 5-HTTLPR moderates this relationship. Specifically, frequently bullied children with the SS genotype were at greater risk of developing emotional problems at age 12 than children with the SL or LL genotype. Furthermore, we demonstrated that this genetic moderation persisted (a) after controlling for children's pre-victimization emotional problems by assessing intra-individual change in problems between ages 5 and 12 years, and (b) after controlling for other risk factors shared by children growing up in the same family by comparing emotional problems in twins discordant for bullying victimization.
Conclusions
These findings are further evidence that the 5-HTTLPR moderates the risk of emotional disturbance after exposure to stressful events.
Keywords: Bullying, Emotional Problems, Gene-Environment Interaction, Serotonin Transporter, Victimization
Introduction
Bullying is the act of intentionally and repeatedly causing harm (through verbal harassment, coercive actions, or physical assault) to someone who has difficulty defending him or herself.1 Bullying victimization is widespread among school-age children.2 Although bullying is not a new problem, its consequences are not as benign as long presumed: Being the victim of bullying is associated with a broad spectrum of emotional problems,3 and compromises the well being and health of some children and adolescents.
A notable feature of research on the psychological effects of bullying victimization is the wide range of reactions observed among victims, raising the question of what accounts for response variability. Diathesis-stress models of psychopathology suggest the possibility that genetic differences may render some children more vulnerable to the effects of bullying victimization than other children.
In the present study, a functional polymorphism in the promoter region of the serotonin transporter gene (5-HTT) was used to characterize genetic vulnerability to bullying victimization and to test the hypothesis that 5-HTT variation moderates the influence of bullying victimization on children's emotional problems. The 5-HTT maps to chromosome 17, and transcriptional activity is modulated by variation in the length of the serotonin transporter linked polymorphic region (5-HTTLPR) within the gene's promoter. This regulatory region contains two common alleles of which the short (S) allele is associated with lower transcriptional efficiency of the promoter compared with the long (L) allele4, and it has been suggested that this polymorphism contributes to dysregulation of serotonergic neurotransmission. 5-6
Studies of various stress-reactive endophenotypes suggest that S carriers should be most reactive to the effects of stressful experiences such as bullying victimization. We refer to a new generation of research in experimental psychopathology that exposes individuals with different genotypes to stress-inducing situations or affectively-charged stimuli in order to examine genetic control of sensitivity to the environment by measuring their stress reactivity. Of these, five findings are of note.
First, functional magnetic resonance imaging (fMRI) studies have shown that the S allele is associated with exaggerated amygdala response to environmental threat.7-8 This finding suggests that the S allele may influence risk for emotional problems by biasing the response of a key brain region mediating behavioral and physiologic arousal to environmental challenges.6
Second, research on fear conditioning—whose neural mechanisms involve the amygdala—also reveals that variation in the 5-HTTLPR is implicated in how people learn to fear new stimuli. Compared to LL homozygotes, S-allele carriers acquired potentiated startle reactions to stimuli associated with an aversive event, and this acquired fear was more resistant to extinction. This research suggests that S-carriers are more likely pick up and retain fear of stimuli associated with threat.9
Third, research using acute stress-induction paradigms shows that 5-HTTLPR variation is associated with variations in cortisol response to a psychosocial challenge. Adolescents with two copies of the S allele showed a marked increase in cortisol immediately following exposure to stress and a slow return to baseline.10 This research not only suggests that genetic susceptibility to HPA-axis dysregulation is detectable in SS carriers as early as adolescence, but also that those individuals have a higher reactivity to stressors than non-SS individuals.
Fourth, whereas much of the experimental research on 5-HTTLPR variation and stress reactivity has focused on mechanisms by which S-carriage confers risk, an investigation of biased attention provides evidence to suggest why LL homozygotes might be protected from negative events. When exposed to affectively-charged images, LL homozygotes were characterized by selective avoidance of threat and selective attention to positive material.11 This suggests that genetic variation in the tendency to ‘look on the bright side of life’ may be a key mechanism underlying resilience.
Fifth, non-human primate studies also provide a hint as to why aspects of S-carriage may be linked to greater reactivity to bullying victimization. Bullying involves repeated hurtful actions between individuals where there is a power differential between the bully and the victim.12 Research with rhesus macaques has uncovered different reactions among monkeys carrying the S allele versus LL homozygotes when they are confronted with high- versus low-status con-specifics. In particular, S-carrying monkeys were more likely to be threatened by and to avoid high-status dominant con-specifics (e.g., they displayed greater pupil diameter in response to high-status monkeys13). This research suggests that S-carriers may be more sensitive to the threat of confrontation in the context of power imbalance.
In previous research we empirically documented that being bullied is an environmentally-mediated contributing factor to both boys' and girls' emotional problems in this same cohort.14-15 In the present study we tested the hypothesis that genetic variation in the 5-HTTLPR would moderate the link between bullying victimization and the risk of developing these problems.
Method
Participants
Participants were members of the Environmental Risk (E-Risk) Study which tracks the development of a birth cohort of 2,232 British children. The sample was drawn from a larger birth register of twins born in England and Wales in 1994-1995.16 Details about the sample are reported elsewhere.17 Briefly, the E-risk base sample was constructed in 1999-2000, when 1,116 families with same-sex 5-year old twins (93% of those eligible) participated in home-visit assessments. The sample includes 55% monozygotic (MZ) and 45% dizygotic (DZ) twin pairs. Sex is evenly distributed within zygosity (49% male). Follow-up home visits were conducted when the children were aged 7 years (98% participation), 10 years (96% participation), and, most recently, 12 years (96% participation). The Maudsley Hospital Ethics Committee approved each phase of the study.
Measures
Bullying victimization was assessed during private interviews with the children during home visits when they were age 12 years. We explained to them that someone is being bullied when another child (1) says mean and hurtful things, makes fun or calls a person mean and hurtful names; 2) completely ignores or excludes someone from their group of friends or leaves them out of things on purpose; 3) hits, kicks, or shoves a person, or locks them in a room; 4) tells lies or spreads rumors about them; and 5) other hurtful things like these. We call it bullying when these things happen often, and it is difficult for the person being bullied to stop it happening. We do not call it bullying when it is done in a friendly or playful way. Children indicated whether they had been bullied by another child “never”, “sometimes”, or “a lot”. When a child reported being bullied, the interviewer asked the child to describe what happened. Notes taken by the interviewers were later checked by an independent rater to verify that the events described by the child relate to instances of bullying, operationally defined as evidence of repeated harmful actions between children where there is a power differential between the bully and the victim. This was done blind to data on emotional problems and genotype.
Emotional problems were assessed using the Child Behavior Checklist18 for mothers and the Teacher's Report Form19 for teachers, at age 5 years and again at age 12 years. The emotional problems scale is the sum of items on the Withdrawn and Anxious/Depressed scales, including items such as “cries a lot”, “withdrawn, doesn't get involved with others” and “worries” (Somatic Complaints were not included, as this scale was not assessed at age 12). The internal consistency reliability of the mother and the teacher reports at age 5 was .86 and .87 respectively. The internal consistency reliability of the mother and the teacher reports at age 12 was .87 and .89 respectively. Mother and teacher reports at each age were summed and standardized to create cross-informant scales for ages 5 and 12 years. For consistency across our research program on bullying, we use the same outcome measures as in previous reports.15
DNA Extraction and Genotyping
At ages 5 and 7 years, DNA was obtained from 2161 (97%) of the children. DNA samples were obtained via buccal swabs and extracted using an established procedure.20 Primer sequences for 5-HTTLPR are described by Gelernter et al., (1997),21 (forward primer: 5′-ATGCCAGCACCTAACCCCTAATGT-3′; reverse primer: 5′-GGACCGCAAGGTGGGCGGGA-3′). The forward primer was 5′-labeled with a HEX fluorophore. PCR was carried out on a PTC-225 DNA engine (MJ Research, Waltham, MA, USA), using the following cycling conditions: initial 15 min denaturing step at 95°C, followed by 35 cycles of 94°C for 30 sec, 66°C for 30 sec and 72°C for 40 sec, and a final extension phase of 72°C for 15 min. Reactions were performed in 1× reaction Buffer IV (ABgene, Epsom, UK), 1.5 mM MgCl2, 50ng of genomic DNA, 5pmols of each primer, 0.2mM dNTPs and 2 units of Native Taq (Promega, Madison, WI, USA). This amplifies a 419 base pair product for the 16 repeat (L) allele and a 375 base pair product for the 14 repeat (S) allele. PCR products were denatured in highly deionised formamide and analysed by electrophoresis on an Applied Biosystems 3100 genetic analyzer (Applied Biosystems, Foster City, CA, USA), set up in genotyping mode, using POP4 polymer and ROX labeled GS500 size standard (Applied Biosystems). Results were analyzed using GeneScan v3.7 and genotypes called using Genotyper v3.6 software (Applied Biosystems). All genotype calls were reviewed manually. Samples were genotyped in birthdate order and blind to data on bullying victimization and emotional scores.
Statistical Analyses
We tested the gene × environment interaction (G×E) in a hierarchical regression framework, with all main-effect terms entered on the first step of the model and interaction terms entered on the second step:
Emotional problems=a+b1(5-HTTLPR)+b2(Occasional Bullying Victimization)+b3(Frequent Bullying Victimization)+b4(5-HTTLPR × Occasional Victimization)+B5(5-HTTLPR × Frequent Victimization)+e,
where Occasional and Frequent Victimization were coded as dummy variables, respectively, and 5-HTTLPR was coded as 0=no S alleles, 1=1 S allele, and 2=2 S alleles. We tested for change in emotional problems between ages 5 and 12 by creating a variable that is the difference between the child's age-12 and age-5 score, and performed the regression analysis as above. We also performed regression analyses of age-12 emotional problems, controlling for age-5 emotional scores. Reported significance tests are based on the sandwich, or Huber/White, variance estimator,22 a method available in STATA 7.0 (StataCorp, 2001). Application of this technique addresses the assumption of independence of observations. It adjusts estimated standard errors and therefore accounts for the dependence in the data that is due to analyzing sets of twins.
Within-family comparisons were conducted by correlating within-twin pair discordance in bullying victimization with within-twin pair discordance in age-12 emotional problems. To achieve this, we first created two categories of twin pairs; those who were concordant for bullying (i.e. same rate of bullying within twins) or discordant (i.e. the twins experienced different rates of bullying). Twin pairs were selected only if they grew up in the same household. Second, we divided these twin pairs into 3 groups according to their 5-HTTLPR genotype, selecting only pairs in which both twins shared the same 5-HTTLPR genotype. Third, we correlated twin differences in bullying victimization with twin differences in both emotional problems at age 12 and change in emotional problems between ages 5-12 years.
Results
Bullying victimization and genotype data were available for 2017 children (90.4% of the total sample); 46.8% of these individuals had never experienced any bullying victimization by age 12, 41.8% had experienced occasional victimization and 11.4% had experienced frequent victimization. Genotype frequencies were: SS=17.5%, SL=49.2% and LL=33.4% and genotypes were in Hardy-Weinberg equilibrium (χ2=0.17, df=2, p=.918).
Does 5-HTTLPR genotype moderate the association between bullying victimization and children's emotional problems?
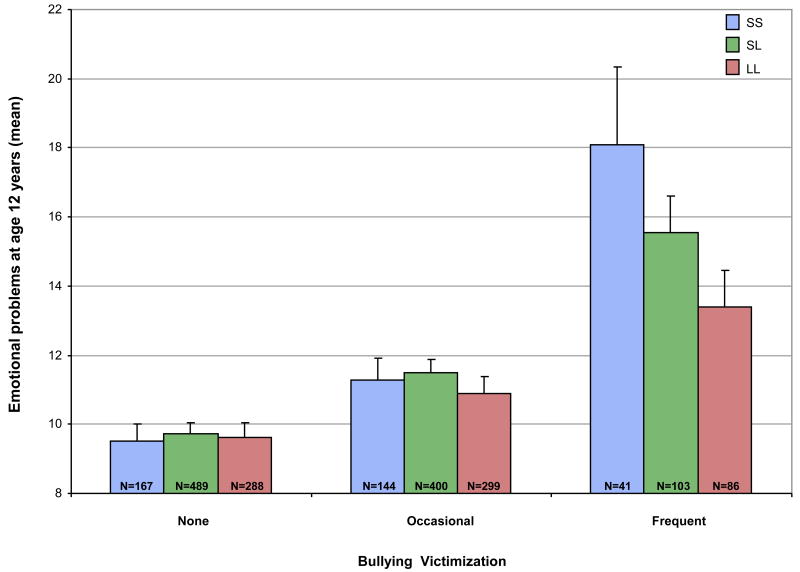
Figure 1 shows children's emotional problems at age 12 years as a function of their 5-HTTLPR genotype and bullying victimization experiences. Children's genotype was not significantly associated with their emotional problems (b=.38, SE=0.31, t =1.23, p =.218) (Table 1; Panel A). In contrast, children's victimization was significantly associated with their emotional problems; occasionally victimized children were significantly more like to experience emotional problems than non-bullied children (b=1.60, SE=.38, t=4.20, p=<.001) and frequently victimized children were at especially pronounced risk (b=5.56, SE=.82, t=6.76, p < .001). The association between bullying victimization and children's emotional problems was moderated, albeit at a trend level, by children's genotype, and this moderation was more pronounced among children who were frequently victimized (b=2.33, SE=1.23, t =1.89, p =.059) rather than occasionally victimized (b=0.30, SE=0.54, t=0.55, p=.582). The statistical effect of frequent bullying victimization on children's emotional problems was strongest among SS homozygotes (b=8.56, SE=2.42, t=3.53, p <.001) followed by SL heterozygotes (b=5.81, SE=1.16, t=5.00, p <.001) and LL homozygotes (b=3.79, SE=1.19, t=3.19, p=.002). Within children who experienced frequent bullying, 31.7% of SS homozygotes had emotional problem scores in the clinical range (i.e. emotional problem scores above 1.3 SD of the mean, the clinically relevant cut-off for the internalizing scale of the CBCL as suggested by Achenbach18), compared with 29.1% of SL heterozygotes and 15.1% of LL homozygotes.
Figure 1.
Children's emotional problems at age 12, as a function of their 5-HTTLPR genotype and bullying victimization experiences. Note: Emotional problems at age 12 were elevated as a function of bullying victimization, but to a greater degree in SS homozygotes than SL heterozygotes and LL homozygotes. Error bars represent standard errors of the mean, Ns represent the number of individuals. 5-HTTLPR = serotonin transporter linked polymorphic region; LL= Long/Long; SL= Short/Long; SS= Short/Short.
Table 1.
Results of regression analyses testing Gene × Environment interaction effects on emotional problems at age 12 years (Panel A) and on within-individual change in emotional problems between ages 5 and 12 years (Panel B).
| Panel A: Emotional problems (age 12 years) |
Panel B: Change in emotional problems (ages 5-12 years) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Predictor variables | b | se | t | p | b | se | t | p |
| Genetic and environmental main effects | ||||||||
| 5-HTTLPR | .38 | .31 | 1.23 | .218 | .49 | .35 | 1.40 | .160 |
| Occasional bullying | 1.60 | .38 | 4.20 | <.001 | 1.47 | .46 | 3.23 | .001 |
| Frequent bullying | 5.56 | .82 | 6.76 | <.001 | 4.09 | .77 | 5.28 | <.001 |
| Gene × Environment interactions | ||||||||
| 5-HTTLPR × Occasional bullying | .30 | .54 | 0.55 | 0.582 | -.44 | .66 | -.66 | .508 |
| 5-HTTLPR × Frequent bullying | 2.33 | 1.23 | 1.89 | 0.059 | 2.90 | 1.17 | 2.48 | .013 |
Note: 5-HTTLPR = serotonin transporter linked polymorphic region
Does 5-HTTLPR genotype confer heightened risk to emotional problems following bullying even after controlling for children's pre-victimization emotional problems?
This evidence that 5-HTTLPR variation moderates the statistical effect of bullying victimization on children's emotional problems does not constitute unambiguous evidence of G×E because some children may evoke bullying victimization; such evocation could be a function of children's 5-HTTLPR genotype or, more generally, of children's pre-existing and partially heritable emotional problems.23 To rule out the possibility of such gene-environment correlations,24 we conducted two tests. First, we tested whether 5-HTTLPR genotype was associated with risk of bullying victimization. There was no significant association between 5-HTTLPR genotype and risk of being bullied (χ2=7.25, df=4, p=.123). The rates of occasional and frequent victimization were 40.9% and 11.6% among SS homozygotes, 40.3% and 10.4% among SL heterozygotes, and 44.4% and 12.8% among LL homozygotes.
Second, we performed longitudinal analyses to test whether genotype moderated the effect of bullying victimization on within-individual change in children's emotional problems from age 5 to age 12. This analysis is important because our longitudinal study revealed that children who were bullied had more emotional problems already at age 5 years, before they entered primary school (the mean age-5 emotional problem scores for children who were not bullied, occasionally bullied, and frequently bullied were 11.88 [SD=8.31], 12.07 [SD=8.16], and 13.29 [SD=8.90], respectively). A multinomial logistic regression (a model which generalizes logistic regression by allowing two discrete outcomes) showed that children's emotional problems at age 5 years predicted their subsequent risk of experiencing frequent bullying victimization (RRR=1.019, SE=.009, z=2.12, p=.034, CI=1.001-1.036), although not occasional bullying victimization (RRR=1.002, SE=.006, z=0.37, p=.710, CI=0.99-1.01). As such, we sought to test (a) whether bullying victimization was associated with the emergence of more emotional problems among children from age 5 to age 12 years and (b) whether 5-HTTLPR genotype moderated the risk of these newly emerging emotional problems, using each child as his or her own control
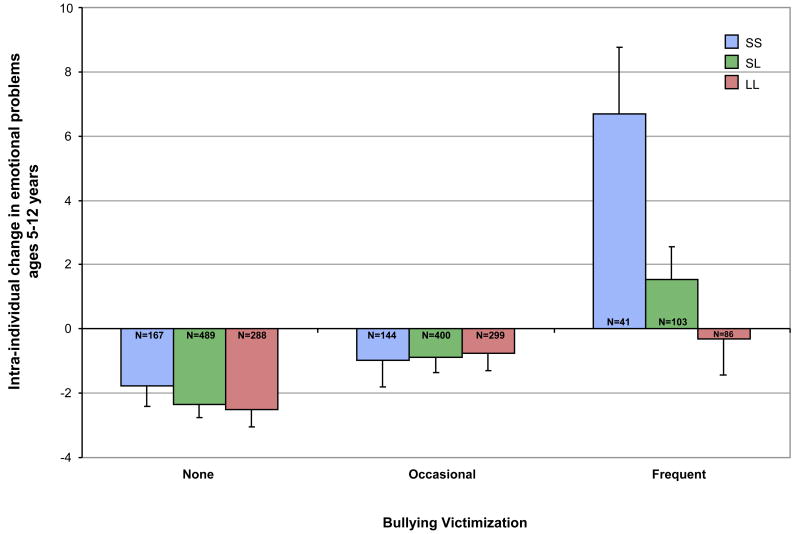
Figure 2 shows the mean change in children's emotional problems from age 5 to age 12 years (calculated as the difference between each child's age-5 and age-12 emotional problem score), as a function of their 5-HTTLPR genotype and bullying victimization experiences. Emotional problems remained fairly stable from age 5 to 12. However, one group of children showed significant increases in emotional problems: Children who were frequently victimized by bullying (Figure 2). Moreover, within the group of frequently victimized children, the results point to significant genetic moderation (b=2.90, SE=1.17, t=2.48, p=.013) (Table 1; Panel B). The statistical effect of frequent bullying victimization on increases in children's emotional problems was strongest among SS homozygotes (b=8.47, SE=2.22, t=3.82, p <.001) followed by SL heterozygotes (b=3.90, SE=1.04, t=3.73, p <.001) and weakest among LL homozygotes (b=2.18, SE=1.20, t=1.82, p=.070). We also tested whether this genetic moderation was observed when predicting age-12 emotional problems after statistically controlling for age-5 emotional problems. In agreement with the change-score analyses, we found a significant interaction effect between 5-HTTLPR and frequency bullying victimization (b=2.52, SE=1.14, t=2.22, p=.027).
Figure 2.
Change in children's emotional problems from age 5 to age 12 years, as a function of their 5-HTTLPR genotype and bullying victimization experiences. Note: Frequent victimization led to significant increases in emotional problems, and this statistical effect is strongest amongst SS homozygotes, followed by SL heterozygotes. Error bars represent standard errors of the mean, Ns represent the number of individuals. 5-HTTLPR = serotonin transporter linked polymorphic region; LL= Long/Long; SL= Short/Long; SS= Short/Short.
Does 5-HTTLPR genotype confer heightened risk to emotional problems following bullying, even after controlling for other common family experiences?
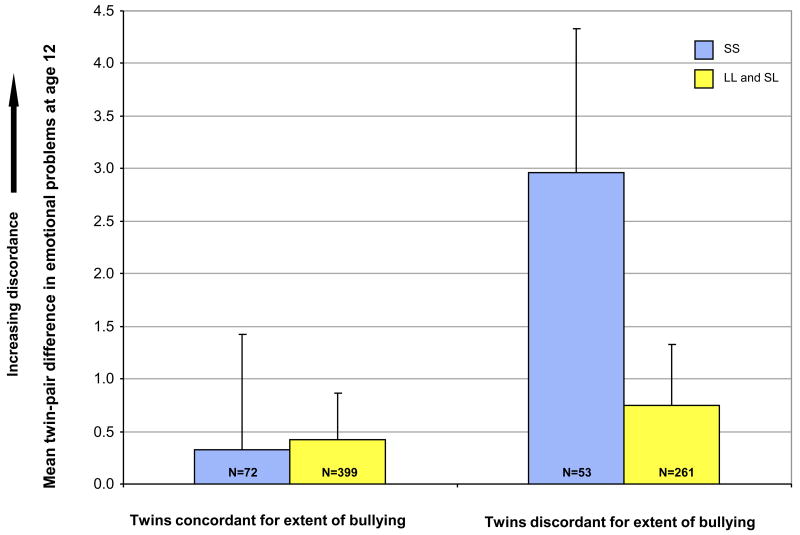
The longitudinal analyses suggest that the experience of being frequently victimized by bullying leads to increases in children's emotional problems from age 5 to age 12 years, and that this increase is especially pronounced among genetically-vulnerable SS homozygotes. However, it is also possible that other factors in children's environments could place them at risk of being both victimized by bullies and developing emotional problems. These family-wide factors—i.e., factors common to members of a family—might include circumstances such as living in a deprived neighborhood or attending a school where bullying is widely accepted, or having neglectful parents who do not teach their children how to avoid bullies.25 This suggests the possibility that it is not the effects of bullying per se that are being genetically moderated, but the effects of other environmental risk factors that are correlated with bullying victimization and that can also affect children's emotional problems. To address this possibility we used our twin design to test whether genotype confers heightened risk to bullying, even after controlling for other family-wide experiences that are shared by the twins. We did this in 3 steps. First, we studied twin pairs who grew up in the same family, and for each pair we identified whether the twins were concordant (N=471 pairs) or discordant for bullying (N=314 pairs). Second, we divided these twin pairs into 3 groups according to their 5-HTTLPR genotype; pairs in which both twins were SS (N=125 pairs, 73% MZ), SL (N=390 pairs, 66% MZ), or LL (N=270 pairs, 67% MZ), excluding pairs with different genotypes. (Ideally, we would have restricted our analysis to MZ twin pairs, but we had to include both MZ and DZ pairs in order to ensure sufficient power for this analysis.) Genotype groups did not differ on their rates of discordance of bullying (% discordance: SS=42%, SL=38%, LL=42%). Third, we correlated twin differences in bullying victimization with twin differences in emotional problems at age 12. If bullying is associated with emotional problems independently of other risk factors shared by children growing up in the same family, the twin who is bullied should have more emotional problems than the non-bullied twin. Moreover, if genotype moderates the association between bullying victimization and emotional problems, the twin who is bullied should have more emotional problems than the non-bullied co-twin if he or she is also genetically stress reactive (i.e., if they carry the SS genotype). The results support this prediction. The correlation between discordance in bullying victimization and corresponding discordance in emotional problems was positive and significant among twin pairs who were SS homozygotes (r=.21, p=.017), but not significant among SL heterozygotes (r=.03, p=.565) or among LL homozygotes (r=.06, p=.333). Both groups of L-carriers appear to be protected from the effect of bullying victimization. Figure 3 shows the twin-pair differences in emotional problems as a function of twin-pair differences in bullying victimization. Among twin pairs discordant for bullying, there were corresponding twin-pair differences in emotional problems, and these twin-pair differences were larger among children carrying two copies of the 5-HTTLPR S alleles than among L-carriers. We repeated this analysis, correlating the discordance in bullying victimization with corresponding discordance in change in emotional problems between ages 5 to 12 years. In agreement with the previous analysis, we found that bullied twins were more likely to experience increases in emotional problems than their non-bullied co-twins, but this was conditioned by genotype. Specifically, the correlation was positive and significant among twin pairs who were SS homozygotes (r=.20, p=.024), less so among SL heterozygotes (r=.10, p=.046), but not significant among LL homozygotes (r=-.01, p=.868). These within-family correlations suggest that genotype has a moderating effect on the association between bullying victimization and children's emotional problems, independent of other risk factors that are shared by children growing up in the same family.
Figure 3.
Twin-pair differences in emotional problems as a function of twin-pair discordance in bullying victimization. Note: Within twin pairs concordant for the extent of bully-victimization, both twins had similar levels of emotional problems; that is, the mean twin-pair difference in emotional problems was near zero regardless of genotype. Within twin pairs discordant for bullying victimization, there were corresponding twin-pair differences in emotional problems; that is, the more victimized twin had more emotional problems than the less victimized twin. These twin-pair differences were noticeable among children carrying two copies of the 5-HTTLPR S alleles but not among L-carriers. Error bars represent standard errors of the mean, Ns represent the number of twin pairs. 5-HTTLPR = serotonin transporter linked polymorphic region; LL= Long/Long; SL= Short/Long; SS= Short/Short.
Discussion
The current study provides evidence (a) that children's bullying victimization leads to their developing emotional problems and (b) that genetic variation in the 5-HTTLPR is a moderator of the link between bullying victimization and children's risk of developing these problems. Specifically, frequently bullied children with the SS genotype are at greater risk of developing emotional problems at age 12 than children with the SL or LL genotype. This genetic moderation persists after controlling (a) for children's pre-victimization emotional problems and (b) other risk factors shared by children growing up within the same family environment.
These findings confirm and add to the body of evidence that victims of bullying are at risk of developing emotional problems. 14-26 However, not every bullied child develops emotional problems, and the present findings offer new clues as to why this might be. First, genetic differences (in the 5-HTTLPR) interact with bullying victimization to exacerbate emotional problems. Second, the strength of this genetically-influenced response is related to the frequency of the bullying experience (i.e., the G×E was strongest for frequently bullied children). The present findings are consistent with the recent report that SS genotype victims of relational aggression are prone to depression.27 Because early-onset emotional problems constitute a risk for developing later mental-health problems,28 strategies to reduce bully victimization in school-age children, especially those with specific genetic vulnerabilities, could help to reduce both childhood emotional problems and subsequent psychiatric difficulties.
Since the original report of an interaction between life stress and the 5-HTT gene,29 there have been multiple positive replications of this G×E in prospective-cohort (e.g.30), cross-sectional (e.g.31), and case-only (e.g.32) designs. Additional G×E studies have documented that variation in the 5-HTTLPR is related to other stress-reactive phenotypes, including PTSD33 and anxious mood.34 There have also been failures to replicate.35 This body of research has been difficult to summarize because of cross-study inconsistency in measurement; in particular, practically every study has measured stress exposure differently. Unfortunately, this has not stopped some meta-analysts from failing to take differences in exposure measurement seriously, and from generating un-interpretable summary statistics by averaging findings across different studies of uneven quality.36-37 Some reviewers have cautioned that many of these different studies cannot be treated as replications, positive or negative, because of their ad hoc approaches to measuring life stress.38 For example, whereas some researchers have studied maltreated children,39 others have treated large family size as a chronic stressor.40 As an analogy, would two independent genetic association studies be considered replications if the positive finding was with different markers of the same gene? Superficially, both markers measure the same thing (the gene in question), but each could be representative of two completely different pieces of information (for example, different haplotypes that have diverse biological consequences). In this example, the claim of valid replication would be met with some caution. If one considers different methods of measuring and defining stress analogous to the two different markers in the previous example, then we should consider claims of replicating 5-HTTLPR × stress interactions with equal prudence.
As such, we do not claim the present study to be a replication of earlier study designs. Instead, we focused on a specific childhood stressor (bullying victimization) and tested the hypothesis that its effects on children's emotional problems are modified by variation in the 5-HTTLPR. Following the lead of experimental research,41 we think that focusing on a specific, developmentally-relevant, and clearly-operationalized stressor (rather than on ad hoc measures of stress) offers a valuable opportunity to study the genetics of stress reactivity and stress resistance.38 Furthermore, utilizing a precise, well-operationalized stressful experience decreases between-subject heterogeneity in the stressful event and thereby increases the internal validity of the study design. In turn, understanding genetic sensitivity to a particular stressor may offer insights about G×E more generally, although it must be understood that generalization to other stressors must be demonstrated rather than assumed.
With a focus on bullying victimization, we employed a number of strategies to ensure the robustness of the observed G×E. First, we used independent assessments of stress exposure and outcome (i.e., we obtained reports about victimization experiences from the children themselves and reports about emotional problems from adults who knew the children). In most previous studies of G×E, the same person who has been the source for acquiring stress data has also been the source for acquiring outcome data.38 In the present study, we used independent sources of measurement in order to control for subjective bias and reduce shared method variance which inflates associations between measures of stress exposure and emotional problems.
Second, we established a temporal relationship between the stressor (bullying victimization) and the outcome (emotional problems) and evaluated the G×E in relation to within-individual change in emotional problems between ages 5 and 12 years. Specifically, we documented that frequent bullying is associated with increases in emotional problems between ages 5 to 12 years among children with the SS genotype independently of pre-victimization emotional problems among these genetically at-risk children. Temporal precision coupled with analysis of developmental change helped to rule out the possibility of reverse causation (i.e., that emotional problems led children to be victimized by bullies42) and the potential confounding of G×E by gene-environment correlation.24
Third, we used within-family comparisons to assess whether the genetic moderation of bullying victimization was independent of other environmental risk factors shared by children growing up in the same family. We correlated twin-pair discordance in bullying experience (the difference in the amount of bullying each twin experiences) with twin-pair discordance in emotional problems. We found that the bullied twin had more emotional problems, but only among those twin pairs where both twins carried the SS genotype. These results suggest that variation in 5-HTTLPR moderates the association between bully victimization and emotional problems independent of other risk factors shared by twins.
The present study also has limitations. First, although we carried out within-family comparisons of twins discordant for bullying victimization in order to control for family-wide factors that might influence the observed G×E, we are not able to rule out the residual influence of child-specific environmental experiences. That is, we established that the G×E persists irrespective of experiences shared by children in the same family but it remains possible that the interaction may depend on experiences unique to the bullied versus non-bullied child in the family. Furthermore, the influence of shared environmental factors may not be stable throughout childhood and early adolescence. 43-44 Second, the interaction between 5-HTTLPR and bullying victimization observed here may be modified by other unmeasured genetic characteristics.45-46 One way to test this possibility is to conduct within-family tests using exposure-discordant but genetically-identical (MZ) twin pairs; however, we did not have sufficient power or variation to carry out analyses on MZ twin pairs alone. Third, we cannot entirely rule out the possibility that children with emotional problems over-reported victimization experiences. However, we attempted to control for this by utilizing independent assessments of exposure and outcome, as recommended in stress research.38
In conclusion, this study adds to an accumulating body of observational studies showing that emotional disturbance is jointly influenced by stressful events and 5-HTTLPR genotype, and that this G×E is observable in childhood. This observational evidence is buttressed by a wide range of emerging experimental G×E studies of stress reactivity (highlighted in the introduction) and G×E animal models of stress exposure (e.g.47-50), all of which underscore the need for studies that can elucidate the mechanism of the contribution of 5-HTTLPR variation to stress reactivity in development.
Acknowledgments
This research received support from UK Medical Research Council grants G9806489, G0100527, and G0601483, and NIH grants MH077874 and HD061298.
The authors acknowledge the study families for their help and participation. We also thank Renate Houts and Stephen Ross with the Departments of Psychology & Neuroscience and Psychiatry & Behavioral Sciences, Duke University for their help with manuscript preparation.
Footnotes
This article represents one of several articles published in the xxx issue of the Journal of the American Academy of Child and Adolescent Psychiatry that explores the intersection of genetics and mental health disorders in children and adolescents. The editors invite the reader to investigate the additional articles on this burgeoning area of psychiatric medicine.
Disclosure: Dr. Arseneault is supported by a Career Scientist Award from the Department of Health, United Kingdom. Dr. Caspi is a Royal Society-Wolfson Merit Award holder. Drs. Caspi and Moffitt, through the Wisconsin Alumni Research Foundation, have applied for a patent entitled ‘Method for Assessing a Behavioral Disposition’, US Patent Office Serial Number 10/889,450. Dr. Sugden, Ms. Harrington, and Mr. Williams report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Karen Sugden, Duke University.; Social, Genetic, and Developmental Psychiatry Centre, Institute of Psychiatry, King's College London
Louise Arseneault, Social, Genetic, and Developmental Psychiatry Centre, Institute of Psychiatry, King's College London
HonaLee Harrington, Duke University
Terrie E. Moffitt, Duke University.; Social, Genetic, and Developmental Psychiatry Centre, Institute of Psychiatry, King's College London
Benjamin Williams, Duke University.; Social, Genetic, and Developmental Psychiatry Centre, Institute of Psychiatry, King's College London
Avshalom Caspi, Duke University.; Social, Genetic, and Developmental Psychiatry Centre, Institute of Psychiatry, King's College London
References
- 1.Olweus D. Bullying at school : what we know and what we can do. Blackwell; Oxford, UK; Cambridge, USA: 1993. pp. xii–140. [Google Scholar]
- 2.Nansel TR, Craig W, Overpeck MD, Saluja G, Ruan WJ. Cross-national consistency in the relationship between bullying behaviors and psychosocial adjustment. Arch Pediatr Adolesc Med. 2004;158(8):730–736. doi: 10.1001/archpedi.158.8.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arseneault L, Bowes L, Shakoor S. Bullying victimization in youths and mental health problems: ‘Much ado about nothing’? Psychol Med. 2009 doi: 10.1017/S0033291709991383. published on-line ahead of print Sept 29. [DOI] [PubMed] [Google Scholar]
- 4.Murphy DL, Lesch KP. Targeting the murine serotonin transporter: insights into human neurobiology. Nat Rev Neurosci. 2008;9(2):85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- 5.Holmes A, Hariri AR. The serotonin transporter gene-linked polymorphism and negative emotionality: placing single gene effects in the context of genetic background and environment. Genes Brain Behav. 2003;2(6):332–335. doi: 10.1046/j.1601-1848.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- 6.Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends in Cognitive Sciences. 2006;10(4):182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 8.Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Archives of General Psychiatry. 2005;62(2):146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 9.Lonsdorf TB, Weike AI, Nikamo P, Schalling M, Hamm AO, Ohman A. Genetic gating of human fear learning and extinction: possible implications for gene-environment interaction in anxiety disorder. Psychol Sci. 2009;20(2):198–206. doi: 10.1111/j.1467-9280.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- 10.Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiatry. 2008;63(9):847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox E, Ridgewell A, Ashwin C. Looking on the bright side: biased attention and the human serotonin transporter gene. Proc Biol Sci. 2009;276(1663):1747–1751. doi: 10.1098/rspb.2008.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rigby K. Consequences of bullying in schools. Can J Psychiatry. 2003;48(9):583–590. doi: 10.1177/070674370304800904. [DOI] [PubMed] [Google Scholar]
- 13.Watson KK, Ghodasra JH, Platt ML. Serotonin transporter genotype modulates social reward and punishment in rhesus macaques. PLoS ONE. 2009;4(1):e4156. doi: 10.1371/journal.pone.0004156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arseneault L, Walsh E, Trzesniewski K, Newcombe R, Caspi A, Moffitt TE. Bullying victimization uniquely contributes to adjustment problems in young children: a nationally representative cohort study. Pediatrics. 2006;118(1):130–138. doi: 10.1542/peds.2005-2388. [DOI] [PubMed] [Google Scholar]
- 15.Arseneault L, Milne BJ, Taylor A, Adams F, Delgado K, Caspi A, et al. Being bullied as an environmentally mediated contributing factor to children's internalizing problems: A study of twins discordant for victimization. Archives of Pediatrics and Adolescent Medicine. 2008;162:145–150. doi: 10.1001/archpediatrics.2007.53. [DOI] [PubMed] [Google Scholar]
- 16.Trouton A, Spinath FM, Plomin R. Twins early development study (TEDS): a multivariate, longitudinal genetic investigation of language, cognition and behavior problems in childhood. Twin Res. 2002;5(5):444–448. doi: 10.1375/136905202320906255. [DOI] [PubMed] [Google Scholar]
- 17.Moffitt TE. Teen-aged mothers in contemporary Britain. Journal of child psychology and psychiatry, and allied disciplines. 2002;43(6):727–742. doi: 10.1111/1469-7610.00082. [DOI] [PubMed] [Google Scholar]
- 18.Achenbach TM. Manual for the child behavior checklist/4-18 and 1991 profile. Burlington: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- 19.Achenbach TM. Manual for the Teacher's Report Form and 1991 Profile. Burlington: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- 20.Freeman B, Smith N, Curtis C, Huckett L, Mill J, Craig IW. DNA from buccal swabs recruited by mail: evaluation of storage effects on long-term stability and suitability for multiplex polymerase chain reaction genotyping. Behav Genet. 2003;33(1):67–72. doi: 10.1023/a:1021055617738. [DOI] [PubMed] [Google Scholar]
- 21.Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Hum Genet. 1997;101(2):243–246. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- 22.Gould W, Scribney W. Maximum likelihood estimation with STATA. Stata Press; College Station, TX: 1999. [Google Scholar]
- 23.Ball HA, Arseneault L, Taylor A, Maughan B, Caspi A, Moffitt TE. Genetic and environmental influences on victims, bullies and bully-victims in childhood. Journal of child psychology and psychiatry, and allied disciplines. 2008;49(1):104–112. doi: 10.1111/j.1469-7610.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- 24.Jaffee SR, Price TS. Gene-environment correlations: a review of the evidence and implications for prevention of mental illness. Molecular Psychiatry. 2007;12(5):432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowes L, Arseneault L, Maughan B, Taylor A, Caspi A, Moffitt TE. School, neighborhood, and family factors are associated with children's bullying involvement: a nationally representative longitudinal study. J Am Acad Child Adolesc Psychiatry. 2009;48(5):545–553. doi: 10.1097/CHI.0b013e31819cb017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bond L, Carlin JB, Thomas L, Rubin K, Patton G. Does bullying cause emotional problems? A prospective study of young teenagers. BMJ. 2001;323(7311):480–484. doi: 10.1136/bmj.323.7311.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benjet C, Thompson RJ, Gotlib IH. 5-HTTLPR moderates the effect of relational peer victimization on depressive symptoms in adolescent girls. Journal of child psychology and psychiatry, and allied disciplines. 2009;51(2):173–179. doi: 10.1111/j.1469-7610.2009.02149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R. Prior juvenile diagnoses in adults with mental disorder: Developmental follow-back of a prospective-longitudinal cohort. Archives of General Psychiatry. 2003;60(7):709–717. doi: 10.1001/archpsyc.60.7.709. [DOI] [PubMed] [Google Scholar]
- 29.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 30.Kendler KS, Kuhm JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. American Journal of Psychiatry. 2005;62(5):529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- 31.Lazary J, Lazary A, Gonda X, Benko A, Molnar E, Juhasz G, et al. New evidence for the association of the serotonin transporter gene (SLC6A4) haplotypes, threatening life events, and depressive phenotype. Biological Psychiatry. 2008;64(6):498–504. doi: 10.1016/j.biopsych.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 32.Drachmann Bukh J, Bock C, Vinberg M, Werge T, Gether U, Vedel Kessing L. Interaction between genetic polymorphisms and stressful life events in first episode depression. J Affect Disord. 2009;119(1-3):107–115. doi: 10.1016/j.jad.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 33.Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Brady K, et al. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Arch Gen Psychiatry. 2009;66(11):1201–1209. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunthert KC, Conner TS, Armeli S, Tennen H, Covault J, Kranzler HR. Serotonin transporter gene polymorphism (5-HTTLPR) and anxiety reactivity in daily life: A daily process approach to gene-environment interaction. Psychosomatic Medicine. 2007;69(8):762–768. doi: 10.1097/PSY.0b013e318157ad42. [DOI] [PubMed] [Google Scholar]
- 35.Gillespie NA, Whitfield JB, Williams B, Heath AC, Martin NG. The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychological Medicine. 2005;35(1):101–111. doi: 10.1017/s0033291704002727. [DOI] [PubMed] [Google Scholar]
- 36.Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh JH, et al. Interaction Between the Serotonin Transporter Gene (5-HTTLPR), Stressful Life Events, and Risk of Depression. JAMA. 2009;301(23):2462. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutter M, Thapar A, Pickles A. Gene-Environment Interactions: Biologically Valid Pathway or Artifact? Arch Gen Psychiatry. 2009;66(12):1287–1289. doi: 10.1001/archgenpsychiatry.2009.167. [DOI] [PubMed] [Google Scholar]
- 38.Monroe SM, Reid MW. Gene-environment interactions in depression research: genetic polymorphisms and life-stress polyprocedures. Psychol Sci. 2008;19(10):947–956. doi: 10.1111/j.1467-9280.2008.02181.x. [DOI] [PubMed] [Google Scholar]
- 39.Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proceedings of the National Academy of Sciences. 2004;101(49):17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chipman P, Jorm AF, Prior M, Sanson A, Smart D, Tan X, et al. No interaction between the serotonin transporter polymorphism (5-HTTLPR) and childhood adversity or recent stressful life events on symptoms of depression: Results from two community surveys. American Journal of Medical Genetics Part B-Neuropsychiatric Genetics. 2007;144B(4):561–565. doi: 10.1002/ajmg.b.30480. [DOI] [PubMed] [Google Scholar]
- 41.Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: Joining forces with neuroscience. Nature Reviews Neuroscience. 2006;7(7):583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- 42.Susser ES. Psychiatric epidemiology : searching for the causes of mental disorders. Oxford University Press; Oxford; New York: 2006. pp. xxii–516. [Google Scholar]
- 43.Boomsma DI, van Beijsterveldt CE, Hudziak JJ. Genetic and environmental influences on Anxious/Depression during childhood: a study from the Netherlands Twin Register. Genes Brain Behav. 2005;4(8):466–481. doi: 10.1111/j.1601-183X.2005.00141.x. [DOI] [PubMed] [Google Scholar]
- 44.Hoekstra RA, Bartels M, Hudziak JJ, Van Beijsterveldt TC, Boomsma DI. Genetic and environmental influences on the stability of withdrawn behavior in children: a longitudinal, multi-informant twin study. Behav Genet. 2008;38(5):447–461. doi: 10.1007/s10519-008-9213-4. [DOI] [PubMed] [Google Scholar]
- 45.Kaufman J, Yang BZ, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, et al. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biological Psychiatry. 2006;59(8):673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 46.Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Kim YH, et al. Interactions between life stressors and susceptibility genes (5-HTTLPR and BDNF) on depression in Korean elders. Biol Psychiatry. 2007;62(5):423–428. doi: 10.1016/j.biopsych.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 47.Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, et al. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol Psychiatry. 2004;55(7):733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 48.Carola V, Frazzetto G, Pascucci T, Audero E, Puglisi-Allegra S, Cabib S, et al. Identifying molecular substrates in a mouse model of the serotonin transporter × environment risk factor for anxiety and depression. Biological Psychiatry. 2008;63(9):840–846. doi: 10.1016/j.biopsych.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 49.Kalin NH, Shelton SE, Fox AS, Rogers J, Oakes TR, Davidson RJ. The serotonin transporter genotype is associated with intermediate brain phenotypes that depend on the context of eliciting stressor. Molecular Psychiatry. 2008;13(11):1021–1027. doi: 10.1038/mp.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, et al. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. Journal of Neuroscience. 2007;27(3):684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]