SUMMARY
Arousal is fundamental to many behaviors, but whether it is unitary, or whether there are different types of behavior-specific arousal, has not been clear. In Drosophila, dopamine promotes sleep-wake arousal. However there is conflicting evidence regarding its influence on environmentally stimulated arousal. Here we show that loss-of-function mutations in the D1 dopamine receptor DopR enhance repetitive startle-induced arousal, while decreasing nocturnal arousal (i.e., increasing sleep). These two types of arousal are also inversely influenced by cocaine, whose effects in each case are opposite to, and abrogated by, the DopR mutation. Selective restoration of DopR function in the central complex rescues the enhanced stimulated arousal but not the increased sleep phenotype of DopR mutants. These data provide evidence for at least two different forms of arousal, which are independently regulated by dopamine in opposite directions, via distinct neural circuits.
INTRODUCTION
“Arousal,” a state characterized by increased activity, sensitivity to sensory stimuli and certain patterns of brain activity (Coull, 1998), accompanies many different behaviors, including circadian rhythms, escape, aggression, courtship and emotional responses in higher vertebrates (Cahill and McGaugh, 1998; van Swinderen and Andretic, 2003; Devidze et al., 2006). A key unanswered question is whether arousal is a uni-dimensional, generalized state (Hebb, 1955; Pfaff et al., 2005), or rather multi-dimensional (Robbins, 1997). Biogenic amines, such as dopamine (DA), norepinephrine (NE), serotonin (5-HT) and histamine, as well as cholinergic systems, have all been implicated in arousal in numerous behavioral settings (Robbins et al., 1998; Pfaff et al., 2002; Berridge, 2006; Devidze et al., 2006). For several reasons, however, it is not clear whether these neuromodulators act on a common “generalized arousal” pathway (Pfaff et al., 2005), or rather control distinct arousal pathways that independently regulate different behaviors. This is because a single amine typically acts through multiple receptors. Thus different receptors (or even a single receptor subtype) may act in distinct circuits to control different forms of arousal. Resolving this issue requires identifying the receptors and circuits on which these modulators act, in different behavioral settings of arousal.
Most studies of arousal in Drosophila have focused on spontaneous locomotor activity associated with sleep-wake arousal, a form of “endogenously generated” arousal (van Swinderen and Andretic, 2003). Several lines of evidence point to a role for DA in enhancing this form of arousal in Drosophila (reviewed in (Birman, 2005). Drug-feeding experiments, as well as genetic silencing of dopaminergic neurons, have indicated that DA promotes waking during the subjective night phase of the circadian cycle (Andretic et al., 2005). Similar conclusions were drawn from studying mutations the Drosophila DA transporter (dDAT) (Kume et al., 2005; Wu et al., 2008). Consistent with these data, overexpression of the vesicular monoamine transporter (dVMAT-A), promoted hyperactivity in this species (Chang et al., 2006), as did activation of DA neurons in quiescent flies (Lima and Miesenbock, 2005; Wu et al., 2008).
Evidence regarding the nature of DA effects on “exogenously generated,” or environmentally stimulated arousal (van Swinderen and Andretic, 2003), such as that licited by startle, is less consistent. Classical genetic studies and quantitative trait locus (QTL) analyses have suggested that differences in DA levels may underlie genetic variation in startle-induced locomotor activity (Connolly, 1967; Tunnicliff et al., 1969; Carbone et al., 2006; Jordan et al., 2006). Fmn (dDAT) mutants displayed hyperactivity in response to mechanical shocks, implying a positive-acting role for DA in controlling environmentally-induced arousal (Kume et al., 2005). In contrast, other data imply a negative-acting role for DA in controlling stimulated arousal. Mutants in Tyr-1, which exhibit a reduction in dopamine levels (Burnell and Daly, 1982), show an increased in stimulated but not spontaneous levels of locomotor activity (Meehan and Wilson, 1987). Genetic inhibition of tyrosine hydroxylase-expressing neurons caused hyperactivity in response to mechanical startle (Friggi-Grelin et al., 2003). Finally, transient activation of DA neurons in hyperactive flies inhibited locomotion (Lima and Miesenbock, 2005). Whether these differing results reflect differences in behavioral assays, the involvement of different types of DA receptors, or an “inverted U”-like dosage sensitivity to DA (Birman, 2005), is unclear.
We have developed a novel behavioral paradigm for environmentally induced arousal, using repetitive mechanical startle as a stimulus, and have carried out a screen for mutations that potentiate this response. One such mutation is an hypomorphic allele of the D1 receptor ortholog, DopR. This same mutation caused decreased spontaneous activity during the night phase of the circadian cycle due to increased sleep. In both assays, cocaine influenced behavior in the opposite direction as the DopR mutation, and the effect of cocaine was abolished in DopR mutant flies, supporting the idea that DA inversely regulates these two forms of arousal. Genetic rescue experiments, using Gal4 drivers with restricted CNS expression, indicate that these independent and opposite influences of DopR are exerted in different neural circuits. These data suggest the existence of different types of arousal states mediated by distinct neural circuits in Drosophila, which can be inversely regulated by DA acting via the same receptor subtype.
RESULTS
Repetitive stress induces an extended state of locomotor hyperactivity
In an effort to develop a Drosophila model of cumulative stress-induced arousal, we tested whether closely spaced repetitive startle stimuli could produce an extended period of hyperactivity. We delivered a succession of brief air puffs (200 msec duration at 5 sec intervals, 35 psi), to adult flies placed in horizontal plastic tubes (10 flies/tube) (Fig. 1A), in an 8-tube manifold (the “puff-o-mat”) based on a device developed by Heberlein and colleagues (Wolf et al., 2002; Rothenfluh et al., 2006). These airpuffs, while relatively gentle, were strong enough to blow the flies against the mesh at the back of the tube, from which they immediately rebounded (Supplemental Movie SM1). Application of 6 successive puffs produced an extended period of hyperactivity, which lasted 7-10 minutes (Fig. 1B). We call this behavioral response Repetitive Startle-induced Hyperactivity (ReSH).
Figure 1. Stress-induced locomotor hyperactivity.
(A) Schematic illustrating experimental set-up. (B) Mechanical stress induced by successive airpuffs (vertical arrows) causes persistent locomotor hyperactivity. Solid line represents mean velocity (n = 8 tubes, each containing 10 flies). Thin lines indicate traces from each tube, gray envelope S.E.M. (C) Initial acceleration computed during the interpuff-interval (5 sec following each airpuff). (D) Walking bout frequency prior to and following 6 airpuffs (pink line). (E) Exponential curve-fit to post-puff decay data. See Supplemental Methods for further details. (F) Puff “dose-response” curves. “1p” “2p” etc. indicates number of puffs (1p n=68 tubes, 2p n=64, 3p n=80, 4p n=72, 6p n=84). (G-J) Parameter values extracted from the data in (F) using the equation in (E). “Distance traveled” (J) is computed by integrating the area under the post-puff curve, after subtracting the pre-puff baseline. Lower-case letters indicate statistically significant differences (p<0.005; Kruskal-Wallis ANOVA followed by Mann-Whitney U-test, with Bonferroni correction for multiple comparisons).
To characterize ReSH behavior more quantitatively, we developed custom software to record the position, velocity, acceleration and trajectories of the flies in response to the airpuffs (see Supplementary Information). The acceleration of the flies, in the 5-second period immediately following each puff, increased steeply during the presentation of the first three puffs (Fig. 1C), suggesting a cumulative effect of the stimuli. Following a 6-puff exposure, the average velocity of the flies was elevated almost 10-fold, relative to pre-stimulus baseline, and gradually declined thereafter (Fig. 1B).
Flies walk intermittently in bouts of activity interrupted by periods of immobility (Martin et al., 1999a; Wolf et al., 2002). An increase in average velocity could, in principle, reflect a change in bout duration, bout frequency or walking speed during the bout. Our analysis indicated that the airpuffs caused little change in the average duration of walking bouts (Supplemental Figure S1A), but instead transiently increased both the bout frequency (Figure 1D), and average speed during the bouts (Supplemental Figure S1C).
The gradual decline in locomotor activity during the post-puff period appeared to follow exponential decay kinetics. Indeed, the response profile was fit well by a modified exponential function (Fig. 1E; see Experimental Procedures). This model permitted us to extract a number of parameters, including the peak height, decay constant tau (τ), and the total distance traveled following the puffs (see Experimental Procedures), and to determine the effect of varying different stimulus properties on these response parameters. As the number of puffs was systematically varied from 1-6 (Fig. 1F), the net increase in peak velocity, and the total distance traveled after the puffs, increased up to an apparent saturation point at 4 puffs (Fig. 1H,J). The magnitude of τ also increased with increasing puff number, although this was more variable between experiments (Fig. 1I). Peak velocity and distance traveled also increased as a function of stimulus intensity (psi/puff) (Supplemental Fig. S7G, J). These data suggest that the ReSH response scales in proportion to the amount of stimulation, and therefore that it indeed reflects a response to repetitive, cumulative stress.
The ReSH response also exhibited sensitization. Flies were exposed to 6 puffs, and allowed 10 minutes to recover. Subsequently, they were exposed to a single puff, and their responses compared to those of naïve flies exposed to a single puff. Both τ and the total distance traveled during the post-puff period were significantly higher in flies that had previously been exposed to 6 puffs (Supplemental Figure S2). However, if flies were allowed to rest for 30 minutes after the 6 puffs, there was no statistically significant difference in their subsequent response to a single air puff (data not shown), implying that the sensitization state undergoes time-dependent extinction.
The sensitization induced by repeated air-puff exposure generalized to at least one other sensory modality, olfaction. When flies are briefly exposed to a high concentration of an odor, they exhibit a transient increase in locomotor activity, a response termed olfactory startle (Wolf et al., 2002; Cho et al., 2004). Following recovery from ReSH, the olfactory startle response to methyl cyclohexanol (MCH) was significantly enhanced (Supplemental Figure S3). These data suggest that repetitive startle induces an extended state of elevated arousal, which is manifested by: 1) increased acceleration following each successive stimulus; 2) a protracted period of locomotor hyperactivity in the post-stimulus period; and 3) sensitization to a subsequent low-intensity stimulus, a state which persists even after the overt locomoter hyperactivity phase has subsided, and which extends to at least one other sensory modality.
DopR negatively regulates repetitive startle-induced hyperactivity
To identify genes controlling ReSH behavior, we screened several hundred lines from a collection of transposon insertion mutants (Artavanis-Tsakonas, 2004), focusing on genes with neurobiological relevance. The response of each mutant to a sub-saturating (2-puff) stimulus was analyzed using our software, and compared to the average of the entire collection. Candidates were identified by >2 standard deviations from the mean population values of parameters such as τ (Fig. 1E), or as outliers in Principal Component space (Fig. 2A). Lines exhibiting both diminished and exaggerated responses were identified; we focused on those lines exhibiting hyperactive responses. One such mutant was a piggyBac transposon insertion in the dDA1/DopR1 locus (Gotzes et al., 1994; Sugamori et al., 1995), DopRf02676 (Fig. 2A; hereafter referred to as DopR). The τ of this line was almost ten-fold higher than that of the mean of the collection of lines screened.
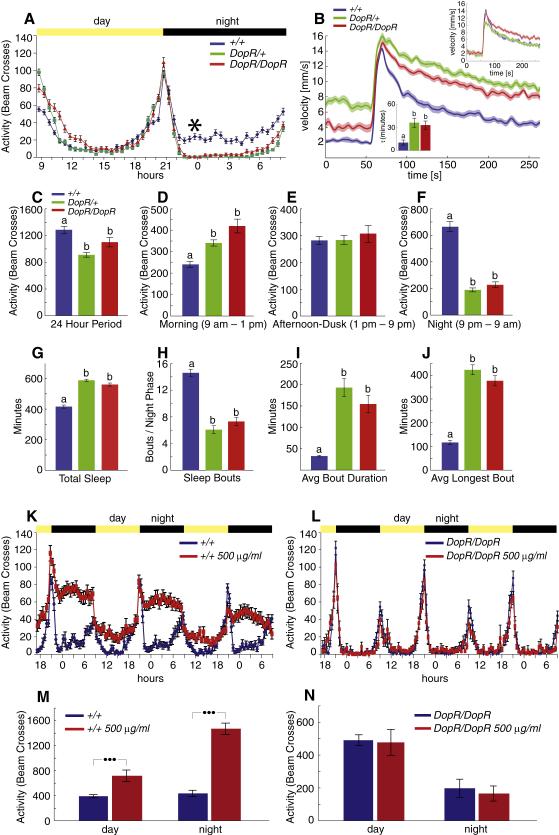
Figure 2. DopR negatively regulates stress-induced locomotor hyperactivity.
(A) Sample traces from mutant lines screened. Magenta curve represents the mean response of the piggyBac collection. Plots are ranked as outliers in principal component (PC) space. The plot encircled in red is the DopR mutation. (B) Upper, structure of the DopRf02676 insertional allele; note the UAS in the first intron. Lower, levels of DopR mRNA determined by Q-RT-PCR in different genotypes. (C) Phenotype of DopR/DopR and DopR/+ flies in comparison to CS (+/+) controls (n=40 tubes per genotype). (D, E) Behavioral parameters in DopR mutants, calculated from the data in (C). (F) Phenotypic reversion of flies carrying a precise excision of the f02676 insertion (n=24 tubes per genotype). (G, H) Behavioral parameters in excision line, calculated from the data in (E). Insets in (C) and (F), comparison of genotypes after normalization to +/+ baseline. Additional behavioral parameters of (C) and (F) are available in Supplemental figure S5. In this and all following figures, •, p<.017; ••, p<.003; •••, p<.0003; Kruskal-Wallis ANOVA followed by Mann-Whitney U-test with Bonferroni correction for multiple comparisons.
The DopR insertion was back-crossed into a Canton-S (CS) background for six generations for further analysis. DopR/+ and DopR/DopR flies exhibited both elevated pre-puff baseline and post-puff velocities (Fig. 2C), reflecting an increase in locomotor bout frequency and bout velocity (Supplemental Figure S1B). When the pre-puff baseline velocity of the mutant was normalized to that of wild-type CS flies, both DopR/+ and DopR/DopR flies still showed an extended period of post-puff hyperactivity (Fig. 2C, inset; D, E). This suggested that the DopR mutation does not cause simply a “shift-up” in the puff-response curve, due to an increase in spontaneous locomotor activity, but rather that the flies take longer to “calm down” following the repetitive startle stimulus. This enhanced reactivity to mechanical startle is also reflected in the elevated pre-puff activity of the flies immediately following introduction into the apparatus (Connolly, 1967; Burnell and Daly, 1982). This interpretation was confirmed by additional controls in which the flies were anaesthetized prior to introduction to the puff-o-mat (Supplemental Footnote S1 and Supplemental Figure S6).
To confirm that the phenotype of DopR flies was indeed due to a disruption of the DopR gene, we first measured the levels of DopR mRNA in different genotypes by quantitative RT-PCR. There was an approximately 50% reduction in the amount of DopR mRNA in DopR/+ flies, and an ~95% reduction in DopR/DopR files (Fig. 2B). These data confirm prior analysis of the DopRf02676/dumb2 allele (Kim et al., 2007), as well as independent studies by Wolf et al. (submitted), and indicate that this allele is a strong molecular hypomorph. We further confirmed that this insertion caused the ReSH phenotype by isolating revertant flies bearing a precise excision of the piggyBac transposon (Thibault et al., 2004) (see Supplemental Methods). When crossed into a w+ background, the puff-response of these excision flies was indistinguishable from that of w+ CS controls, by all parameters measured (Fig. 2F-H, orange vs. blue curves, Supplemental Figure S4 D-F).
Further confirmation that the phenotype is due to disruption of the DopR gene was provided by analyzing trans-heterozygous flies bearing an independent transposon insertion in the DopR locus (DopRPL00420) over a deficiency spanning the locus (Df(3R)ED5364) (Figure S5), as well as by genetic rescue experiments (see below). Taken together, these data indicate that the ReSH phenotype is due to a reduction in DopR function. The similar ReSH phenotypes of DopR/+ and DopR/DopR flies (Fig. 2C-E) suggests that this behavior is sensitive to DopR gene dosage in this genetic background. However, it should be noted that the magnitude of the kinetic parameters characterizing both the wild-type response, and the DopR mutant phenotype, varied with genetic background, consistent with evidence that startle-induced locomotor activity is controlled by complex genetic networks (Jordan et al., 2007; Yamamoto et al., 2008).
The exaggerated ReSH response in DopR flies exposed to 2 puffs (Fig. 2D, E) suggested that the mutants might be hypersensitive to the airpuff stimulus. To investigate this in more detail, we systematically varied the puff number and intensity. In response to small numbers of puffs, or to low puff intensities, DopR flies showed much stronger increases in τ and post-puff distance traveled, than did wild-type flies (Fig. 3A-D, and Supplemental Figure S7). Furthermore, DopR/DopR flies appeared to reach saturation in their post-stimulus activity after a smaller number of puffs, in comparison to CS controls (Fig. 3B). These data support the idea that the DopR mutation causes hyper-reactivity to the puff stimulus, as well as an increased time to recover from repetitive puffs.
Figure 3. DopR mutant flies are hypersensitive to the airpuff stimulus.
(A, B) Puff-titration of CS (A) and DopR/DopR (B) flies (n=44 tubes for each genotype). DopR/DopR flies show heightened responses relative to CS at all puff conditions, and reach a peak response after 2 puffs (green). (C, D) Single-puff trials at the indicated puff intensities for wild-type CS (C, +/+) and DopR/DopR (D) flies (n=24 tubes for each condition). DopR/DopR mutants show increased responses at low puff intensities (D). (E, F) Cocaine suppresses stress-induced locomotor hyperactivity in a DopR-dependent manner (n=32 tubes for each condition). Flies fed with the indicated doses of cocaine were subjected to 2-puff trials. Note the suppression of post-puff activity by cocaine in CS (E) but not DopR/DopR (F) flies. Parametric analysis of data in (A-F) available in Supplemental Figure S6.
If an hypomorphic mutation in DopR enhances ReSH behavior, then one might predict that elevating DA should, conversely, suppress ReSH behavior. To test this, we examined the effect of cocaine, which elevates synaptic DA. Previous studies have indicated that cocaine can cause hyperactivity in Drosophila (McClung and Hirsh, 1998; Bainton et al., 2000; Li et al., 2000). While cocaine indeed promoted spontaneous activity measured in a circadian monitor (see below), it suppressed the ReSH response at the same doses (Fig. 3E). Parameter analysis indicated a significant depression of τ and post-puff distance traveled in wild-type flies (Supplemental Figure S7N-O; white bars). Strikingly this effect was eliminated in DopR/DopR mutants (Fig. 3F, Supplemental Figure S7N-O; black bars), as well as in DopR/+ heterozygotes (data not shown). A similar result was obtained using Df(3R)ED5364/DopRPL00420 transheterozygotes (data not shown). Taken together, these data indicate both that the effect of cocaine in this assay is opposite to that of the DopR mutation, and that DopR is the major receptor mediating this effect.
DopR flies exhibit decreased spontaneous activity during the night
To examine directly the effect of the DopR mutation on spontaneous (unstimulated) locomotion, we measured circadian activity using a standard Drosophila Activity Monitoring System (DAMS) (TriKinetics, Inc.). Under these conditions, both DopR/+ and DopR/DopR flies showed substantially decreased activity, in comparison to wild-type controls, during the night phase (Fig. 4A, F). This phenotype is consistent with the results of previous pharmacological and genetic studies indicating that DA positively regulates spontaneous locomotor activity (Bainton et al., 2000; Andretic et al., 2005; Kume et al., 2005; Chang et al., 2006). DopR/+ and DopR/DopR flies showed a modest increase in activity during the morning phase (Fig. 4D), an effect that declined as the day progressed (Fig. 4A). This morning hyperactivity, however, cannot account for the DopR ReSH phenotype, since this phenotype was observed even when the puff-o-mat assays were performed at midnight (Fig. 4B), a time when spontaneous locomotor activity is strongly decreased in the mutant (Fig. 4A, asterisk). These data provide further evidence that the ReSH phenotype of the DopR mutation does not simply reflect a general elevation of spontaneous locomotor activity. To the contrary, they suggest that DA regulates ReSH and spontaneous nocturnal activity in opposite directions, via DopR.
Figure 4. DopR mutant flies show nocturnal hypoactivity and increased sleep.
(A) Circadian activity of CS and DopR mutant flies (n=70 individuals per genotype). Note reduced activity of DopR/+ (green) and DopR/DopR (red) flies, relative to +/+ (blue), during the night phase. (B) DopR mutants still show an enhanced ReSH response during the night phase (n=25 tubes per genotype). 2-puff trials were performed at midnight (position of asterisk in 4A). Upper inset, comparison of curves after normalization to +/+ median baseline; Lower inset, τ values. (C-F) Total activity in the circadian monitor during different time periods. Letters on all bar graphs indicate significant differences (p<.017 after Bonferroni correction for multiple comparisons). (G-J) Sleep parameters in wild-type vs. DopR mutant flies during the night phase. (K-N) Cocaine stimulation of circadian activity is DopR-dependent. (K, L) activity plots of wild-type (K) or DopR/DopR (L) flies assayed without (blue) or with (red) 500 μg/ml cocaine. (M, N) Summary of data in (K, L). Note that the strongest effect of cocaine is at night (M) and is suppressed in DopR/DopR mutants (N). n=20 flies per genotype or drug treatment condition in (K-N). •••, p<.01.
We examined in more detail the behavioral basis of the decreased spontaneous nocturnal activity in DopR flies, by measuring various sleep parameters. Sleep in Drosophila has been defined as discrete periods of inactivity lasting 5 minutes or longer, during which time the flies show increased arousal thresholds (Shaw et al., 2000; Nitz et al., 2002; Andretic and Shaw, 2005). Strikingly, both DopR/+ and DopR/DopR flies showed increased overall sleep in comparison to wild-type (Fig. 4G). Analysis of sleep bout structure (Andretic and Shaw, 2005) indicated that the average sleep bout duration, and the length of the longest bout, were both significantly greater in DopR heterozygous and homozygous mutant flies, than in wild-type (Fig. 4I, J) . Thus, DopR flies are less active at night and sleep more, supporting a positive-acting role for DA in controlling sleep-wake arousal (Andretic et al., 2005).
We next investigated whether cocaine inversely influenced ReSH and sleep, but in the opposite direction as the DopR mutation. Cocaine increased activity in CS flies in the circadian monitor during both the day and night phases (Fig. 4K), but its effect was much more pronounced at night (Fig. 4M). Surprisingly, the effect of cocaine was entirely abolished in DopR/DopR flies (Fig. 4L, N), indicating that none of the other three Drosophila DA receptors (Feng et al., 1996; Han et al., 1996; Hearn et al., 2002; Srivastava et al., 2005) mediates the influence of the drug in this assay. The opposite effects of cocaine on spontaneous vs. environmentally stimulated locomotor activity (Fig. 3E), taken together with the fact that both effects of the drug are abolished by the DopR mutation, provides further evidence that the phenotype of the mutation in both assays is due to alterations in DA sensitivity.
Selective rescue of the DopR ReSH phenotype in subsets of CNS neurons
We next sought to determine the neural substrates of DopR action in controlling ReSH behavior. To do this, we tested various Gal4 enhancer trap lines for their ability to rescue the ReSH phenotype, taking advantage of the Gal4 UAS element in the first intron of the DopRf02676 allele (Fig. 2B). Transcription from this site is predicted to produce a truncated protein(s) with a shortened extracellular domain, presumably translated from one of several internal methionines; this N-terminal domain is non-essential for DopR function, at least in cell culture (Gotzes and Baumann, 1996). This strategy has also been used successfully to rescue the learning and memory deficit observed in homozygous DopR/DopR flies (Kim et al., 2007), as well as the reduced ethanol sensitivity of these flies (Wolf et al., submitted). The fact that DopR/+ heterozygotes show dominant phenotypes in both the ReSH and sleep assays (Fig. 2C-E), afforded the opportunity to test the ability of different Gal4 lines to rescue the phenotype in Gal4/+; DopR/+ flies. To control for genetic background effects, Gal4/+; DopR/+ flies were always compared to controls (Gal4/+ or DopR/+) in an F1 hybrid background derived from the same parental DopR and Gal4 strains (see Experimental Procedures).
Initial experiments indicated that the DopR ReSH phenotype could be rescued by Elav-Gal4 (Supplemental Figure S8), a pan-neuronal driver (Robinow and White, 1988), suggesting that this phenotype reflects a requirement for DopR function in the nervous system. All behavioral parameters were rescued, including pre-puff baseline and post-puff peak velocities, τ, and distance traveled (Fig. S8B-F). Importantly, Elav-Gal4 also restored (and even enhanced) the ability of cocaine to suppress the ReSH response (data not shown). The observations that Gal4-driven expression of DopR rescued both the ReSH phenotype, and the sensitivity of ReSH behavior to cocaine, provide additional evidence that the ReSH phenotype is indeed due to a reduction in DopR function.
In order to localize further the site of DopR function in the nervous system, we sought to rescue the phenotype using Gal4 lines with more restricted CNS expression (Manseau et al., 1997). DopR has previously been reported to be expressed at highest levels in two major brain structures: the mushroom body (MB) and central complex (CC) (Kim et al., 2003). Twenty-four different Gal4 lines were tested for their ability to rescue the ReSH phenotype of DopR flies. Of these, 8 lines expressing in the CC rescued the phenotype, while none of the lines with MB expression yielded rescue (Table I).
Table I. Summary of DopR genetic rescue experiments.
All rescue experiments were performed in Gal4/+; DopR/+ double-heterozygous flies.
| Gal4 line | Rescue of DopR PoSH | Expression Pattern |
|---|---|---|
| MB247 | fail | MB, surface glia |
| 201y | fail | MB, PI |
| 17D | fail | MB, PI |
| c739 | fail | MB, weak CC, AL, optic lobes, PI |
| 95y | fail | Optic Lobes |
| 78y | fail | Small Field CC, PI |
| c161 | fail | Small Field CC, PI |
| 11-3f | fail | PI, AL |
| 10-4m | fail | PI |
| Cha-Gal4 | fail | Cholinergic Neurons |
| TH-Gal4 | fail | Dopaminergic Neurons |
| Ddc-Gal4 | fail | Serotenergic and Dopaminergic Neurons |
| nan-Gal4 | fail | Antenna |
| Tim-Gal4 | fail | Timeless clock Neurons |
| Per-Gal4 | fail | Period clock Neurons |
| PDF-Gal4 | fail | PDF clock Neurons |
| Elav-Gal4 | rescue | Pan-Neuronal |
| GAD1-Gal4 | rescue | GABA-ergic Neurons |
| 189y | rescue | CC (R3), MB (weak), PI |
| c761 | rescue | CC (R3), AL, Lateral Horn, PI |
| c547 | rescue | CC (R2,R4), AL, PI |
| 30y | rescue | CC, MB (strong), PI |
| 5.30* | rescue | CC (R2, R4), PI |
| 11.148* | rescue | CC (R2, R4), PI |
MB, mushroom body; PI, pars intercerebralis; AL, antennal lobe; CC, central complex.
These experiments were performed in a Berlin:CS F1 hybrid genetic background and therefore the details of the parameters rescued differed slightly from that obtained with other drivers that were tested in a different F1 hybrid background.
Detailed analysis of the rescue obtained with several lines is shown in Fig. 5 and Supplemental Figure S9. Line c547, which (like lines 11.148 and 5.30) expresses in R4m/R2 neurons of the ellipsoid body (EB), a CC substructure (Fig. 6A, A2), (Renn et al., 1999), rescued the DopR mutant phenotype as effectively as Elav-Gal4 (Fig. 5A-A2). Immunostaining with an anti-DopR antibody (see Supplemental Fig. S10 for specificity) confirmed that endogenous DopR expression overlaps that of c547 in the EB (Fig. 6A1, A3). Line c547 also rescued the phenotype of DopR/DopR homozygous flies (Supplemental Figure S11). Rescue in these homozygous flies was associated with re-expression of DopR protein in the EB (Supplemental Figure S12). Rescue was also obtained when expression of Gal4 in c547 was restricted to the adult phase, using a temperature-sensitive version of the Gal4 inhibitor, Gal80ts (McGuire et al., 2003) (Fig. 5D-D2; E-E2). The partial rescue at 18°C likely reflects incomplete suppression of Gal4 by Gal80ts, due to leaky Gal80 inactivation (Kamikouchi et al., 2009); therefore we cannot completely exclude some developmental contribution of DopR function in mediating the puff response. Nevertheless, these data indicate that full rescue of the DopR ReSH phenotype requires expression of the receptor in the adult CNS, and also confirm that rescue requires Gal4 activity (McGuire et al., 2003).
Figure 5. Neuroanatomical mapping of DopR function.
(A-C) Selective rescue of the ReSH phenotype by restoration of DopR function in the Ellipsoid Body by different Gal4 lines. 2-puff trials applied to DopR/+ heterozygotes (red, “DopR”), Gal4/+ controls (blue), and Gal4/+; DopR/+ double heterozygotes (green). Superposition of the green and blue curves indicates phenotypic rescue. (A1-C2) Parametric analysis of the data in (A-C), respectively. “•••,” p<.0003, “••,” p<.003, “•,” p<.017 (n=40 tubes per genotype in A, n=44 tubes per genotype in B,C). (D, E) Conditional rescue of DopR in adult flies using c547-Gal4 and tubulin-Gal80ts. “DopR,” DopR/DopR mutants; “Rescue,” c547-Gal4, DopR/DopR; “Gal80ts,”tub-Gal80ts; c547-Gal4, DopR/DopR. Flies were raised at 18°C and tested at either 18° (D) or 30° (E). The partial rescue at 18° likely reflects incomplete suppression of Gal4 by Gal80ts. (D1-D2, E1-E2) Parameter plots of curves in (D) and (E), respectively (n=38 tubes per genotype in D, n = 32 tubes per genotype in E). (F-H) Double-dissociation of DopR function in learning vs. the ReSH assay. (F) MB247 Gal4 fails to rescue DopR (n=30 tubes per genotype). (G) MB247 rescues the DopR/DopR learning phenotype (green bar), while c547 does not (orange bar) (n=4 replicate experiments per genotype). (H) Expression of GFP in the mushroom body (MB247/+;UAS-mCD8-GFP/+).
Figure 6. Expression of DopR in the ellipsoid body of the central complex.
(A-C) Confocal z-series of wholemount brains double-labeled with antibodies to nc82 (red) and GFP (green). Arrows indicate the ellipsoid body. (A) UAS-mCD8-GFP/+ ; c547/+. (B) 189y/UAS-mCD8-GFP. (C) c761/UAS-mCD8-GFP. (A1-A3), (B1-B3) and (C1-C3) illustrate double-labeling with antibodies to DopR (Green, panels A1, B1, C1) and to GFP (Magenta, panels A2, B2, C2). (D1-D3) illustrates relationship of DopR (D1) to TH+ dopaminergic fibers (D2) in the EB. All images represent single 1.7 μm optical sections of whole-mount stained brains. Panels (A1-A3) and (D1-D3) illustrate the same focal plane of the same specimen triply-labeled for DopR, GFP and TH, at low (A1-A3) and high (D1-D3) magnification. MB, mushroom body; EB, ellipsoid body; NOD, noduli. Open arrowheads in (A3) and (D3) indicate the ring of DopR expression in the R3 domain. Scale bars (A-C) = 80 μm, (A1-C3) = 40 μm, (D1-D3) = 20 μm.
Several other Gal4 lines whose expression overlaps with that of DopR in the EB, including 189y, c761 and 30y, also rescued the DopR ReSH phenotype (Fig. 5B-B2; C-C2 and Table I). Interestingly, lines 189y and c761 express in R3 neurons of the EB, rather than in R2/R4m neurons (Renn et al., 1999). However, they also overlap DopR expression within this structure (Fig. 6B1-B3, C1-C3). Thus, DopR expression within the EB is widespread. Moreover, it is juxtaposed with dense varicosities of TH+ fibers (Fig. 6D1-D3), supporting the idea that the EB receives dopaminergic innervation (see also Wolf et al., submitted). The Gal4 line c232, which expresses very strongly in R4d neurons (Renn et al. 1999), was also tested but was hyperactive on its own, and therefore uninformative.
Most of the Gal4 lines that rescue are expressed in other sites besides the CC. While many of these extra-CC sites do not overlap, some of them do. For example, lines c547 and 189y also express in median bundle neurons of the pars intercerebralis (PI; Fig. 6A, B). However, seven other Gal4 lines that express in the PI, but not in R2/R3/R4m neurons of the EB, did not rescue the phenotype (Table I). Furthermore, immunocytochemical double-labeling experiments indicated that DopR is normally not expressed by PI neurons (Supplemental Figure S14). These data argue that rescue is unlikely due to expression in the PI. Lines c547, 189y, c761, and 11-3f also expressed, to different extents, in the antennal lobe (AL). However, line c739, which expresses in the AL but not the CC, failed to rescue, as did nanchung-Gal4, a line that expresses in ntennal mechanosensory neurons (Table I). These data argue against the AL as a site of rescue. Finally, several of the rescuing Gal4 lines also express in the thoracic ganglia (TG). One such line, 189y, overlaps with GABA in EB neurons but not in the TG (Supplemental Figure S15). Importantly, rescue of the ReSH phenotype was obtained with a GAD1-Gal4 line, (Supplemental Figure S13), but not with a Cha-Gal4 line (Table I). Since rescue requires DopR expression in GABAergic neurons, and since the thoracic neurons that express 189y are not GABAergic, it is unlikely that 189y-driven expression of DopR in the TG is responsible for rescue.
DA is involved in learning and memory (Neckameyer, 1998; Schwaerzel et al., 2003; Riemensperger et al., 2005; Schroll et al., 2006). Recent studies indicate that mutants homozygous for dumb2, a DopR allele, exhibit deficits in olfactory associative learning that reflect a requirement for this receptor in the MB (Kim et al., 2007). A MB requirement for DopR also underlies the effect of DA to promote learning in sleep-deprived flies (Seugnet et al., 2008). Interestingly, Gal4 line c547 (which rescued the ReSH phenotype of DopR/DopR flies) did not rescue the olfactory learning deficit of DopR mutants (Fig. 5G, red vs. orange bars). In contrast, line MB247 (Fig. 5H), which rescued the learning phenotype of DopR/DopR mutants (Fig. 5G, green bar and (Kim et al., 2007)), failed to rescue the ReSH phenotype of DopR/+ and DopR/DopR flies (Fig. 5F). These data demonstrate a double-dissociation between the requirement for DopR in associative learning vs. ReSH behavior, in the MB and EB, respectively.
Independent requirements for DopR in spontaneous vs. stimulated activity
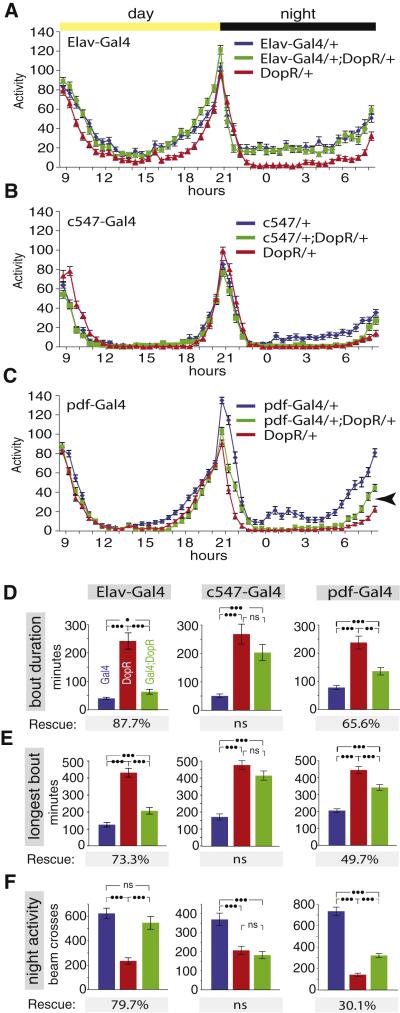
The opposite effects of the DopR mutation on spontaneous activity in the circadian monitor vs. ReSH behavior raised the question of whether these functions are exerted in distinct neural circuits. To address this question, we examined the ability of different Gal4 lines to rescue the DopR sleep phenotype. Restoration of DopR expression throughout the CNS, using the pan-neuronal driver Elav-Gal4, resulted in a strong rescue of the nocturnal hypoactivity phenotype (Fig. 7A, F). Analysis of sleep parameters indicated that Elav-Gal4 restored their values to levels close to those of control Elav-Gal4/+ flies (Fig. 7D-E, green vs. blue bars). Thus, DopR controls sleep by acting in the nervous system.
Figure 7. Rescue of the nocturnal hypoactivity phenotype of DopR flies.
(A-C) represent circadian activity of control (Gal4/+; blue curves), mutant (DopR/+; red curves) and rescue (Gal4/+; DopR/+, green curves), using Elav-Gal4, c547-Gal4 and pdf-Gal4 drivers, respectively (n=66 flies per genotype in A, n=46 flies per genotype in B, n=87 flies per genotype in C). Arrowhead in (C) indicates partial rescue of pre-dawn activity by pdf-Gal4. (D-F) Sleep (D, E) and nighttime activity (F) parameters calculated from the data in (A-C). The rescue percentages indicate potency of rescue calculated as [(PGal4;DopR - PDopR)/(PGal4 - PDopR)] × 100%, where P is the value of a given parameter for the genotype indicated by the subscript. “ns,” not significantly different (p>.017, using the Bonferroni correction for multiple comparisons).
In contrast to Elav-Gal4, line c547, which effectively rescued the ReSH phenotype of the DopR mutant, did not rescue the nocturnal hypoactivity phenotype (Fig. 7B, F). There was no statistically significant difference between DopR/+ and c547; DopR/+ flies for any sleep parameters measured (Fig. 7D, E; red vs. green bars). These data suggest that DopR is unlikely to control sleep-wake arousal by acting in the EB. We therefore sought other Gal4 lines that might rescue the sleep phenotype of DopR mutants. Pigment-Dispersing Factor (PDF)-expressing neurons are circadian pacemaker neurons that regulate the pre-dawn activity phase of the circadian cycle (Stoleru et al., 2004; Parisky et al., 2008), and arousal during the night phase (Shang et al., 2008). pdf-Gal4/DopR flies showed a significant, albeit partial, rescue of both average sleep bout duration, and the length of the longest sleep bout (Fig. 7C-E, green vs. blue bars). Similar results were obtained with line c929, an independent Gal4 driver that also expresses in circadian pacemaker neurons (Shang et al., 2008) (data not shown). Importantly, the pdf-Gal4 driver failed to rescue the ReSH phenotype of DopR flies (Supplementary Figure S16). Taken together, these data provide a double-dissociation suggesting that the inverse effects of the DopR mutation on ReSH behavior, and on sleep-wake transitions, reflect independent functions for the receptor in distinct neuronal circuits.
DISCUSSION
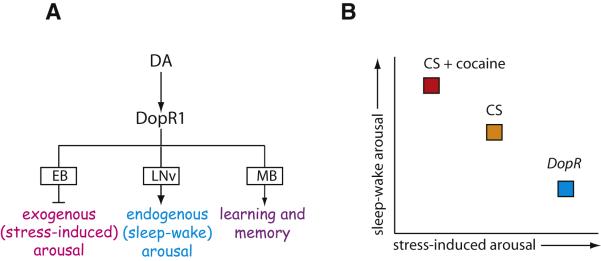
Previous studies of arousal in Drosophila have focused on sleep-wake transitions (Nitz et al., 2002; van Swinderen et al., 2004; Andretic et al., 2005; Kume et al., 2005; Sheeba et al., 2008), a form of “endogenous” arousal (van Swinderen and Andretic, 2003). Here we introduce a quantitative behavioral assay for repetitive startle-induced hyperactivity, which displays properties of an environmentally induced (“exogenous”) arousal state. We have conducted a screen for mutations affecting this behavior, characterized the phenotype of one such mutation (DopR), and mapped the neural substrates of its action by cell-specific genetic rescue experiments. Our results reveal that DopR independently regulates ReSH and sleep in opposite directions, by acting on distinct neural substrates. Negative regulation of the ReSH response requires DopR function in the EB of the CC, while positive regulation of waking reflects a function in other populations of neurons, including PDF-expressing circadian pacemaker cells (Stoleru et al., 2004; Parisky et al., 2008; Shang et al., 2008). Both of these functions, moreover, are independent of the function of DopR in learning and memory, which is required in the mushroom body (Kim et al., 2007) (Fig. 8A). Our data suggest that ReSH behavior and sleep-wake transitions reflect distinct forms of arousal that are genetically, anatomically and behaviorally separable.
Figure 8. DopR1 exerts opposite effects on different types of arousal by acting in different circuits.
(A) Summary of genetic rescue data indicating loci of DopR1 action in Drosophila. EB, ellipsoid body; LNv, lateral-ventral neurons (PDF+); MB, mushroom body. (B) Schematic illustrating how cocaine and the DopR mutation influence noctural arousal (sleep-wake transitions) and stress-induced arousal (ReSH behavior) in Drosophila.
ReSH behavior expresses an environmentally induced arousal state
Early genetic studies of Drosophila locomotion have suggested that spontaneous and environmentally stimulated locomotor activity reflect “distinct behavioral systems” (Connolly, 1967; Burnell and Daly, 1982). Several lines of evidence suggest that ReSH behavior represents a form of environmentally stimulated arousal. First, hyperactivity is an evolutionarily conserved expression of increased arousal (van Swinderen et al., 2004; Devidze et al., 2006). Although not all arousal is necessarily expressed as hyperactivity, electrophysiological studies indicate that mechanical startle evokes increases in 20-30 Hz and 80-90 Hz brain activity, which have been suggested to reflect a neural correlate of arousal in flies (Nitz et al., 2002; van Swinderen et al., 2004). Second, ReSH does not immediately dissipate following termination of the stimulus, as would be expected for a simple reflexive stimulus-response behavior, but rather persists for an extended period of time, suggesting that it reflects a change in internal state. Third, this state, like arousal, is scalable: more puffs, or more intense puffs, produce a stronger and/or longer-lasting state of hyperactivity. Fourth, this state exhibits sensitization: even after overt locomotor activity has recovered to pre-puff levels, flies remain hypersensitive to a single puff for several minutes. Fifth, this sensitization state generalizes to a startle stimulus of at least one other sensory modality (olfactory). In Aplysia, sensitization of the gill/siphon withdrawal reflex has been likened to behavioral arousal (Kandel and Schwartz, 1982). Taken together, these features strongly suggest that ReSH represents an example of environmentally stimulated (“exogenous”) arousal in Drosophila (Fig. 8A).
DA inversely and independently regulates environmentally stimulated and sleep-wake arousal
DopR mutant flies exhibited longer sleep bouts during their subjective night phase, suggesting that DopR normally promotes waking. These data are consistent with earlier studies indicating that DA promotes sleep-wake arousal (Andretic et al., 2005; Kume et al., 2005; Wu et al., 2008). In contrast, prior evidence regarding the role of DA in startle-induced arousal is conflicting. Some studies have suggested that DA negatively regulates locomotor reactivity to environmental stimuli (Burnell and Daly, 1982; Friggi-Grelin et al., 2003), consistent with our observations, while others have suggested that it positively regulates this response (McClung and Hirsh, 1998; Bainton et al., 2000; Kume et al., 2005). Even within the same study, light-stimulated activation of TH+ neurons produced opposite effects on locomotor activity, depending on the pre-stimulus level of locomotor activity (Lima and Miesenbock, 2005).
We find that DA and DopR negatively regulate environmentally stimulated arousal: the DopR mutation enhanced ReSH, while cocaine suppressed it. Furthermore, the effect of cocaine in the ReSH assay was eliminated in the DopR mutant but could be rescued by Gal4-driven DopR expression, confirming that the effect of the drug is mediated by DA. Taken together, our results reconcile apparently conflicting data on the role of DA in “arousal” in Drosophila, by identifying two different forms of arousal—repetitive startle-induced arousal and sleep-wake arousal--that are regulated by DA in an inverse manner. Whether these two forms of arousal are controlled by the same subset of DA neurons is an interesting question for future study.
Neural substrates of DA action in repetitive startle-induced arousal
Several lines of evidence suggest that endogenous DopR likely acts in the ellipsoid body (EB) of the central complex (CC) to regulate repetitive startle-induced arousal. First, multiple Gal4 lines that drive expression in the EB rescued the ReSH phenotype of DopR mutants. Second, endogenous DopR is expressed in EB neurons, including those in which the rescuing Gal4 drivers are expressed (Fig. 6). Third, the domain of DopR expression in the EB overlaps the varicosities of TH+ fibers. In the accompanying manuscript, Wolf et al. (2009) identify TH+ neurons that are a likely source of these projections to the EB. Fourth, rescue of the ReSH phenotype is associated with re-expression of DopR in EB neurons. Finally, rescue is observed using conditional DopR expression in adults. Taken together, these data argue that rescue of the ReSH phenotype by the Gal4 lines tested reflects their common expression in the EB, and that this is a normal site of DopR action in adult flies.
A requirement for DopR in the EB in regulating ReSH behavior is consistent with the fact that the CC is involved in the control of walking activity (Strauss and Heisenberg, 1993; Martin et al., 1999b; Strauss, 2002; Neuser et al., 2008). However, the mushroom body has also been implicated in the control of locomotor behavior (Martin et al., 1998; Helfrich-Forster et al., 2002), and DopR is strongly expressed in this structure as well (Kim et al., 2003). Our rescue data argue against the MB and in favor of the CC as a neural substrate for the ReSH phenotype of DopR mutants. Unexpectedly, the nocturnal hypoactivity phenotype of DopR mutants was not rescued by restoration of DopR expression to the CC. Thus, not all locomotor activity phenotypes of the DopR mutant necessarily reflect a function for the gene in the CC.
Interestingly, Gal4 line c547 expresses in R2/R4m neurons of the EB, while lines 189y and c761 express in R3 neurons (Renn et al., 1999), yet both rescued the ReSH phenotype of DopR mutants. Similar results have been obtained in experiments to rescue the deficit in ethanol-induced behavior exhibited by the DopR mutant (Wolf et al., submitted; see below). Double-labeling experiments suggest that endogenous DopR is expressed in all of these EB neuronal subpopulations (Fig. 6 and Wolf et al., submitted). Perhaps the receptor functions in parallel or in series in R4m and R3 neurons, so that restoration of DopR expression in either population can rescue the ReSH phenotype. Whether these DopR-expressing EB subpopulations are synaptically interconnected is an interesting question for future investigation.
In the accompanying manuscript, Wolf et al. show that DopR acts in the EB to positively regulate locomotor hyperactivity induced by chronic ethanol exposure (Wolf et al., submitted). There are several explanations for this difference in the “polarity” of the influence of the DopR mutation in the EB. First, the two behavioral paradigms are very different: hyperactivity in the ReSH paradigm is expressed immediately upon stimulus exposure and persists following stimulus cessation, while ethanol-induced hyperactivity develops more slowly and requires chronic ethanol exposure (Wolf et al., 2002; Cho et al., 2004). Second, the polarity of DA/DopR regulation of activity in the EB may itself be influenced by ethanol. Finally, DopR activation in the CC in response to different types of stimuli may either potentiate or suppress activity, depending on the initial state of the circuit and ambient levels of DA (Birman, 2005; Lima and Miesenbock, 2005). Whatever the explanation, the role of DopR and DA in regulating locomotor activity in the EB is clearly complex, and a deeper understanding will require more detailed anatomical mapping of EB circuitry and functional analysis.
Modeling “emotional” behaviors in Drosophila
Despite its power as a system for studying neural development, function and behavior, Drosophila has not been extensively used in affective neuroscience (Iliadi, 2009), in part because of uncertainty as to whether this model organism exhibits states related to emotion or affect. Increased arousal is a key component of many emotional or affective behaviors (Russell, 1980). The data presented here establish a quantitative behavioral paradigm for studying the genetic and neural circuit basis of a persistent arousal state elicited by repetitive traumatic startle. ReSH behavior exhibits several features that distinguish it from reflexive stimulus-response paradigms: scalability, persistence following stimulus termination, and sensitization. In addition, the observation that mechanical trauma promotes release from Drosophila of an odorant that repels other flies (Suh et al., 2004) suggests that the arousal state underlying ReSH behavior may have a negative “ affective valence” as well (Robbins et al., 1998; Calder et al., 2001). These considerations, together with the fact that ReSH is influenced by genetic and pharmacologic manipulations of DA, a biogenic amine implicated in emotional behavior in humans, support the idea that ReSH behavior may represent an evolutionary precursor of emotional responses in higher organisms.
The phenotype of DopR flies, which includes hyper-reactivity to environmental stimuli, is reminiscent of some symptoms of Attention-Deficit Hyperactivity Disorder (ADHD), an affective disorder linked to dopamine (Levy, 1991; Solanto, 2002; Bobb et al., 2005). If humans, like flies, have distinct brain circuits mediating environmentally stimulated and endogenous arousal, then it is possible given our data that ADHD may specifically involve dopaminergic dysfunction in circuits mediating the former rather than the latter type of arousal. The fact that DA can negatively regulate stimulated arousal circuits could then explain why treatment with DA reuptake inhibitors such as methylphenidate (Ritalin), which increases synaptic levels of DA, can ameliorate symptoms of ADHD (Arnsten, 2006).
Consistent with this view, in vertebrates DA is thought to promote waking activity via D1 receptors in the nucleus accumbens (Monti and Monti, 2007), while D1 receptors in the pre-frontal cortex (PFC) have been proposed to negatively regulate activity (Vezina et al., 1991; Heijtz et al., 2007). Numerous studies have linked dopaminergic dysfunction in the PFC to ADHD (reviewed in (Brennan and Arnsten, 2008)). While most research has focused on the role of the PFC in attention and cognition, rather than in environmentally stimulated arousal per se, dysfunction of PFC circuits mediating phasic DA release has been invoked to explain behavioral hypersensitivity to environmental stimuli in ADHD (Sikstrom and Soderlund, 2007). This view of ADHD as a specific disorder of stimulated arousal circuits suggests that further elucidation of such circuits in humans and vertebrate animal models, as well as in Drosophila, may deepen our understanding of this disorder and potentially lead to more targeted therapies.
EXPERIMENTAL PROCEDURES
Genetics
Homozygous viable insertional piggyBac alleles from the Exelixis collection were acquired from the Harvard Stock Center and tested in a pilot screen. The DopRf02676 allele was backcrossed to a standardized CS background for 6 generations prior to behavioral testing. Details of the excision of the piggyBac DopRf02676 allele are described in Supplementary Methods. Rescue experiments using DopRf02676/+ flies were performed in an F1 hybrid background that reflects equal contribution of the Gal4 genetic background and the DopRf02676 allele.
Behavioral Assays
Flies assayed were males (2-4 days old) CO2 anaesthetized and allowed to recover for 2 days prior to testing. Flies were reared on a 12 hr day-night cycle at 25°. Temperature for behavioral experiments was maintained at 23-25°. For the standard ReSH assay, 10 flies were manually loaded into tubes and allowed to acclimatize for 10 min prior to filming. Activity was recorded beginning at one minute before delivery of the puff stimuli, until 3.5 minutes after stimulus termination. Each air puff (35 psi unless otherwise indicated) lasted 200ms with a 5s inter-puff interval. Movies were analyzed using custom locomotor tracking software (described in Supplemental methods). Cocaine feeding and learning and memory protocols are described in Supplemental Methods.
Trikinetics Individual Drosophila Activity Monitors and Trikinetics software were used for all circadian/sleep observations (see Supplemental Methods for details). A period of sleep was defined as 5 minutes of inactivity (Shaw et al., 2000; Andretic and Shaw, 2005).
For Gal80ts experiments, flies were crossed and kept at 18° until eclosion. Flies (2-4 days old) were collected and maintained at 18° or shifted to 30° for 48 hrs prior to testing. Animals from both rearing conditions were acclimated to a 25° behavioral room for one hr prior to testing.
Immunohistochemistry
A polyclonal DopR antibody was raised in guinea pigs against the peptide CIKAVTRPGEVAEKQRYKSIR, derived from the third cytoplasmic loop (Kim et al., 2003). Antibodies were affinity purified. Antibody staining procedures and sources of other immune reagents are described in Supplemental Methods.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Fred Wolf and Ulrike Heberlein for sharing data and early drafts of their manuscript as well as providing the manifold for the puff-o-mat, Martin Heisenberg and members of the Anderson lab for helpful comments on the manuscript, Eric Hoopfer for help with imaging, Michael Reiser for contributions to software development, Mary Wahl for assistance with screening, Tim Tayler for sharing Gal4 stocks prior to publication, Shilpa Jeeda for maintenance of stocks, Gaby Mosconi for lab management and Gina Mancuso for Administrative Support. Fly stocks were obtained from the Bloomington and Harvard stock centers, and also generously provided by Doug Armstrong. T.L. was a fellow of the Jane Coffin Childs Foundation and H.D. a fellow of the Alexander von Humboldt Association. Supported in part by an NSF FIBR grant to D.J.A., P.P. and Michael Dickinson. D.J.A. is an Investigator of the Howard Hughes Medical Institute. This paper is dedicated to the late Seymour Benzer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andretic R, Shaw PJ. Essentials of sleep recordings in Drosophila: moving beyond sleep time. Meth Enzymol. 2005;393:759–772. doi: 10.1016/S0076-6879(05)93040-1. [DOI] [PubMed] [Google Scholar]
- Andretic R, van Swinderen B, Greenspan RJ. Dopaminergic modulation of arousal in Drosophila. Curr Biol. 2005;15:1165–1175. doi: 10.1016/j.cub.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31:2376–2383. doi: 10.1038/sj.npp.1301164. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S. Accessing the Exelixis collection. Nature genetics. 2004;36:207. doi: 10.1038/ng1316. [DOI] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LT, Singh CM, Moore MS, Neckameyer WS, Heberlein U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr Biol. 2000;10:187–194. doi: 10.1016/s0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Berridge CW. Neural substrates of psychostimulant-induced arousal. Neuropsychopharmacology. 2006;31:2332–2340. doi: 10.1038/sj.npp.1301159. [DOI] [PubMed] [Google Scholar]
- Birman S. Arousal mechanisms: speedy flies don’t sleep at night. Curr Biol. 2005;15:R511–513. doi: 10.1016/j.cub.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Bobb AJ, Castellanos FX, Addington AM, Rapoport JL. Molecular genetic studies of ADHD: 1991 to 2004. Am J Med Genet B Neuropsychiatr Genet. 2005;132B:109–125. [PubMed] [Google Scholar]
- Brennan AR, Arnsten AF. Neuronal mechanisms underlying attention deficit hyperactivity disorder: the influence of arousal on prefrontal cortical function. Annals of the New York Academy of Sciences. 2008;1129:236–245. doi: 10.1196/annals.1417.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell AM, Daly BA. Spontaneous activity an dopamine levels in Tyr-1 mutants of Drosophila melangoaster. In: Lakovaara S, editor. Advanes in Genetics, Development and Evolution of Drosophila. Plenum; New York: 1982. [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Lawrence AD, Young AW. Neuropsychology of fear and loathing. Nature reviews. 2001;2:352–363. doi: 10.1038/35072584. [DOI] [PubMed] [Google Scholar]
- Carbone MA, Jordan KW, Lyman RF, Harbison ST, Leips J, Morgan TJ, DeLuca M, Awadalla P, Mackay TF. Phenotypic variation and natural selection at catsup, a pleiotropic quantitative trait gene in Drosophila. Curr Biol. 2006;16:912–919. doi: 10.1016/j.cub.2006.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Grygoruk A, Brooks ES, Ackerson LC, Maidment NT, Bainton RJ, Krantz DE. Overexpression of the Drosophila vesicular monoamine transporter increases motor activity and courtship but decreases the behavioral response to cocaine. Mol Psychiatry. 2006;11:99–113. doi: 10.1038/sj.mp.4001742. [DOI] [PubMed] [Google Scholar]
- Cho W, Heberlein U, Wolf FW. Habituation of an odorant-induced startle response in Drosophila. Genes Brain Behav. 2004;3:127–137. doi: 10.1111/j.1601-183x.2004.00061.x. [DOI] [PubMed] [Google Scholar]
- Connolly K. Locomotor activity in Drosophila. 3. A distinction between activity and reactivity. Animal behaviour. 1967;15:149–152. doi: 10.1016/s0003-3472(67)80026-5. [DOI] [PubMed] [Google Scholar]
- Coull JT. Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol. 1998;55:343–361. doi: 10.1016/s0301-0082(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Devidze N, Lee A, Zhou J, Pfaff D. CNS arousal mechanisms bearing on sex and other biologically regulated behaviors. Physiology & Behavior. 2006;88:283–293. doi: 10.1016/j.physbeh.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Feng G, Hannan F, Reale V, Hon YY, Kousky CT, Evans PD, Hall LM. Cloning and functional characterization of a novel dopamine receptor from Drosophila melanogaster. J Neurosci. 1996;16:3925–3933. doi: 10.1523/JNEUROSCI.16-12-03925.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. Targeted gene expression inDrosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- Gotzes F, Balfanz S, Baumann A. Primary structure and functional characterization of a Drosophila dopamine receptor with high homology to human D1/5 receptors. Receptors & channels. 1994;2:131–141. [PubMed] [Google Scholar]
- Gotzes F, Baumann A. Functional properties of Drosophila dopamine D1-receptors are not altered by the size of the N-terminus. Biochemical and biophysical research communications. 1996;222:121–126. doi: 10.1006/bbrc.1996.0708. [DOI] [PubMed] [Google Scholar]
- Han KA, Millar NS, Grotewiel MS, Davis RL. DAMB, a novel dopamine receptor expressed specifically in Drosophila mushroom bodies. Neuron. 1996;16:1127–1135. doi: 10.1016/s0896-6273(00)80139-7. [DOI] [PubMed] [Google Scholar]
- Hearn MG, Ren Y, McBride EW, Reveillaud I, Beinborn M, Kopin AS. A Drosophila dopamine 2-like receptor: Molecular characterization and identification of multiple alternatively spliced variants. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14554–14559. doi: 10.1073/pnas.202498299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. Drives and the C.N.S. (conceptual nervous system) Psychol REv. 1955;62:243–254. doi: 10.1037/h0041823. [DOI] [PubMed] [Google Scholar]
- Heijtz RD, Kolb B, Forssberg H. Motor inhibitory role of dopamine D1 receptors: implications for ADHD. Physiol Behav. 2007;92:155–160. doi: 10.1016/j.physbeh.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Helfrich-Forster C, Wulf J, de Belle JS. Mushroom body influence on locomotor activity and circadian rhythms in Drosophila melanogaster. Journal of neurogenetics. 2002;16:73–109. doi: 10.1080/01677060213158. [DOI] [PubMed] [Google Scholar]
- Iliadi KG. The genetic basis of emotional behavior: has the time come for a Drosophila model? Journal of neurogenetics. 2009;23:136–146. doi: 10.1080/01677060802471650. [DOI] [PubMed] [Google Scholar]
- Jordan KW, Carbone MA, Yamamoto A, Morgan TJ, Mackay TF. Quantitative genomics of locomotor behavior in Drosophila melanogaster. Genome biology. 2007;8:R172. doi: 10.1186/gb-2007-8-8-r172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan KW, Morgan TJ, Mackay TF. Quantitative trait loci for locomotor behavior in Drosophila melanogaster. Genetics. 2006;174:271–284. doi: 10.1534/genetics.106.058099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikouchi A, Inagaki HK, Effertz T, Hendrich O, Fiala A, Gopfert MC, Ito K. The neural basis of Drosophila gravity-sensing and hearing. Nature. 2009;458:165–171. doi: 10.1038/nature07810. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH. Molecular biology of learning: modulation of transmitter release. Science. 1982;218:433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Kim YC, Lee HG, Han KA. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J Neurosci. 2007;27:7640–7647. doi: 10.1523/JNEUROSCI.1167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Lee HG, Seong CS, Han KA. Expression of a D1 dopmaine receptor dDA1/DmDOP1 in the central nervous system of Drosophila melanogaster. Gene Expr Patterns. 2003;3:237–245. doi: 10.1016/s1567-133x(02)00098-4. [DOI] [PubMed] [Google Scholar]
- Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit fly. J Neurosci. 2005;25:7377–7384. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy F. The dopamine theory of attention deficit hyperactivity disorder (ADHD) The Australian and New Zealand journal of psychiatry. 1991;25:277–283. doi: 10.3109/00048679109077746. [DOI] [PubMed] [Google Scholar]
- Li H, Chaney S, Forte MA, Hirsh J. Ectopic G-protein expression in dopmaine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. Curr Biol. 2000;11:211–214. doi: 10.1016/s0960-9822(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Lima SQ, Miesenbock G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;121:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Manseau L, Baradaran A, Brower D, Budhu A, Elefant F, Phan H, Philp AV, Yang M, Glover D, Kaiser K, et al. GAL4 enhancer traps expressed in the embryo, larval brain, imaginal discs, and ovary of Drosophila. Dev Dyn. 1997;209:310–322. doi: 10.1002/(SICI)1097-0177(199707)209:3<310::AID-AJA6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Martin JR, Ernst R, Heisenberg M. Mushroom bodies suppress locomotor activity in Drosophila melanogaster. Learning & memory (Cold Spring Harbor, NY. 1998;5:179–191. [PMC free article] [PubMed] [Google Scholar]
- Martin JR, Ernst R, Heisenberg M. Temporal pattern of locomotor activity in Drosophila melanogaster. Journal of comparative physiology. 1999a;184:73–84. doi: 10.1007/s003590050307. [DOI] [PubMed] [Google Scholar]
- Martin JR, Raabe T, Heisenberg M. Central complex substructures are required for the maintenance of locomotor activity in Drosophila melanogaster. Journal of comparative physiology. 1999b;185:277–288. doi: 10.1007/s003590050387. [DOI] [PubMed] [Google Scholar]
- McClung C, Hirsh J. Stereotypic behavioral responses to free-base cocaine and the development of behavioral sensitization in Drosophila. Curr Biol. 1998;8:109–112. doi: 10.1016/s0960-9822(98)70041-7. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- Meehan MJ, Wilson R. Locomotor activity in the Tyr-1 mutant of Drosophila melanogaster. Behavior genetics. 1987;17:503–512. doi: 10.1007/BF01073117. [DOI] [PubMed] [Google Scholar]
- Monti JM, Monti D. The involvement of dopamine in the modulation of sleep and waking. Sleep medicine reviews. 2007;11:113–133. doi: 10.1016/j.smrv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS. Dopamine and mushroom bodies in Drosophila: experience-dependent and -independent aspects of sexual behavior. Learning & memory (Cold Spring Harbor, NY. 1998;5:157–165. [PMC free article] [PubMed] [Google Scholar]
- Neuser K, Triphan T, Mronz M, Poeck B, Strauss R. Analysis of a spatial orientation memory in Drosophila. Nature. 2008;453:1244–1247. doi: 10.1038/nature07003. [DOI] [PubMed] [Google Scholar]
- Nitz DA, van Swinderen B, Tononi G, Greenspan RJ. Electrophysiological correlates of rest and activity in Drosophila melanogaster. Curr Biol. 2002;12:1934–1940. doi: 10.1016/s0960-9822(02)01300-3. [DOI] [PubMed] [Google Scholar]
- Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ, Kang K, Liu X, Garrity PA, Rosbash M, et al. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–682. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff D, Frohlich J, Morgan M. Hormonal and genetic influences on arousal--sexual and otherwise. Trends Neurosci. 2002;25:45–50. doi: 10.1016/s0166-2236(00)02084-1. [DOI] [PubMed] [Google Scholar]
- Pfaff D, Westberg L, Kow L. Generalized arousal of mammalian central nervous system. J Comp Neurol. 2005;493:86–91. doi: 10.1002/cne.20720. [DOI] [PubMed] [Google Scholar]
- Renn SCP, Armstrong JD, Yang M, Wang Z, An X, Kaiser K, Taghert PH. Genetic analysis of the Drosophila ellipsoid body neuropil: organization and development of the central complex. J Neurobiol. 1999;41:189–207. [PubMed] [Google Scholar]
- Riemensperger T, Voller T, Stock P, Buchner E, Fiala A. Punishment prediction by dopaminergic neurons in Drosophila. Curr Biol. 2005;15:1953–1960. doi: 10.1016/j.cub.2005.09.042. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Arousal systems and attentional processes. Biological psychology. 1997;45:57–71. doi: 10.1016/s0301-0511(96)05222-2. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Granon S, Muir JL, Durantou F, Harrison A, Everitt BJ. Neural systems underlying arousal and attention. Implications for drug abuse. Annals of the New York Academy of Sciences. 1998;846:222–237. [PubMed] [Google Scholar]
- Robinow S, White K. The locus elav of Drosophila melanogaster is expressed in neurons at all developmental stages. Dev Biol. 1988;126:294–303. doi: 10.1016/0012-1606(88)90139-x. [DOI] [PubMed] [Google Scholar]
- Rothenfluh A, Threlkeld RJ, Bainton RJ, Tsai LT, Lasek AW, Heberlein U. Distinct behavioral responses to ethanol are regulated by alternate RhoGAP18B isoforms. Cell. 2006;127:199–211. doi: 10.1016/j.cell.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Russell JA. A circumplex model of affect. J Personality Social Psychol. 1980;39:1161–1178. [Google Scholar]
- Schroll C, Riemensperger T, Bucher D, Ehmer J, Voller T, Erbguth K, Gerber B, Hendel T, Nagel G, Buchner E, et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol. 2006;16:1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seugnet L, Suzuki Y, Vine L, Gottschalk L, Shaw PJ. D1 receptor activation in the mushroom bodies rescues sleep-loss-induced learning impairments in Drosophila. Curr Biol. 2008;18:1110–1117. doi: 10.1016/j.cub.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Griffith LC, Rosbash M. Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19587–19594. doi: 10.1073/pnas.0809577105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- Sheeba V, Fogle KJ, Kaneko M, Rashid S, Chou YT, Sharma VK, Holmes TC. Large Ventral Lateral Neurons Modulate Arousal and Sleep in Drosophila. Curr Biol. 2008;18:1537–1545. doi: 10.1016/j.cub.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikstrom S, Soderlund G. Stimulus-dependent dopamine release in attention-deficit/hyperactivity disorder. Psychological review. 2007;114:1047–1075. doi: 10.1037/0033-295X.114.4.1047. [DOI] [PubMed] [Google Scholar]
- Solanto MV. Dopamine dysfunction in AD/HD: integrating clinical and basic neuroscience research. Behavioural brain research. 2002;130:65–71. doi: 10.1016/s0166-4328(01)00431-4. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Yu EJ, Kennedy K, Chatwin H, Reale V, Hamon M, Smith T, Evans PD. Rapid, nongenomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. J Neurosci. 2005;25:6145–6155. doi: 10.1523/JNEUROSCI.1005-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- Strauss R. The central complex and the genetic dissection of locomotor behaviour. Current Opinion in Neurobiology. 2002;12:633–638. doi: 10.1016/s0959-4388(02)00385-9. [DOI] [PubMed] [Google Scholar]
- Strauss R, Heisenberg M. A higher control center of locomotor behavior in the Drosophila brain. J Neurosci. 1993;13:1852–1861. doi: 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugamori KS, Demchyshyn LL, mcConkey F, Forte MA, Niznik HB. A primordial dopmaine D-like adenylyl cclase-linked receptor from Drosophila melanogaster displaying poor affinity for benzazepines. FEBS Lett. 1995;362:131–138. doi: 10.1016/0014-5793(95)00224-w. [DOI] [PubMed] [Google Scholar]
- Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- Thibault ST, Singer MA, Miyazaki WY, Milash B, Dompe NA, Singh CM, Buchholz R, Demsky M, Fawcett R, Francis-Lang HL, et al. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nature genetics. 2004;36:283–287. doi: 10.1038/ng1314. [DOI] [PubMed] [Google Scholar]
- Tunnicliff G, Rick JT, Connolly K. Locomotor acitvity in Drosophila. V. A comparative biochemical study of selectively bred populations. Comparative biochemistry and physiology. 1969;29:1239–1245. doi: 10.1016/0010-406x(69)91028-7. [DOI] [PubMed] [Google Scholar]
- van Swinderen B, Andretic R. Arousal in Drosophila. Behavioural Processes. 2003;64:133–144. doi: 10.1016/s0376-6357(03)00131-1. [DOI] [PubMed] [Google Scholar]
- van Swinderen B, Nitz DA, Greenspan RJ. Uncoupling of brain activity from movement defines arousal States in Drosophila. Curr Biol. 2004;14:81–87. [PubMed] [Google Scholar]
- Vezina P, Blanc G, Glowinski J, Tassin JP. Opposed Behavioural Outputs of Increased Dopamine Transmission in Prefrontocortical and Subcortical Areas: A Role for the Cortical D-1 Dopamine Receptor. The European journal of neuroscience. 1991;3:1001–1007. doi: 10.1111/j.1460-9568.1991.tb00036.x. [DOI] [PubMed] [Google Scholar]
- Wolf FW, Rodan AR, Tsai LT, Heberlein U. High-resolution analysis of ethanol-induced locomotor stimulation in Drosophila. J Neurosci. 2002;22:11035–11044. doi: 10.1523/JNEUROSCI.22-24-11035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MN, Koh K, Yue Z, Joiner WJ, Sehgal A. A genetic screen for sleep and circadian mutants reveals mechanisms underlying regulation of sleep in Drosophila. Sleep. 2008;31:465–472. doi: 10.1093/sleep/31.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Zwarts L, Callaerts P, Norga K, Mackay TF, Anholt RR. Neurogenetic networks for startle-induced locomotion in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12393–12398. doi: 10.1073/pnas.0804889105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.