Synopsis
The intestine plays a central role in the pathophysiology of critical illness and is frequently called the “motor” of the systemic inflammatory response. Perturbations to the intestinal barrier can lead to distant organ damage and multiple organ failure. Therefore, identifying ways to preserve intestinal integrity may be of paramount importance. Growth factors and other peptides have emerged as potential tools for modulation of intestinal inflammation and repair due to their roles in cellular proliferation, differentiation, migration, and survival. In this review, we will examine the involvement of growth factors and other peptides in intestinal epithelial repair during critical illness and their potential use as therapeutic targets.
Keywords: critical illness, intestine, growth factors
Introduction
For more than two decades, the gut has been hypothesized to be the “motor” of the systemic inflammatory response syndrome. As critical care research has evolved, numerous studies have defined how the gut plays a role in the origin and propagation of critical illness. During shock, intestinal hypoperfusion followed by reperfusion leads to production of proinflammatory mediators that can amplify the systemic inflammatory response1. Interactions between host and bacterial pathogens in the intestine contribute to gut-derived sepsis2. Intestinal permeability in critical illness, as a result of compromised epithelial tight junctions, leads to persistent activation of systemic inflammation3–5. Toxic gut-derived substances enter the mesenteric lymph leading to lung damage, and distant organ injury can be prevented by ligating the mesenteric lymph duct in hemorrhagic shock6. Intestinal epithelial apoptosis is elevated following sepsis, and prevention of sepsis-induced intestinal apoptosis by overexpression of the anti-apoptotic protein Bcl-2 improves survival in multiple animal models of sepsis7,8.
Since perturbations to the intestinal epithelium can cause distant organ damage and development of multiple organ dysfunction syndrome, identifying ways to preserve intestinal integrity may be of paramount importance in the treatment of critical illness. Growth factors have emerged as potential tools for modulation of intestinal inflammation and repair, playing important roles in cellular proliferation, differentiation, migration, and survival. In this review, we will examine the involvement of growth factors and other peptides in intestinal mucosal repair during critical illness and their potential use as therapeutic targets.
Mucosal repair in the gastrointestinal tract
The mucosal lining of the gastrointestinal tract represents the largest body surface in contact with the outside world (approximately 300 m2, roughly the area of a tennis court). The intestinal epithelium consists of a single layer of columnar epithelial cells that are constantly renewed from multipotent stem cells originating in the crypts of Lieberkühn. These stem cells give rise to four major epithelial lineages: absorptive enterocytes, goblet cells, enteroendocrine cells, and Paneth cells9. Over the course of a three to five day lifespan, enterocytes, goblet cells, and enteroendocrine cells migrate upwards along the crypt-villus axis where they differentiate and ultimately die of apoptosis or are exfoliated whole into the lumen10. In contrast, Paneth cells migrate downward over the course of five to eight days to the crypt base where they reside for approximately three weeks. Each epithelial cell is in intimate contact with its neighbors, and the integrity of the epithelium is maintained by apical junctional complexes11. Tight junctions are the most apical components of the complex and create a dynamic barrier to the paracellular movement of water, solutes, and immune cells12,13.
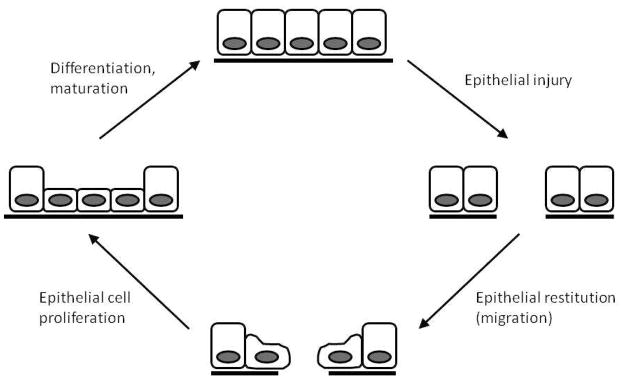
While minor breaches in epithelial integrity occur daily due to mechanical strain associated with intestinal motility and physiologic digestive trauma, more extensive disruption of epithelial continuity can result from bacterial invasion, chemical injury, or tissue destruction due to ischemic, septic, and inflammatory enteropathies14. Rapid resealing of the intestinal barrier is essential to prevent systemic penetration of toxins, immunogens, and other factors that can lead to activation of the systemic inflammatory response. The gastrointestinal tract utilizes at least three distinct mechanisms to re-establish epithelial continuity (Figure 1)15,16. Within minutes after injury, epithelial cells bordering the zone of injury migrate into the wound to cover the denuded area. During this process, termed epithelial restitution, epithelial cells adjacent to the injury undergo a striking change in cell shape and phenotype. Instead of their normal columnar shape, the cells flatten and adopt a squamoid appearance, followed by extension of lammelipodia. In addition, the cells undergo brush border and junctional disassembly and become polarized along the leading-trailing edge axis. After the wound is sealed, the cells reorganize their cytoskeleton and redifferentiate into mature enterocytes. Epithelial cell proliferation is also stimulated in order to restore the functional capacity of the mucosa. Finally, undifferentiated epithelial cells undergo maturation and differentiation to maintain normal mucosal epithelial function. It is important to note that when an epithelial defect is large, stimulation of cell proliferation is crucial for restoration of normal mucosal architecture. If the lesion is deep or penetrating, additional repair mechanisms are required, such as angiogenesis and deposition of extracellular matrix components to form granulation tissue.
Figure 1.
A simplified model of epithelial injury and restitution. Following epithelial injury, cells depolarize, dedifferentiate, and migrate to cover the denuded area (restitution). Once the epithelial defect is sealed, epithelial cell proliferation is stimulated to replace the cell pool. Epithelial cells then differentiate and mature to become an intact epithelial layer again.
Regulation of intestinal epithelial repair by growth factors
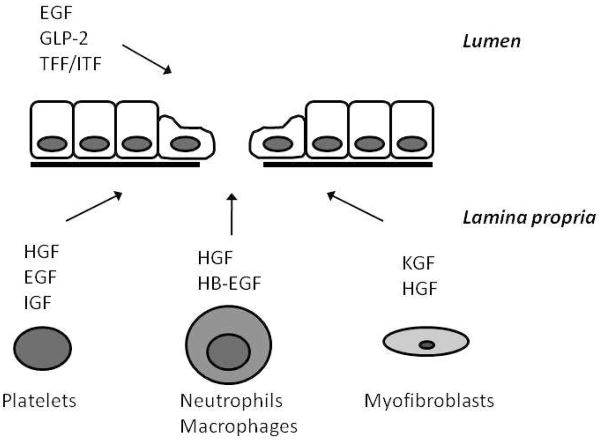
Numerous growth factors regulate the process of epithelial repair (Figure 2)14,17. Growth factors control a wide variety of activities, including stimulation of proliferation and migration, cell differentiation, acceleration of angiogenesis and extracellular matrix remodeling, as well as promotion of epithelial mucosal repair14,17. These factors can either be derived from the luminal environment as the result of intrinsic secretions from epithelial cells, or they can be produced by a wide variety of mucosal and submucosal cells14. Myofibroblasts beneath a mucosal injury secrete hepatocyte growth factor (HGF) and keratinocyte growth factor (KGF), both of which stimulate migration and proliferation of epithelial cells14. Neutrophils also release HGF18. Platelets also release growth factors in response to tissue injury, including epidermal growth factor (EGF)19, insulin-like growth factor (IGF-I)20, and HGF 21. These growth factors interact predominantly with receptors on the basolateral membrane of epithelial cells. In contrast, other growth factors including intestinal trefoil factor (ITF) and glucagon-like peptide-2 (GLP-2) are secreted into the lumen and act primarily at the apical surface of epithelial cells. Of note, EGF can also be secreted into the lumen and act on the apical surface. While a complete understanding of the complex interrelationships and redundancy of growth factors in epithelial repair remains to be determined, multiple studies have shed light on how these peptides protect the intestine during injury.
Figure 2.
Several growth factors are involved in preventing or enhancing intestinal epithelial repair. HGF, hepatocyte growth factor; EGF, epidermal growth factor; IGF, insulin-like growth factor; HB-EGF, heparin binding EGF-like growth factor; KGF, keratinocyte growth factor; GLP-2, glucagon-like peptide-2; TFF, trefoil factor family; ITF, intestinal trefoil factor
Epidermal Growth Factor
EGF is a potent 53 amino acid cytoprotective peptide that exhibits trophic and healing effects on the intestinal mucosa22,23. As a mitogen, EGF is involved with the regulation of cellular proliferation, survival, and migration. Under basal conditions, the EGF signaling pathway is crucial for intestinal epithelial proliferation and cell survival24. EGF receptor (EGF-R) deficient mice die early in postnatal life and exhibit severe defects in intestinal morphology, including fewer and shorter villi25. Activation of EGF-R following binding of EGF in the intestine can lead to increased blood flow26, increased cell survival27,28, decreased inflammation29, and improved barrier function30,31.
There is significantly more preclinical data on the use of EGF in adult critical illness than other growth factors. Circulating EGF levels are decreased while intestinal EGF and EGF-R levels are increased following cecal ligation and puncture (CLP), a preclinical model of peritonitis-induced sepsis32. Animals subjected to CLP have increased sepsis-induced apoptosis, and this is associated with increased expression of Bid, FADD and p21. Apoptosis is normalized to sham levels in mice treated with exogenous EGF after the onset of sepsis, as are the levels of Bid, FADD and p21. Septic mice also have decreased intestinal proliferation and villus length, while giving exogenous EGF after the onset of sepsis restores proliferation to levels seen in sham animals and nearly normalizes villus length. Importantly, giving exogenous EGF after CLP results in a 2-fold improvement in survival in septic mice.
Since EGF can have a number of extra-intestinal effects, it was unclear whether the benefits conferred by exogenous EGF were enterocyte-specific. Therefore, similar experiments were performed using transgenic mice with enterocyte-specific overexpression of EGF31. Intestine-specific EGF overexpression is sufficient to prevent sepsis-induced decreases in intestinal proliferation and villus length and sepsis-induced increases in gut epithelial apoptosis. Further, intestinal permeability is markedly increased following CLP in wild type mice but permeability is normalized to sham levels in septic transgenic mice that overexpress EGF. This change in barrier function is associated with normalization of claudin-2 expression and localization in transgenic mice that overexpress EGF in their intestinal epithelium. Importantly, enterocyte-specific overexpression of EGF confers a marked improvement in survival in CLP-induced sepsis, suggesting the protective effects of systemic EGF in septic peritonitis are mediated in a gut-specific fashion.
In addition, to improving survival in CLP, systemic administration of EGF has also been demonstrated to be beneficial in other models of adult critical illness. Specifically, exogenous EGF reduces intestinal injury and improves host survival in animal models of ischemia-reperfusion injury33,34 and thermal injury35.
Several lines of evidence have demonstrated an important role for EGF in intestinal repair as well. In a neonatal rat model of necrotizing enterocolitis (NEC), EGF-R is significantly upregulated in the intestinal epithelium, and supplementation of milk formula with EGF decreases the incidence and severity of disease36. This protection is associated with decreased intestinal epithelial apoptosis and restoration of intestinal barrier function28,37. The EGF/EGF-R signaling axis has also been shown to play a critical role in the adaptive response following short bowel resection since administration of either exogenous EGF or enterocyte-specific overexpression of EGF enhance the adaptive response following short bowel resection38,39. On the other hand, this adaptive response is severely impaired in mice that lack functional EGF-R or following pharmacological inhibition of EGF-R40. Finally, in patients with peptic ulcer disease, salivary levels of EGF are significantly reduced, and EGF-R expression is 75-fold higher in rats with chemically induced ulcers compared to untreated controls41. Patients with peptic ulcer disease treated intravenously with EGF also have improved ulcer healing compared to patients treated with cetraxate hydrochloride42.
Exogenous EGF appears to be an attractive candidate for clinical trials in critically ill patients. EGF and EGF-R have been targeted for therapeutic use in a large number of diseases, and a federal government registration of clinical trials lists over 200 trials involving or targeting EGF and/or EGF-R43. While many of these trials target extraintestinal effects of EGF, beneficial effects in the gut have been noted in clinical trials with EGF. For instance, in patients with ulcerative colitis, treatment with EGF-containing enemas significantly improved scoring of disease activity, sigmoidoscopic findings, and histological grading of injury when compared with placebo44. Similarly, a prospective, randomized trial with recombinant EGF in a small group of premature neonates with evidence of NEC demonstrated improved intestinal repair as determined by rectal biopsy specimens45. Importantly, no toxicities were reported after EGF administration to these infants. Based upon the benefits of EGF in preclinical trials and its apparent safety when used for short-term therapy in patients, EGF treatment may represent a novel therapeutic in critical illness.
Growth Hormone and Insulin-like Growth Factor-I
Critical illness alters the body’s metabolic rate, and a prolonged hypercatabolic state is associated with increased morbidity and mortality46. Critical illness is also often associated with alterations in the circulating concentrations or a diminished responsiveness of tissues to anabolic proteins such as IGF-I and growth hormone (GH)47.
GH is a 22-kDa anabolic protein that can antagonize some of the deleterious effects of hypercatabolism48. In critically ill patients, the circulating concentration of GH is markedly elevated. Despite this, there is paradoxical GH resistance, in which GH fails to stimulate IGF-I synthesis in the liver. This has been demonstrated in preclinical trials in sheep that were injected with endotoxin49, as well as in septic patients who were given exogenous GH but failed to increase circulating IGF-I levels to the same extent as in controls50.
The receptor for GH is expressed throughout the intestine, which suggests that GH may act to promote epithelial repair during intestinal injury51. However, the response to GH in the intestine under both basal and pathophysiologic conditions is incompletely understood. Potent trophic effects of GH have been demonstrated in the intestine of unmanipulated transgenic mice that overexpress GH52. When these transgenic mice are subjected to dextran sodium sulfate (DSS)-induced colitis, they exhibit increased crypt cell proliferation resulting in improved intestinal structure53. However, studies examining GH in animal models of short bowel syndrome have shown conflicting results with varying effects on mucosal mass54,55. Further, a rat total parenteral nutrition (TPN) model failed to demonstrate a trophic effect of GH on the intestine despite normalized body weight gain and increased plasma IGF-I levels56,57. Similarly, rats given GH after severe thermal injury have improved villus morphology compared to controls, but this effect is not mediated by either increased crypt cell proliferation or inhibition of epithelial apoptosis58.
Critical illness decreases circulating levels of IGF-I59. IGF-I is a small polypeptide (70 amino acids) with considerable homology to insulin. The primary biological effect of IGF-I is to stimulate cellular growth and differentiation60,61. Multiple studies have demonstrated that IGF-I has beneficial effects on intestinal homeostasis, and specific receptors for IGF-I are present in the gastrointestinal tract of humans and animals. Under normal conditions, transgenic mice that overexpress IGF-I exhibit increased crypt cell mitosis and increased growth of the small intestine62. In rats subjected to small bowel resection, administration of IGF-I augments compensatory mucosal hyperplasia and epithelial restitution63. Further, IGF-I administration decreases bacterial translocation after severe thermal injury by maintaining intestinal integrity64,65. In addition to its effects on intestinal proliferation, IGF-I has also been shown to attenuate intestinal epithelial apoptosis in a murine model of NEC66 and in vitro following H2O2-induced injury67.
Both GH and IGF-1 have been used in clinical trials. Importantly, GH increased morbidity and mortality in critically ill patients in a large prospective, randomized trial68. While GH has recently been hypothesized to be of potential benefit in refractory critical illness69, its utility in this setting is not proven. Long-term GH may be of benefit in patients recovering from critical illness, as opposed to patients who are acutely critically ill. A recent prospective, randomized trial of long-term GH in severely burned children with greater than 40% body surface burn showed improved growth and lean body mass two years after the initial insult70. However, GH was initiated after hospital discharge in this study, so they were no longer critically ill by the time GH was initiated.
Therapeutic use of IGF-I has been has not been possible because of adverse side effects such as hypoglycemia, electrolyte imbalances, and cardiac arrest71,72. However, when IGF-I is bound to its principle binding protein (IGFBP-3), it has been shown to be safe and efficacious in humans73–76. Although IGF-1/IGFBP-3 would be expected to have extraintestinal effects, limited preclinical data suggests it also has beneficial effects on gut integrity. In a rat model of severe thermal injury, intravenous administration of IGF-I in combination with IGFBP-3 stimulated small intestinal epithelial proliferation and increased villus length, crypt depth, and cell number. In addition, IGF-I/IGFBP-3 significantly decreased burn-induced intestinal epithelial apoptosis77. These data suggest that IGF-I/IGFBP-3 may be a potential therapeutic agent to improve intestinal integrity in critically ill patients.
Keratinocyte Growth Factor
KGF is a member of the fibroblast growth factor family that stimulates growth and differentiation of epithelial cells in the gastrointestinal tract, lung, and kidney78. The receptor for KGF has been found exclusively in the intestinal epithelium, suggesting that KGF acts in a paracrine manner to stimulate epithelial repair in the gut. KGF expression is markedly increased in the mucosa and submucosa of patients with inflammatory bowel disease, and KGF overexpression correlates with the degree of inflammation79. The fact that KGF is upregulated following intestinal injury suggests it plays an important role in normal tissue repair. Administration of KGF to unmanipulated rats causes a marked increase in epithelial proliferation as well as a selective induction of mucin-producing goblet cells throughout the gastrointestinal tract80. This induction is associated with increased expression of intestinal trefoil factors, which also play a role in epithelial repair (discussed in more detail below). Intraperitoneal administration of KGF also reduces the extent of intestinal injury in several animal models of colitis81 while KGF knockout mice subjected to DSS-induced colitis exhibit more severe colonic inflammation and delayed tissue repair than wild-type mice subjected to the same insult82. Exogenous KGF also promotes cell survival, as mice subjected given TPN exhibit decreased apoptosis and increased expression of anti-apoptotic Bcl-2 proteins83.
Chemotherapy and irradiation can compromise epithelial integrity by rapidly killing dividing cells in the mucosa, thereby impairing normal epithelial cell renewal. These treatments are often associated with mucositis, a condition which is characterized by mucosal atrophy, ulceration, barrier dysfunction, and infection84. KGF has been successfully used as a pretreatment in animal models of gastrointestinal injury induced by radiation85,86, chemotherapy86, or a combination of both86. In these models, KGF increases intestinal epithelial cell survival and mucosal thickness which is associated with decreased mortality. Importantly, KGF does not effect the growth rate of epithelial tumors, suggesting it may be a good therapeutic agent to prevent intestinal damage in patients receiving cancer therapy86. In contrast, intravenous administration of recombinant KGF failed to induce remission in a Phase II study of patients with active ulcerative colitis87 although the dose of KGF may have been too low for any beneficial effect to be seen. The effects of KGF in critical illness are unknown.
Hepatocyte Growth Factor
HGF is a mesenchymal-derived pleiotropic protein that regulates cell proliferation, cell survival, motility, morphogenesis, anti-inflammation, and angiogenesis in a wide variety of cells, including gastrointestinal epithelial cells88,89. HGF has been shown to accelerate epithelial remodeling after injury by stimulating intestinal epithelial proliferation90. Administration of HGF increases mucosal mass and enhances intestinal substrate absorption in rats following small bowel resection91. Similarly, HGF stimulates intestinal proliferation leading to preserved villus structure in an animal model of severe thermal injury92. The effect of HGF on apoptosis is more variable. HGF administration inhibits intestinal epithelial apoptosis during ischemia-reperfusion injury93, but has no effect on burn-induced intestinal apoptosis92.
Several studies have demonstrated that HGF promotes colonic mucosal repair in animal models of colitis. However, the mechanisms underlying protection vary depending on the model and route of HGF treatment. In rats subjected to DSS-induced colitis, continuous intraperitoneal administration of recombinant human HGF reduces colitis-associated weight loss, colonic shortening, and improved colonic erosions, and this is associated with enhanced epithelial regeneration and cellular proliferation 94. Similarly, daily intravenous administration of recombinant human HGF to rats with TNBS-induced colitis causes a significant reduction in colonic ulcer coverage and colonic shortening, and this is associated with increased epithelial proliferation and decreased inflammatory cell infiltrate in the inflamed colon 95. The improvements noted with intraperitoneal administration of recombinant human HGF in these models is associated with inhibition of intestinal epithelial apoptosis rather than stimulation of proliferation 96. Several studies have reported that colitis can also be ameliorated when adenoviral-mediated, liposome-formulated, or naked HGF gene is administered intrarectally, intramuscularly, or intravenously 97–100. A potential roadbloack towards using HGF in clinical trials is the observation that it may be a carcinogen since transgenic mouse strains that overexpress HGF exhibit increased rates of benign and malignant liver and mammary gland tumors101. The benefits and/or risks of short term usage of HGF in critically ill patients remains to be determined.
Heparin-binding EGF-like Growth Factor
Heparin-binding epidermal growth factor-like growth factor (HB-EGF) was first identified as a 22-kDa glycoprotein in the conditioned medium of cultured human macrophages102. A member of the EGF family, HB-EGF is a potent mitogen for a number of cell types, including epithelial cells, fibroblasts, smooth muscle cells, keratinocytes, and renal tubule cells103. Expression of endogenous HB-EGF is significantly increased in response to tissue damage, hypoxia, oxidative stress, and during wound healing and regeneration104. In cell culture, HB-EGF has been shown to protect intestinal epithelial cells from pro-inflammatory cytokine-induced apoptosis105. Pretreatment of intestinal epithelial cells with HB-EGF in vitro leads to decreased necrosis, preserved cytoskeletal structure, higher adenosine triphosphate levels, and improved proliferative capacity during recovery from hypoxia106. HB-EGF decreases the generation of reactive oxygen species in intestinal epithelial cells after ischemia-reperfusion injury107. HB-EGF also preserves the crypt proliferative response and decreases bacterial translocation across intestinal epithelial cell monolayers after ischemia-reperfusion injury, indicating preservation of epithelial integrity108.
HB-EGF has also been shown to protect the intestine in vivo. In a neonatal rat model of NEC, HB-EGF treatment caused increased intestinal proliferation and migration as well as preservation of intestinal epithelial barrier function when compared with untreated animals109. Further, in a neonatal hemorrhagic shock model, HB-EGF treatment resulted in increased intestinal blood flow and microcirculatory flow to levels greater than basal pre-shock levels110. While these findings are encouraging, the mechanisms for the beneficial effects of HB-EGF remain to be elucidated and its effects in adult models of critical illness have yet to be determined.
Glucagon-like Peptide-2
GLP-2 is a 33 amino acid peptide that is secreted from intestinal endocrine cells in response to nutrient ingestion, which acts as a potent growth factor for the small intestinal epithelium and, to a lesser extent, the large intestinal epithelium 111. GLP-2 administration significantly improves morbidity and enhances epithelial repair in a diverse number of intestinal injury models, including small bowel resection112,113, colitis114,115, and enteritis116. The protective effects of GLP-2 are thought to be due to its ability to stimulate crypt cell proliferation, prevent epithelial apoptosis, enhance epithelial barrier function, and reduce intestinal permeability117–119.
Administration of GLP-2 or a degradation-resistant analogue h[Gly2]GLP-2 has been shown to attenuate intestinal injury in a number of preclinical models of acute disease, including necrotizing pancreatitis119, burn injury120, and ischemia-reperfusion injury121. In addition, it has been shown to be beneficial in inflammatory bowel disease114,116,122. Mice treated with h[Gly2]GLP-2 have preserved mucosal integrity with an increase in intestinal mass as a result of increased proliferation in DSS-induced colitis122. Additionally, in a murine model of indomethacin-induced enteritis, h[Gly2]GLP-2 not only stimulated proliferation but also reduced intestinal epithelial apoptosis116. Treatment was also associated with decreased mucosal cytokine expression, decreased myeloperoxidase activity, and a marked diminution in bacterial translocation116. The trophic and anti-apoptotic activities of GLP-2 have also been demonstrated in rodents and pigs following withdrawal of enteral nutrition where GLP-2 infusion prevents the development of mucosal atrophy, reduces proteolysis, and decreases crypt cell apoptosis in the small intestine123,124.
In contrast to the significant amount of evidence supporting GLP-2’s usage in preclinical models of gut injury, very limited information is available about its safety and efficacy in humans. In a small pilot study, patients with intestinal failure secondary to short bowel syndrome treated with GLP-2 had improved nutrient absorption, increased body weight, and delayed gastric emptying125. Further clinical evaluation of GLP-2 in humans is needed to determine if GLP-2 is effective in reducing intestinal injury or enhancing gut repair in critically ill patients.
Intestinal trefoil factor
The trefoil factor family (TFF) is a group of small protease-resistant peptides that are expressed in mucus-secreting epithelial cells, especially in the gastrointestinal tract. To date, three mammalian TFF members have been identified: TFF1, expressed by surface and pit mucus cells in the stomach; TFF2, expressed by mucus neck and glandular mucus cells of the stomach and Brunner’s glands of the proximal duodenum; and TFF3 (also called intestinal trefoil factor, ITF), expressed by goblet cells of the intestine and colon126.
The trefoil factors have been shown to play an important role in the protection and repair of the gastrointestinal mucosa. Oral administration of TFF2 protects against ethanol-, indomethacin-, and aspirin-induced gastric injury in rats127,128 and accelerates healing and reduces inflammation in a rat model of inflammatory bowel disease129. ITF also promotes epithelial cell migration and inhibits intestinal epithelial apoptosis130,131. Mice deficient in ITF are extremely sensitive to mucosal injury and fail to undergo any epithelial repair132. Increased ITF expression has been observed in proximity to sites of injury in the gastrointestinal tract, including peptic ulcers and active inflammatory bowel disease. Oral and subcutaneous administration of ITF has also been shown to protect the intestinal epithelium from a variety of insults including ethanol, nonsteroidal anti-inflammatory drugs, and restraint stress. In addition, administration of ITF ameliorates the severity of intestinal injury in a rat model of NEC133. Further, ITF has been shown to be effective in both prevention of and healing from acute DSS-induced colitis134. ITF also plays a role in protection against and recovery from intestinal mucositis induced by radiation and chemotherapy135. Finally, oral administration of either TFF2 or ITF has been shown to significantly reduce mucosal lesions following severe thermal injury136,137. These studies show the trefoil factors are important regulators of intestinal epithelial repair in preclinical studies but these have not been translated into clinical findings at the bedside.
Synergism between growth factors
There is some evidence that growth factors may act synergistically to prevent gut injury. When given in isolation, both EGF and growth hormone releasing peptide (GHRP)-6 have beneficial effects in animal models of intestinal injury and repair. However, this effect is additive when EGF and GHRP-6 are given together in an ischemia-reperfusion injury model138. In addition, combining glutamine with either GH, IGF-1, or EGF has been demonstrated to have additive or synergistic effects on intestinal growth and adaptation139–142. Whether a combination of growth factors listed above will be more effective than a single growth factor in isolation in critical illness has yet to be determined.
Conclusions
In critical illness, the gut functions as the “motor” of the systemic inflammatory response, and maintaining gut barrier function may be a key toward preventing multiple organ dysfunction syndrome. Growth factors have been shown to play a central role in protecting the gut against injury under both basal conditions and in chronic disease, and increasing evidence suggests they may play a role in acute critical illness as well. Although many of the agents described above have potential therapeutic benefits, EGF is the best studied and may be the most attractive candidate for clinical trials. A synergistic approach combining growth factors may also have significant utility. A more complete understanding of the mechanisms through which growth factors protect the gut is needed, as are strategies for translating preclinical findings to the bedside.
Acknowledgments
This work was supported by from the National Institutes of Health (GM66202, GM072808, F32 GM082008) and the Shock Society Research Fellowship for Early Career Investigator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1–10. doi: 10.1097/00024382-200115010-00001. [DOI] [PubMed] [Google Scholar]
- 2.Alverdy JC, Laughlin RS, Wu L. Influence of the critically ill state on host-pathogen interactions within the intestine: gut-derived sepsis redefined. Crit Care Med. 2003;31:598–607. doi: 10.1097/01.CCM.0000045576.55937.67. [DOI] [PubMed] [Google Scholar]
- 3.Fink MP. Intestinal epithelial hyperpermeability: update on the pathogenesis of gut mucosal barrier dysfunction in critical illness. Curr Opin Crit Care. 2003;9:143–151. doi: 10.1097/00075198-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Han X, Fink MP, Yang R, Delude RL. Increased iNOS activity is essential for intestinal epithelial tight junction dysfunction in endotoxemic mice. Shock. 2004;21:261–270. doi: 10.1097/01.shk.0000112346.38599.10. [DOI] [PubMed] [Google Scholar]
- 5.Fink MP, Delude RL. Epithelial Barrier Dysfunction: A Unifying Theme to Explain the Pathogenesis of Multiple Organ Dysfunction at the Cellular Level. Crit Care Clin. 2005;21:177–196. doi: 10.1016/j.ccc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Deitch EA, Xu D, Kaise VL. Role of the gut in the development of injury- and shock induced SIRS and MODS: the gut-lymph hypothesis, a review. Front Biosci. 2006;11:520–528. doi: 10.2741/1816. [DOI] [PubMed] [Google Scholar]
- 7.Coopersmith CM, Stromberg PE, Dunne WM, et al. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA. 2002;287:1716–1721. doi: 10.1001/jama.287.13.1716. [DOI] [PubMed] [Google Scholar]
- 8.Coopersmith CM, Chang KC, Swanson PE, et al. Overexpression of Bcl-2 in the intestinal epithelium improves survival in septic mice. Crit Care Med. 2002;30:195–201. doi: 10.1097/00003246-200201000-00028. [DOI] [PubMed] [Google Scholar]
- 9.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 10.Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107 (Pt 12):3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 11.Utech M, Bruwer M, Nusrat A. Tight junctions and cell-cell interactions. Methods Mol Biol. 2006;341:185–195. doi: 10.1385/1-59745-113-4:185. [DOI] [PubMed] [Google Scholar]
- 12.Han X, Fink MP, Yang R, Delude RL. Increased iNOS activity is essential for intestinal epithelial tight junction dysfunction in endotoxemic mice. Shock. 2004;21:261–270. doi: 10.1097/01.shk.0000112346.38599.10. [DOI] [PubMed] [Google Scholar]
- 13.Johnson LG. Applications of imaging techniques to studies of epithelial tight junctions. Adv Drug Deliv Rev. 2005;57:111–121. doi: 10.1016/j.addr.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Mammen JMVM, Matthews JBM. Mucosal repair in the gastrointestinal tract. [Miscellaneous] Critical Care Medicine Healing Responses in Critical Illness. 2003;31:S532–S537. doi: 10.1097/01.CCM.0000081429.89277.AF. [DOI] [PubMed] [Google Scholar]
- 15.Nusrat A, Delp C, Madara JL. Intestinal epithelial restitution. Characterization of a cell culture model and mapping of cytoskeletal elements in migrating cells. J Clin Invest. 1992;89:1501–1511. doi: 10.1172/JCI115741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore R, Carlson S, Madara JL. Rapid barrier restitution in an in vitro model of intestinal epithelial injury. Lab Invest. 1989;60:237–244. [PubMed] [Google Scholar]
- 17.Dignass AU. Mechanisms and modulation of intestinal epithelial repair. Inflamm Bowel Dis. 2001;7:68–77. doi: 10.1097/00054725-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Grenier A, Chollet-Martin S, Crestani B, et al. Presence of a mobilizable intracellular pool of hepatocyte growth factor in human polymorphonuclear neutrophils. Blood. 2002;99:2997–3004. doi: 10.1182/blood.v99.8.2997. [DOI] [PubMed] [Google Scholar]
- 19.Oka Y, Orth DN. Human plasma epidermal growth factor/beta-urogastrone is associated with blood platelets. J Clin Invest. 1983;72:249–259. doi: 10.1172/JCI110964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karey KP, Marquardt H, Sirbasku DA. Human platelet-derived mitogens. I. Identification of insulinlike growth factors I and II by purification and N alpha amino acid sequence analysis. Blood. 1989;74:1084–1092. [PubMed] [Google Scholar]
- 21.Nakamura T, Nawa K, Ichihara A, Kaise N, Nishino T. Purification and subunit structure of hepatocyte growth factor from rat platelets. FEBS Lett. 1987;224:311–316. doi: 10.1016/0014-5793(87)80475-1. [DOI] [PubMed] [Google Scholar]
- 22.Playford RJ, Wright NA. Why is epidermal growth factor present in the gut lumen? Gut. 1996;38:303–305. doi: 10.1136/gut.38.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones MK, Tomikawa M, Mohajer B, Tarnawski AS. Gastrointestinal mucosal regeneration: role of growth factors. Front Biosci. 1999;4:D303–D309. doi: 10.2741/a428. [DOI] [PubMed] [Google Scholar]
- 24.Abud HE, Watson N, Heath JK. Growth of intestinal epithelium in organ culture is dependent on EGF signalling. Exp Cell Res. 2005;303:252–262. doi: 10.1016/j.yexcr.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Miettinen PJ, Berger JE, Meneses J, et al. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995;376:337–341. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- 26.Tepperman BL, Soper BD. Effect of epidermal growth factor, transforming growth factor alpha and nerve growth factor on gastric mucosal integrity and microcirculation in the rat. Regul Pept. 1994;50:13–21. doi: 10.1016/0167-0115(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 27.Bernal NP, Stehr W, Coyle R, Erwin CR, Warner BW. Epidermal growth factor receptor signaling regulates Bax and Bcl-w expression and apoptotic responses during intestinal adaptation in mice. Gastroenterology. 2006;130:412–423. doi: 10.1053/j.gastro.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Clark JA, Lane RH, Maclennan NK, et al. Epidermal growth factor reduces intestinal apoptosis in an experimental model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G755–G762. doi: 10.1152/ajpgi.00172.2004. [DOI] [PubMed] [Google Scholar]
- 29.Halpern MD, Dominguez JA, Dvorakova K, et al. Ileal cytokine dysregulation in experimental necrotizing enterocolitis is reduced by epidermal growth factor. J Pediatr Gastroenterol Nutr. 2003;36:126–133. doi: 10.1097/00005176-200301000-00024. [DOI] [PubMed] [Google Scholar]
- 30.Clark JA, Doelle SM, Halpern MD, et al. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol. 2006;291:G938–G949. doi: 10.1152/ajpgi.00090.2006. [DOI] [PubMed] [Google Scholar]
- 31.Clark JA, Gan H, Samocha AJ, Fox AC, Buchman TG, Coopersmith CM. Enterocyte-specific epidermal growth factor prevents barrier dysfunction and improves mortality in murine peritonitis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G471–G479. doi: 10.1152/ajpgi.00012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark JA, Clark AT, Hotchkiss RS, Buchman TG, Coopersmith CM. Epidermal growth factor treatment decreases mortality and is associated with improved gut integrity in sepsis. Shock. 2008;30:36–42. doi: 10.1097/shk.0b013e31815D0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berlanga J, Prats P, Remirez D, et al. Prophylactic use of epidermal growth factor reduces ischemia/reperfusion intestinal damage. Am J Pathol. 2002;161:373–379. doi: 10.1016/S0002-9440(10)64192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishikawa T, Tarnawski A, Sarfeh IJ, Stachura J. Epidermal growth factor protects gastric mucosa against ischemia-reperfusion injury. J Clin Gastroenterol. 1993;17 (Suppl 1):S104–S110. doi: 10.1097/00004836-199312001-00019. [DOI] [PubMed] [Google Scholar]
- 35.Berlanga J, Lodos J, Lopez-Saura P. Attenuation of internal organ damages by exogenously administered epidermal growth factor (EGF) in burned rodents. Burns. 2002;28:435–442. doi: 10.1016/s0305-4179(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 36.Dvorak B, Halpern MD, Holubec H, et al. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol. 2002;282:G156–G164. doi: 10.1152/ajpgi.00196.2001. [DOI] [PubMed] [Google Scholar]
- 37.Clark JA, Doelle SM, Halpern MD, et al. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol. 2006;291:G938–G949. doi: 10.1152/ajpgi.00090.2006. [DOI] [PubMed] [Google Scholar]
- 38.Erwin CR, Helmrath MA, Shin CE, Falcone RA, Jr, Stern LE, Warner BW. Intestinal overexpression of EGF in transgenic mice enhances adaptation after small bowel resection. Am J Physiol. 1999;277:G533–G540. doi: 10.1152/ajpgi.1999.277.3.G533. [DOI] [PubMed] [Google Scholar]
- 39.Chaet MS, Arya G, Ziegler MM, Warner BW. Epidermal growth factor enhances intestinal adaptation after massive small bowel resection. J Pediatr Surg. 1994;29:1035–1038. doi: 10.1016/0022-3468(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 40.Helmrath MA, Erwin CR, Warner BW. A defective EGF-receptor in waved-2 mice attenuates intestinal adaptation. J Surg Res. 1997;69:76–80. doi: 10.1006/jsre.1997.5033. [DOI] [PubMed] [Google Scholar]
- 41.Tarnawski A, Stachura J, Durbin T, Sarfeh IJ, Gergely H. Increased expression of epidermal growth factor receptor during gastric ulcer healing in rats. Gastroenterology. 1992;102:695–698. doi: 10.1016/0016-5085(92)90123-g. [DOI] [PubMed] [Google Scholar]
- 42.Itoh M, Matsuo Y. Gastric ulcer treatment with intravenous human epidermal growth factor: a double-blind controlled clinical study. J Gastroenterol Hepatol. 1994;9 (Suppl 1):S78–S83. doi: 10.1111/j.1440-1746.1994.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 43.Clinical trials. Other. 10-19-0009. 10-13-0009. Ref Type: Electronic Citation
- 44.Sinha A, Nightingale J, West KP, Berlanga-Acosta J, Playford RJ. Epidermal growth factor enemas with oral mesalamine for mild-to-moderate left-sided ulcerative colitis or proctitis. N Engl J Med. 2003;349:350–357. doi: 10.1056/NEJMoa013136. [DOI] [PubMed] [Google Scholar]
- 45.Sullivan PB, Lewindon PJ, Cheng C, et al. Intestinal mucosa remodeling by recombinant human epidermal growth factor(1–48) in neonates with severe necrotizing enterocolitis. J Pediatr Surg. 2007;42:462–469. doi: 10.1016/j.jpedsurg.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 46.Teng CT, Hinds CJ. Treatment with GH and IGF-1 in critical illness. Crit Care Clin. 2006;22:29–40. vi. doi: 10.1016/j.ccc.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Lang CH, Frost RA. Role of growth hormone, insulin-like growth factor-I, and insulin-like growth factor binding proteins in the catabolic response to injury and infection. [Miscellaneous Article] Current Opinion in Clinical Nutrition & Metabolic Care. 2002;5:271–279. doi: 10.1097/00075197-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Lal SO, Wolf SE, Herndon DN. Growth hormone, burns and tissue healing. Growth Horm IGF Res. 2000;10 (Suppl B):S39–S43. doi: 10.1016/s1096-6374(00)80008-8. [DOI] [PubMed] [Google Scholar]
- 49.Briard N, Dadoun F, Pommier G, et al. IGF-I/IGFBPs system response to endotoxin challenge in sheep. J Endocrinol. 2000;164:361–369. doi: 10.1677/joe.0.1640361. [DOI] [PubMed] [Google Scholar]
- 50.Dahn MS, Lange MP. Systemic and splanchnic metabolic response to exogenous human growth hormone. Surgery. 1998;123:528–538. doi: 10.1067/msy.1998.86924. [DOI] [PubMed] [Google Scholar]
- 51.ehaye-Zervas MC, Mertani H, Martini JF, Nihoul-Fekete C, Morel G, Postel-Vinay MC. Expression of the growth hormone receptor gene in human digestive tissue. J Clin Endocrinol Metab. 1994;78:1473–1480. doi: 10.1210/jcem.78.6.8200952. [DOI] [PubMed] [Google Scholar]
- 52.Ulshen MH, Dowling RH, Fuller CR, Zimmermann EM, Lund PK. Enhanced growth of small bowel in transgenic mice overexpressing bovine growth hormone. Gastroenterology. 1993;104:973–980. doi: 10.1016/0016-5085(93)90263-c. [DOI] [PubMed] [Google Scholar]
- 53.Williams KL, Fuller CR, Dieleman LA, et al. Enhanced survival and mucosal repair after dextran sodium sulfate-induced colitis in transgenic mice that overexpress growth hormone. Gastroenterology. 2001;120:925–937. doi: 10.1053/gast.2001.22470. [DOI] [PubMed] [Google Scholar]
- 54.Gu Y, Wu ZH, Xie JX, Jin DY, Zhuo HC. Effects of growth hormone (rhGH) and glutamine supplemented parenteral nutrition on intestinal adaptation in short bowel rats. Clin Nutr. 2001;20:159–166. doi: 10.1054/clnu.2000.0379. [DOI] [PubMed] [Google Scholar]
- 55.Waitzberg DL, Cukier C, Mucerino DR, Logulo AF, Torrinhas RS, de CI. Small bowel adaptation with growth hormone and glutamine after massive resection of rat’s small bowel. Nutr Hosp. 1999;14:81–90. [PubMed] [Google Scholar]
- 56.Peterson CA, Gillingham MB, Mohapatra NK, et al. Enterotrophic effect of insulin-like growth factor-I but not growth hormone and localized expression of insulin-like growth factor-I, insulin-like growth factor binding protein-3 and -5 mRNAs in jejunum of parenterally fed rats. JPEN J Parenter Enteral Nutr. 2000;24:288–295. doi: 10.1177/0148607100024005288. [DOI] [PubMed] [Google Scholar]
- 57.Peterson CA, Carey HV, Hinton PL, Lo HC, Ney DM. GH elevates serum IGF-I levels but does not alter mucosal atrophy in parenterally fed rats. Am J Physiol. 1997;272:G1100–G1108. doi: 10.1152/ajpgi.1997.272.5.G1100. [DOI] [PubMed] [Google Scholar]
- 58.Jeschke MG, Herndon DN, Finnerty CC, et al. The effect of growth hormone on gut mucosal homeostasis and cellular mediators after severe trauma. J Surg Res. 2005;127:183–189. doi: 10.1016/j.jss.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 59.Frost RA, Lang CH. Growth factors in critical illness: regulation and therapeutic aspects. [Miscellaneous Article] Current Opinion in Clinical Nutrition & Metabolic Care. 1998;1:195–204. doi: 10.1097/00075197-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 60.Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- 61.Entingh-Pearsall A, Kahn CR. Differential roles of the insulin and insulin-like growth factor-I (IGF-I) receptors in response to insulin and IGF-I. J Biol Chem. 2004;279:38016–38024. doi: 10.1074/jbc.M313201200. [DOI] [PubMed] [Google Scholar]
- 62.Ohneda K, Ulshen MH, Fuller CR, D’Ercole AJ, Lund PK. Enhanced growth of small bowel in transgenic mice expressing human insulin-like growth factor I. Gastroenterology. 1997;112:444–454. doi: 10.1053/gast.1997.v112.pm9024298. [DOI] [PubMed] [Google Scholar]
- 63.Zhang W, Frankel WL, Adamson WT, et al. Insulin-like growth factor-I improves mucosal structure and function in transplanted rat small intestine. Transplantation. 1995;59:755–761. doi: 10.1097/00007890-199503150-00020. [DOI] [PubMed] [Google Scholar]
- 64.Huang KF, Chung DH, Herndon DN. Insulinlike growth factor 1 (IGF-1) reduces gut atrophy and bacterial translocation after severe burn injury. Arch Surg. 1993;128:47–53. doi: 10.1001/archsurg.1993.01420130051009. [DOI] [PubMed] [Google Scholar]
- 65.Fukushima R, Saito H, Inoue T, et al. Prophylactic treatment with growth hormone and insulin-like growth factor I improve systemic bacterial clearance and survival in a murine model of burn-induced gut-derived sepsis. Burns. 1999;25:425–430. doi: 10.1016/s0305-4179(98)00188-0. [DOI] [PubMed] [Google Scholar]
- 66.Ozen S, Akisu M, Baka M, et al. Insulin-like growth factor attenuates apoptosis and mucosal damage in hypoxia/reoxygenation-induced intestinal injury. Biol Neonate. 2005;87:91–96. doi: 10.1159/000081897. [DOI] [PubMed] [Google Scholar]
- 67.Baregamian N, Song J, Jeschke MG, Evers BM, Chung DH. IGF-1 protects intestinal epithelial cells from oxidative stress-induced apoptosis. J Surg Res. 2006;136:31–37. doi: 10.1016/j.jss.2006.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takala J, Ruokonen E, Webster NR, et al. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999;341:785–792. doi: 10.1056/NEJM199909093411102. [DOI] [PubMed] [Google Scholar]
- 69.Taylor BE, Buchman TG. Is there a role for growth hormone therapy in refractory critical illness? Curr Opin Crit Care. 2008;14:438–444. doi: 10.1097/MCC.0b013e328306a965. [DOI] [PubMed] [Google Scholar]
- 70.Branski LK, Herndon DN, Barrow RE, et al. Randomized Controlled Trial to Determine the Efficacy of Long-Term Growth Hormone Treatment in Severely Burned Children. Ann Surg. 2009 doi: 10.1097/SLA.0b013e3181b8f9ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jabri N, Schalch DS, Schwartz SL, et al. Adverse effects of recombinant human insulin-like growth factor I in obese insulin-resistant type II diabetic patients. Diabetes. 1994;43:369–374. doi: 10.2337/diab.43.3.369. [DOI] [PubMed] [Google Scholar]
- 72.Bondy CA, Underwood LE, Clemmons DR, Guler HP, Bach MA, Skarulis M. Clinical uses of insulin-like growth factor I. Ann Intern Med. 1994;120:593–601. doi: 10.7326/0003-4819-120-7-199404010-00011. [DOI] [PubMed] [Google Scholar]
- 73.DebRoy MA, Wolf SE, Zhang XJ, et al. Anabolic effects of insulin-like growth factor in combination with insulin-like growth factor binding protein-3 in severely burned adults. J Trauma. 1999;47:904–910. doi: 10.1097/00005373-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 74.Herndon DN, Ramzy PI, DebRoy MA, et al. Muscle protein catabolism after severe burn: effects of IGF-1/IGFBP-3 treatment. Ann Surg. 1999;229:713–720. doi: 10.1097/00000658-199905000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jeschke MG, Barrow RE, Suzuki F, Rai J, Benjamin D, Herndon DN. IGF-I/IGFBP-3 equilibrates ratios of pro- to anti-inflammatory cytokines, which are predictors for organ function in severely burned pediatric patients. Mol Med. 2002;8:238–246. [PMC free article] [PubMed] [Google Scholar]
- 76.Jeschke MG, Barrow RE, Herndon DN. Insulinlike growth factor I plus insulinlike growth factor binding protein 3 attenuates the proinflammatory acute phase response in severely burned children. Ann Surg. 2000;231:246–252. doi: 10.1097/00000658-200002000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jeschke MG, Bolder U, Chung DH, et al. Gut mucosal homeostasis and cellular mediators after severe thermal trauma and the effect of insulin-like growth factor-I in combination with insulin-like growth factor binding protein-3. Endocrinology. 2007;148:354–362. doi: 10.1210/en.2006-0883. [DOI] [PubMed] [Google Scholar]
- 78.Finch PW, Rubin JS, Miki T, Ron D, Aaronson SA. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989;245:752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- 79.Brauchle M, Madlener M, Wagner AD, et al. Keratinocyte growth factor is highly overexpressed in inflammatory bowel disease. Am J Pathol. 1996;149:521–529. [PMC free article] [PubMed] [Google Scholar]
- 80.Housley RM, Morris CF, Boyle W, et al. Keratinocyte growth factor induces proliferation of hepatocytes and epithelial cells throughout the rat gastrointestinal tract. J Clin Invest. 1994;94:1764–1777. doi: 10.1172/JCI117524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zeeh JM, Procaccino F, Hoffmann P, et al. Keratinocyte growth factor ameliorates mucosal injury in an experimental model of colitis in rats. Gastroenterology. 1996;110:1077–1083. doi: 10.1053/gast.1996.v110.pm8612996. [DOI] [PubMed] [Google Scholar]
- 82.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci U S A. 2002;99:14338–14343. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wildhaber BE, Yang H, Teitelbaum DH. Keratinocyte growth factor decreases total parenteral nutrition-induced apoptosis in mouse intestinal epithelium via Bcl-2. J Pediatr Surg. 2003;38:92–96. doi: 10.1053/jpsu.2003.50018. [DOI] [PubMed] [Google Scholar]
- 84.Finch PW, Rubin JS. Keratinocyte growth factor/fibroblast growth factor 7, a homeostatic factor with therapeutic potential for epithelial protection and repair. Adv Cancer Res. 2004;91:69–136. doi: 10.1016/S0065-230X(04)91003-2. [DOI] [PubMed] [Google Scholar]
- 85.Khan WB, Shui C, Ning S, Knox SJ. Enhancement of murine intestinal stem cell survival after irradiation by keratinocyte growth factor. Radiat Res. 1997;148:248–253. [PubMed] [Google Scholar]
- 86.Farrell CL, Bready JV, Rex KL, et al. Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer Res. 1998;58:933–939. [PubMed] [Google Scholar]
- 87.Sandborn WJ, Sands BE, Wolf DC, et al. Repifermin (keratinocyte growth factor-2) for the treatment of active ulcerative colitis: a randomized, double-blind, placebo-controlled, dose-escalation trial. Aliment Pharmacol Ther. 2003;17:1355–1364. doi: 10.1046/j.1365-2036.2003.01589.x. [DOI] [PubMed] [Google Scholar]
- 88.Kanayama M, Takahara T, Yata Y, et al. Hepatocyte growth factor promotes colonic epithelial regeneration via Akt signaling. Am J Physiol Gastrointest Liver Physiol. 2007;293:G230–G239. doi: 10.1152/ajpgi.00068.2007. [DOI] [PubMed] [Google Scholar]
- 89.Ido A, Numata M, Kodama M, Tsubouchi H. Mucosal repair and growth factors: recombinant human hepatocyte growth factor as an innovative therapy for inflammatory bowel disease. J Gastroenterol. 2005;40:925–931. doi: 10.1007/s00535-005-1705-x. [DOI] [PubMed] [Google Scholar]
- 90.Nishimura S, Takahashi M, Ota S, Hirano M, Hiraishi H. Hepatocyte growth factor accelerates restitution of intestinal epithelial cells. J Gastroenterol. 1998;33:172–178. doi: 10.1007/s005350050066. [DOI] [PubMed] [Google Scholar]
- 91.Schwartz MZ, Kato Y, Yu D, Lukish JR. Growth-factor enhancement of compromised gut function following massive small-bowel resection. Pediatr Surg Int. 2000;16:174–175. doi: 10.1007/s003830050716. [DOI] [PubMed] [Google Scholar]
- 92.Jeschke MG, Bolder U, Finnerty CC, et al. The effect of hepatocyte growth factor on gut mucosal apoptosis and proliferation, and cellular mediators after severe trauma. Surgery. 2005;138:482–489. doi: 10.1016/j.surg.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 93.Kuenzler KA, Pearson PY, Schwartz MZ. Hepatocyte growth factor pretreatment reduces apoptosis and mucosal damage after intestinal ischemia-reperfusion. J Pediatr Surg. 2002;37:1093–1097. doi: 10.1053/jpsu.2002.33884. [DOI] [PubMed] [Google Scholar]
- 94.Tahara Y, Ido A, Yamamoto S, et al. Hepatocyte growth factor facilitates colonic mucosal repair in experimental ulcerative colitis in rats. J Pharmacol Exp Ther. 2003;307:146–151. doi: 10.1124/jpet.103.054106. [DOI] [PubMed] [Google Scholar]
- 95.Numata M, Ido A, Moriuchi A, et al. Hepatocyte growth factor facilitates the repair of large colonic ulcers in 2,4,6-trinitrobenzene sulfonic acid-induced colitis in rats. Inflamm Bowel Dis. 2005;11:551–558. doi: 10.1097/01.mib.0000164192.71381.5c. [DOI] [PubMed] [Google Scholar]
- 96.Ohda Y, Hori K, Tomita T, et al. Effects of hepatocyte growth factor on rat inflammatory bowel disease models. Dig Dis Sci. 2005;50:914–921. doi: 10.1007/s10620-005-2664-z. [DOI] [PubMed] [Google Scholar]
- 97.Mukoyama T, Kanbe T, Murai R, et al. Therapeutic effect of adenoviral-mediated hepatocyte growth factor gene administration on TNBS-induced colitis in mice. Biochem Biophys Res Commun. 2005;329:1217–1224. doi: 10.1016/j.bbrc.2005.01.166. [DOI] [PubMed] [Google Scholar]
- 98.Kanbe T, Murai R, Mukoyama T, et al. Naked gene therapy of hepatocyte growth factor for dextran sulfate sodium-induced colitis in mice. Biochem Biophys Res Commun. 2006;345:1517–1525. doi: 10.1016/j.bbrc.2006.05.084. [DOI] [PubMed] [Google Scholar]
- 99.Hanawa T, Suzuki K, Kawauchi Y, et al. Attenuation of mouse acute colitis by naked hepatocyte growth factor gene transfer into the liver. J Gene Med. 2006;8:623–635. doi: 10.1002/jgm.880. [DOI] [PubMed] [Google Scholar]
- 100.Oh K, Iimuro Y, Takeuchi M, et al. Ameliorating effect of hepatocyte growth factor on inflammatory bowel disease in a murine model. Am J Physiol Gastrointest Liver Physiol. 2005;288:G729–G735. doi: 10.1152/ajpgi.00438.2004. [DOI] [PubMed] [Google Scholar]
- 101.Sakata H, Takayama H, Sharp R, Rubin JS, Merlino G, LaRochelle WJ. Hepatocyte growth factor/scatter factor overexpression induces growth, abnormal development, and tumor formation in transgenic mouse livers. Cell Growth Differ. 1996;7:1513–1523. [PubMed] [Google Scholar]
- 102.Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- 103.vis-Fleischer KM, Besner GE. Structure and function of heparin-binding EGF-like growth factor (HB-EGF) Front Biosci. 1998;3:d288–d299. doi: 10.2741/a241. [DOI] [PubMed] [Google Scholar]
- 104.Cribbs RK, Harding PA, Luquette MH, Besner GE. Endogenous production of heparin-binding EGF-like growth factor during murine partial-thickness burn wound healing. J Burn Care Rehabil. 2002;23:116–125. doi: 10.1097/00004630-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 105.Michalsky MP, Kuhn A, Mehta V, Besner GE. Heparin-binding EGF-like growth factor decreases apoptosis in intestinal epithelial cells in vitro. J Pediatr Surg. 2001;36:1130–1135. doi: 10.1053/jpsu.2001.25730. [DOI] [PubMed] [Google Scholar]
- 106.Pillai SB, Turman MA, Besner GE. Heparin-binding EGF-like growth factor is cytoprotective for intestinal epithelial cells exposed to hypoxia. J Pediatr Surg. 1998;33:973–978. doi: 10.1016/s0022-3468(98)90517-6. [DOI] [PubMed] [Google Scholar]
- 107.Kuhn MA, Xia G, Mehta VB, Glenn S, Michalsky MP, Besner GE. Heparin-binding EGF-like growth factor (HB-EGF) decreases oxygen free radical production in vitro and in vivo. Antioxid Redox Signal. 2002;4:639–646. doi: 10.1089/15230860260220148. [DOI] [PubMed] [Google Scholar]
- 108.Xia G, Martin AE, Michalsky MP, Besner GE. Heparin-binding EGF-like growth factor preserves crypt cell proliferation and decreases bacterial translocation after intestinal ischemia/reperfusion injury. J Pediatr Surg. 2002;37:1081–1087. doi: 10.1053/jpsu.2002.33881. [DOI] [PubMed] [Google Scholar]
- 109.Feng J, Besner GE. Heparin-binding epidermal growth factor-like growth factor promotes enterocyte migration and proliferation in neonatal rats with necrotizing enterocolitis. J Pediatr Surg. 2007;42:214–220. doi: 10.1016/j.jpedsurg.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 110.El-Assal ON, Radulescu A, Besner GE. Heparin-binding EGF-like growth factor preserves mesenteric microcirculatory blood flow and protects against intestinal injury in rats subjected to hemorrhagic shock and resuscitation. Surgery. 2007;142:234–242. doi: 10.1016/j.surg.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 111.Brubaker PL, Drucker DJ. Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145:2653–2659. doi: 10.1210/en.2004-0015. [DOI] [PubMed] [Google Scholar]
- 112.Scott RB, Kirk D, MacNaughton WK, Meddings JB. GLP-2 augments the adaptive response to massive intestinal resection in rat. Am J Physiol. 1998;275:G911–G921. doi: 10.1152/ajpgi.1998.275.5.G911. [DOI] [PubMed] [Google Scholar]
- 113.Sigalet DL, Martin GR. Hormonal therapy for short bowel syndrome. J Pediatr Surg. 2000;35:360–363. doi: 10.1016/s0022-3468(00)90041-1. [DOI] [PubMed] [Google Scholar]
- 114.Alavi K, Schwartz MZ, Palazzo JP, Prasad R. Treatment of inflammatory bowel disease in a rodent model with the intestinal growth factor glucagon-like peptide-2. J Pediatr Surg. 2000;35:847–851. doi: 10.1053/jpsu.2000.6861. [DOI] [PubMed] [Google Scholar]
- 115.L’Heureux MC, Brubaker PL. Glucagon-like peptide-2 and common therapeutics in a murine model of ulcerative colitis. J Pharmacol Exp Ther. 2003;306:347–354. doi: 10.1124/jpet.103.051771. [DOI] [PubMed] [Google Scholar]
- 116.Boushey RP, Yusta B, Drucker DJ. Glucagon-like peptide 2 decreases mortality and reduces the severity of indomethacin-induced murine enteritis. Am J Physiol. 1999;277:E937–E947. doi: 10.1152/ajpendo.1999.277.5.E937. [DOI] [PubMed] [Google Scholar]
- 117.Cameron HL, Yang PC, Perdue MH. Glucagon-like peptide-2-enhanced barrier function reduces pathophysiology in a model of food allergy. Am J Physiol Gastrointest Liver Physiol. 2003;284:G905–G912. doi: 10.1152/ajpgi.00231.2002. [DOI] [PubMed] [Google Scholar]
- 118.Benjamin MA, McKay DM, Yang PC, Cameron H, Perdue MH. Glucagon-like peptide-2 enhances intestinal epithelial barrier function of both transcellular and paracellular pathways in the mouse. Gut. 2000;47:112–119. doi: 10.1136/gut.47.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kouris GJ, Liu Q, Rossi H, et al. The effect of glucagon-like peptide 2 on intestinal permeability and bacterial translocation in acute necrotizing pancreatitis. Am J Surg. 2001;181:571–575. doi: 10.1016/s0002-9610(01)00635-3. [DOI] [PubMed] [Google Scholar]
- 120.Chance WT, Sheriff S, McCarter F, Ogle C. Glucagon-like peptide-2 stimulates gut mucosal growth and immune response in burned rats. J Burn Care Rehabil. 2001;22:136–143. doi: 10.1097/00004630-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 121.Rajeevprasad R, Alavi K, Schwartz MZ. Glucagonlike peptide-2 analogue enhances intestinal mucosal mass and absorptive function after ischemia-reperfusion injury. J Pediatr Surg. 2000;35:1537–1539. doi: 10.1053/jpsu.2000.18301. [DOI] [PubMed] [Google Scholar]
- 122.Drucker DJ, Yusta B, Boushey RP, DeForest L, Brubaker PL. Human [Gly2]GLP-2 reduces the severity of colonic injury in a murine model of experimental colitis. Am J Physiol. 1999;276:G79–G91. doi: 10.1152/ajpgi.1999.276.1.G79. [DOI] [PubMed] [Google Scholar]
- 123.Burrin DG, Stoll B, Jiang R, et al. GLP-2 stimulates intestinal growth in premature TPN-fed pigs by suppressing proteolysis and apoptosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1249–G1256. doi: 10.1152/ajpgi.2000.279.6.G1249. [DOI] [PubMed] [Google Scholar]
- 124.Burrin DG, Stoll B, Guan X, Cui L, Chang X, Hadsell D. GLP-2 rapidly activates divergent intracellular signaling pathways involved in intestinal cell survival and proliferation in neonatal piglets. Am J Physiol Endocrinol Metab. 2007;292:E281–E291. doi: 10.1152/ajpendo.00129.2006. [DOI] [PubMed] [Google Scholar]
- 125.Jeppesen PB, Hartmann B, Thulesen J, et al. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology. 2001;120:806–815. doi: 10.1053/gast.2001.22555. [DOI] [PubMed] [Google Scholar]
- 126.Thim L. Trefoil peptides: a new family of gastrointestinal molecules. Digestion. 1994;55:353–360. doi: 10.1159/000201165. [DOI] [PubMed] [Google Scholar]
- 127.Babyatsky MW, deBeaumont M, Thim L, Podolsky DK. Oral trefoil peptides protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology. 1996;110:489–497. doi: 10.1053/gast.1996.v110.pm8566596. [DOI] [PubMed] [Google Scholar]
- 128.Cook GA, Thim L, Yeomans ND, Giraud AS. Oral human spasmolytic polypeptide protects against aspirin-induced gastric injury in rats. J Gastroenterol Hepatol. 1998;13:363–370. doi: 10.1111/j.1440-1746.1998.tb00647.x. [DOI] [PubMed] [Google Scholar]
- 129.Tran CP, Cook GA, Yeomans ND, Thim L, Giraud AS. Trefoil peptide TFF2 (spasmolytic polypeptide) potently accelerates healing and reduces inflammation in a rat model of colitis. Gut. 1999;44:636–642. doi: 10.1136/gut.44.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Taupin DR, Kinoshita K, Podolsky DK. Intestinal trefoil factor confers colonic epithelial resistance to apoptosis. Proc Natl Acad Sci U S A. 2000;97:799–804. doi: 10.1073/pnas.97.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sands BE, Podolsky DK. The trefoil peptide family. Annu Rev Physiol. 1996;58:253–273. doi: 10.1146/annurev.ph.58.030196.001345. [DOI] [PubMed] [Google Scholar]
- 132.Mashimo H, Wu DC, Podolsky DK, Fishman MC. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274:262–265. doi: 10.1126/science.274.5285.262. [DOI] [PubMed] [Google Scholar]
- 133.Shi L, Zhang BH, Yu HG, Yu JP, Xi JL. Intestinal trefoil factor in treatment of neonatal necrotizing enterocolitis in the rat model. J Perinat Med. 2007;35:443–446. doi: 10.1515/JPM.2007.096. [DOI] [PubMed] [Google Scholar]
- 134.Vandenbroucke K, Hans W, Van HJ, et al. Active delivery of trefoil factors by genetically modified Lactococcus lactis prevents and heals acute colitis in mice. Gastroenterology. 2004;127:502–513. doi: 10.1053/j.gastro.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 135.Beck PL, Wong JF, Li Y, et al. Chemotherapy- and radiotherapy-induced intestinal damage is regulated by intestinal trefoil factor. Gastroenterology. 2004;126:796–808. doi: 10.1053/j.gastro.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 136.Sun YM, Wu WM, Zhang YM, Lv SM, Wang SM, Peng XM. Intestinal Trefoil Factor Produced in Escherichia coli Promotes the Healing of Rat Burn-Induced Acute Gastric Mucosal Lesions. [Article] Journal of Trauma-Injury Infection & Critical Care. 2008;65:163–169. doi: 10.1097/TA.0b013e318076b49f. [DOI] [PubMed] [Google Scholar]
- 137.Yong S, Wu W, Zhang Y, et al. Stability analysis of recombinant human TFF2 and its therapeutic effect on burn-induced gastric injury in mice. Burns. 2009;35:869–874. doi: 10.1016/j.burns.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 138.Cibrian D, Ajamieh H, Berlanga J, et al. Use of growth-hormone-releasing peptide-6 (GHRP-6) for the prevention of multiple organ failure. Clin Sci (Lond) 2006;110:563–573. doi: 10.1042/CS20050374. [DOI] [PubMed] [Google Scholar]
- 139.Jacobs DO, Evans DA, Mealy K, O’Dwyer ST, Smith RJ, Wilmore DW. Combined effects of glutamine and epidermal growth factor on the rat intestine. Surgery. 1988;104:358–364. [PubMed] [Google Scholar]
- 140.Ko TC, Beauchamp RD, Townsend CM, Jr, Thompson JC. Glutamine is essential for epidermal growth factor-stimulated intestinal cell proliferation. Surgery. 1993;114:147–153. [PubMed] [Google Scholar]
- 141.Zhang W, Bain A, Rombeau JL. Insulin-like growth factor-I (IGF-I) and glutamine improve structure and function in the small bowel allograft. J Surg Res. 1995;59:6–12. doi: 10.1006/jsre.1995.1124. [DOI] [PubMed] [Google Scholar]
- 142.Byrne TA, Morrissey TB, Nattakom TV, Ziegler TR, Wilmore DW. Growth hormone, glutamine, and a modified diet enhance nutrient absorption in patients with severe short bowel syndrome. JPEN J Parenter Enteral Nutr. 1995;19:296–302. doi: 10.1177/0148607195019004296. [DOI] [PubMed] [Google Scholar]