Abstract
Background
Cystinosis is an autosomal recessive disorder characterised by an intralysosomal accumulation of cystine, and affected individuals progress to end-stage renal failure before the age of ten. The causative gene, CTNS, was cloned in 1998 and the encoded protein, cystinosin, was predicted to be a lysosomal membrane protein.
Results
We have cloned the murine homologue of CTNS, Ctns, and the encoded amino acid sequence is 92.6% similar to cystinosin. We localised Ctns to mouse chromosome 11 in a region syntenic to human chromosome 17 containing CTNS. Ctns is widely expressed in all tissues tested with the exception of skeletal muscle, in contrast to CTNS.
Conclusions
We have isolated, characterised and localised Ctns, the murine homologue of CTNS underlying cystinosis. Furthermore, our work has brought to light the existence of a differential pattern of expression between the human and murine homologues, providing critical information for the generation of a mouse model for cystinosis.
Background
Cystinosis is an autosomal recessive disorder characterised by an intralysosomal accumulation of cystine. The underlying metabolic defect is a defective transport of cystine across the lysosomal membrane [1, 2]. Cystine is poorly soluble and forms crystals in the lysosomes as its concentration increases. Affected individuals develop a de Toni-Debré-Fanconi syndrome around six-eight months and suffer a progressive decline of glomerular filtration rate with end-stage renal failure occuring before the age of ten [3]. Other clinical signs, such as severe growth retardation, ocular anomalies, diabetes, portal hypertension, hypothyroidism as well as muscular and neurological deterioration, appear due to the accumulation of cystine in different organs [2, 4]. Two other less severe forms (juvenile and ocular nonnephropathic) have also been described and shown to be allelic by complementation studies [5]. To date, the most effective treatment of cystinosis is by the drug cysteamine which reduces intracellular levels of cystine [6, 7]. However, this treatment needs to be installed early on in the disease and at high doses, in order to be effective, and has a number of undesirable side effects.
The cystinosis gene was mapped to a 1 cM interval on the short arm of chromosome 17 between the markers D17S1798 and D17S1828 [8]. The identification of a 57 kb [9] deletion, encompassing the marker D17S829 in 30% of affected individuals, allowed us to delineate the gene interval to within the boundaries of this deletion [10]. Via a combinaton of large-scale sequencing and exon-trapping techniques, a novel gene was identified in the minimum deletion interval. The detection of various mutations within the coding region of this gene, named CTNS, validated it as responsible for all three forms of cystinosis [11, 12, 13]. CTNS is composed of 12 exons and the predicted translation start site is situated in exon 3. The 2.7 kb CTNS transcript contains a 1101 bp open reading frame (ORF) predicted to encode a protein of 367 amino acids, named cystinosin. Computer modelling using several hydrophobicity algorithms predicts that cystinosin is an integral membrane-spanning protein with seven transmembrane domains preceded by seven potential N-glycosylation sites and an uncleavable signal peptide at the amino-teminal end, and followed by a lysosomal targetting signal (GY-DQ-L) at the carboxy-terminal end [10]. Taken together, these results suggest that cystinosin is a lysosomal membrane protein. However, as yet, the exact role of cystinosin in cystine transport is unknown and no animal model for cystinosis exists. Thus, as a first step towards the creation of such a model, we report here the isolation and characterisation of the murine homologue of CTNS.
Results and Discussion
In order to identify the murine homologue of CTNS, Ctns, a E11.5 mouse embryonic cDNA library (Clontech) was screened with a probe spanning the entire ORF of the human CTNS gene. Three overlapping cDNA clones were isolated and completely sequenced. The reconstructed sequence was homologous to a region of the human CTNS cDNA from base pair 565 to 2388. RACE (rapid amplification of cDNA ends) PCR of an E13.5 adaptor ligated-cDNA library (kindly provided by D. Weil, Institut Pasteur, Paris, France) was employed to isolate the remainder of the 5' and 3' murine cDNA sequences using the Ctns cDNA primers 1.1 (5'-ACC AAG AAC CGG ATC CTG GGG CA-3') and 2.1 (5'-GCC CTC TTT CCT ACC TCC ACT TTC TGA-3'), coupled with the adaptor primer AP1, and the nested primers 1.2 (5'-GCA TTT TTC TCT CCG CGA GGC AC-3') and 2.2 (5'-GTC AAT CAG TAA GCT GCC CTG GAT G-3'), coupled with AP2, for the amplification of the 5' and 3' ends, respectively (Marathon-Ready cDNA kit, Clontech).
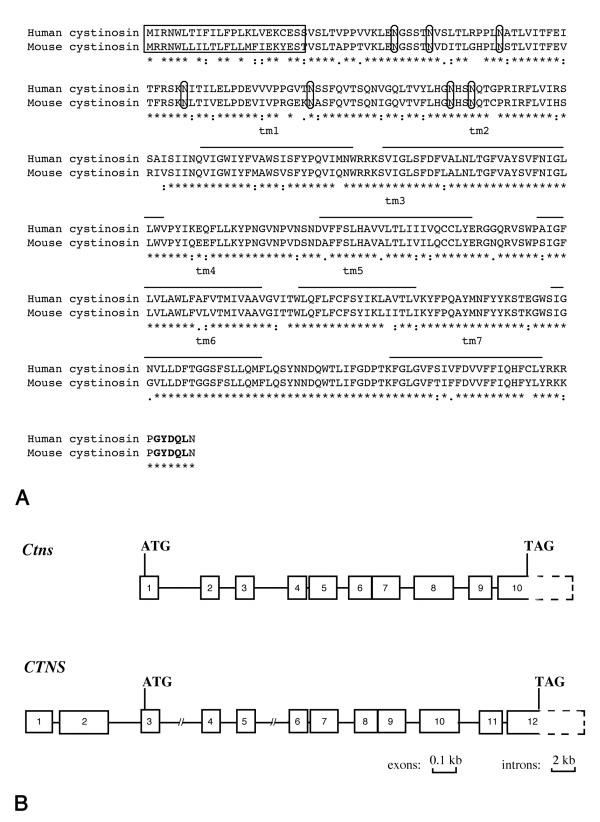
The resulting consensus Ctns cDNA sequence is 2464 bp long (GenBank accession number-AJ250670) with a 19 bp 5' non-coding region, a 1344 bp 3' non-coding region and a 1101 bp ORF which, like CTNS, is predicted to encode a 367 aa protein. The Ctns translation start site, which likewise lies within a Kozak consensus sequence (GAGACCAatgA), and the polyadenylation site are predicted to be equivalent to those deduced for CTNS. All of the 5' cDNA sequences amplified by RACE-PCR began at the same position, i.e. with a sequence homologous to the beginning of the CTNS exon 3. No sequences homologous to the CTNS untranslated exons 1 and 2 were detected. Moreover, two EST clones (GenBank accession numbers gi9165096 and gi6083297) containing 69 and 126 bp of upstream sequence, respectively, preceding the beginning of the 5' non coding region that we identified, also do not show homology with the CTNS exons 1 and 2. At the nucleotide level, Ctns shows 83% homology with CTNS for the ORF and 58.8% homology over 1257 bp for the 3' untranslated region. The predicted encoded amino acid sequence shows 83.9% identity and 92.6% similarity with cystinosin (Figure 1A). The uncleavable signal peptide, seven amino-terminal N-glycosylation sites, seven transmembrane domains and lysosomal targetting signal of cystinosin are conserved in the murine sequence.
Figure 1.
Comparison of the human and murine CTNS genes and encoded products. (A) Alignment of the human and murine cystinosin sequences. Asterisks indicate identical amino acid residues. Colons indicate residues of high similarity and fullstops residues of low similarity. The uncleavable signal peptide is boxed, the N-glycosylation sites circled, the transmembrane domains overlined and the lysosomal targetting signal is in bold. (B) Schematic representation of the gene structure of CTNS and Ctns. The exons are indicated by solid boxes, the 3' non coding regions by a dashed box and the introns by a solid line. Ctns exon sizes are identical to those reported for CTNS [9]. Intron sizes are as follows: i) Ctns intron 1-3.5 kb; 2-1.5; 3-3; 4-0.127; 5-0.8; 6-0.095; 7-1.1; 8-1.15 and 9-0.35. ii) CTNS intron 1-0.257 kb; 2-2.8; 3-8; 4-1.54; 5-6; 6-0.115; 7-1.2; 8-0.085; 9-1.2; 10-1.683 and 11-0.26.
In order to determine the exon/intron structure of Ctns, the 129/Sv Ev Tac flor DNA genomic library (Stratagene) was screened with a cDNA probe homologous to the CTNS exons 7 to 12 and four clones were isolated. Sequence analysis of their inserts demonstrated that the four overlapping clones spanned all the Ctns exons. The Ctns gene spans 14 kb is composed of ten exons and its exon/intron boundaries are identical to that of CTNS (Figure 1B). A combination of Long Range PCR (Expand Long Template PCR System, Boehringer Mannheim) and sequencing permitted an estimation of intron sizes. A comparison of the sizes of CTNS and Ctns introns showed that, with the exception of the human introns 3 and 5, intron sizes were similar between both species (Figure 1B).
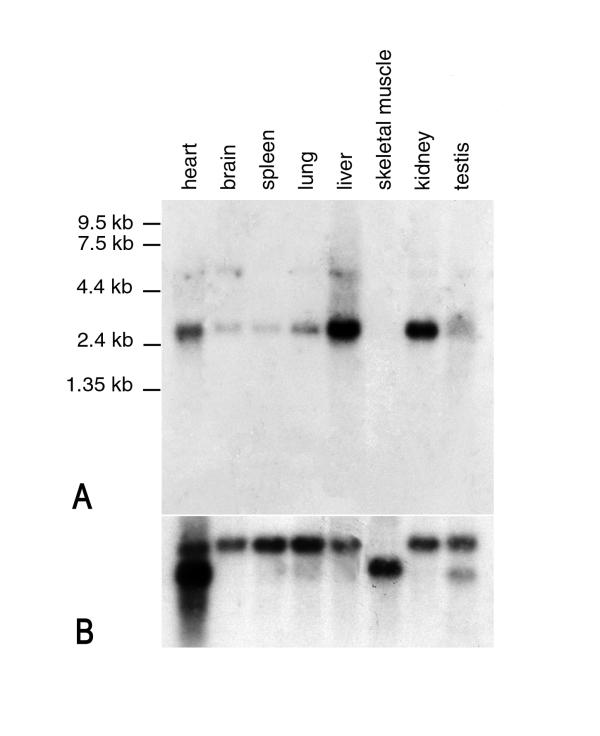
The Ctns expression pattern was determined by hybridisation of a probe spanning exons 4 and 5 to a Clontech multiple tissue northern blot (MTN 7762-1). A transcript with an expected size of 2.5 kb, highly expressed in kidney and liver, less expressed in heart, and weakly expressed in brain, spleen, lung and testis was detected (Figure 2); a proportionally faint approximately 4.4 kb transcript could also be seen, reminiscent of the additional 4.4 kb CTNS transcript detected in human pancreas [10]. In contrast to the CTNS transcript, no expression was detected in skeletal muscle (Fig. 2A) whereas hybridisation with a β-actin cDNA control probe confirms the loading of RNA for all tissues (Fig. 2B). A possible explanation for this differential pattern of expression is that the promoter situated upstream of the CTNS exon 1 has been lost during the course of evolution and the working murine promoter does not direct the same tissue expression, as has been proposed for IGF II [14]. Similarly, it is possible that sequences corresponding to the CTNS exons 1 and 2 have also been lost during evolution or, alternatively, they exist in the form of pseudo-exons in the upstream genomic sequence. However, low stringency hybridisation of the genomic clones containing theCtns gene with a probe corresponding to the CTNS exons 1 and 2, did not detect any homologous sequences thus providing support for the former hypothesis. Moreover, comparison of 1.1 kb of genomic sequence immediately upstream of the murine exon 1, which is hypothesised to contain the Ctns promoter, with the CTNS intron 2 sequence detected a region of significant homology (66.7% over 306 bp). These results suggest the possible presence of an alternative CTNS promoter. Such regions of homology between the regulatory sequences of other human and murine homologue, such as WT1 orPAX-2, have also been described [15, 16].
Figure 2.
Northern blot analysis of Ctns expression. (A) A 2.5 kb transcript, accompanied by a faint 4.4 kb transcript, can be seen in all tissues except skeletal muscle upon hybridisatin with a Ctns specific probe. (B) Following hybridisation with a β-actin cDNA control probe, a 2.0 kb transcript can be seen for brain, spleen, lung, liver and kidney, a 2.0 kb and a 1.8 kb transcript can be seen for heart and testis, and a 1.8 kb transcript can be seen for skeletal muscle.
The chromosomal localisation of the Ctns gene was determined by PCR amplification of the mouse T31 irradiation hybrid panel [17] with the primers LOC/1 (5'-TGT GGC CCA TGG ACT TGA AG-3') and LOC/2 (5'-CAA TCT GGC AGG CAC CTC A-3') situated in the 3' non-coding region. Two point lod score analysis resulted in a significant score of 5.37 and 5.36 with the chromosome 11 markers D11Mit279 and D11Mit7, respectively. A highly significant score of 12.25 and 11.09 was obtained with the ESTs AA589579 and R74628, respectively. R74628 maps distal to D11Mit7 and both of these markers are located within two contigs which lie in the region of synteny with human chromosome 17. Hence, this leads us to assume that Ctns also lies in the region of chromosome 11 syntenic with chromosome 17 which contains the human CTNS gene.
Conclusions
Although, the encoded products of CTNS, the gene underlying nephropathic cystinosis, and its murine homologue, Ctns, are highly conserved between man and mouse, they have a differential expression pattern. This information is crucial for the analysis of a mouse model for cystinosis which, in the absence of a spontaneous mouse mutant, we are currently generating in order to study the pathogenesis of cystinosis in vivo.
Acknowledgments
Acknowledgments
The chromosomal localisation of the murine Ctns gene was part of a service offered by the EEC Mouse Mapping Consortium led by Philip Avner (Institut Pasteur, Paris, France). V. Kalatzis was supported by the Institut Electricité Santé. The work presented here was supported by grants from Vaincre les Maladies Lysosomales, INSERM (programme APEX), Association Française contre les Myopathies and Association pour l'Utilisation du Rein Artificel.
Contributor Information
Stéphanie Cherqui, Email: cherqui@necker.fr.
Vasiliki Kalatzis, Email: kalatzis@necker.fr.
Lionel Forestier, Email: lionel.forestier@unilim.fr.
Isabelle Poras, Email: poras@genoscope.cns.fr.
Corinne Antignac, Email: antignac@necker.fr.
References
- Gahl WA, Bashan N, Tietze F, Bernardini I, Schulman JD. Cystine transport is defective in isolated leukocyte lysosomes from patients with cystinosis. Science. 1982;217:1263–1265. doi: 10.1126/science.7112129. [DOI] [PubMed] [Google Scholar]
- Gahl WA, Schneider JA, Aula PP. Lysosomal transport disorders: cystinosis and sialic acid storage disorders. In The metabolic and molecular basis of inherited disease Edited by Scriver CR, Beaudet AL, Sly WS, and Valle D New York: McGraw-Hill, 1995. pp. 3763–3797.
- Lemire J, Kaplan BS. The various manifestations of the nephropathic form of cystinosis. Am J Nephrol. 1984;4:81–85. doi: 10.1159/000166782. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Katz B, Melles RB. Update on nephropathic cystinosis. Pediatr Nephrol. 1990;4:645–653. doi: 10.1007/BF00858644. [DOI] [PubMed] [Google Scholar]
- Pellett OL, Smith ML, Greene AA, Schneider JA. Lack of complementation in somatic cell hybrids between fibroblasts from patients with different forms of cystinosis. Proc Natl Acad Sci USA. 1988;85:3531–3534. doi: 10.1073/pnas.85.10.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahl WA, Reed GF, Thoene JG, Schulman JD, Rizzo WB, Jonas AJ, Denman DW, Schlesseman JJ, Corden BJ, Schneider JA. Cysteamine therapy for children with nephropatic cystinosis. N Engl J Med. 1987;316:971–977. doi: 10.1056/NEJM198704163161602. [DOI] [PubMed] [Google Scholar]
- Markello TC, Bernardini IM, Gahl WA. Improved renal function in children with cystinosis treated with cysteamine. N Engl J Med. 1993;328:1157–1162. doi: 10.1056/NEJM199304223281604. [DOI] [PubMed] [Google Scholar]
- Jean G, Fuchshuber A, Town MM, Gribouval O, Schneider JA, Broyer M, van't Hoff W, Niaudet P, Antignac C. High-resolution mapping of the gene for cystinosis, using combined biochemical and linkage analysis. Am J Hum Genet. 1996;58:535–543. [PMC free article] [PubMed] [Google Scholar]
- Touchman JW, Anikster Y, Dietrich NL, Braden Maduro VV, McDowell G, Shotelersuk V, Bouffard GG, Beckstrom-Sternberg M, Gahl WA, Green ED. The Genomic Region Encompassing the Nephropathic Cystinosis Gene (CTNS): Complete Sequencing of a 200-kb Segment and Discovery of a Novel Gene within the Common Cystinosis-Causing Deletion. Genome Res. 2000;10:165–173. doi: 10.1101/gr.10.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town M, Jean G, Cherqui S, Attard M, Forestier L, Whitmore SA, Callen DF, Gribouval O, Broyer M, Bates GP, van't Hoff W, Antignac C. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nature Genet. 1998;18:319–324. doi: 10.1038/ng0498-319. [DOI] [PubMed] [Google Scholar]
- Theone J, Lemons R, Anikster Y, Mullet J, Paelicke K, Lucero C, Gahl W, Schneider J, Shu SG, Campbell HT. Mutations of CTNS Causing Intermediate Cystinosis. Mol Genet Metab. 1999;67:283–293. doi: 10.1006/mgme.1999.2876. [DOI] [PubMed] [Google Scholar]
- Attard M, Jean G, Forestier L, Cherqui S, van't Hoff W, Broyer M, Antignac C, Town M. Severity of phenotype in cystinosis varies with mutations in the CTNS gene: predicted effect on the model of cystinosin. Hum Mol Genet. 1999;8:2507–2514. doi: 10.1093/hmg/8.13.2507. [DOI] [PubMed] [Google Scholar]
- Anikster YA, Lucero C, Guo J, Huizing M, Shotelersuk V, Bernardini I, McDowell G, Iwata F, Kaiser-Kupfer MI, Jaffe R, et al. Ocular Nonnephropathic Cystinosis: Clinical, Biochemical, and Molecular Correlations. Pediatr Res. 2000;47:17–23. doi: 10.1203/00006450-200001000-00007. [DOI] [PubMed] [Google Scholar]
- Rotwein P, Hall LJ. Evolution of insulin-like growth factor II: characterization of the mouse IGF-II gene and identification of two pseudo-exons. DNA Cell Biol. 1990;9:725–735. doi: 10.1089/dna.1990.9.725. [DOI] [PubMed] [Google Scholar]
- Gessler M, Bruns GA. Sequence of the WT1 upstream region including the Wit-1 gene. Genomics. 1993;17:499–501. doi: 10.1006/geno.1993.1355. [DOI] [PubMed] [Google Scholar]
- Stayner CK, Cunliffe HE, Ward TA, Eccles MR. Cloning and characterization of the human PAX2 promoter. J Biol Chem. 1998;273:25472–25479. doi: 10.1074/jbc.273.39.25472. [DOI] [PubMed] [Google Scholar]
- McCarthy LC, Terrett J, Davis ME, Knights CJ, Smith AL, Critcher R, Schmitt K, Hudson J, Spurr NK, Goodfellow PN. A first generation whole genome-radiation hybrid map spanning the mouse genome. Genome Res. 1997;7:1153–1161. doi: 10.1101/gr.7.12.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]