Abstract
Dopaminergic neurons in the substantia nigra produce dopamine for the nigrostriatal pathway that facilitates motor function. Post mortem examinations demonstrate an age-related loss of cells in the substantia nigra, with most of the cell loss focused on the dorsal substantia nigra compared with the ventral substantia nigra. The current study used DTI to provide the first in-vivo assessment of age-related degeneration in specific segments of the substantia nigra of humans. Measures extracted from DTI of 16 young adults (19–27 years) and 15 older adults (55–71 years) showed that in the dorsal substantia nigra, fractional anisotropy was reduced and radial diffusivity was increased with age. In the ventral substantia nigra and red nucleus, there were no differences across age for the DTI measures. DTI provides a non-invasive technique that accurately reflects the established pattern of age-related cell loss in the dorsal and ventral substantia nigra, further suggesting the robust potential for using DTI to characterize degeneration in the nigrostriatal pathway in both health and disease.
Keywords: Diffusion tensor imaging, substantia nigra, aging, biomarker, human, fractional anisotropy
Introduction
It is well known that the aging process is accompanied by alterations in structure, function, neurotransmitters, and physiology of the human brain. Advancing age is also associated with reduction in fine motor performance, coordination, and movement slowing (Krampe, 2002; Serrien et al., 2000). Of particular relevance to motor function, reductions in dopamine and N-methyl-D-aspartate (NMDA) receptors in aging samples are well established (Ossowska et al., 2001; Roth and Joseph, 1994). Consistent with the decrease in dopamine and NMDA receptors, there is also an age-related loss of cells in the substantia nigra pars compacta in post mortem examinations (Hirai, 1968). In-vivo, the caudate, putamen, and globus pallidus have been studied in older adults (Pfefferbaum et al., 2008), but the substantia nigra has not been studied in detail. Beyond the theoretical importance to develop in-vivo markers of degeneration in the substantia nigra of normal aging, there is also a clinical rationale for developing in-vivo measures of the substantia nigra for the diagnosis and monitoring of treatment efficacy in individuals with Parkinson’s disease (Auer, 2009).
Although diffusion tensor imaging (DTI) has typically been used to study white matter tracts (Kraus et al., 2007; Mori and Zhang, 2006), it also holds promise for studying abnormalities in areas such as the substantia nigra and other basal ganglia nuclei (Pfefferbaum et al., 2008; Yoshikawa et al., 2004). For example, after administering 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in a murine model of Parkinson’s disease, fractional anisotropy (FA) was decreased and radial diffusivity (RD) was increased when compared to saline controls (Boska et al., 2007). In addition, the authors found that RD was significantly correlated with the number of dopaminergic neurons. The authors suggested that DTI provides an indirect measure of dopaminergic degeneration within the substantia nigra, presumably because cell loss in the substantia nigra alters the microstructural integrity and directionally dependent diffusivity of water molecules. Building on the findings from the murine model of Parkinson’s disease, our laboratory used high resolution DTI of the substantia nigra to assess alterations in FA and RD in early stage, de novo patients with Parkinson’s disease relative to age-matched control subjects (Vaillancourt et al., 2009). FA was reduced and RD was increased in the ventrolateral segment of the substantia nigra in patients with Parkinson’s disease, and the clinically relevant finding was that FA values in the ventrolateral substantia nigra revealed 100% sensitivity and specificity for distinguishing Parkinson’s disease patients from healthy subjects at the individual patient level.
Whereas cell loss determined ex-vivo in Parkinson’s disease is greater in the ventral substantia nigra compared with the dorsal substantia nigra, the cell loss observed in normal human aging is greater in the dorsal substantia nigra than the ventral substantia nigra (Fearnley and Lees, 1991). Thus, the current study tests the hypothesis that in the dorsal substantia nigra FA values will be reduced and RD values will be increased in older adults compared with young adults. In contrast, in the ventral substantia nigra we test the hypothesis that FA and RD values will either not be affected by age or will only show a small difference in older adults compared with young adults. In summary, the current study used high resolution DTI and hand drawn regions of interest to determine how aging affects segments within substantia nigra of humans.
Methods
Subjects
Thirty-two neurologically healthy subjects were recruited in a young group (N = 16; mean age = 23.6 years; range = 19 – 27 years) and older group (N = 16; mean age = 61.2 years; range = 55–71 years). The young and older subjects were recruited from advertisements in the Chicago-land area. Fifteen of sixteen older participants had no history of neuropsychiatric or neurological disease. On the day of scanning the older subjects were also evaluated using questions 20, 21, 23, 24, 27, 28, and 29 from the Unified Parkinson’s Disease Rating Scale (UPDRS) by a trained clinician who has passed the UPDRS certification provided through Rush University Medical Center. These questions assess bradykinesia, rigidity, and tremor. Fifteen of sixteen older subjects scored at a 0 on these questions indicating that these older healthy subjects did not express symptoms of Parkinsonism. One of the older subjects had a prominent bilateral hand tremor and head tremor. Upon further inquiry, this subject had previously been diagnosed with essential tremor and we confirmed this with the patient’s physician. This individual was removed from further analysis. All younger adults underwent a detailed medical history interview. None reported any history of neurologic, psychiatric, or major medical illness that might affect cerebral function or structure (e.g. meningitis, high blood pressure). All subjects gave written informed consent consistent with the Declaration of Helsinki, which was approved by the local Institutional Review Board at the University of Illinois at Chicago.
Diffusion Tensor Imaging Acquisition
Studies were acquired on a General Electric 3.0-Tesla Signa HDx scanner (General Electric Healthcare, Waukesha, WI) using a customized DTI pulse sequence to reduce eddy current induced distortion (Poonawalla and Zhou, 2001). All images were acquired using an 8-channel phased-array head coil, together with parallel imaging (acceleration factor = 2) to increase the spatial resolution. The key data acquisition parameters were TR = 4500ms, TE = 82 ms, b values = 0, 1000 s/mm2, directions = 27, FOV = 20cm × 20cm, k-space matrix = 192 × 128; image space matrix = 256×256, NEX = 4, slice thickness = 4mm, slice skip = 1 mm, slice number = 15. The top slice was placed approximately 4mm superior to the superior edge of the corpus callosum. All images were acquired axially parallel with the AC-PC plane. The diffusion gradient orientations were designed based on the electrostatic repulsion model proposed by Jones and colleagues (1999).
Diffusion Tensor Imaging Analysis
Fractional anisotropy (FA) is a measure of the degree of diffusion anisotropy and is represented by:
where λ1, λ2, and λ3 are the eigenvalues of the diffusion tensor matrix (Basser and Pierpaoli, 1996). FA was the primary dependent measure in this study. FA values close to 0 indicate isotropic diffusion. Comparatively, FA values near 1 represent highly anisotropic diffusion. Highly organized white matter tracts have high FA because water molecular diffusion is considerably more restricted in the direction perpendicular to the fiber tract than the other directions. In regions such as the substantia nigra, FA values are considerably lower than in white matter regions such as the internal capsule. In this study, FA was used as one indicator of degeneration in the substantia nigra, and was calculated using singular value decomposition from the diffusion-weighted images acquired with the aforementioned protocol. All FA calculations were carried out using DTI Studio (Wakana et al., 2004).
To help assess the factors that contribute to alterations in FA, we calculated the radial diffusivity (RD) (λR = (λ2 +λ3)/2) and longitudinal diffusivity (LD) (λL = λ1) as secondary measures where λ1, λ2, and λ3 are in descending order. Both RD and LD contribute to FA with radial diffusivity differentially reflecting average diffusivity perpendicular to axonal fibers and longitudinal diffusivity reflects diffusivity parallel to axonal fibers. We also quantified the mean diffusivity (MD) because MD provides additional information than FA (Hasan and Narayana, 2006). For each DTI dependent measure, the average value was extracted from each ROI that is described below. The average DTI value was then used in the statistical analysis. In order to assure image quality and the reliability of the diffusion measures, head motion of the DTI data set was examined using AFNI and was found to be less than 0.5mm for all subjects. We therefore did not implement a head motion correction. A standard noise threshold was applied to all images in DTI Studio. Each image was visually inspected after application of this threshold to make certain that signal loss did not occur in our regions of interest or in surrounding tissue.
Regions of Interest in the Substantia Nigra and Red Nucleus
Regions of interest (ROI) were hand drawn in the substantia nigra and red nucleus based upon the B0 images as shown in Figure 1. The red nucleus was also examined because it is anatomically close to the substantia nigra and is located on the same slice as the dorsal substantia nigra. Figure 1 shows the B0 image (which is essentially a T2-weighted image) for dorsal and ventral substantia nigra and red nucleus. The corresponding FA images are also shown. Since previous studies suggest that degeneration in the substantia nigra may occur differently in the dorsal and ventral segments (Fearnley and Lees, 1991; Martin et al., 2008), we drew separate ROIs for the dorsal and ventral substantia nigra slices. Pfefferbaum and colleagues (2009) (see Figure 2, page 495) have shown on a coronal slice that the dorsal substantia nigra can be visualized as lateral to the red nucleus, whereas the ventral substantia nigra is inferior to the red nucleus. Also, as previously suggested using a T2-weighted image (Sohmiya et al., 2001), the dorsal substantia nigra can be visualized on an axial slice as lateral to the red nucleus on the same slice as the red nucleus. The dorsal slice in our Figure 1A indicates that the red nucleus and substantia nigra can be seen bilaterally as a hypointense signal (darker on B0). We recognize that substantia nigra pars compacta is difficult to differentiate from substantia nigra pars reticulata using T2 weighted imaging in-vivo, and we therefore interpret our regions to be within the substantia nigra rather than just in substantia nigra pars compacta. Also, previous studies have indicated that the rostral part of the hypointense area of the substantia nigra on a T2 weighted image includes both the cerebral peduncle and substantia nigra (Oikawa et al., 2002). As shown in Figure 1B on the ventral slice, our most rostral region of interest was drawn caudal to where the cerebral peduncle could occur.
Figure 1.
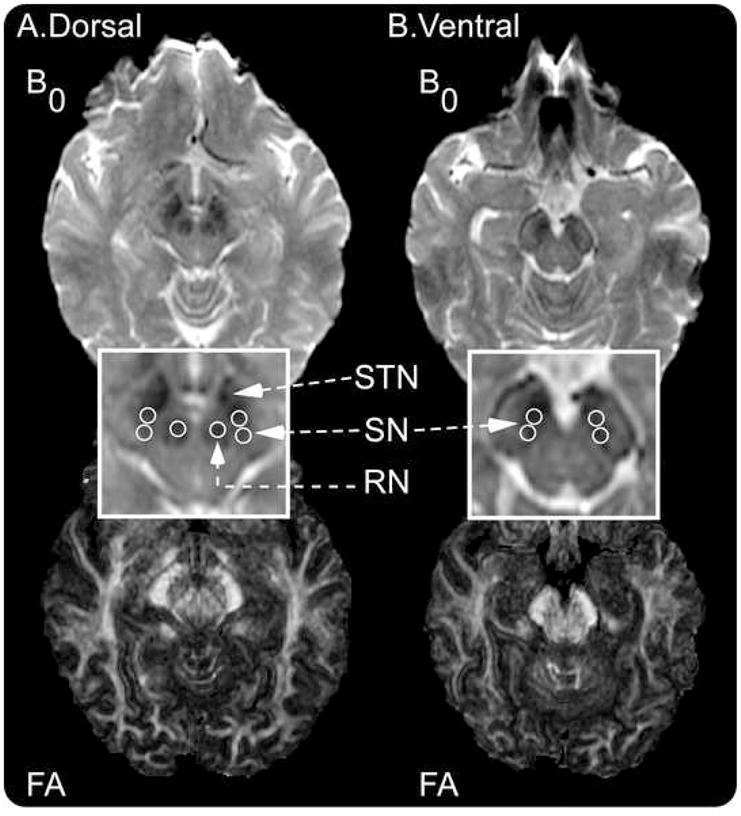
Procedure used to draw regions of interest in the dorsal and ventral substantia nigra (SN). A, the top panel shows the dorsal B0 image with the red nucleus (RN), subthalamic nucleus (STN), and substantia nigra. The bottom panel shows the FA image from the same dorsal slice. B, the top panel shows the ventral B0 image where the substantia nigra can be visualized. In this image, the RN and STN are no longer visible. The bottom panel shows the FA image from the same ventral slice. All images were generated in DTI Studio. The regions of interest were drawn on the B0 images prior to examining the FA images.
Figure 2.
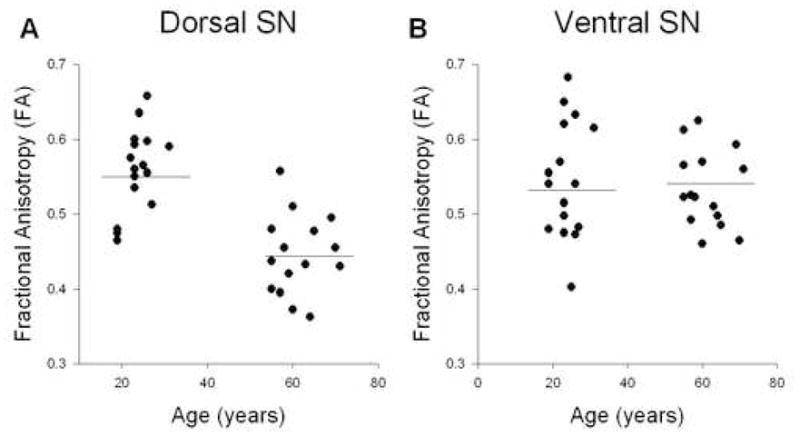
Analysis for FA and age. A, shows FA across age for the dorsal substantia nigra. B, shows FA across age for the ventral substantia nigra. There was a significant reduction in FA in the dorsal substantia nigra. The horizontal line indicates the mean for each group.
The following method was used to draw ROIs for the substantia nigra and red nucleus. Each ROI was 4 voxels in diameter, and each voxel was 0.781 mm. This provides an ROI volume equal to 30.6 mm3. First, on the B0 image we identified the slice where the red nucleus, subthalamic nucleus, and substantia nigra were prominent (Figure 1A). The ROIs for the red nucleus were drawn for the left and right sides, and each ROI was within the hypointense area of the RN (Figure 1). This slice with the red nucleus was labeled the dorsal slice. As shown in Figure 1, for the dorsal slice two substantia nigra ROIs were drawn on both the left and right substantia nigra. Since the subthalamic nucleus is anterior to the substantia nigra (Lucerna et al. 2002), the rostral ROI for the substantia nigra was drawn lateral from the red nucleus so as to capture the posterior portion of the hypointense region. The caudal ROI was drawn just posterior to the rostral ROI in the hypointense area. Second, we moved one slice inferior because much of the substantia nigra is below the red nucleus (Pfefferbaum et al., 2009). This was labeled the ventral substantia nigra. In this slice, the substantia nigra was always visible, and the red nucleus was either no longer visible or was only faintly visible (Figure 1A). This method was used to focus on the ventral substantia nigra. The first ROI was placed in the hypointense part of the middle segment of the substantia nigra while making sure to stay within the brainstem. The caudal ROI was placed lateral and posterior to the first circle such that the two circles did not overlap (Figure 1A). When placing all regions of interest in the substantia nigra, we used the FA map and color map to make sure that our regions of interest were not in the cerebral peduncle laterally. The same procedure was carried out for the right and left substantia nigra (Figure 1A).
All substantia nigra and red nucleus ROIs were drawn independently by two raters. Both raters agreed upon the common method a priori that was described above. All regions of interest were drawn blinded to the age group status.
Statistical Analysis
On each dependent measure (FA, RD, LD, MD), we conducted separate linear regression analysis for these measures in the dorsal and ventral substantia nigra and red nucleus. The predictor variable was age and the dependent measure was one of the four DTI measures. When analyzing the data in the regression analysis, the average DTI value was computed across ROIs for the left and right sides for the dorsal substantia nigra. The same procedure was performed for the ventral substantia nigra and red nucleus. Significant effects were interpreted at p < 0.05. Inter-rater reliability between Raters 1 and 2 was examined using intraclass correlation coefficients (Maclennan, 1993). All statistics were performed using SPSS 15.0.
Results
Figure 2 shows how FA changes with age across the young and older adults in the dorsal (2A) and ventral (2B) substantia nigra. Table 1 shows the complete results of the regression analysis for all dependent measures. In the dorsal substantia nigra, FA decreased with age and this was significant in the regression analysis (R2 = 0.46; p < 0.0001). In the ventral substantia nigra, Figure 2B indicates that the slope was relatively flat between FA and age, and this was supported by the non-significant regression analysis (R2 = 0.009; p = 0.59).
Table 1.
Regression analysis results
| P | R2 | Young (mean ± SD) | Older (mean ± SD) | |
|---|---|---|---|---|
| SNd | ||||
| FA | <0.001 | 0.46 | 0.55 ± 0.05 | 0.44 ± 0.05 |
| RD | <0.001 | 0.42 | 0.00052 ± 0.000062 | 0.00063 ± .000051 |
| LD | <.01 | 0.20 | 0.0014 ± 0.000096 | 0.0013 ± 0.00010 |
| MD | 0.12 | 0.07 | 0.00085 ± 0.000052 | 0.00082 ± 0.000053 |
| SNv | ||||
| FA | 0.59 | 0.009 | 0.53 ± 0.07 | 0.54 ± 0.05 |
| RD | 0.56 | 0.011 | 0.00058 ± 0.000088 | 0.00056 ± 0.000087 |
| LD | 0.79 | 0.002 | 0.00148 ± 0.00010 | 0.00148 ± 0.00014 |
| MD | 0.73 | 0.004 | 0.00088 ± 0.000093 | 0.00086 ± 0.000056 |
| RN | ||||
| FA | 0.37 | 0.027 | 0.42 ± 0.08 | 0.39 ± 0.07 |
| RD | 0.79 | 0.002 | 0.000595 ± 0.000097 | 0.000582 ± .000074 |
| LD | 0.14 | 0.072 | 0.0011 ± 0.000093 | 0.0010 ± 0.000096 |
| MD | 0.48 | 0.017 | 0.00077 ± 0.000079 | 0.00075 ± 0.000063 |
Figure 3 shows how RD changes with age across the young and older adults for the dorsal (3A) and ventral (3B) substantia nigra. RD increased with age in the dorsal substantia nigra (R2 = 0.42; p < 0.0001). However, in the ventral substantia nigra RD values remained unchanged as a function of age (R2 = 0.01; p = 0.56). Figure 4 shows how LD changes across age in the dorsal (4A) and ventral (4B) substantia nigra. The LD values in the dorsal substantia nigra decreased with age (R2 = 0.20; p < 0.01), whereas the LD values in the ventral substantia nigra did not change across age (R2 = 0.002; p = 0.79). Finally, the MD values in the dorsal substantia nigra (R2 = 0.07; p = 0.12) and ventral substantia nigra (R2 = 0.004; p = 0.73) did not change significantly as a function of age (Table 1).
Figure 3.
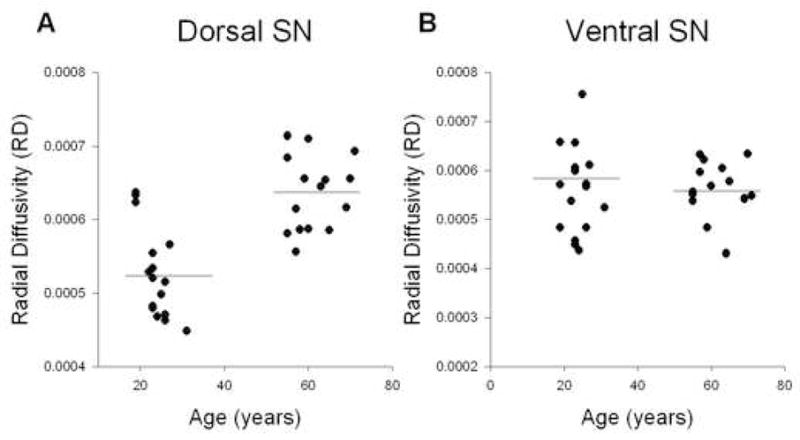
Analysis for RD and age. A, shows RD across age for the dorsal substantia nigra. B, shows RD across age for the ventral substantia nigra. There was a significant increase in RD in the dorsal substantia nigra. The horizontal line indicates the mean for each group.
Figure 4.
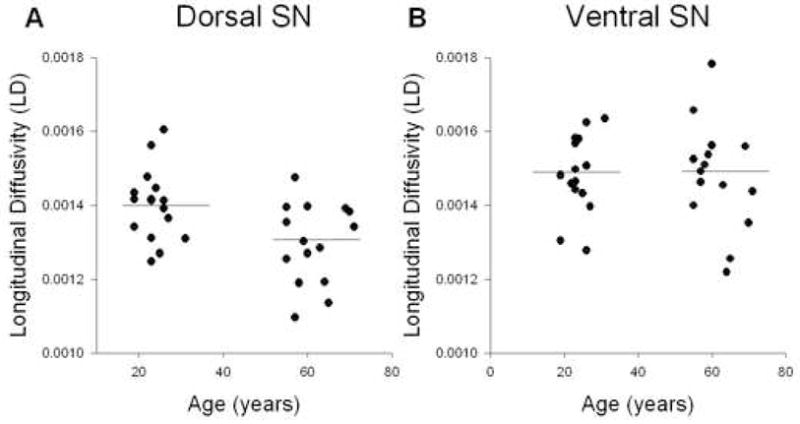
Analysis for LD and age. A, shows LD across age for the dorsal substantia nigra. B, shows LD across age for the ventral substantia nigra. There was a significant reduction in LD in the dorsal substantia nigra. The horizontal line indicates the mean for each group.
The red nucleus was also examined to determine the specificity of the findings related to the substantia nigra. Table 1 indicates that the regression analyses for FA, RD, LD, and MD were not significant for the red nucleus. This suggests that the altered DTI values in the dorsal substantia nigra are specific to this structure.
Inter-rater reliability analysis was performed on the FA values for the dorsal and ventral substantia nigra and the red nucleus. The intraclass correlation coefficient from the dorsal substantia nigra was 0.91, and the ventral substantia nigra was 0.89. The intraclass correlation coefficient from the red nucleus was 0.93. Thus, there was strong agreement between the two raters.
Discussion
The findings from the current study provide the first in-vivo evidence that the microstructural integrity of the dorsal substantia nigra is affected by age, whereas the ventral substantia nigra is not affected by age. In particular, FA was reduced and RD was increased in older adults compared with young adults in the dorsal substantia nigra. It was also found that LD was reduced in the older adults compared with young adults in the dorsal substantia nigra, but there were no significant changes in the ventral substantia nigra. MD in the ventral and dorsal substantia nigra was not affected by age. Also, the FA, RD, LD, and MD values in the red nucleus were not altered by age, consistent with previous work using T2-weighted estimates of the area of red nucleus (Sohmiya et al., 2001). These findings expand our understanding of the age-related degeneration of the substantia nigra of humans in-vivo and provide important implications for clinical neurology.
One interpretation of these data is that iron deposition might be correlated with the FA changes. However, previous work using DTI in young and older adults has shown that older adults (65–79 years) have increased FA and MD in the caudate and putamen compared with a young control group (22–37 years) (Pfefferbaum et al., 2008). This pattern of findings in nuclei of the basal ganglia was interpreted to partly represent increased iron deposition because the field-dependent relaxation rate (FDRI) also increased in the basal ganglia regions in older adults compared with young adults. In addition, the findings in deep gray matter areas were distinctly different from previous studies of white matter changes with aging, which typically find lower FA and higher diffusivity in frontal white matter areas (Sullivan and Pfefferbaum, 2006). As such, these authors have argued that increased FA corresponds with increased iron deposition. Other studies have argued that decreased FA corresponds with increased iron deposition.
For example, a previous study of early stage, untreated Parkinson’s disease used a multiple gradient echo sequence for rapid mapping of the proton transverse relaxation rate (Martin et al. 2008). The authors used hand-drawn regions of interest in the dorsal and ventral substantia nigra pars compacta and found that the ventral substantia nigra pars compacta of Parkinson’s disease patients had an increased proton transverse relaxation rate. Since the proton transverse relaxation rate was correlated with iron levels from fresh tissue in the basal ganglia (Hallgren and Sourander, 1958), Martin and colleagues (2008) concluded that the substantia nigra pars compacta has increased iron content in early stage patients with Parkinson’s disease. A previous DTI study of early stage untreated Parkinson’s disease found reduced FA in the ventral and lateral substantia nigra (Vaillancourt et al., 2009). In a commentary of the paper by Vaillancourt and colleagues (2009), Lang and Mikulis (2009) suggested that reduced FA could be caused by the susceptibility effect of water diffusing through iron-induced field gradients in the tissue. Typically, diffusion through magnetic susceptibility-induced gradients would result in an elevated signal loss, leading to a high MD (Majumdar and Gore, 1988). This argues that the iron content should be positively correlated with MD. However, the fact that no significant MD changes were observed suggests that such a correlation was not particularly strong. Instead, the effect of iron content on diffusivity may have a spatial variation due to either the spatial distribution of iron or the specific orientation of magnetic dipoles, which lead to changes in FA and RD, as observed in this study.
An alternative explanation that is not mutually exclusive from iron deposition is that cell loss in the substantia nigra alters the microstructural integrity and alters DTI measures such as FA, RD, and LD. Previously, DTI was used to assess nigrostriatal degeneration in the MPTP mouse model of Parkinson’s disease, and the study found that RD increased, MD increased, and FA reduced in the substantia nigra of 5 to 7 day MPTP-treated animals compared with saline controls (Boska et al., 2007). The authors concluded that DTI may provide a surrogate marker of dopaminergic neuronal degeneration in the substantia nigra.
Consistent with this prediction, DTI has been used to show in-vivo that the ventral substantia nigra, which is mostly inferior to the red nucleus, of early stage, untreated patients with Parkinson’s disease had the most consistent reduction in FA values compared with a control group (Vaillancourt et al., 2009). The study also found that a receiver operator characteristics analysis of FA values from hand-drawn regions of interest in the regions of the ventral substantia nigra revealed a sensitivity gradient [rostral (7.1%), middle (35%), and caudal (100%)] for separating control subjects from patients with Parkinson’s disease. Menke and colleagues (2009) used DTI and a T1-weighted protocol to assess changes in the connectivity between the substantia nigra and targets in the basal ganglia and thalamus, and volume changes in the substantia nigra of Parkinson’s disease and control participants. The authors found that FA values were similar between patients with Parkinson’s disease and controls, which is different from the findings of three other groups that have used DTI to find reduced FA values in the substantia nigra of Parkinson’s disease (Chan et al., 2007; Vaillancourt et al., 2009; Yoshikawa et al., 2004). Menke and colleagues (2009) did find that the volume of the substantia nigra was reduced in Parkinson’s disease and control subjects, and that the connectivity between the substantia nigra and thalamus was abnormal in Parkinson’s disease. The authors suggested that the reason they did not find a reduction in FA could be due to the way that the ROI is defined. Since the current study found that age affects the dorsal substantia nigra but not the ventral substantia nigra, these findings provide further evidence that where the ROI is defined is critical in finding degenerative changes.
The current findings have important implications for differentiating normal aging from that of Parkinson’s disease. For instance, using haematoxylin and eosin stained slice preparations from the caudal substantia nigra of 36 healthy control subjects aged 20 to 90 years, there was a linear fallout of pigmented neurons with advancing age in the substantia nigra pars compacta at a rate of 4.7% per decade (Fearnley and Lees, 1991). Using a subdivided substantia nigra, the authors also found that the ventral and lateral tier was relatively spared (2.1% loss per decade) compared with the dorsal tier (6.9% per decade). This pattern of cell loss was also studied in 20 patients with Parkinson’s disease with varying disease duration. It was found that an exponential loss of pigmented neurons occurred in the patients, with a 45% loss in the first decade since diagnosis. The cell loss in patients with Parkinson’s disease was greatest in the ventral and lateral tier of the substantia nigra pars compacta compared with the dorsal tier. The authors concluded that pigmented nigral cells in the ventral substantia nigra pars compacta were most affected by Parkinson’s disease, whereas aging affected pigmented nigral cells in the dorsal substantia nigra.
Since it is estimated that greater than 50% of the cells are lost before motor symptoms are present and a patient is subsequently diagnosed with Parkinson’s disease (Braak et al., 2003; Hodaie et al., 2007), one potential interpretation of the findings by Vaillancourt and colleagues (2009) is that the DTI technique was able to detect microstructural degeneration in the substantia nigra once there was greater than 50% cell loss. What makes the current findings on healthy older adults so important is that Fearnley and Lees (1991) found that the dorsal substantia nigra loses pigmented nigral neurons between 30–50% in healthy older adults from ages 20 to 90 years. Thus, since the current study found reduced FA and increased RD in the dorsal substantia nigra of individuals aged 19–71 years, then it is possible that DTI could detect microstructural degeneration in the substantia nigra of patients with Parkinson’s disease before motor symptoms are present and before a patient is typically diagnosed clinically.
Acknowledgments
This research was supported in part by grants from the National Institutes of Health (R01-NS-52318, R01-NS58487, R21-AG28662) and Department of the Army PT 075675.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auer DP. In vivo imaging markers of neurodegeneration of the substantia nigra. Exp Gerontol. 2009;44:4–9. doi: 10.1016/j.exger.2008.08.051. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–19. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Boska MD, Hasan KM, Kibuule D, Banerjee R, McIntyre E, Nelson JA, Hahn T, Gendelman HE, Mosley RL. Quantitative diffusion tensor imaging detects dopaminergic neuronal degeneration in a murine model of Parkinson’s disease. Neurobiol Dis. 2007;26:590–6. doi: 10.1016/j.nbd.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Chan LL, Rumpel H, Yap K, Lee E, Loo HV, Ho GL, Fook-Chong S, Yuen Y, Tan EK. Case control study of diffusion tensor imaging in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:1383–6. doi: 10.1136/jnnp.2007.121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem. 1958;3:41–51. doi: 10.1111/j.1471-4159.1958.tb12607.x. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Narayana PA. Retrospective measurement of the diffusion tensor eigenvalues from diffusion anisotropy and mean diffusivity in DTI. Magn Reson Med. 2006;56:130–7. doi: 10.1002/mrm.20935. [DOI] [PubMed] [Google Scholar]
- Hirai S. Histochemical study on regressive degeneration of the senile brain with special reference to the aging of the substantia nigra. Adv Neurol Sci. 1968;12:845–849. [PubMed] [Google Scholar]
- Hodaie M, Neimat JS, Lozano AM. The dopaminergic nigrostriatal system and Parkinson’s disease: molecular events in development, disease, and cell death, and new therapeutic strategies. Neurosurgery. 2007;60:17–28. doi: 10.1227/01.NEU.0000249209.11967.CB. discussion 28–30. [DOI] [PubMed] [Google Scholar]
- Jones DK, Simmons A, Williams SC, Horsfield MA. Non-invasive assessment of axonal fiber connectivity in the human brain via diffusion tensor MRI. Magn Reson Med. 1999;42:37–41. doi: 10.1002/(sici)1522-2594(199907)42:1<37::aid-mrm7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Krampe RT. Aging, expertise and fine motor movement. Neurosci Biobehav Rev. 2002;26:769–76. doi: 10.1016/s0149-7634(02)00064-7. [DOI] [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130:2508–19. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- Lang AE, Mikulis D. A new sensitive imaging biomarker for Parkinson disease? Neurology. 2009;72:1374–1375. doi: 10.1212/01.wnl.0000343512.36654.41. [DOI] [PubMed] [Google Scholar]
- Lucerna S, Salpietro FM, Alafaci C, Tomasello F. In Vivo Atlas of Deep Brain Structures. Springer-Verlag; Berlin, Germany: 2002. [Google Scholar]
- Maclennan RN. Interrater reliability with SPSS for Windows 5.0. The American Statistician. 1993;47:292–296. [Google Scholar]
- Majumdar S, Gore JC. Studies of diffusion in random fields produced by variations in susceptibility. Journal of Magnetic Resonance. 1988;78:41–55. [Google Scholar]
- Martin WR, Wieler M, Gee M. Midbrain iron content in early Parkinson disease: a potential biomarker of disease status. Neurology. 2008;70:1411–7. doi: 10.1212/01.wnl.0000286384.31050.b5. [DOI] [PubMed] [Google Scholar]
- Menke RA, Scholz J, Miller KL, Deoni S, Jbabdi S, Matthews PM, Zarei M. MRI characteristics of the substantia nigra in Parkinson’s disease: a combined quantitative T1 and DTI study. Neuroimage. 2009;47:435–41. doi: 10.1016/j.neuroimage.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Oikawa H, Sasaki M, Tamakawa Y, Ehara S, Tohyama K. The substantia nigra in Parkinson disease: proton density-weighted spin-echo and fast short inversion time inversion-recovery MR findings. AJNR Am J Neuroradiol. 2002;23:1747–56. [PMC free article] [PubMed] [Google Scholar]
- Ossowska K, Wolfarth S, Schulze G, Wardas J, Pietraszek M, Lorenc-Koci E, Smialowska M, Coper H. Decline in motor functions in aging is realted to the loss of NMDA receptors. Brain Research. 2001;907:71–83. doi: 10.1016/s0006-8993(01)02601-4. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Rohlfing T, Sullivan EV. Diffusion tensor imaging of deep gray matter brain structures: Effects of age and iron concentration. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Rohlfing T, Sullivan EV. MRI estimates of brain iron concentration in normal aging: comparison of field-dependent (FDRI) and phase (SWI) methods. Neuroimage. 2009;47:493–500. doi: 10.1016/j.neuroimage.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poonawalla AH, Zhou XJ. Correction of eddy currents in EPI-based diffusion tensor imaging. Proc Intl Soc Magn Resan Med; Glasgow, Scotland. 2001. p. 736. [Google Scholar]
- Roth G, Joseph J. Cellular and molecular mechanisms of impaired dopaminergic function during aging. Ann NY Acad Sci. 1994;719:129–135. doi: 10.1111/j.1749-6632.1994.tb56824.x. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Swinnen SP, Stelmach GE. Age-related deterioration of coordinated interlimb behavior. J Gerontol B Psychol Sci Soc Sci. 2000;55:P295–303. doi: 10.1093/geronb/55.5.p295. [DOI] [PubMed] [Google Scholar]
- Sohmiya M, Tanaka M, Aihara Y, Hirai S, Okamoto K. Age-related structural changes in the human midbrain: an MR image study. Neurobiol Aging. 2001;22:595–601. doi: 10.1016/s0197-4580(01)00227-5. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 2006;30:749–61. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Spraker MB, Prodoehl J, Abraham I, Corcos DM, Zhou XJ, Comella CL, Little DM. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology. 2009;72:1378–84. doi: 10.1212/01.wnl.0000340982.01727.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K, Nakata Y, Yamada K, Nakagawa M. Early pathological changes in the parkinsonian brain demonstrated by diffusion tensor MRI. J Neurol Neurosurg Psychiatry. 2004;75:481–4. doi: 10.1136/jnnp.2003.021873. [DOI] [PMC free article] [PubMed] [Google Scholar]


