Abstract
Purpose
To conduct a controlled trial of bevacizumab for the treatment of symptomatic radiation necrosis of the brain.
Methods and Materials
Fourteen patients were entered into a placebo-controlled randomized double-blind study of bevacizumab for the treatment of central nervous system (CNS) radiation necrosis. All patients were required to have radiographic or biopsy proof of CNS radiation necrosis and progressive neurological symptoms or signs. Eligible patients received irradiation for head and neck carcinomas, meningioma, or low- to mid-grade gliomas. Patients were randomized to receive IV saline or bevacizumab at 3-week intervals. MRI 3-weeks after the second treatment and clinical signs and symptoms defined response or progression.
Results
The volumes of necrosis estimated on T2FLAIR and T1-weighted gadolinium-enhanced MRI demonstrated that, while no patient receiving placebo responded (0/7), all bevacizumab-treated patients did (5/5 randomized and 7/7 cross-over) with decreases in T2FLAIR and T1-weighted gadolinium-enhanced volumes and decrease Ktrans. All bevacizumab-treated patients – and none of the placebo-treated patients - showed improvement in neurological symptoms or signs. At a median of 10 months after the last dose of bevacizumab in patients receiving all 4 study doses, only 2 patients had experienced a recurrence of MRI changes consistent with progressive radiation necrosis, and this was the only patient to receive only 2 treatments with bevacizumab.
Conclusions
This class I evidence of bevacizumab efficacy in the treatment of CNS radiation necrosis justifies consideration of this treatment option for people who suffer radiation necrosis secondary to the treatment of head and neck and brain cancers.
Keywords: brain edema, magnetic resonance imaging, volumetric MRI changes, Ktrans, neurotoxicity
INTRODUCTION
Radiation therapy, while helpful in the management of central nervous system (CNS) and head and neck tumors, can cause devastating radiation necrosis of normal CNS tissues. At present, we believe this damage is results from local cytokine release, increase in capillary permeability and extracellular edema, and loss of the myelin covering of neurons. If allowed to progress, radiation necrosis can lead to small vessel occlusive disease and bleeding from friable small vessels(4, 5). These changes combine to cause a definable worsening in patients' neurological signs and symptoms(1–4).
Traditionally, physicians have tried to combat CNS radiation necrosis with corticosteroids, antiplatelet agents, anticoagulants, hyperbaric oxygen, high-dose vitamins, and surgery(2, 6). However, none of these approaches has proven effective in controlled clinical trials.
The development of effective therapy for CNS radiation necrosis is complicated by the fact that the mechanisms of radiation-induced injury are not completely understood. Current dogma views radiation necrosis as a continuous process from endothelial cell dysfunction to tissue hypoxia and necrosis, with the concomitant liberation of a vasoactive compound such as the vascular endothelial growth factor (VEGF) that can lead to progressive blood-brain barrier dysfunction and brain edema(7–11). We hypothesized that blocking VEGF from reaching its capillary targets was a logical treatment strategy for radiation necrosis to reduce the movement of plasma and plasma water through leaky brain capillary endothelium to the extracellular space.. In fact, early descriptions of VEGF by Dvorak and Senger's group(12–15) used the term “vascular permeability factor” to recognize VEGF's ability to dramatically increase vascular permeability.
Recently, we observed that in 8 patients with CNS radiation necrosis bevacizumab, alone or with anticancer agents, markedly reduced apparent lesion extent on T2-weighted fluid attenuated inversion recovery (T2 FLAIR) and T1-weighted gadolinium-enhanced magnetic resonance imaging (MRI), as well as reducing the patients' dexamethasone dependence (17). Others have reported similar observations(18). Thus, to better determine whether bevacizumab can effectively treat symptomatic and progressive CNS radiation necrosis, we conducted a placebo-controlled double-blind study to determine the extent intravenous bevacizumab, administered every 3 weeks reduced active radiation necrosis in the CNS.
METHODS AND MATERIALS
Study design
Eligible patients were randomized to Group A to receive intravenous bevacizumab at a dose of 7.5 mg/kg at 3-week intervals for 2 treatments, or to Group B to receive intravenous placebo at 3-week intervals for 2 treatments. It was planned that all patients would undergo a MRI scan prior to beginning treatment and 3 weeks after the second dose of placebo/bevacizumab, i.e., 6 weeks after study entry. At that time, patients responding to the treatment/placebo and showing no adverse effects that would require treatment discontinuation would receive 2 more cycles of the same treatment and be evaluated by MRI 3 weeks after the fourth treatment. Responding patients were to be monitored for at least 24 weeks.
Failure of treatment would allow a patient to cross over from one treatment arm to the other at or before the time of the 6-week MRI; patients randomized to Group A or Group B cross-over patients also had the possibility of later treatment with bevacizumab in the event that radiation necrosis returned at any time after receiving the planned 4 doses. Failure was defined as either no or insufficient reduction in the volume of radiation necrosis on MRI and worsening neurological symptoms and signs, or MRI evidence of an increase in radiation necrosis in an area of the brain that is relatively “silent” in terms of patient functioning and therefore not likely to produce noticeable neurological symptoms or signs. If, in the opinion of the patient's physician, progression of neurological symptoms warranted, then the patient was scheduled for an MRI sooner than the usual schedule and the randomization code was broken. The radiologist and radiation physicist were unaware of the treatment status of the patient at the time of MRI read and interpretation. Patients with documented treatment failure from either arm of the study would be allowed to receive 2 cycles of treatment from the other arm.
An increase in dexamethasone daily dose would not, in itself, be insufficient for study re-entry in patients who had already received 4 doses of bevacizumab. Group A patients who developed adverse events requiring discontinuation of bevacizumab during the trial were not considered likely to need treatment with bevacizumab if the radiation necrosis returned at a later date (e.g., after week 24).
Eligibility criteria
Patients must have received cranial irradiation for histologically confirmed WHO grade 2–3 primary brain neoplasm, meningioma, or head and neck cancer ≥ 6 months prior to study entry.
MRI evidence to support the diagnosis of radiation necrosis and/or surgical biopsy evidence of necrosis without tumor ≤ 2 months of study entry.
Karnofsky performance status ≥ 60
Evidence of progressive neurologic signs or symptoms appropriate to the location of the radiation necrosis.
No prior bevacizumab therapy.
Patients allowed to receive prior chemotherapy for their tumor. Patients who have been treated with tyrosine kinase inhibitors of VEGFR will be permitted.
Routine laboratory studies with bilirubin ≤1.5 × ULN, AST and/or ALT< 2.5 × ULN, creatinine <1.0 × ULN, granulocytes >1500, platelets> 75,000; Hb ≥9.0, diarrhea must be < grade 1.
If history of seizures, patients should be on anticonvulsant therapy and be seizure-free for ≥1 week prior to study. Patients can be on enzyme-inducing anticonvulsants, however, preference will be for enzyme-non-inducing anticonvulsants.
Patients can be on dexamethasone but must be on a stable dose for ≥ 1 week prior to study MRI.
Because no dosing or adverse event data are currently available on the use of bevacizumab in patients <18 years of age, children are excluded from this study.
Ability to understand and the willingness to sign a written informed consent document.
In addition, patients receiving full-dose anticoagulants (e.g., warfarin) with a prothrombin international normalized ratio >1.5 were eligible provided that the ratio was considered in-range for their indication (between 2 and 3) and had been maintained on a stable dose of oral anticoagulant or on a stable dose of low-molecular-weight heparin for ≥ 1 week.
Excluded from study were patients with:
Active bleeding or a pathological condition that carried a high risk of bleeding.
Head and neck cancer with metastatic disease, invasion to major vessels, or a history of bleeding related to tumor or radiation during or after completion of radiotherapy.
CNS hemorrhage or bleeding diathesis or coagulopathy, abdominal fistula, abscess, or gastrointestinal tract perforation occurring ≤ 28 days of study entry.
Major surgical procedure or significant traumatic injury ≤ 28 days of study entry.
Clinically significant cardiovascular disease such as hypertension, cerebrovascular event, myocardial infarction, cardiac arrhythmias, unstable angina, or congestive heart failure ≤ 6 months of study entry or with an aortic aneurysm, aortic dissection, or significant peripheral vascular disease at time of study.
MRI studies
The MRI evaluation for radiation necrosis included both pre- and post-gadolinium administration sequences. Images obtained before administration of the contrast agent were as follows:
Axial T1- and T2-weighted images (5-mm sections, 1.5-mm gap)
Axial and coronal thin-section images (3-mm, contiguous) T2FLAIR-weighted images
Axial diffusion-weighted images (b=1000 s/mm2, 5-mm sections, 1.5-mm gap)
Axial T2* (susceptibility)-weighted images
Axial diffusion tensor images (b=1200 s/mm2, 27 diffusion encoding directions, 3.5-mm contiguous sections)
Images obtained after the administration of the contrast agent were as follows:
Three-dimensional (3D) dynamic contrast-enhanced MRI (DCE-MRI) images(14 5-mm contiguous sections, fast spoiled gradient recalled echo acquisition, temporal resolution: <10 s)
Axial and sagittal T1-weighted images (5-mm sections, 1.5-mm gap)
3D coronal T1-weighted images (2-mm sections, reconstructed at 1 mm)
All MRI studies were to be done by the same team of medical imaging technologists, imaging physicists, and neuroradiologist and were to be performed on 1 of 2 Excite HD 3.0-T scanners (GE Healthcare, Waukesha, WI). The expectation of the protocol was that MRI disease parameters would be monitored in up to five measurable target lesions that could, at the very least, be measured in 2 dimensions. By these measures, it was our expectation and initial study goal to define response to treatment as a reduction in bidirectional measurements on T2FLAIR images by at least 25% in the product of the two measures. Also measured were 1) the gadolinium-enhancement volume on the 3D coronal T1-weighted images, 2) the volumes of edema and disease on the T2FLAIR images, and 3) the DCE-MRI pharmacokinetic modeling parameters (described below). Early in the study, it was determined that more reliable characterization could be achieved if we measured T2FLAIR and 3D T1-weighted gadolinium contrast-enhanced volumes using independent computer methods (described below, in place of bidirectional measurements and as an improvement over items 1 and 2 above). As a result, we recorded the official neuroradiology reading as well as the independently computed volumes.
Volume measurements
Because of the relatively low spatial resolution--and the ensuing partial-volume effects at the edge of structures and lesions--of the clinical MRI data sets used in this study, we chose to rely on manual and semi-automatic approaches that are available on most commercial workstations and biomedical imaging software for the segmentation of visible lesions on both T2FLAIR and 3D T1-weighted gadolinium-enhanced images. After segmentation, the total volume was estimated as the sum of all voxels that had been identified as being part of the lesion, with the volume of an individual voxel being derived from the spatial characteristics of the source images (e.g., pixel size and thickness) stored in the corresponding Digital Imaging and Communications in Medicine (DICOM) tags. To reduce operator variance and increase the consistency of measurements throughout the study(19), a single team of cross-trained technologists performed all segmentations under close expert guidance. The results were then independently checked by a trained neuroradiologist before being included in the database and subjected to subsequent analysis.
DCE measurements
The DCE-MRI data were analyzed using the 2-compartment generalized kinetic model(20). In the analysis, the vascular input function was sampled from the sagittal sinus and the Ktrans (endothelial transfer constant, min−1), kep (contrast agent reflux rate, min−1), and ve (extravascular, extracellular volume fraction, unitless) parameters were computed on a pixel-by-pixel basis from regions of interest defined on each section by the imaging physicist and reviewed by the study neuroradiologist. As the volume of contrast enhancement changed significantly following therapy, the Ktrans values were normalized to number of gadolinium-enhanced pixels within the DCE-MRI volume, i.e., Ktransnormalized = Ktrans ([number of enhanced pixels at time t] / [number of enhanced pixels at baseline]). Baseline was defined as the initial DCE-MRI examination done prior to the administration of any bevacizumab or placebo or, for those patients initially showing disease progression on placebo, the DCE-MRI examination done at the time of placebo failure and, therefore, prior to bevacizumab administration.
Other studies
We obtained serial neurological examinations, formal neurocognitive testing, dexamethasone dosing records, and self-reports of symptoms using the M. D. Anderson Symptom Inventory (MDASI). Patients underwent neuropsychological testing and completed the MDASI right before randomization to placebo or bevacizumab and also after 6 weeks of treatment. Prior to analyses, no subsets of any tests were arbitrarily chosen for comparison. The neurocognitive testing required about 30 minutes to complete and included the Trail Making Test Part A and Part B (21), Hopkin's Verbal Learning Test-Revised (Total Recall, Delayed Recall, Delayed Recognition) (22), and the Controlled Oral Word Association subtest of the Multilingual Aphasia Examination (23). Neurocognitive testing was conducted by a licensed neuropsychologist or a trained neuropsychological technician under the supervision of a neuropsychologist. Test results were kept in a Neuropsychology Section database prior to analysis.
Statistical considerations
The primary endpoint was the change in edema volume on MRI from baseline to the first evaluation at 6 weeks, with the volume estimated on T2FLAIR images being the selected as the primary endpoint of interest. A reduction in volume of 25% or more constituted a response On the basis of results from our first study (17), we designed the current study such that alpha = 0.10, power = 0.95 and we assumed that the response rate for the placebo group was equal to 0.1, while the response rate for the bevacizumab group was equal to 0.8. These estimates yielded a sample size of 8 patients per arm to detect differences using a continuity corrected chi-sq test.
Summary statistics of demographic characteristics were provided. The Wilcoxon rank sum test was used to compare differences in T2FLAIR, T1-weighted gadolinium enhancement volume, and Ktrans between the two treatment groups. The Wilcoxon signed rank test was used to compare within group changes in T2FLAIR, and T1-weighted gadolinium enhancement volume The Fisher's exact test was used to evaluate the association between treatment and response.
After 4 cycles (12 weeks), treatments were discontinued and the patients were monitored by MRI at 3-month intervals for evidence of renewed radiation necrosis activity. Differences between groups in terms of dexamethasone dosing beginning at 12 weeks were evaluated by t-test. Between-group differences in neurocognitive test and symptom inventory findings were also evaluated by t-test. All neurocognitive test scores were transformed to standardized z-scores using demographically adjusted published normative data from healthy controls (22–24). In addition to examining changes in individual neurocognitive test results, a composite summary variable (NCF6Z) was calculated for each patient by calculating the average z-score across all six neurocognitive subtests. SPSS 16.0 was used for all neuropsychological analyses
RESULTS
Patient demographics
Table 1 summarizes the demographic data for the patients entered into this study. The majority of patients did not have primary CNS tumors. Symptoms varied from headache to hemiparesis and decreasing macular vision. In the process of screening patients for eligibility for this study, we found that not all patients with obvious MRI evidence of radiation necrosis had neurological symptoms or signs. Of the group, 2 patients were followed by serial clinical visits with MRI until they developed signs/symptoms allowing entry into the study.
Table 1.
Demographics of randomized patients that registered for study.
| Pt | Age | Sex | Reason for irradiation | Site of radiation necrosis | Months from RT to study entry | Symptom or sign of RT necrosis |
|---|---|---|---|---|---|---|
| 1 | 44 | F | Anaplastic astrocytoma, right temporal | Right periventricular/right trigone | 17 mo from R | Headache and fatigue |
| 2 | 43 | F | Anaplastic astrocytoma, left frontal | Left frontal | 25 mo from R | Worsened concentration |
| 3 | 40 | M | Malignent schwannoma, left inferior alveolar nerve | Left temporal | 54 mo from R | Increased blurred vision |
| 4 | 41 | M | Astrocytoma, left frontal | Left anterior frontal | 82 mo from R | Seizure |
| 5 | 55 | F | Ologodendriglioma, right temporal | Right temporal | 8 mo from R | Left-side weakness |
| 6 | 59 | M | Ologodendriglioma, right frontal | Right frontal | 11 mo from R | Seizure |
| 7 | 56 | F | left orbital squamous cell carcinoma | Left frontal | 21 mo from R | Pain behind left eye |
| 8 | 43 | F | Nasopharyngeal carcinoma | Right temporal | 40 mo from R | Headache |
| 9 | 53 | M | Nasopharyngeal carcinoma | Right temporal | 44mo from R, 43 mo from Brachytherapy | Memory loss |
| 10 | 66 | M | Squamous cell carcinoma, left cheek | Left temporal | 39 mo from R | Word finding difficulty |
| 11 | 49 | M | posterior fossa hemangiopericytoma | Bilateral occipital lobes | 47 mo from R | Decreased peripheral vision |
| 12 | 47 | F | pituitary adenoma | Bilateral anterior temporal lobes | 21 yrs from R, 39 mo from SRS | Confusion |
| 13 | 48 | M | left frontal-temporal oligodendroglioma | Periventricular white matter | 88 mo from R, 8 mo from definite R | Right-sided weakness, dysphasia, change of mental status |
| 14 | 39 | M | Left frontal-temporal anaplastic astrocytoma | Left inferior parietal lobe | 9 mo from R | Neurocognitive slowing, decreased concentration, word finding problems, headache |
R = conventional external beam irradiation; SRS = stereotactic radiosurgery
Neurological signs and symptoms
Of the seven patients randomized to placebo, 71% (5/7) had worsening neurological from 3.1 to 8.8 weeks after receiving the first placebo dose, while 29% (2/7) showed only MRI progression of radiation necrosis by MRI. While all placebo patients showed worsening neurological signs and symptoms, we did not foresee that they might experience marked deterioration prior to the 6-week MRI. Such observations, however, led us to sometimes repeat the MRI shortly after the second dose and break the randomization code so that the patient could be moved to the bevacizumab arm of the study. All patients receiving bevacizumab showed improvements in neurological signs and symptoms by the 6-week clinic visit.
MRI observations
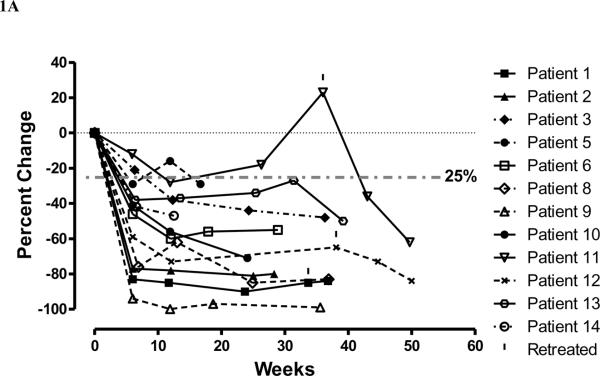
For all patients randomized to bevacizumab we observed MRI responses at six weeks, while for those patients receiving placebo we observed progressive disease by six weeks (p-value: 0.0013) No patient receiving placebo who showed worsening of neurological signs and symptoms at 6 weeks showed a decrease in lesion volume on MRI (Table 2). However, for the 7 patients in the placebo arm, MRI showed an increase in the volume of T2FLAIR edema (median change in volume of T2FLAIR edema = +14%), while the patients randomized to bevacizumab showed a median % change decrease in T2FLAIR edema of −59% (p-value: 0.0149). In addition, for the placebo group we observed a median percent change increase in T1-weighted gadolinium enhancement volume of +17%, compared to a median decrease in T1-weighted gadolinium enhancement volume in the bevacizumab arm of −63%. (p-value:0.0058). Lastly, for Ktransnormalized, we observed a median increase of 49% in the placebo arm and a median decrease of 99% in the bevacizumab (p-value: 0.023)., We graphically show the reductions for all patients receiving bevacizumab for T2FLAIR volume (Fig. 1A) and the volume of T1-weighted gadolinium enhancement (Fig. 1B). We observe that within treatment group changes in T2FLAIR volume at 6 weeks are not statistically significant for the placebo group (p-value=0.5781). Similarly within treatment group changes in T1-weighted gadolinium enhancement were not significant (p-value=0.3125). In contrast the within treatment group changes for the bevacizumab arm were marginally statistically significant for both T2FLAIR volume and T1-weighted gadolinium enhancement volume (both p-values = 0.0625). Furthermore, for six patients who were initially randomized to placebo then crossed over to receive bevacizumab treatment after progression we observed statistically significant decreases in volume as measured by the two volume endpoints (both p-values = 0.0313). Figure 1C shows that the Ktransnormalized value over time for all patients treated with bevacizumab decreased on 90–100% on average at 6 and 12 weeks after starting bevacizumab.
Table 2.
MRI T2 FLAIR and T1 Gd enhancement volumes for placebo treatment group
| Identifier | Weeks after study entry | T2 FLAIR volume | % change from baseline | Gd-contrast T1 volume | % change from baseline |
|---|---|---|---|---|---|
| 1 | 0 | 81.2 | 7.8 | ||
| 1 | 5.6 | 201 | +148% | 40.6 | +421% |
| 2 | 0 | 158 | 38.9 | ||
| 2 | 6.1 | 171 | +8% | 39.8 | +2% |
| 6 | 0 | 134.7 | 31.2 | ||
| 6 | 6.7 | 140 | +4% | 36.5 | +17% |
| 10 | 0 | 60.4 | 32,4 | ||
| 10 | 5.6 | 53.3 | –12% | 23.7 | –27% |
| 11 | 0 | 61.7 | 7.4 | ||
| 11 | 4.7 | 42.6 | –31% | 8.7 | +18% |
| 13 | 0 | 76.0 | 18.2 | ||
| 13 | 7.4 | 65.5 | –14% | 18.2 | 0% |
| 14 | 0 | 76.3 | 13.3 | ||
| 14 | 6.0 | 103.8 | +36% | 18.9 | +43% |
| Median | 5.8 | +4% | +17% |
Fig. 1.
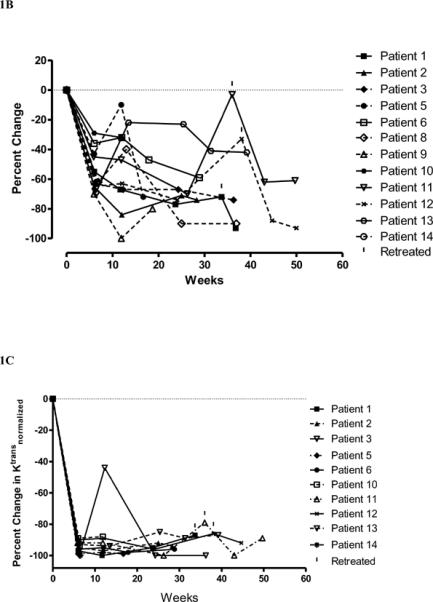
Plot of percentage change in necrosis volumes on (A) T2FLAIR MRI and (B) T1-weighted gadolinium contrast-enhanced MRI and (C) percentage change in Ktransnormalized, over time, for evaluable patients randomized to bevacizumab (solid line) and those who received it at cross-over (dashed line) at failure to placebo. Patients 1 received one additional treatment, patient 11 received four additional treatments, and patient 12 two additional treatments with bevacizumab beginning at the time indicated by the vertical mark above the symbol.
Figure 2 shows the series of MRI studies done for patient 1, who received placebo originally, had radiation necrosis progression evidenced by increasing T2FLAIR volume (+148%), T1-weighted gadolinium enhancement volume (+421%), and Ktransnormalized (+260%). The patient then has a response to bevacizumab, with reductions in neurological signs and symptoms, T2FLAIR volume (−85%), T1-weighted gadolinium enhancement volume (−67%), and Ktransnormalized (−100%).
Fig. 2.
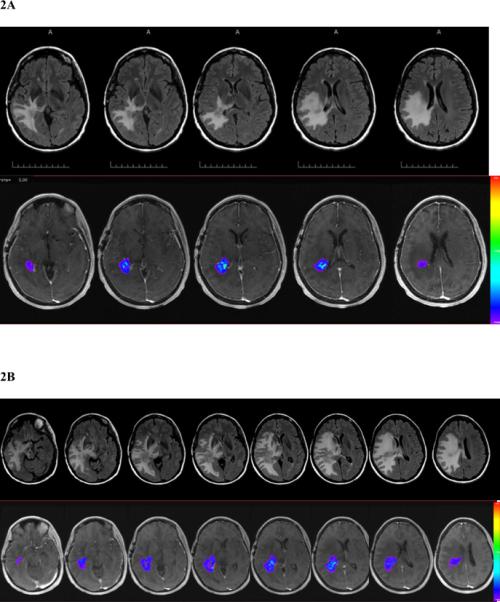
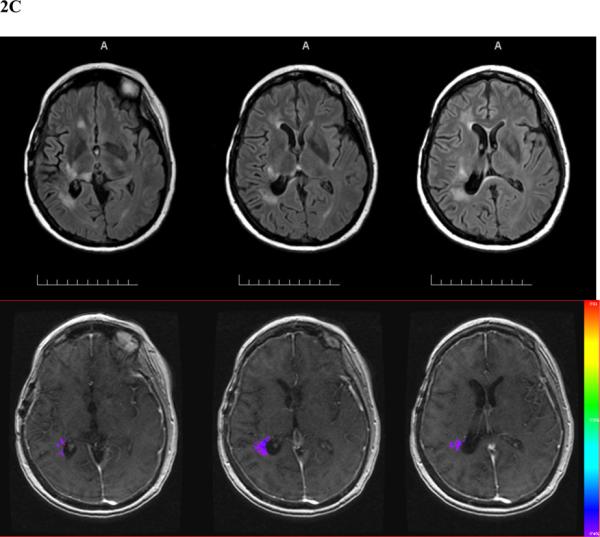
Patient 1 prior to receiving placebo at study entry (A), 5 weeks after the initiation of placebo and prior to bevacizumab (B), and 3 weeks after the 4th bevacizumab treatment (C). In each series, the upper set shows T2FLAIR MR images and the lower set T1-weighted gadolinium contrast-enhanced images with colorized Ktrans overlays (red high, blue low).
While follow-up continues, at a median 10 months after the end of treatment, 25% (3/12) patients receiving bevacizumab has had worsening of radiation necrosis leading to additional doses of bevacizumab. Patient 1 had a modest deterioration in MRI on week 33.7 that led her physician to treat with an additional dose of bevacizumab. This improved the MRI and further therapy has not been needed over the next year. Patient 11 was treated with only 2 doses of bevacizumab because he developed superior sagittal sinus thrombosis. The volume of necrosis on T2FLAIR and T1-weighted gadolinium contrast-enhanced images had increased at about 7 months after bevacizumab treatment. At that time the patient was receiving oral anti-coagulation with warfarin and was given 4 doses of bevacizumab; he then showed again volume reduction on T2FLAIR (−36% at 7 weeks −62% at 13.7 weeks) and gadolinium-contrast MRI (−62% at 7 weeks and −61% at 13.7 weeks). The MRI performed on Patient 12 showed some worsening at week 38.1 and the patient was treated with two additional doses of bevacizumab. In summary, all patients showed improvement in T2FLAIR and T1-weighted gadolinium contrast-enhanced volume and Ktransnormalized (see figures 1A, 1B, 1C for the relationship of MRI changes to retreatment).
Neurocognitive testing and symptom self-reports
Since there appeared to be no differences in neurocognitive test results in the randomized versus cross-over bevacizumab treated patients, all bevacizumab patients were pooled into one group. The mean (±SD) follow-up intervals for completion of neuropsychological testing and completion of the MDASI were 40 (±9) days for patients receiving placebo and 44 (±15)_days for those receiving bevacizumab. The difference in days is not statistically significant. Two patients at baseline and 1 at follow-up had missing informative data on the Trail Making Test. In all 3 cases, the patients were unable to complete the test because of neurological deficits. These patients were assigned the maximum allowable score for the purpose of all analyses. Effect sizes ranged from small (i.e., Cohen's d ≥ 0.2) to large (i.e., Cohen's d ≥ 1.0). Comparison of change scores on the composite measure of neurocognitive function (NCF6Z) yielded only small effect size differences (Cohen's d=0.21). The mean NCF6Z score changed from −1.27 at baseline to −1.34 after 6 weeks in the placebo-treated patients, whereas it went from −1.08 to −1 after 6 weeks in the bevacizumab-treated group. Examination of tests and measures demonstrating between group differences in change scores that were of medium to large effect-size (Cohen's d ≥ 0.5) suggested bevacizumab-treated patients exhibited greater improvement than placebo patients on the learning trials (HVLT-R Total Recall) and the delayed recognition trial (HVLT-R Delayed Recognition) of a memory test as well as improved symptom severity ratings on the MDASI (MDASI-Severity). However, bevacizumab-treated patients evidenced worsened performance on the delayed free recall trial of the memory measure (HVLT-R Delayed Recall), whereas placebo-treated patients improved. Independent sample t-tests on change scores demonstrated that this difference was of borderline statistical significance (p=0.052). No other statistically significant trends were found (Table 3). Bevacizumab patients also reported developing greater interference in their everyday activities from their symptoms (MDASI-Interference) than placebo-treated patients.
Table 3.
Changes in neurocognitive function and symptoms at 6-weeks for patients receiving placebo and bevacizumab. Some placebo treated patients will appear twice, once in the placebo group and once in the bevacizumab-treated group.
| Test / Measure | N | Mean | SD | t -test | P | Cohen's d |
|---|---|---|---|---|---|---|
| NCF6Z Δ | ||||||
| Bevacizumab | 11 | 0.08 | 0.69 | 0.43 | 0.67 | 0.21 |
| Placebo | 7 | −0.07 | 0.82 | |||
|
| ||||||
| HVLT-R Total Recall Δ | ||||||
| Bevacizumab | 11 | −0.22 | 0.90 | 1.17 | 0.26 | 0.59 |
| Placebo | 7 | −0.66 | 0.57 | |||
|
| ||||||
| HVLT-R Delayed Recall Δ | ||||||
| Bevacizumab | 11 | −0.58 | 1.32 | −2.10 | 0.051 | −1.05 |
| Placebo | 7 | 0.74 | 1.26 | |||
|
| ||||||
| HVLT-R Delayed Recognition Δ | ||||||
| Bevacizumab | 11 | 0.64 | 2.28 | 1.84 | 0.08 | 0.92 |
| Placebo | 7 | −1.43 | 2.40 | |||
|
| ||||||
| TMTA Δ | ||||||
| Bevacizumab | 11 | 0.48 | 1.29 | 0.41 | 0.67 | 0.21 |
| Placebo | 7 | 0.26 | 0.80 | |||
|
| ||||||
| TMTB Δ | ||||||
| Bevacizumab | 11 | 0.11 | 1.07 | −0.65 | 0.52 | −0.33 |
| Placebo | 7 | 0.44 | 1.04 | |||
|
| ||||||
| COWA Δ | ||||||
| Bevacizumab | 11 | 0.03 | 0.54 | −0.72 | 0.48 | −0.36 |
| Placebo | 7 | 0.21 | 0.49 | |||
|
| ||||||
| MDASI-Severity Δ | ||||||
| Bevacizumab | 11 | 0.52 | 1.49 | 1.36 | 0.19 | 0.68 |
| Placebo | 7 | −0.45 | 1.47 | |||
|
| ||||||
| MDASI-Interference Δ | ||||||
| Bevacizumab | 11 | −0.63 | 2.17 | −1.61 | 0.13 | −0.81 |
| Placebo | 7 | 1.04 | 2.11 | |||
Note: Means were coded such that negative values represent worse function, worse symptom severity and greater interference.
Glucocorticoid (dexamethasone) requirements
Of the 13 evaluable patients only 5 (38%) were receiving dexamethasone at the time of study registration. This low percentage likely reflects the fact that patients eligible for the trial were required to have progressive neurological signs or symptoms and were referred shortly after symptom development. All but 1 of the patients had a dose reduction in dexamethasone by 12 weeks after starting bevacizumab. Patient 4 had a good response to bevacizumab based on his lesions' reduction in T2FLAIR and T1 gadolinium enhancement volumes, but tapering dexamethasone required conversion to prednisone and then a slow taper over months. This patient had transformation of his astrocytoma to glioblastoma 6 months after receiving the first dose of bevacizumab, and thus he was not fully evaluable and is missing from figure 1.
Adverse events
Table 4 summarizes adverse events. There were no adverse events in 7/7 placebo patients, but 6/11 patients on bevacizumab had adverse events. Three adverse events were serious: 1 had aspiration pneumonia, 1 pulmonary embolus secondary to deep vein thrombosis, and 1 superior sagittal sinus thrombosis. Three additional patients had ischemic changes considered to be due to small vessel thrombosis. Since small vessel occlusion sometimes occurs with radiation necrosis (25, 26), we were uncertain whether the ischemic episodes were secondary to radiation necrosis or caused and/or abetted by the bevacizumab treatment.
Table 4.
Adverse events observed in 11 patients receiving bevacizumab.
| Patient | Observation |
|---|---|
| 1 | 3 wks after 2nd cycle developed ischemic changes with worsening of visual field – off study but no SAE |
| 5 | 3 wks after 2nd cycle had possible ischemic changes and worsening of hemiplegia but no SAE |
| 6 | Week 24 visit had ischemic changes but no SAE |
| 9 | Pt admitted for pneumonia and aspiration pneumonia – SAE filed |
| 11 | 3 wks after 2nd cycle showed dramatic improvement in RT necrosis but developed superior sagittal sinus thrombosis – SAE filed |
| 13 | Developed DVT and PE within 1 wk of BVZ – SAE filed but continues with Lovenox |
SAE = serious adverse event
DISCUSSION
This study demonstrates class I evidence (27) of the efficacy of bevacizumab therapy for CNS radiation necrosis. Only bevacizumab-treated patients showed improvement in clinical symptoms and signs and reduction in the volume of necrosis on T2FLAIR and T1-weighted gadolinium-contrast MRI, as well as a 90–100% reduction in Ktransnormalized. Formal neurocognitive testing revealed a mixed pattern of findings from the objective tests of neurocognitive function and the self-reported measure of symptoms. There was a trend toward improvement in aspects of learning and memory after 6 weeks of therapy that suggested improved memory encoding and retention despite increasing deficits in memory retrieval. This pattern has been associated with dysfunction in frontal subcortical systems (28). Similarly, there was mixed evidence suggesting a reduction in symptom severity for patients treated with bevacizumab but increased interference in everyday functioning related to these symptoms. These results should be interpreted cautiously as the study was likely underpowered to detect statistically significant changes in neurocognitive function and symptoms and the confidence intervals for the estimates of effect size were large due to the small sample size.
While this study demonstrates that bevacizumab can reduce progressive CNS radiation necrosis, it still leaves some questions to be answered. What is the lowest dose of bevacizumab and longest interval between doses needed to achieve resolution of CNS radiation necrosis? While it appears that 4 treatments with bevacizumab at 7.5 mg/kg are sufficient to attain long-term resolution, it may be that 3 doses at 4 week intervals would suffice -especially if the dose were higher (e.g., 10 mg/kg) than that tested here or possibly 4 doses at a lower dose (e.g., 5 mg/kg). While we are reasonably confident that 4 doses of bevacizumab over 12 weeks we be sufficient in most patients, two patients who had received 4 doses of bevacizumab required 1 to 2 additional doses of bevacizumab 36 to 38 weeks after in initiating bevacizumab treatment in order to reestablish a reduction in both enhancing and FLAIR abnormality on MRI. Last, it may be appropriate to treat patients with radiation necrosis with low-dose anticoagulation while they receive bevacizumab. At least for the present, one can be reasonably optimistic that bevacizumab at a dose of 7.5 mg/kg every 3 weeks for 12 weeks can stop the progression of radiation necrosis in most patients. In addition, we would expect this treatment to help control progressive radiation necrosis of the brainstem and spinal cord.
Acknowledgements
The MRI data acquisition contributions by David T. Evans, R.T., are gratefully acknowledged. The authors also acknowledge the support of Sandeep N. Gupta, Ph.D. (GE Global Research) for his support of the CineTool software package used for the DCE-MRI analyses. The study was supported in part by NIH N01 Phase II contract, N01-CM-62202 and from The University of Texas M. D. Anderson Cancer Center institutional funds and Core Grant CA 16672 to support clinical trials.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Notification: Victor Levin – none
Luc Bidaut – none
Ping Hou - none
Ashok Kumar – none
Jeffrey Wefel – Neuropsychology consultant to Genentech
Benjamin Bekele – none
Sujit Prabhu – none
Monica Loghin – none
Mark Gilbert – Consultant to Genentech
Edward Jackson – none
REFERENCES
- 1.Cheung M-C, Chan AS, Law SC, et al. Impact of radionecrosis on cognitive dysfunction in patients after radiotherapy for nasopharyngeal carcinoma. Cancer. 2003;97:2019–2026. doi: 10.1002/cncr.11295. [DOI] [PubMed] [Google Scholar]
- 2.Giglio P, Gilbert MR. Cerebral radiation necrosis. Neurolog. 2003;9:180–188. doi: 10.1097/01.nrl.0000080951.78533.c4. [DOI] [PubMed] [Google Scholar]
- 3.Crossen JR, Garwood D, Glatstein E, et al. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. Journal of Clinical Oncology. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- 4.Leibel SA, Sheline GE. Tolerance of the brain and spinal cord to conventional irradiation. In: PH G, SA L, GE S, editors. Radiation injury to the nervous system. Raven Press; New York: 1991. pp. 211–239. [Google Scholar]
- 5.Levin VA, Leibel SA, Gutin PH. Neoplasms of the central nervous system. In: DeVita VTJ, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 6 ed. Lippincott-Raven; Philadelphia: 2001. pp. 2100–2160. [Google Scholar]
- 6.Glantz MJ, Burger PC, Friedman AH, et al. Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology. 1994;44:2020–2027. doi: 10.1212/wnl.44.11.2020. [DOI] [PubMed] [Google Scholar]
- 7.Kim JH, Chung YG, Kim CY, et al. Upregulation of VEGF and FGF2 in normal rat brain after experimental intraoperative radiation therapy. Journal of Korean Medical Science. 2004;19:879–886. doi: 10.3346/jkms.2004.19.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y-Q, Ballinger JR, Nordal RA, et al. Hypoxia in radiation-induced blood-spinal cord barrier breakdown. Cancer Research. 2001;61:3348–3354. [PubMed] [Google Scholar]
- 9.sao MN, Li YQ, Lu G, et al. Upregulation of vascular endothelial growth factor is associated with radiation-induced blood-spinal cord barrier breakdown. J Neuropathol Exp Neurol. 1999;58:1051–1060. doi: 10.1097/00005072-199910000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Chung YG, Kim CY, et al. Upregulation of VEGF and FGF2 in normal rat brain after experimental intraoperative radiation therapy. J Korean Med Sci. 2004;19:879–886. doi: 10.3346/jkms.2004.19.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gridley DS, Loredo LN, Slater JD, et al. Pilot evaluation of cytokine levels in patients undergoing radiotherapy for brain tumor. Cancer Detect Prev. 1998;22:20–29. doi: 10.1046/j.1525-1500.1998.00010.x. [DOI] [PubMed] [Google Scholar]
- 12.Dvorak HF, Sioussat TM, Brown LF, et al. Distribution of vascular permeability factor (vascular endothelial growth factor) in tumors: concentration in tumor blood vessels. Journal of Experimental Medicine. 1991;174:1275–1278. doi: 10.1084/jem.174.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 14.Senger DR, Perruzzi CA, Feder J, et al. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 1986;46:5629–5632. [PubMed] [Google Scholar]
- 15.Senger DR, Van de Water L, Brown LF, et al. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer & Metastasis Reviews. 1993;12:303–324. doi: 10.1007/BF00665960. [DOI] [PubMed] [Google Scholar]
- 16.Presta LG, Chen H, O'Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–4599. [PubMed] [Google Scholar]
- 17.Gonzalez J, Kumar AJ, Conrad CA, et al. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67:323–326. doi: 10.1016/j.ijrobp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Torcuator R, Zuniga R, Mohan YS, et al. Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol. 2009 doi: 10.1007/s11060-009-9801-z. [DOI] [PubMed] [Google Scholar]
- 19.Meyer CR, Johnson TD, McLennan G, et al. Evaluation of lung MDCT nodule annotation across radiologists and methods. Acad Radiol. 2006;13:1254–1265. doi: 10.1016/j.acra.2006.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging. 1997;7:91–101. doi: 10.1002/jmri.1880070113. [DOI] [PubMed] [Google Scholar]
- 21.Reitan RM. Trail Making Test Manual for Administration and Scoring. Reitan Neuropsychology Laboratory; Tucson: 1992. [Google Scholar]
- 22.Benedict RHB, Schretlen D, Groninger L, et al. Hopkins verbal learning test – revised: normative data and analysis of inter-form and test-retest reliability. The Clin Psychologist. 1998;12:43–55. [Google Scholar]
- 23.Benton AL, Hamsher K. Multilingual Aphasia Examination. AJA Associates; Iowa City: 1983. [Google Scholar]
- 24.Heaton RK, Miller SW, Taylor MJ, et al. Revised comprehensive norms for an expanded Halstead-Reitan battery. Psychological Assessment Resources; Odessa, FL: 2004. [Google Scholar]
- 25.Leeds NE, Kumar AJ, Jackson EF. Diagnostic Imaging. In: Levin VA, editor. Cancer in the Nervous System. Second ed. Oxford University Press; New York: 2002. pp. 3–59. [Google Scholar]
- 26.Kumar AJ, Leeds NE, Fuller GN, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217:377–384. doi: 10.1148/radiology.217.2.r00nv36377. [DOI] [PubMed] [Google Scholar]
- 27.Sackett D. Evidence-based medicine. Lancet. 1995;346:1171. [PubMed] [Google Scholar]
- 28.Welsh-Bohmer KA, Ogrock PK. Clinical differentiation of memory disorders in neurodegenerative disease. In: Troster AI, editor. Memory in neurodegenerative disease: biological, cognitive, and clinical perspectives. Cambridge University Press; Cambridge, UK: 1998. pp. 290–314. [Google Scholar]