Abstract
Deep brain stimulation (DBS) for the treatment of advanced Parkinson’s disease involves implantation of a lead with four small contacts usually within the subthalamic nucleus (STN) or globus pallidus internus (GPi). While generally safe from a cognitive standpoint, STN DBS has been commonly associated with a decrease in the speeded production of words, a skill referred to as verbal fluency. Virtually all studies comparing pre-surgical to post-surgical verbal fluency performance have detected a decrease with DBS. The decline may be attributable in part to the surgical procedures, yet the relative contributions of stimulation effects are not known. In the present study, we used patient-specific DBS computer models to investigate the effects of stimulation on verbal fluency performance. Specifically, we investigated relationships of the volume and locus of activated STN tissue to verbal fluency outcome. Stimulation of different electrode contacts within the STN did not affect total verbal fluency scores. However, models of activation revealed subtle relationships between the locus and volume of activated tissue and verbal fluency performance. At ventral contacts, more tissue activation inside the STN was associated with decreased letter fluency performance. At optimal contacts, more tissue activation within the STN was associated with improved letter fluency performance. These findings suggest subtle effects of stimulation on verbal fluency performance, consistent with the functional non-motor subregions/somatopy of the STN.
Keywords: verbal fluency, DBS, cognition, mood, microlesion
Introduction
Deep brain stimulation (DBS) surgery, a well-accepted treatment for medication-refractory Parkinson’s disease, improves the cardinal motor symptoms and reduces the complications that may accompany dopaminergic therapies (Pahwa, 2006). However, the underlying bases for these dramatic motor improvements are not fully understood. One technique to quantify the effects of DBS on the nervous system is to create patient-specific models of the spread of stimulation (Butson et al., 2007; Maks et al., 2009). Patient-specific models take into account the anatomical placement of the electrode in relation to the surrounding brain structures, the electrical signals generated by the DBS device, and the neural activation of affected cells. In turn, these models provide a means of relating DBS activation information to clinical outcome. Previous patient-specific modeling efforts focused on motor outcomes; however, cognitive outcomes represent another important aspect of DBS. Although DBS appears to be relatively safe from a cognitive standpoint, long-term declines in speeded word fluency tasks have consistently been reported (Ardouin et al., 1999; Dujardin et al., 2001; Dujardin et al., 2000; Rodriguez et al., 2005; Saint-Cyr and Albanese, 2006; Saint-Cyr et al., 2000). Verbal fluency tasks require subjects to produce as many words as possible within 60 seconds, according to specific letters (Benton, 1968; Borkowski et al., 1967) or semantic categories such as animals or vegetables (Newcombe, 1969). Both tasks are time-limited, requiring self-generation of words under constrained search conditions. According to a meta-analysis of DBS outcome studies, significant declines on tests of verbal fluency reach moderate effect sizes (Parsons et al., 2006).
The extent to which stimulation contributes to verbal fluency declines has not been clearly determined. The findings of studies investigating stimulation alone (ON versus OFF stimulation studies) have been variable. At least one study has shown a decline in verbal fluency when PD patients were tested ON compared to OFF stimulation (Schroeder et al., 2003). Additionally, Wojtecki et al. (2006) showed better verbal fluency performance with low frequency compared to high frequency stimulation. Others have not discovered ON versus OFF stimulation differences (Jahanshahi et al., 2000; Morrison et al., 2004; Pillon et al., 2000; Witt et al., 2004). In two studies, investigators directly examined pre-post verbal fluency performance in relation to that obtained during ON and OFF stimulation conditions (Morrison et al., 2004; Pillon et al., 2000). In these studies, fluency was unaffected by bilateral DBS stimulation per se (ON-OFF comparison), but there was a decline in performance following DBS surgery (pre-post comparison). In particular, Morrison et al. (Morrison et al., 2004) noted a decline in verbal fluency performance in an assessment designed to test the effects of surgery (pre-surgery compared to post-surgery OFF DBS) but not in the ON versus OFF stimulation condition (which assesses the effects of stimulation only).
A recent prospective randomized trial of DBS outcome, named the COMPARE trial, provides further support for surgery-related effects on verbal fluency performance in STN DBS (Okun et al., 2009). In this study, 52 subjects were randomized to receive unilateral STN or GPi DBS. At 7 months post-DBS, subjects were tested in four randomized/counterbalanced conditions: with stimulation of the contact providing optimal motor benefit, with stimulation of the contacts ventral and dorsal to that, and off DBS). Forty-five subjects (23 GPi, 22 STN) completed the protocol. Comparison of verbal fluency performance at each of the DBS testing conditions to pre-DBS performance showed a deterioration of letter fluency in the STN group, especially when off DBS. The persistence of deterioration in verbal fluency in the off STN DBS state suggested a surgical rather than a stimulation-induced effect. This observation and those reported earlier suggest that the decline in verbal fluency following DBS may have more to do with the surgical intervention than the effects of stimulation. However, further studies are needed, particularly studies involving direct examination of the effects of stimulation.
The STN is thought to contain separate functional subregions, which presents an important consideration when targeting stimulation. Movement-related cells have been observed to be segregated within the dorsolateral STN in both the human (Rodriguez-Oroz et al., 2001; Theodosopoulos et al., 2003) and nonhuman primate (DeLong et al., 1985; Matsumura et al., 1992; Wichmann et al., 1994). DBS of the dorsolateral sensorimotor area is thought to result in the best motor outcome (Herzog et al., 2004; Richardson et al., 2009). In addition to the dorsolateral sensorimotor territory, primate studies have revealed functional territories of the STN consisting of a more central associative territory, and a smaller medial limbic area (Parent and Hazrati, 1995; Yelnik, 2002). One explanation that has been proposed for verbal fluency declines following STN DBS surgery is that current spread to nonmotor regions of the STN affects cognitive functions (Saint-Cyr et al., 2000; Woods et al., 2002).
The present study employed computer modeling to depict the volume and location of activated tissue in the STN patients from the COMPARE trial. Our goal was to better characterize relationships between verbal fluency performance and stimulation of functional subregions of the STN. Although neurosurgeons generally attempt to target the lateral sensorimotor subregions of the STN, it is possible that electrical current may affect neural activity in the associative subregions which are directly adjacent to the sensorimotor subregions. Patient-specific DBS models provide an opportunity to quantify the spread of stimulation within the context of the STN anatomy and to address relationships between different stimulation conditions and clinical outcomes.
STN patients from the COMPARE study underwent verbal fluency testing in four randomized conditions (OFF stimulation and with stimulation of the optimal, dorsal, and ventral contacts) (Okun et al., 2009). Patient-specific DBS models were created for each stimulation condition (Butson et al., 2007). Four indices were generated from the patient-specific models, including: a) total volume of activated tissue (VTA); b) volume of activated tissue within the STN, c) volume of activated tissue outside of the STN (i.e., white matter), and d) proportion of volume overlap of activated tissue within the STN (PVO-STN). We investigated whether these indices were related to verbal fluency performance with stimulation at each contact. We hypothesized that direct stimulation of the ventral region would have adverse effects on verbal fluency performance, while stimulation of optimal or dorsal regions would either result in improvements or no change in verbal fluency performance.
Methods
Participants
The present study drew STN patients from the COMPARE clinical trial conducted at the University of Florida (Okun et al., 2009). The trial recruited 52 individuals with a diagnosis of idiopathic PD to undergo surgery for DBS of the STN (N=26) or GPi (N=26) as well as 10 PD control subjects who did not undergo surgery. Prior to DBS, all participants underwent intensive baseline screening that included a diagnosis of PD by strict UK Brain Bank criteria, consultation with a neurology movement disorders specialist for medication optimization, consultation with a movement disorders neurosurgeon, a complete neuropsychology profile, and a psychiatry consultation.
Inclusion criteria for the clinical trial consisted of: 1) Hoehn and Yahr stage II or worse when “off” medication; 2) Intractable, disabling motor fluctuations, dyskinesias, or freezing episodes; 3) Age between 30–75 years; 4) Unsatisfactory clinical response to maximal medical management (with trials of both higher and lower doses of antiparkinsonian drugs); 5) A stable and optimal medical regimen of antiparkinsonian drug therapy for at least three months prior to surgery; and 6) Right handedness.
Exclusion criteria included the following: 1) Clinically significant medical disease that would increase the risk of developing pre- or postoperative complications (e.g. significant cardiac or pulmonary disease, uncontrolled hypertension); 2) Evidence of secondary or atypical parkinsonism as suggested by the presence of a history of stroke(s), encephalitis, exposure to toxins or neuroleptics, neurological signs of upper motor neuron disease, cerebellar involvement, supranuclear gaze palsy, or significant orthostatic hypotension; 3) MRI scan with significant evidence of brain atrophy (i.e., enough atrophy to suspect a possibility of complications such as subdural hematoma, as determined by the neurosurgeon) or other abnormalities (e.g. lacunar infarcts or iron deposits in the putamen); 4) Dementia as evidenced by impairment in two neuropsychological domains and impaired or borderline neuropsychological function in one additional domain; 5) A major psychiatric disorder on the Structured Clinical Interview for DSM-IV (SCID-IV) including current Major Depression.
Seven months after unilateral DBS implantation, patients underwent neuropsychological and motor testing. Testing for the study was performed with stimulation at the clinically defined optimal contact (the one yielding the best improvement in motor symptoms upon UPDRS motor examination and the one selected by the clinical team to use for long-term stimulation) as well as with stimulation of the dorsal and ventral contacts and OFF stimulation. Testing was conducted off medications (withdrawn 12 hours prior to testing). The order of the testing conditions was chosen by a computer-generated random sequence. Aside from the DBS programmer, all investigators were blind to the sequence of conditions. There was a standardized ten-minute delay from the time of setting the stimulator to the time of testing in order to allow the patients time to adjust to each of the new settings.
Forty-five subjects (23 GPi and 22 STN) completed the clinical trial protocol. After initial DBS activation, repeated follow-up evaluations were performed, as needed, until the optimal chronic stimulation parameters and adjunctive PD medication regimen were determined. The present study analyzed each STN patient who had all the necessary data for model creation and good agreement between the intended stereotactic DBS electrode implantation location and the location determined from post-operative CT. Patient demographic characteristics are shown in Table 1. The measured lead locations from computed tomography/magnetic resonance imaging fusion and the mean chronic deep brain stimulation parameters are described by Okun et al. (2009).
Table 1.
Demographic and disease characteristics of the study participants. Means (±standard deviation) are shown.
| STN DBS | |
|---|---|
| Age | 59.8 (10.0) |
| Gender (% Male) | 69.2 |
| Disease Duration (years) | 13.3 (4.0) |
| Hoehn and Yahr “off” stage (percent) | 2: 8.3 |
| 2.5: 29.2 | |
| 3: 37.5 | |
| 4: 25.0 | |
| 5: 0.0 | |
| Mean LED before surgery | 935.9 (373.9) |
| Preoperative “on” UPDRS III score | 22.5 (8.2) |
| Preoperative “off” UPDRS III score | 45.2 (12.6) |
| Mini-Mental State Exam (max: 30) | 28.0 (1.8) |
| Dementia Rating Scale (max: 144) | 136.5 (7.0) |
| Beck Depression Inventory (max: 63) | 10.4 (5.9) |
| Verbal Fluency Task Scores (raw) | |
| Letter | 38.1 (11.7) |
| Semantic | 18.3 (4.7) |
LED- Levodopa equivalent dosage
Verbal Fluency Tasks
Verbal fluency tasks consist of letter fluency and semantic fluency. Letter fluency tasks require subjects to rapidly produce words beginning with a particular letter of the alphabet, excluding proper nouns and the same word with a different suffix (Benton et al., 1994). The test allows 60 seconds to generate words beginning with a particular letter. Semantic fluency tasks require subjects to rapidly produce words belonging to a particular category (e.g., animals). For each testing session, the total number of words produced under each of three letter conditions and one semantic condition was recorded. Alternate forms of the verbal fluency tasks (i.e., different letter combinations and different semantic categories) were employed in order to minimize practice effects. The order of the four stimulation testing conditions was randomized and the alternate forms were counterbalanced across the testing conditions.
Imaging and Surgical Procedure
Each patient received four MRI scans on the day prior to surgery: a three plane localizing scout, a T1-weighted 3D Magnetization Prepared-Rapid Acquisition Gradient Echo (MP-RAGE), a 3D T2-weighted Fluid Attenuated Inversion Recovery (FLAIR), and a T1-weighted 3D FGATIR (Sudhyadhom et al., 2009). The last three scans were each single volume whole brain scans). All scans were acquired on a clinical Siemens Allegra 3T MRI using a quadrature birdcage headcoil. The total scanning time for all four scans was 30 minutes. On the morning of the operation, a Cosman–Roberts–Wells (CRW) head ring was applied under local anesthesia and a high resolution stereotactic head computed tomography (CT) scan was performed.
During the surgical procedure, microelectrode recording (MER) techniques were used to develop a 3D map of the target structures. For each MER pass, cellular activity was recorded at millimeter intervals beginning at 30 mm above the selected target, and at submillimeter intervals as the microelectrode approached the target region. At each interval, the encountered region was determined by the recording neurologist (Dr. Okun) based on the sound and appearance of the recording and the depth at which it was observed. Each determination was represented in real-time as a color-coded point overlaid on an individual patient’s MRI at the corresponding stereotactic coordinates. In addition to single cell recordings, cellular firing in response to passive motion and sensory stimulation was used to delineate the somatotopic organization of the target structure. MER passes were translated into a linear map of structure and somatotopy that was then overlaid on the patient’s MRI. A final decision, based on this aggregate map, was made as to the best location to place the permanent DBS electrode. The decision-making process took into account both electrode tip location within the target region and the electrode’s proximity to regions near the target that might result in side effects when stimulated. Recording data from the MER passes were saved in a spreadsheet for later use in development of the patient-specific DBS models.
Patient Specific DBS Models
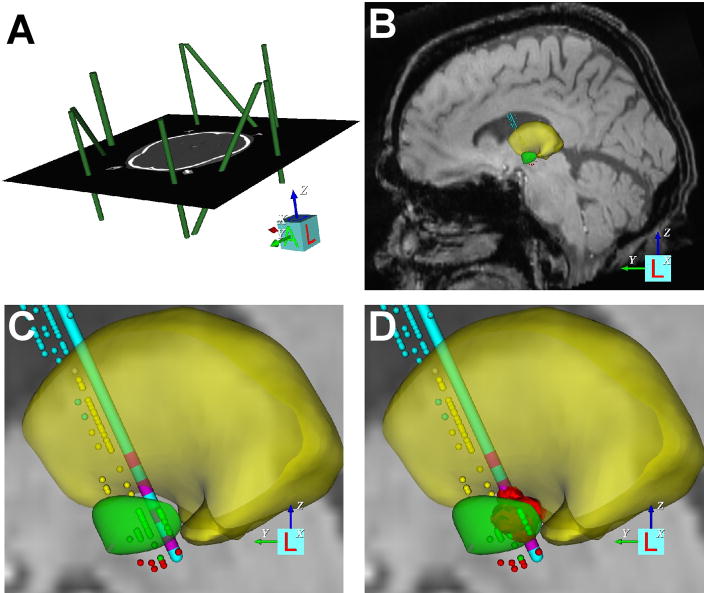
Each patient-specific DBS model was generated at the University of Florida (UF) with the use of a modified version of Human Cicerone v1.2 that was able to account for the CRW frame system (Miocinovic et al., 2007). The stimulation predictions were generated at the Cleveland Clinic Foundation (CCF) using a diffusion-tensor-based finite element model of the neural activation induced by DBS (Butson et al., 2007). Each patient-specific DBS model included co-registration of the magnetic resonance images (MRI), computed tomography (CT) scans, a 3D brain atlas, neurophysiological microelectrode recording (MER) data, DBS electrode, and the volume of tissue activated (VTA) for each clinically evaluated stimulation parameter setting (Fig. 1).
Figure 1.
Patient-specific DBS model. (A) The CRW stereotactic coordinate system (green imaging fiducials within the context of the pre-operative CT) was defined relative to the imaging data. (B) Microelectrode recording data were entered into the model (thalamic cells, yellow dots; subthalamic cells, green dots; substantia nigra cells, red dots), and a 3D brain atlas was fitted to both the neuroanatomy and neurophysiology (yellow volume, thalamus; green volume, subthalamic nucleus). (C) The implanted DBS electrode location was defined in the model. (D) The VTA (red volume) for each clinically evaluated stimulation parameter settings was calculated.
Creation of each patient-specific DBS model followed a step-by-step procedure. First, the pre-operative CT image with the stereotactic frame was loaded into the software (Figure 1A). A virtual replica of the frame fiducial system (CRW frame) was positioned within the context of the CT scan, thereby defining the stereotactic coordinate system relative to the imaging data. The pre-operative CT scan was then co-registered with the pre-operative MRI scan. The next step consisted of scaling and positioning 3D anatomical representations of nuclei of interest (e.g. subthalamic nucleus, globus pallidus, thalamus, etc.) (Figure 1B). The anatomical nuclei were originally positioned within the context of the MRI based on the stereotactic definition of the AC and PC points. However, the atlas was typically translated, rotated, and/or scaled along the anterior/posterior, dorsal/ventral, and medial/lateral axes to enable the most accurate match possible with the MRI.
The stereotactic location of each intra-operative MER data point was entered into the model. The microelectrode recording locations were displayed in Cicerone as small spheres and color-coded according to the anatomical structure they represented (Figure 1C). Once MER data were entered, the 3D brain atlas of anatomical nuclei was further adjusted via 9 degrees of freedom. The goal was to use the anatomical information provided in the pre-operative MRI along with the neurophysiological information provided by the MER tracks to achieve the best possible fit. In situations where both MER and MRI data could not be maximally accommodated, the MER data took precedence. This decision was justified because the overriding goal was to orient atlas surfaces as accurately as possible with respect to the patient’s DBS electrode, and the relative position of the MER data was the basis for the surgical DBS electrode placement.
Completion of the preceding steps produced an anatomical model of the DBS electrode placement in accordance with the patient’s pre-operative MRI scan and MER data. This anatomical model of electrode placement was checked against the post-operative CT scan to verify correspondence between the surgically intended placement and the final resting place of the electrode relative to the skull. Many factors can impact discrepancies between these two measures of electrode placement (brain shift, lead migration, etc.) that are beyond the scope of this study. Nonetheless, 3 of the 22 patients we analyzed had poor correspondence, even when considering the errors associated with image registration. Therefore, these 3 subjects were excluded from further analysis.
The next step in the process was to calculate the volume of tissue activated (VTA) for each stimulation parameter setting evaluated in each patient. This was accomplished by transferring the patient-specific anatomical models from UF to CCF and then performing the DBS neural activation calculations using the DBS model described by Butson et al. (Butson et al., 2007). The 3D atlas surfaces used in Cicerone were originally fitted to a diffusion tensor atlas brain image (DTI brain atlas) (Wakana et al., 2004). This DTI brain atlas represents the basis for the Butson et al. (2007) 3D finite element model of the electric field generated by DBS. The fitted 3D brain atlas surfaces used in each patient’s anatomical model, and their corresponding electrode location, were reverse transformed into the context of the DTI brain atlas and calculations of the volume of tissue activated (VTA) were performed (Maks et al., 2009). The VTA can be interpreted as a region where the axons that pass through the volume will generate propagating action potentials at the stimulation frequency (Figure 1D). The VTA methodology used in Butson et al. (2007) is principally applicable to monopolar stimulation. Two subjects from the COMPARE trial were clinically programmed with bipolar stimulation; these two subjects were excluded from the analysis in this study.
The patient-specific DBS models generated quantitative predictions on the spread of stimulation for each clinically evaluated stimulation parameter setting (Figure 2). The specific predictions consisted of the total VTA, and the VTA that occurred inside and outside of the STN. Finally, there may be some variation in total volumes of the STN between patients, because the brain atlas was scaled to fit the nuances of each patient. Thus, we calculated the VTA as a proportion of the total STN volume (i.e. proportion of VTA overlap with the STN, PVO-STN).
Figure 2.
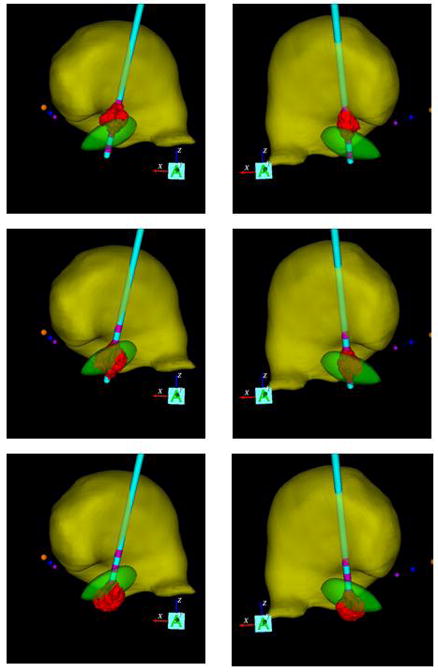
Coronal view of two patient-specific DBS models. The left and right columns show patients with their DBS electrode implanted in their left or right hemisphere, respectively. The red volumes of activation generated by DBS at the dorsal, optimal, and ventral electrode contacts are displayed in each column. Anatomical nuclei (yellow volume – thalamus; green volume – STN) are also depicted relative to the AC (yellow sphere), PC (purple sphere), and MCP (blue sphere).
Results
Patient-specific DBS models were created and analyzed for 17 of the 22 STN DBS patients in the COMPARE trial (Okun et al., 2009). Three patients were excluded because of discrepancies in their post-operative electrode location, and two patients were excluded due to their use of bipolar stimulation settings. Nine of the 17 patients underwent left STN DBS and 8 underwent right DBS. Figure 2 displays example patient-specific models for a patient with left STN DBS and a patient with right STN DBS.
Relationship of the VTA to Verbal Fluency Performance
Verbal fluency indices of interest included the total words generated in both letter and semantic conditions. Verbal fluency indices are expressed as standardized residual change scores with stimulation (dorsal, ventral, or optimal contact) relative to OFF stimulation. Positive values reflect higher than predicted scores with stimulation; negative values reflect lower than predicted scores. Standardized residual change scores were chosen over raw change scores to control for baseline differences in verbal fluency performance. Mean standardized residual change scores for each of the stimulation conditions are displayed in Table 2. Univariate ANOVAs revealed that there were no differences in verbal fluency performance among the three electrode contacts [letter fluency: F (2,56) = 0.043, p = 0.96, ηp2 =0.01, power =0.06; semantic fluency: F (2,56) =0.03, p = 0.97, ηp2 = 0.01, power = 0.05].
Table 2.
Mean (SD) standardized residual change scores of verbal fluency performance at each of the three stimulation conditions relative to OFF stimulation. Negative values indicate lower than predicted scores obtained with stimulation relative to OFF stimulation, and positive values indicate higher than predicted scores.
| Optimal | Dorsal | Ventral | |
|---|---|---|---|
| Letter Fluency | 0.17 (3.00) | −0.63 (2.57) | 0.05 (3.46) |
| Semantic Fluency | −0.09 (3.56) | −0.17 (3.67) | −0.73 (2.96) |
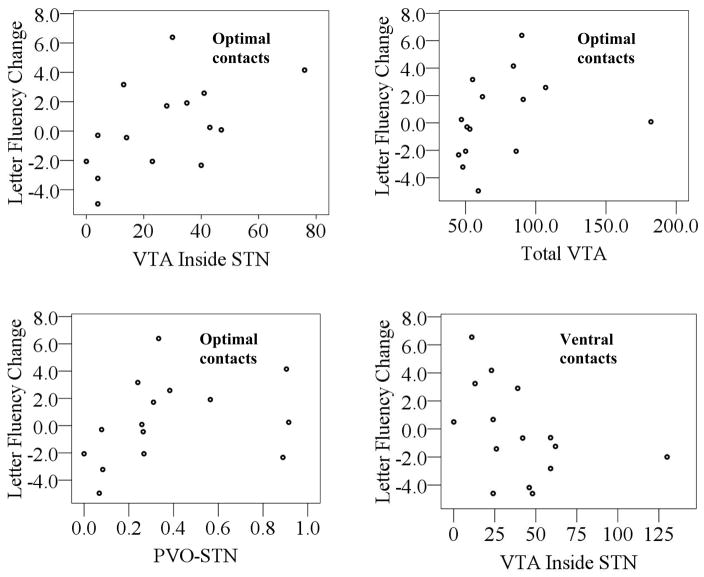
Mean values for patient-specific DBS modeling indices at each of the three contacts are shown in Table 3. Spearman’s correlations were conducted to examine the relationship between verbal fluency performance and these modeling indices, because the data were not normally distributed. Correlation coefficients are shown in Table 4, and the significant relationships are displayed as scatter plots in Figure 3. With optimal stimulation, there was a positive correlation between the VTA inside the STN and letter fluency change scores. That is, larger VTA inside the STN for optimal stimulation was associated with improved letter fluency performance relative to OFF stimulation. This relationship remained at trend level after removal of the outlier apparent in Figure 3 (Spearman’s rho = 0.44, p = 0.10). Additionally, at optimal contacts letter fluency change scores were positively correlated with the total VTA and the percent of VTA overlap with the STN. The relationship between letter fluency change scores and the total VTA remained at trend level after removal of the outliers apparent in Figure 3 (Spearman’s rho = 0.50, p = 0.06).
Table 3.
Mean (standard deviation) values for volume of tissue activated (VTA) variables, expressed in mm3.
| VTA in the STN | VTA outside STN | Total VTA | PVO-STN | |
|---|---|---|---|---|
| Dorsal (N=17) | 9.06 (16.52) | 56.65 (37.91) | 65.71 (29.72) | 0.18 (0.33) |
| Optimal (N=17) | 29.78 (21.47) | 42.33 (31.96) | 72.11 (32.94) | 0.43 (0.33) |
| Ventral (N=17) | 36.39 (30.08) | 22.17 (20.98) | 58.56 (44.73) | 0.63 (0.25) |
Table 4.
Spearman’s rho correlation coefficients for the relationship of verbal fluency performance with stimulation at different contacts to patient-specific modeling indices.
| VTA inside STN | VTA outside STN | Total VTA | PVO-STN | |
|---|---|---|---|---|
| Letter fluency standardized residual change | ||||
| Dorsal (N=14) | 0.19 | 0.02 | 0.09 | 0.18 |
| Optimal (N=16) | 0.54* | 0.02 | 0.52* | 0.51* |
| Ventral (N=15) | −0.60* | 0.57 | −0.24 | −0.31 |
| Category fluency standardized residual change | ||||
| Dorsal (N=14) | 0.37 | −0.42 | −0.34 | 0.36 |
| Optimal (N=16) | 0.42 | −0.09 | 0.27 | 0.35 |
| Ventral (N=15) | 0.10 | −0.15 | −0.06 | 0.18 |
Indicates significance at the p<0.05 level.
Note. Negative change values indicate lower than predicted scores obtained with stimulation relative to OFF stimulation, and positive values indicate higher than predicted scores.
Figure 3.
Scatter plots of significant relationships between VTA indices and verbal fluency change scores relative to OFF stimulation. Negative change values indicate lower than predicted scores obtained with stimulation relative to OFF stimulation, and positive values indicate higher than predicted scores.
With ventral stimulation, there was a negative correlation between the VTA inside the STN and letter fluency change scores. That is, more VTA inside the STN for ventral stimulation was associated with worse letter fluency performance relative to OFF stimulation. This relationship remained significant after removal of the outlier apparent in Figure 3 (Spearman’s rho = −0.60, p = 0.024).
Discussion
Declines in verbal fluency tasks following STN DBS are thought to be due, in part, to a surgical/lesional effect (Morrison et al., 2004; Okun et al., 2009; Pillon et al., 2000). There is a lack of existing evidence in the literature supporting stimulation effects on verbal fluency performance. Indeed, in the present study, total verbal fluency output did not differ with stimulation of each of four conditions (dorsal, ventral, optimal, off). It must be noted that testing ON stimulation reveals the effect of stimulation as well as surgery and is not purely a measure of stimulation. Nevertheless, relationships between tissue activation and letter fluency performance were detected in the present study through the use of patient-specific stimulation models. Specifically, with ventral stimulation, a larger volume of tissue activated (VTA) inside the STN was associated with worse letter fluency performance relative to the OFF stimulation condition. On the other hand, with optimal stimulation, more VTA inside of the STN was associated with better letter fluency performance relative to the OFF stimulation condition.
Verbal Fluency and DBS
Despite previous observations that the predominant effect underlying DBS associated verbal fluency declines were lesional (Morrison et al., 2004; Okun et al., 2009; Pillon et al., 2000), the data from this study suggest that there may be additional stimulation-induced effects depending on the locus and volume of activated tissue. However, the lack of support for stimulation effects may have to do with the nature of the testing procedure, and also whether stimulation was applied unilaterally or bilaterally. For instance, Alberts et al. (Alberts et al., 2008) tested bilateral STN DBS patients on a cognitive test (n-back) under single task and dual task conditions. The dual task condition involved performing the cognitive task simultaneously with a motor task (force tracking) and was meant to better represent the conditions under which individuals performed cognitive activities in their daily lives. Under relatively simple dual-task conditions there were no differences in cognitive or motor performance with unilateral or bilateral stimulation. As dual-task complexity increased, cognitive and motor performances were worse with bilateral compared to unilateral stimulation or when compared to OFF stimulation. These findings suggested that it is probably important to assess verbal fluency performance under conditions that may include increased task complexity, a dual task paradigm involving a motor component, and bilateral stimulation. These more rigorous assessments may reveal that stimulation can directly affect cognition and specifically verbal fluency.
The patients in the present study underwent unilateral DBS surgery, which is important because it provided confidence that the behavior effects of DBS were the result of the single site of stimulation. However, sample size limitations prevented direct comparison of left and right DBS patient groups. Zahodne et al. (Zahodne et al., 2009) showed in a larger sample size that left sided surgery was associated with a greater risk for decline in semantic fluency performance when compared to right. Therefore, future larger studies should examine the relationship between laterality, tissue activation and verbal fluency performance.
In the present study, the relationships of certain tissue activation indices were noted with letter fluency performance, but not with category fluency. It has been proposed that category fluency relies more on temporal lobe functioning and letter fluency more on frontal-subcortical structures (Baldo et al., 2006; Birn et al., 2009; Rascovsky et al., 2007). Given that many of the cortical afferents to STN originate from motor and frontal areas (Inase et al., 1999; Nambu et al., 1997), letter fluency may be more susceptible to STN stimulation effects than category fluency.
Sub-Divisions of STN
Observations of cognitive change following STN DBS (Parsons et al., 2006; Rothlind et al., 2007; Saint-Cyr et al., 2000; Smeding et al., 2006) have raised questions about whether cognitive effects result from spread of stimulation to neighboring subcortical structures or whether these changes can be traced to within the STN itself. Primate studies have revealed functional territories of the STN consisting of a dorsolateral sensorimotor area, a more central associative territory, and a smaller medial limbic area (Parent and Hazrati, 1995; Yelnik, 2002). The STN, as a primary relay in the indirect pathway, is thought to exert powerful excitatory action upon basal ganglia output structures through these separate functional subsystems (Parent and Hazrati, 1995). Our findings support the notion that functional subregions of the STN itself exert an influence on cognitive performance. In particular, activation in the ventral associative region resulted in negative effects on verbal fluency performance, while activation of the dorsolateral sensorimotor area, associated with the best motor outcome, was also positively associated with letter fluency performance. Thus, our findings were consistent with the known functional architecture of the STN.
York et al. (York et al., 2009) also found supportive evidence for the hypothesis that the STN itself may be responsible for cognitive and behavioral change following DBS. They found that patients whose active electrodes were closer to the approximated STN seemed more likely to show verbal learning, memory, and verbal fluency declines at six months following surgery. However, they attempted to derive multiple comparisons on only 17 patients, and therefore were underpowered to draw firm conclusions on the effects of trajectory and lead location to cognitive outcomes. Additionally, localization was performed by one rater using only imaging data and was not confirmed by physiology or any other measures. The methods of our study attempted to integrate a number of additional techniques (i.e. imaging data, intra-operative mapping data, and a 3D brain atlas) to better define the relative location between the electrode and the STN. Further, we utilized detailed predictions of the VTA to identify statistically significant relationships between STN DBS and verbal fluency.
Our findings may be relevant to the clinician charged with DBS programming. While previous research seems to indicate that it is the surgical procedure, and not stimulation per se that adversely affects verbal fluency performance, our findings indicate that there may be subtle effects of stimulation consistent with the STN’s functional architecture. Stimulation within the region that yields optimal motor benefits may also yield the best outcome for verbal fluency performance. On the other hand, stimulation within the ventral associative region may have adverse effects on verbal fluency performance. Thus, if a patient demonstrates significant declines in verbal fluency performance following STN DBS surgery, it may be useful to evaluate whether optimal motor outcome is being achieved, and to move one contact dorsally.
Limitations
The patient-specific DBS models used in this study relied on the most advanced neurostimulation prediction techniques currently available. However, DBS modeling technology is in its infancy, and therefore has numerous limitations. For example, 3 of the 22 patients were excluded from analysis because their models revealed poor correspondence between the surgically intended placement and the final position of the electrode relative to the skull as revealed by CT scan. Many factors can impact discrepancies between these two measures of electrode placement (brain shift, lead migration, etc.), and further work is necessary to clarify their nature. It should also be noted that the co-registration of multiple images and atlas representations of the patient creates spatial variability that cannot be ignored. It is difficult to estimate the exact errors associated with our models, but we attempted to minimize co-registration error by using easily identifiable landmarks such as the AC/PC and intense visual inspection of our co-registration results. Another issue is that, outside of histological reconstruction, it is impossible to know the exact size, shape, and location of the STN in a given patient (Hardman et al., 2002; Nowinski et al., 2006; Yelnik et al., 2007). We relied on a 3D atlas volume to represent the STN in our analysis. Clearly this simplifying assumption introduces some degree of error; however, we went to great efforts to fit each brain atlas to the recorded neurophysiology and MRI neuroanatomy of each patient. The VTA prediction functions used in our patient-specific DBS models were derived from the activation of straight, relatively large diameter (5.7 μm) myelinated axons, and may not be representative of the response of other neuron types surrounding the electrode (local projection neurons, local interneurons, afferent inputs, etc.). Given that myelinated axons are the most excitable neuron type to extracellular electrical stimulation (Ranck, 1975); our VTA predictions should be considered an upper limit in terms of stimulation spread. However, the stimulation predictions of our DBS models have been validated by both indirect (Butson et al., 2007) and direct (Miocinovic et al., 2009) measurements, and we have recently shown that prospective stimulation predictions from the models are capable of improving outcomes relative to traditional clinical DBS parameter selection (Frankemolle et al., 2010).
The present study contains a limited sample size, a problem inherent to many DBS outcome studies that limits the power to detect anything but very large effect sizes (Woods et al., 2006). Thus, our null findings may have been due to limited power. Additionally, we employed a 10-minute interval between the changing of stimulator settings and the onset of testing. This may have limited the ability to detect cognitive effects of stimulator settings, given that the time course of DBS effects is not entirely known.
In summary, the present study investigated stimulation effects on the verbal fluency task, which has been noted previously to consistently decline following STN DBS surgery. Stimulation of different electrode contacts within the STN did not affect total verbal fluency scores. However, models of activation revealed subtle relationships between the locus and volume of activated tissue and verbal fluency performance that were consistent with functional architecture of the STN. At ventral contacts, more tissue activation inside the STN was associated with worse letter fluency performance, but at optimal contacts, more tissue activation within the STN was associated with better letter fluency performance. Future studies should investigate stimulation effects on verbal fluency with regard to laterality, as well as to surgery-related factors such as the number of microelectrode passes. Future studies should also assess verbal fluency performance in the context of increased task complexity and dual task paradigms that more closely approximate daily functioning. These more stressful cognitive conditions will help to better characterize the contribution of the STN to cognitive performance.
Supplementary Material
Acknowledgments
This research was supported by a Center of Excellence grant from the National Parkinson Foundation, NIH/NINDS (PI: Okun, the COMPARE Trial, K23 NS044997; PI Bowers, R01 NS60533; PI McIntyre, R01 NS059736), the American Psychological Foundation (Benton-Meier Neuropsychology Scholarship), and the APA Divisions 40 and 20 (Walter G. McMillen Memorial Award for Parkinson’s Research).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberts JL, Voelcker-Rehage C, Hallahan K, Vitek M, Bamzai R, Vitek JL. Bilateral subthalamic stimulation impairs cognitive-motor performance in Parkinson’s disease patients. Brain. 2008;131:3348–3360. doi: 10.1093/brain/awn238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardouin C, Pillon B, Peiffer E, Bejjani P, Limousin P, Damier P, Arnulf I, Benabid AL, Agid Y, Pollak P. Bilateral subthalamic or pallidal stimulation for Parkinson’s disease affects neither memory nor executive functions: a consecutive series of 62 patients. Annals of Neurology. 1999;46:217–223. doi: 10.1002/1531-8249(199908)46:2<217::aid-ana11>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Baldo J, Schwartz S, Wilkins D, Dronkers N. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. Journal of the International Neuropsychological Society. 2006;12:896–900. doi: 10.1017/S1355617706061078. [DOI] [PubMed] [Google Scholar]
- Benton AL. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. 3. AJA Associates; Iowa City, IA: 1994. [Google Scholar]
- Birn R, Kenworthy L, Case L, Caravella R, Jones T, Bandettini P, Martin A. Neural systems supporting lexical search guided by letter and semantic category cues: a self-paced overt response fMRI study of verbal fluency. Neuroimage. 2009;49:1099–1107. doi: 10.1016/j.neuroimage.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5:135–140. [Google Scholar]
- Butson CR, Cooper SE, Henderson JM, McIntyre CC. Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage. 2007;34:661–670. doi: 10.1016/j.neuroimage.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR, Crutcher MD, Georgopoulos AP. Primate globus pallidus and subthalamic nucleus: functional organization. Journal of Neurophysiology. 1985;53:530–543. doi: 10.1152/jn.1985.53.2.530. [DOI] [PubMed] [Google Scholar]
- Dujardin K, Defebvre L, Krystkowiak P, Blond S, Destee A. Influence of chronic bilateral stimulation of the subthalamic nucleus on cognitive function in Parkinson’s disease. Journal of Neurology. 2001;248:603–611. doi: 10.1007/s004150170139. [DOI] [PubMed] [Google Scholar]
- Dujardin K, Krystkowiak P, Defebvre L, Blond S, Destee A. A case of severe dysexecutive syndrome consecutive to chronic bilateral pallidal stimulation. Neuropsychologia. 2000;38:1305–1315. doi: 10.1016/s0028-3932(00)00027-0. [DOI] [PubMed] [Google Scholar]
- Frankemolle A, Wu J, Noecker A, Voelcker-Rehage C, Ho J, Vitek J, McIntyre C, Alberts J. Reversing cognitive-motor impairments in Parkinson’s disease patients using a computational modelling approach to deep brain stimulation programming. Brain. 2010 doi: 10.1093/brain/awp315. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman C, Henderson J, Finkelstein D, Horne M, Paxinos G, Halliday G. Comparison of the basal ganglia in rats, marmosets, macaques, baboons, and humans: volume and neuronal number for the output, internal relay, and striatal modulating nuclei. The Journal of Comparative Neurology. 2002;445:238–255. doi: 10.1002/cne.10165. [DOI] [PubMed] [Google Scholar]
- Herzog J, Fietzek U, Hamel W, Morsnowski A, Steigerwald F, Schrader B, Weinert D, Pfister G, Muller D, Mehdorn HM, Deuschl G, Volkmann J. Most effective stimulation site in subthalamic deep brain stimulation for Parkinson’s disease. Movement Disorders. 2004;19:1050–1054. doi: 10.1002/mds.20056. [DOI] [PubMed] [Google Scholar]
- Inase M, Tokuno H, Nambu A, Akazawa T, Takada M, Miocinovic S, Lempka SF, Russo GS, Maks CB, Butson CR, Sakaie KE, Vitek JL, McIntyre CC. Corticostriatal and corticosubthalamic input zones from the presupplementary motor area in the macaque monkey: comparison with the input zones from the supplementary motor area. Brain Research. 1999;833:191–201. doi: 10.1016/s0006-8993(99)01531-0. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Ardouin CM, Brown RG, Rothwell JC, Obeso J, Albanese A, Rodriguez-Oroz MC, Moro E, Benabid AL, Pollak P, Limousin-Dowsey P. The impact of deep brain stimulation on executive function in Parkinson’s disease. Brain. 2000;123:1142–1154. doi: 10.1093/brain/123.6.1142. [DOI] [PubMed] [Google Scholar]
- Maks CB, Butson CR, Walter BL, Vitek JL, McIntyre CC. Deep brain stimulation activation volumes and their association with neurophysiological mapping and therapeutic outcomes. Journal of Neurology, Neurosurgery and Psychiatry. 2009;80:659–666. doi: 10.1136/jnnp.2007.126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura M, Kojima J, Gardiner TW, Hikosaka O. Visual and oculomotor functions of monkey subthalamic nucleus. Journal of Neurophysiology. 1992;67:1615–1632. doi: 10.1152/jn.1992.67.6.1615. [DOI] [PubMed] [Google Scholar]
- Miocinovic S, Lempka SF, Russo GS, Maks CB, Butson CR, Sakaie KE, Vitek JL, McIntyre CC. Experimental and theoretical characterization of the voltage distribution generated by deep brain stimulation. Experimental Neurology. 2009;216:166–176. doi: 10.1016/j.expneurol.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miocinovic S, Noecker AM, Maks CB, Butson CR, McIntyre CC. Cicerone: stereotactic neurophysiological recording and deep brain stimulation electrode placement software system. Acta Neurochirurgica Supplement. 2007;97:561–567. doi: 10.1007/978-3-211-33081-4_65. [DOI] [PubMed] [Google Scholar]
- Morrison CE, Borod JC, Perrine K, Beric A, Brin MF, Rezai A, Kelly P, Sterio D, Germano I, Weisz D, Olanow CW. Neuropsychological functioning following bilateral subthalamic nucleus stimulation in Parkinson’s disease. Archives of Clinical Neuropsychology. 2004;19:165–181. doi: 10.1016/S0887-6177(03)00004-0. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Inase M, Takada M, Akazawa T, Miocinovic S, Lempka SF, Russo GS, Maks CB, Butson CR, Sakaie KE, Vitek JL, McIntyre CC. Corticosubthalamic input zones from forelimb representations of the dorsal and ventral divisions of the premotor cortex in the macaque monkey: comparison with the input zones from the primary motor cortex and the supplementary motor area. Neuroscience Letters. 1997;239:13–16. doi: 10.1016/s0304-3940(97)00877-x. [DOI] [PubMed] [Google Scholar]
- Newcombe F. Missile Wounds of the Brain. Oxford University Press; London: 1969. [Google Scholar]
- Nowinski W, Liu J, Thirunavuukarasuu A. Quantification and visualization of the three-dimensional inconsistency of the subthalamic nucleus in the Schaltenbrand-Wahren brain atlas. Stereotactic and Functional Neurosurgery. 2006;84:46–55. doi: 10.1159/000093722. [DOI] [PubMed] [Google Scholar]
- Okun MS, Fernandez HH, Wu SS, Kirsch-Darrow L, Bowers D, Bova F, Suelter M, Jacobson CEt, Wang X, Gordon CW, Jr, Zeilman P, Romrell J, Martin P, Ward H, Rodriguez RL, Foote KD. Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: The COMPARE Trial. Annals of Neurology. 2009;65:586–595. doi: 10.1002/ana.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahwa R. Understanding Parkinson’s disease: an update on current diagnostic and treatment strategies. Journal of the American Medical Directors Association. 2006;7:4–10. [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Research. Brain Research Reviews. 1995;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Rogers SA, Braaten AJ, Woods SP, Troster AI. Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson’s disease: a meta-analysis. Lancet Neurology. 2006;5:578–588. doi: 10.1016/S1474-4422(06)70475-6. [DOI] [PubMed] [Google Scholar]
- Pillon B, Ardouin C, Damier P, Krack P, Houeto JL, Klinger H, Bonnet AM, Pollak P, Benabid AL, Agid Y. Neuropsychological changes between “off” and “on” STN or GPi stimulation in Parkinson’s disease. Neurology. 2000;55:411–418. doi: 10.1212/wnl.55.3.411. [DOI] [PubMed] [Google Scholar]
- Ranck JB., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Research. 1975;98:417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Salmon D, Hansen L, Thal L, Galasko D. Disparate letter and semantic category fluency deficits in autopsy-confirmed frontotemporal dementia and Alzheimer’s disease. Neuropsychology. 2007;21:20–30. doi: 10.1037/0894-4105.21.1.20. [DOI] [PubMed] [Google Scholar]
- Richardson R, Ostrem J, Starr P. Surgical repositioning of misplaced subthalamic electrodes in Parkinson’s disease: location of effective and ineffective leads. Stereotactic and Functional Neurosurgery. 2009;87:297–303. doi: 10.1159/000230692. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, Rodriguez M, Guridi J, Mewes K, Chockkman V, Vitek J, DeLong MR, Obeso JA. The subthalamic nucleus in Parkinson’s disease: somatotopic organization and physiological characteristics. Brain. 2001;124:1777–1790. doi: 10.1093/brain/124.9.1777. [DOI] [PubMed] [Google Scholar]
- Rodriguez RL, Miller K, Bowers D, Crucian G, Wint D, Fernandez H, Foote KD, Okun MS. Mood and cognitive changes with deep brain stimulation. What we know and where we should go. Minerva Medicine. 2005;96:125–144. [PubMed] [Google Scholar]
- Rothlind JC, Cockshott RW, Starr PA, Marks WJ., Jr Neuropsychological performance following staged bilateral pallidal or subthalamic nucleus deep brain stimulation for Parkinson’s disease. Journal of the International Neuropsychological Society. 2007;13:68–79. doi: 10.1017/S1355617707070105. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA, Albanese A. STN DBS in PD: selection criteria for surgery should include cognitive and psychiatric factors. Neurology. 2006;66:1799–1800. doi: 10.1212/01.wnl.0000227468.17113.07. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA, Trepanier LL, Kumar R, Lozano AM, Lang AE. Neuropsychological consequences of chronic bilateral stimulation of the subthalamic nucleus in Parkinson’s disease. Brain. 2000;123 ( Pt 10):2091–2108. doi: 10.1093/brain/123.10.2091. [DOI] [PubMed] [Google Scholar]
- Schroeder U, Kuehler A, Lange KW, Haslinger B, Tronnier VM, Krause M, Pfister R, Boecker H, Ceballos-Baumann AO. Subthalamic nucleus stimulation affects a frontotemporal network: a PET study. Annals of Neurology. 2003;54:445–450. doi: 10.1002/ana.10683. [DOI] [PubMed] [Google Scholar]
- Smeding HM, Speelman JD, Koning-Haanstra M, Schuurman PR, Nijssen P, van Laar T, Schmand B. Neuropsychological effects of bilateral STN stimulation in Parkinson disease: a controlled study. Neurology. 2006;66:1830–1836. doi: 10.1212/01.wnl.0000234881.77830.66. [DOI] [PubMed] [Google Scholar]
- Sudhyadhom A, Haq I, Foote K, Okun M, Bova F. A high resolution and high contrast MRI for differentiation of subcortical structures for DBS targeting: the Fast Gray Matter Acquisition T1 Inversion Recovery (FGATIR) Neuroimage. 2009;47:T44–52. doi: 10.1016/j.neuroimage.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Theodosopoulos P, Marks WJ, Christine C, Starr P. Locations of movement-related cells in the human subthalamic nucleus in Parkinson’s disease. Movement Disorders. 2003;18:791–798. doi: 10.1002/mds.10446. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus. I. Functional properties in intact animals. Journal of Neurophysiology. 1994;72:494–506. doi: 10.1152/jn.1994.72.2.494. [DOI] [PubMed] [Google Scholar]
- Witt K, Pulkowski U, Herzog J, Lorenz D, Hamel W, Deuschl G, Krack P. Deep brain stimulation of the subthalamic nucleus improves cognitive flexibility but impairs response inhibition in Parkinson disease. Archives of Neurology. 2004;61:697–700. doi: 10.1001/archneur.61.5.697. [DOI] [PubMed] [Google Scholar]
- Wojtecki L, Timmermann L, Jorgens S, Sudmeyer M, Maarouf M, Treuer H, Gross J, Lehrke R, Koulousakis A, Voges J, Sturm V, Schnitzler A. Frequency-dependent reciprocal modulation of verbal fluency and motor functions in subthalamic deep brain stimulation. Archives of Neurology. 2006;63:1273–1276. doi: 10.1001/archneur.63.9.1273. [DOI] [PubMed] [Google Scholar]
- Woods SP, Fields JA, Troster AI. Neuropsychological sequelae of subthalamic nucleus deep brain stimulation in Parkinson’s disease: a critical review. Neuropsychology Review. 2002;12:111–126. doi: 10.1023/a:1016806711705. [DOI] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Conover E, Carey CL, Parsons TD, Troster AI. Statistical power of studies examining the cognitive effects of subthalamic nucleus deep brain stimulation in Parkinson’s disease. The Clinical Neuropsychologist. 2006;20:27–38. doi: 10.1080/13854040500203290. [DOI] [PubMed] [Google Scholar]
- Yelnik J. Functional anatomy of the basal ganglia. Movement Disorders. 2002;17:S15–21. doi: 10.1002/mds.10138. [DOI] [PubMed] [Google Scholar]
- Yelnik J, Bardinet E, Dormont D, Malandain G, Ourselin S, Tande D, Karachi C, Ayache N, Cornu P, Agid Y. A three-dimensional, histological and deformable atlas of the human basal ganglia. I. Atlas construction based on immunohistochemical and MRI data. Neuroimage. 2007;34:618–638. doi: 10.1016/j.neuroimage.2006.09.026. [DOI] [PubMed] [Google Scholar]
- York MK, Wilde EA, Simpson R, Jankovic J. Relationship between neuropsychological outcome and DBS surgical trajectory and electrode location. Journal of the Neurological Sciences. 2009;287:159–171. doi: 10.1016/j.jns.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Okun MS, Foote KD, Fernandez HH, Rodriguez RL, Kirsch-Darrow L, Bowers D. Cognitive declines one year after unilateral deep brain stimulation surgery in parkinson’s disease: A controlled study using reliable change. The Clinical Neuropsychologist. 2009;23:385–405. doi: 10.1080/13854040802360582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.