Abstract
This is the first report on the use of the Social Attribution Task – Multiple Choice (SAT-MC) to assess social cognitive impairments in schizophrenia. The SAT-MC was originally developed for autism research, and consists of a 64-second animation showing geometric figures enacting a social drama, with 19 multiple choice questions about the interactions. Responses from 85 community-dwelling participants and 66 participants with SCID confirmed schizophrenia or schizoaffective disorders (Scz) revealed highly significant group differences. When the two samples were combined, SAT-MC scores were significantly correlated with other social cognitive measures, including measures of affect recognition, theory of mind, self-report of egocentricity and the Social Cognition Index from the MATRICS battery. Using a cut-off score, 53% of Scz were significantly impaired on SAT-MC compared with 9% of the community sample. Most Scz participants with impairment on SAT-MC also had impairment on affect recognition. Significant correlations were also found with neurocognitive measures but with less dependence on verbal processes than other social cognitive measures. Logistic regression using SAT-MC scores correctly classified 75% of both samples. Results suggest that this measure may have promise, but alternative versions will be needed before it can be used in pre-post or longitudinal designs.
Keywords: social attribution, Theory of Mind, mentalizing, social cognition, Schizophrenia, neuropsychology
1. Introduction
Social functioning deficits are among the most prominent features of schizophrenia and play a large role in the individual’s level of disability and the likelihood of relapse. Social cognition, or how an individual processes, interprets, and responds to social information, has repeatedly been shown to be impaired in schizophrenia (Bora et al., 2009; Brune and Brune, 2005; Corcoran et al., 1995a; Edwards et al., 2002; Fiszdon et al., 2009; Penn et al., 2008; Silverstein, 1997) and to be associated with various components of functioning (Cohen et al., 2009; Couture et al., 2006; Hooker and Park, 2002; Mueser et al., 1996; Penn et al., 1996; Pinkham and Penn, 2006). While some studies suggest that social cognition may mediate the relationship between other variables (e.g. neurocognition) and functional outcomes, other studies indicate that social cognition may also account for a unique portion of the variance in predicting functional outcomes (Addington et al., 2006; Bell et al., 2009; Brekke et al., 2005; Brekke et al., 2007; Dickinson et al., 2007; Kee et al., 2003; Meyer and Kurtz, 2009; Vauth et al., 2004). The interrelationship between social cognition and functional outcomes has led researchers to suggest that social cognition may be a good proximal treatment target for interventions aimed at improving functional outcomes in schizophrenia (Horan et al., 2008).
A number of laboratory measures have been developed to assess social cognitive function. Most of these measures have focused on narrowly defined social cognitive processes such as ability to recognize affect, identify interrelationships and clues in social situations, gauge social rules and expectations, draw inferences about the causes of events, or identify the intentions, dispositions and mental states of other people (Green et al., 2008). While the majority of affect recognition measures have focused on an individual’s ability to recognize or differentiate emotions from photograph stills, other social cognitive measures most frequently rely on written or videotaped vignettes of social situations, where the examinee is asked to make guesses about the relationship of characters to each other, make guesses about how characters may be feeling or what they may be thinking, or make guesses about what caused specific events.
Recently experts concluded that most measures of social cognition have poor or unknown psychometric properties (Green et al., 2008). Specifically, it has been suggested that variables such as dependence on verbal ability (for example having to read short stories and answer questions about them), the explicit nature of tasks, and scoring issues reduce their usefulness in capturing impairments in understanding spontaneous complex social situations (Klin, 2000). It has also been suggested that many of the existing social cognitive tasks fail to measure some variables that do in fact affect real-life performance, such as whether specific skills are actually employed in social situations, whether the individual is able to focus on relevant aspects of social situations, and whether the individual is capable of assimilating various pieces of social information (D’Zurilla and Maydeu-Olivares, 1995; Klin, 2000). Finally, existing social cognitive measures have also been critiqued for how narrowly they target specific social cognitive skills. This may limit their ecological validity, since real-world situations require a combination of different social cognitive processes as well as allow for informational redundancies occurring through multiple sources of information and multiple modes of presentation (Bazin et al., 2009; Bellack et al., 1996; Yager et al., 2006).
The Social Attribution Task (SAT) is a measure of social inference that has been proposed to overcome some of the weaknesses of social cognitive assessments noted above. The stimulus for the SAT is based on a 1944 Heider and Simmel (Heider and Simmel, 1944) silent cartoon animation showing moving geometric figures. Because the task is a silent cartoon, it does not reply on verbal ability, which may offer a purer characterization of social cognitive deficits separate from linguistic skill or verbal memory. When originally developed and tested, it was noted that nearly every subject experienced the cartoon figures as animate beings enacting a social drama. This task has since been adapted by Klin, who created a scoring procedure for narratives made to this animation, which has been shown sensitive to social cognitive deficits in adolescents and adults with Asperger’s Syndrome and high functioning autism (Klin, 2000; Klin et al., 2006). These deficits were unrelated to age, verbal IQ, or metalinguistic skill. Based on these results, Klin created a multiple choice version of the task (SAT-MC), which further reduced the task’s dependence on verbal ability.
This is the first study using SAT-MC in adults with schizophrenia. The multiple-choice version was selected over the narrative approach because it eliminates rating error and allows for easier use across studies. To determine whether this task could be useful for schizophrenia research, we wished to determine its discriminant validity by comparing scores from an urban community mental health center (CMHC) sample with those of an urban community-dwelling sample. We also wished to determine its degree of convergent validity with other measures of social cognition, exploring the extent to which it may share variance with these measures, while perhaps capturing features of the illness that have here-to-fore not been measured. We also wanted to determine its divergent validity from neurocognitive measures. Although we expected that neurocognitive processes would have some association with this new social cognitive measure as they have with other social cognitive measures used in schizophrenia research, we speculated that because the task does not require the examinee to remember verbal content, it might make performance less dependent upon verbal ability, something which we have identified as a problem with other social cognitive measures we have used such as the Hinting Task (Greig et al., 2004), and particularly the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT) (Wexler et al., 2009). Finally, we wished to determine the classification accuracy of this new measure in being able to categorically distinguish our schizophrenia sample from the community comparison group alone and in combination with other social cognitive measures.
Based on these study aims, we hypothesized that: 1) our schizophrenia sample would perform significantly worse than our comparison group on the SAT-MC; 2) SAT-MC performance of our schizophrenia sample would correlate with other social cognitive measures but have only a moderate degree of shared variance; 3) SAT-MC scores for our schizophrenia sample would have modest relationships with neurocognitive measures, particularly with verbal memory tasks; and, 4) schizophrenia and community samples could be accurately classified based on SAT-MC scores alone, and classification accuracy could be increased by using a combination of SAT-MC scores with other social cognitive measures.
2. Methods
2.1 Participants
Participants were 66 adult outpatients with Diagnostic and Statistical Manual of Mental Disorders, 4th revision (DSM-IV) (American Psychiatric Association and Task Force on DSM-IV, 1994) diagnosis of schizophrenia or schizoaffective disorder, as confirmed by the Structured Clinical Interview (SCID) (First, 1996). The participants were recruited from an urban community mental health center for an on-going study of cognitive training and supported employment (Clinical Trials.gov #NCT00339170). Participants were required to meet criteria for clinical stability (no hospitalizations, emergency room visits, homelessness or substance abuse in the past 30 days) and have an interest in returning to work. Other exclusion criteria included evidence of current neurological disease, brain injury or developmental disability. English proficiency was also required. Demographic and illness characteristics of these 66 participants are presented in Table 1. Four subjects were not included in analyses of neurocognition (n = 62) because they were recruited from an earlier version of the parent study that used somewhat different measures. These four subjects plus 1 subject with uncollected symptom data are not included in the symptom analyses (n = 61).
Table 1.
SAT-MC Participants Characteristics
| (n=66) | n (%) | n (%) | |
|---|---|---|---|
| GENDER | ETHNICITY | ||
| Male | 40 (60.6) | African American | 40 (60.6) |
| Female | 26 (39.4) | Caucasian | 24 (36.4) |
| Hispanic | 1 (1.5) | ||
| Other | 1 (1.5) | ||
| SCHIZOPHRENIA DIAGNOSIS | MEDICATIONS | ||
| Disorganized | 2 (3.0) | Atypical | 44 (66.7) |
| Paranoid | 28 (42.4) | Conventional | 8 (12.1) |
| Residual | 11 (16.7) | Both | 5 (7.6) |
| Undifferentiated | 8 (12.1) | None | 9 (13.6) |
| Schizoaffective | 16 (24.2) | ||
| Psychosis Disorder NOS | 1 (1.5) | ||
| (n=65) | MEAN (SD) | MEAN (SD) | |
| PANSS | AGE | 42.73 (10.4) | |
| Total | 64.94 (15.4) | EDUCATION | 12.58 (2.6) |
| Positive | 15.37 (5.1) | AGE AT 1st HOSP | 23.45 (9.2) |
| Negative | 16.20 (6.9) | LIFETIME # HOSP | 8.63 (10.7) |
| Cognitive | 16.52 (4.8) | ||
| Hostility | 6.69 (3.0) | ||
| Emotional Discomfort | 8.75 (3.6) | ||
| SANS | |||
| Total | 35.18 (19.5) | ||
| SAPS | |||
| Total | 29.11 (19.0) | ||
In selecting a comparison group, we sought to recruit participants who were not identified as having a psychiatric illness and who would be similar to our schizophrenia participants in being an ethnically diverse sample of urban dwellers of similar age, education and socioeconomic status. Following approval by our local Institutional Review Board (IRB) we recruited participants who were students in two urban community college classes, and who after reading a description of the study that explained that we were collecting data to represent a non-psychiatric comparison sample, felt that they qualified. Thus, there was no individual screening for psychopathology, and it is likely that had individual screening been performed some of these participants might have been excluded. Therefore, this is an unscreened representative community sample. As a group, they were somewhat younger than our schizophrenia sample (mean age = 31.72 (8.58)), but were similar in education (mean = 13.8 (1.50)) and similar in ethnic composition (42% Caucasian). Unexpectedly, they differed in having a higher percentage of women (88%).
2.2 Measures
Social cognitive measures included:
The Social Attribution Task – Multiple Choice version (SAT-MC)
Developed by Klin (Klin, 2000; Klin et al., 2006) this task is comprised of a 64 second animation created by Heider and Simmel (Heider and Simmel, 1944) in which a large triangle, small triangle and small circle enact a social drama (Interested readers can find the original on YouTube “Heider and Simmel Movie.”). The animation is shown twice and then short segments are presented followed by multiple-choice questions about the actions depicted. In all, 19 questions are asked with 4 possible responses to each. For example, after being shown a short segment, the respondent is presented with the question: What is the little triangle trying to do?” and given these choices: “1. It wants to help the little circle. 2. It wants to help the big triangle. 3. It wants to play with the little circle and with the big triangle. 4. It wants to lock the door.”
The Bell-Lysaker Emotion Recognition Task (BLERT) (Bell et al., 1997)
This affect perception task consists of 21 short video clips in which an actor displays one of seven emotions with three neutral monologues, and the examinee is asked to decide what emotion the actor is portraying. A total correct score of 1–14 is categorized as indicating impairment and 15–21 as unimpaired.
The Hinting Task (Corcoran et al., 1995b)
This is a Theory of Mind measure consisting of 10 brief scenarios that describe an interaction between two people. At the end of the scenario one of the characters drops an obvious hint (e.g. “Jane, I’d love to wear that blue shirt, but it’s very wrinkled”), and the examinee is asked what was meant by the hint. We revised the original Hinting Task to American English. Of relevance to this report, we found the Hinting Task to be significantly correlated with story memory (Wechsler Memory Scale (Wechsler, 1987) Logical Memory I, r = .42, p < .000; Logical Memory II, r = .43, p < .000) and with the cognitive component of the Positive and Negative Syndrome Scale (Kay et al., 1987) (r = .42, p < .000) (Greig et al., 2004).
Bell Object Relations Reality Testing Inventory (BORRTI) (Bell, 1995)
This is a self-report measure with 90 true/false items assessing 4 dimensions of object relations and 3 dimensions of reality testing. It was developed initially for schizophrenia research and has been found to have strong psychometric properties in a wide variety of applications and to have cross-cultural validity (Li and Bell, 2008). The Egocentricity scale, in particular, has been linked with performance measures of social cognition (Bell et al., 2009). Examples of items on this scale include: I believe a good mother should always please her children (True); People are never honest with each other (true); Others frequently try to humiliate me (True).
MATRICS Social Cognition Index
This is comprised of scores from the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT) (Mayer et al., 2002) Emotion Management Task (Section D) and Social Management Task (Section H). The tasks require respondents to evaluate how effective different actions would be in achieving an outcome involving other people (e.g. how effective would calling friends or eating healthy be in making someone feel better). Relevant to this report, we have found that there is a large verbal ability contribution to these scores, with Logical Memory I scores correlating r = 0.63 to both Emotion Management and Social Management (Wexler et al., 2009).
Neurocognitive measures were selected to provide both broad information about separate cognitive domains and overall neurocognitive function as well as provide more specific information about verbal memory function. Neurocognitive and symptom measures included: MATRICS Consensus Cognitive Battery (MCCB) (Nuechterlein and Green, 2006): Index scores for Speed of Processing, Attention and Vigilance, Working Memory, Verbal Learning, Visual Learning, Reasoning, and Neurocognitive Composite Score (average of Index scores excluding Social Cognition).
Wechsler Adult Intelligence Scale-III (WAIS-III) (Wechsler, 1997)
Scaled scores for Vocabulary, Digit Span, Block Design, Matrix Reasoning.
Wechsler Memory Scale, revised (Wechsler, 1987)
Scaled Scores for Logical Memory I, Logical Memory II, and Mental Control.
Wisconsin Card Sorting Task (WCST) (Heaton, 1981)
Scaled scores for Total Errors, Perseverative Errors, Non-perseverative errors, and Conceptual Level.
Symptom assessments were performed at intake by Ph.D. level clinical psychologists who had been trained to high levels of inter-rater agreement (Intra Class Correlation = 0.82 to 0.93). Measures included the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) with 5 component scoring (Bell et al., 1994) and the Schedule for the Assessment of Positive Symptoms (SAPS) (Andreasen, 1984b) and Schedule for the Assessment of Negative Symptoms (SANS) (Andreasen, 1984a).
2.3 Procedures
Following written informed consent, schizophrenia participants were individually administered all measures over several assessment sessions. Neurocognitive assessments were usually performed over at least two testing sessions, but additional breaks were taken if there was concern about the participant’s fatigue or alertness. Symptom evaluations and social cognitive measures were generally performed on different assessment days.
The community sample was provided with a description of the study by their classroom teacher. Group administration of the SAT-MC, BORRTI, BLERT and Hinting Task was done by study personnel during a single classroom session. Community participants were not administered any neurocognitive or symptom measures. For the SAT-MC, a large TV monitor placed at the front of the 40-person classroom displayed the task, and each student had a copy of the answer sheet. The SAT-MC was paused at each questions and administration did not resume until it was clear that all participants had circled a response. All forms were reviewed to determine that they had been filled out correctly and with sincere intent (e.g. not all items scored false).
2.4 Data Analysis
Internal consistency of items on the SAT-MC was assessed using Cronbach’s apha and Spearman-Brown coefficient for split half reliability. Distributions of SAT-MC scores were examined separately for the schizophrenia and community samples. These distributions and a receiver operating characteristic (ROC)curve analysis were used to determine the best SAT-MC cut-off score for sensitivity and specificity to the schizophrenia sample. Demographic variables were compared using t-tests and chi-square analyses. Partial correlations, correcting for group membership, were used to determine the relationship of age, education and gender to SAT-MC scores. Discriminant validity between samples was determined using analysis of covariance (ANCOVA), controlling for age, education and gender. Relationship of convergent and divergent validity measures to SAT-MC scores was determined using bivariate correlations. Only standardized scores were used in correlational analyses and were assumed to meet parametric assumptions. Categorical agreement between BLERT impaired/unimpaired and SAT-MC impaired/unimpaired was determined using chi-square, percent agreement, Kappa and Eta. Logistic regression was used to determine the classification accuracy of SAT-MC and of SAT-MC combined with the other social cognitive measures (BLERT, Hinting Task, Egocentricity). All tests were two-tailed and alpha was set at .05. Because of the exploratory nature of this study, correlations were not corrected for multiple comparisons.
3. Results
3.1 Internal Consistency
SAT-MC items were evaluated for internal consistency for both samples combined. Cronbach’s Alpha for item to scale consistency was .83. Split-half reliability using Spearman-Brown coefficient was .75.
3.21 Discriminant validity
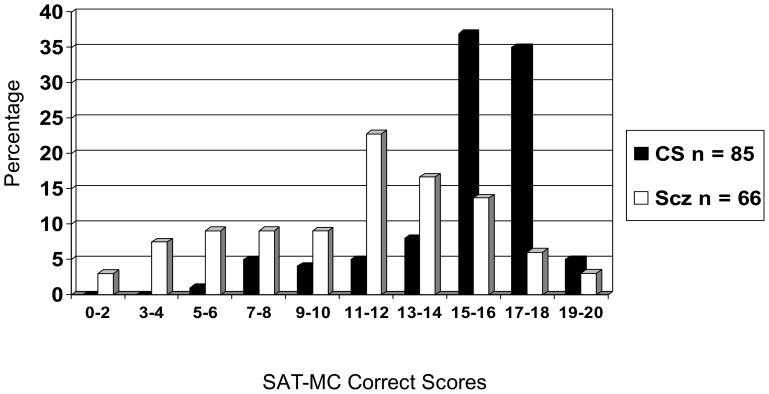
The distributions of SAT-MC scores for both samples are presented in Figure 1.
Figure 1.
SAT-MC Correct Score Distributions for Community Sample (CS) and Schizophrenia Sample (Scz).
Correct scores for the schizophrenia sample are normally distributed with a mean, median and mode of 11, standard deviation of 4, and a range from 2 to 19. The community sample has a mean, median and mode of 15, standard deviation of 3, with 6 outliers with scores below 9.
Demographic differences were not significant for education, but the community comparison sample had a significantly higher proportion of women (88% versus 39%) and were significantly younger (32 versus 43 years of age). Controlling for group membership, ANCOVA did not reveal a significant difference on SAT-MC scores by gender (F(1,147) = 1.36, p = 0.25). Men had a slightly higher mean score (mean = 14.0) than women (mean = 13.11). Age was also not significantly correlated with SAT-MC performance (r = −0.13, p = 0.13). To be conservative, between groups analysis of variance was performed with age, education and gender as covariates. Group differences were statistically significant (F (1,144) = 20.26, p < 0.001). As expected, the covariates in the model were not significant. Removing the demographics from the model, the adjusted R squared was 0.26, indicating that 26% of the between-group variance was explained by SAT-MC performance.
An examination of the distribution of SAT-MC scores and its ROC curve led us to choose the SAT-MC score of 11 as the cut-off for impairment. This score identified 53% of the schizophrenia sample as having an impairment as compared to 9.4% of the community sample (Chi-square(1) = 34.7, p <0.001). A score of 12 also produced good discrimination (61% schizophrenia, 14% community sample), but we felt that specificity was to be preferred over sensitivity.
3.3 Convergent validity
Significant bivariate correlations were found between SAT-MC correct scores for the combined samples with BLERT (r = 0.47, p < 0.001) and with Hinting Task (r = 0.37, p < 0.001). SAT-MC scores were not significantly correlated with the BORRTI subscales Alienation (r = −0.143, p = 0.08), Insecure Attachment (r = −0.05, p = ns) or Social Incompetence (r = −0.07, p = ns). However, they were significantly correlated with Egocentricity (r = −0.37, p < 0.001); that is, better performance on SAT-MC was associated with more pathology on Egocentricity.
For the schizophrenia sample alone, SAT-MC scores had a weaker but similar pattern of correlations to that of the combined samples. They were significantly correlated with BLERT (r = 0.37, p = 0.002), but did not reach significance with Hinting task (r = 0.23, p = 0.07) or with Egocentricity (r = −0.21, p = 0.10). SAT-MC scores were significantly correlated with the Social Cognition index score on the MATRICS (r = 0.29, p = 0.02).
A categorical examination of the relationship between impairment on the SAT-MC and BLERT showed 65% agreement (Chi-square (1) = 5.9, p < 0.05; Kappa = 0.30; Eta = 0.44). Twenty-eight of the 35 (80%) schizophrenia participants identified as SAT-MC impaired were also BLERT impaired, and 15 (48%) of the 31 who were SAT-MC unimpaired were also BLERT unimpaired.
3.4 Divergent Validity
For the schizophrenia sample, correlations between SAT-MC correct scores and neurocognitive variables are presented in Table 2. The strongest correlation is with Matrix Reasoning, a non-verbal problem-solving task; there is also a modest relationship to WCST, which also involves non-verbal problem-solving. SAT-MC scores are about equally correlated with Vocabulary and Block Design, which suggests that IQ (not specifically measured here) probably affects performance. Logical Memory I is significantly correlated, but Logical Memory II, which involves delayed recall, is not. SAT-MC scores have modest significant correlations with MATRICS indices of Working Memory and Reasoning and Problem Solving (based on Mazes, also a non-verbal task) and with the Neurocognitive Composite score. SAT-MC was not significantly correlated with any symptom measures (PANSS Positive, r = 0.08; Negative, r = 0.08; Cognitive, r = 0.07; Hostility, r = 0.16; Emotional Discomfort, r = −0.05; SANS, r = 0.09; SAPS, r = −0.07). SAT-MC was also not significantly correlated with reality testing impairments as measured by the BORRTI (Reality Distortion, r = 0.04; Uncertainty of Perception, r = −0.10; Hallucinations and Delusions, r = −0.10).
Table 2.
Correlations between SAT-MC correct scores and neurocognitive measures in the schizophrenia sample
| WAIS-IV Scaled Scores (n = 62) | Mean | SD | r | p |
|---|---|---|---|---|
| Vocabulary | 8.3 | 3.0 | .38 | .003 |
| Digit Span | 8.8 | 2.7 | .29 | .02 |
| Letter Number Sequencing | 7.3 | 3.3 | .37 | .003 |
| Symbol Search | 6.5 | 2.6 | .18 | ns |
| Digit Symbol Coding | 6.1 | 2.1 | .26 | .04 |
| Block Design | 8.4 | 2.9 | .36 | .004 |
| Matrix Reasoning | 8.7 | 3.3 | .47 | .000 |
| WMS Scaled Scores (n = 62) | ||||
| Logical Memory I | 7.9 | 3.1 | .31 | .01 |
| Logical Memory II | 9.1 | 2.7 | .19 | ns |
| Mental Control | 7.6 | 3.1 | .26 | .05 |
| WCST Standard Scores (n = 62) | ||||
| Total Errors | 85.6 | 16.2 | .31 | .02 |
| Perseverative Errors | 86.5 | 17.4 | .25 | ns |
| Non-perseverative Errors | 87.5 | 14.5 | .34 | .007 |
| Conceptual Level | 86.7 | 15.4 | .32 | .01 |
| *MATRICS T Scores (n =62) | ||||
| Speed of Processing | 35.5 | 10.3 | .22 | ns |
| Attention and Vigilance | 34.0 | 12.3 | .23 | ns |
| Working Memory | 34.3 | 13.7 | .32 | .01 |
| Verbal Learning | 36.0 | 7.6 | .24 | ns |
| Visual Learning | 36.1 | 12.0 | .18 | ns |
| Reasoning and Problem Solving | 41.7 | 9.8 | .28 | .03 |
| Neurocognitive Composite | 36.3 | 8.0 | .34 | .008 |
Social Cognition Index is excluded because it is a social cognition measure. Therefore, overall composite, which includes Social Cognition is also excluded.
3.5 Classification Accuracy
Logistic Regression using SAT-MC scores to predict group membership (Community Comparison sample vs. Schizophrenia) was highly significant (Chi-square (1) = 44.6, p < 0.001). Sensitivity to schizophrenia was 60.6%, specificity was 77.0%, and overall classification accuracy was 74.8% (Table 3). This analysis was repeated adding Hinting Task, BLERT, and Egocentricity scores, which increased classification accuracy slightly to 78%.
Table 3.
Logistic Regression using SAT-MC scores to predict group membership (Community sample (CS) vs. Schizophrenia (Scz)
| Predicted | |||
|---|---|---|---|
| Observed | Scz | CS | Total |
| Scz | 40 | 26 | 66 |
| CC | 12 | 73 | 85 |
| 52 | 99 | 151 | |
Chi-Sq (1) = 44.6, p < 0.001
Sensitivity = 60.6; Specificity = 77.0; Correctly Classified = 74.8%
4. Discussion
This is the first report using the Social Attribution Test in its multiple choice form as a possible measure of social cognition for schizophrenia research. We found that schizophrenia participants had significantly poorer scores than our community sample, who had not been screened for psychopathology. Despite this lack of screening, a cut-off score for impaired functioning on the SAT-MC successfully distinguished our schizophrenia sample from the community sample with more than half the schizophrenia sample designated as having impairment while fewer than 10% of the community sample participants were so designated. We did not expect to find social cognitive deficits in all people with schizophrenia and recognize these deficits as a likely and important source of heterogeneity within the disorder, so it should not be expected that SAT-MC scores would distinguish all schizophrenia participants from the community comparison participants. It is also the case that such deficits are related to other disorders such as Asperger’s Syndrome, so it may be that some of the community comparison participants with poor SAT-MC scores may have a psychiatric condition that affects social cognition and thus were not strictly speaking “false positives”.
Convergent validity for the instrument was sought by examining its relationship to other social cognitive measures and to a self-report measure of object relations. For those measures that were administered to both the community comparison and the schizophrenia participants, the greatest amount of shared variance was with our measure of affect recognition. Most participants with schizophrenia who were SAT-MC impaired were also BLERT impaired, although many who were BLERT impaired were not SAT-MC impaired.
Other highly significant relationships were also found with our theory of mind task and with the self-report measure of Egocentricity. With the more restricted range of scores within the schizophrenia sample, the associations were weaker than for the combined samples but the pattern was similar. In addition, the Social Cognition Index from the MATRICS battery was significantly correlated with SAT-MC scores. These findings lend some support to the construct validity of the SAT-MC as a measure of social cognition, but the associations are moderate at best. This may be because the SAT-MC is capturing features of social cognition (e.g. anthropomorphizing, metacognitive creation of coherent social narrative, and social attribution) that are related to but distinct from affect recognition, theory of mind, social problem-solving, or self-experience.
While it is generally agreed that social cognition relies on some features of neurocognition (e.g. attention, working memory, problem-solving), it is hoped that a social cognitive measure would be relatively independent from basic cognition. This is of particular concern because the Social Cognition Index of the MATRICS and the Hinting Task have both shown highly significant dependence on verbal processes, particularly story memory (Wexler et al., 2009). The SAT-MC had a modest, but significant relationship to Logical Memory I as it did to a number of verbal and non-verbal tasks. Interestingly, the strongest relationship was with Matrix Reasoning, suggesting that correctly interpreting the actions of the geometric figures in the SAT-MC may share some of the same problem-solving processes required for solving the geometric sequences in Matrix Reasoning. Based on the MATRICS index scores, it does appear that the SAT-MC is relatively independent of Speed of Processing, Attention and Vigilance, and Verbal and Visual Learning, and that the total contribution of neurocognition based on the Neurocognitive Composite is relatively modest.
The SAT-MC was found to have no significant correlation with any symptom domain measured by the PANSS and by the SANS and SAPS. These findings are inconsistent with the existing literature linking social cognition to severity of various symptoms such as paranoid delusions (Bentall et al. 2009; Lysaker et al. 2009; Martin and Penn, 2002; Peer et al., 2004) as well as negative and/or disorganized symptoms (Garety and Freeman, 1999; Greig et al., 2004; Corcoran et al., 1995b). It is possible that our divergent results may be due to the relatively low level of symptoms in this outpatient, clinically stable sample, a hypothesis supported by literature indicating that social cognitive impairments are still found in remitted patients (Bora et al., 2008; Bora et al., 2009; Sprong et al., 2007). We found that the SAT-MC was also not significantly correlated with any dimensions of self-reported deficits in reality testing. Thus, the scores of the SAT-MC are indicating a process that distinguishes many people with schizophrenia from the community comparison group but that is relatively unrelated to symptoms.
The robust relationship between SAT-MC scores and schizophrenia was shown in its classification accuracy. When SAT-MC scores were used in a logistic regression to predict group membership, about 75% of participants were correctly classified, a highly significant finding. Adding other social cognitive measures to the model did not increase the prediction very much, suggesting that the SAT-MC on its own is a powerful predictor of group membership.
Taken together, these findings offer encouragement for further use of SAT-MC in schizophrenia research. The instrument showed strong discriminant validity, shared variance with other social cognitive measures, particularly affect recognition, and modest relationships to most measures of neurocognition. In particular, it was relatively independent of story memory as measured by Logical Memory. Thus, the SAT-MC would appear to have promise as an additional tool for exploring social cognition in schizophrenia.
There are a number of important limitations to this study. The community comparison sample was not ideal. Unpredictably, there was a higher proportion of women than men, and although this task does not appear to be sensitive to gender differences, it would have been better to have had no gender differences between samples. Additionally, the administration format (group versus individual) differed between the schizophrenia and community samples. While this potentially may have affected performance, if anything, we would speculate that the individual administration would lead to better performance in the schizophrenia sample and worse performance in the community comparison sample. Thus, if there were an affect, it would have, decreased the true difference in performance of the two samples and makes the differences we did find all the more compelling. We also do not know very much about the psychiatric status of the community sample, except that they did not exclude themselves. Thus, the differences between our samples may be a conservative estimate of the true difference between people with schizophrenia and those without mental illness of any kind. Future studies should explore differences between schizophrenia and other forms of psychopathology where social cognition is not expected to be impaired (e.g. late onset depression) to further clarify how distinctive this impairment is in schizophrenia.
A final consideration is that this is not a repeatable instrument. Once a respondent has answered the 19 questions, he or she may have acquired some ideas about what has occurred in the animation, which may influence subsequent administrations. Therefore, test-retest reliability cannot be easily established. For the same reason, it is likely not useful as a pre-post measure in intervention trials aimed at improving social cognition. In future research, we hope to create a second version of the task, matched for difficulty with the current version, which will extend the SAT-MC’s usefulness for longitudinal studies and interventions research. We also hope to learn whether there are certain patterns of errors that can be associated with deficits in anthropomorphizing, weaknesses in metacognition of social narrative, or attribution bias. This may help us better understand whether the types of errors made by people with schizophrenia are similar to or different from those made by people with other disorders, particularly by those with Autism spectrum disorders.
Acknowledgments
We wish to acknowledge Ami Klin, Ph.D., Director of the Autism Program, Professor of Child Psychology, Yale Child Study Center, for his generously providing us with the SAT-MC for this study.
Role of Funding Source: This study was funded by the NIMH grant R01 MH061493-01 awarded to Bell and Wexler, Research Career Scientist award and Career Development Award from the VA Rehabilitation Research and Development Service, awarded to Bell and Fiszdon, respectively. Neither sponsor contributed in the study design, in the collection, analysis or interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Footnotes
Conflict of Interest: There are no conflicts of interest for any of the authors of this paper. No author has any possible financial gain for the findings presented here.
Contributors: Dr. Bell designed the study, planned and executed the statistical analysis and wrote the first draft. Dr. Fiszdon managed the literature searches, helped plan the study, collected the community control data, and drafted the introduction. Dr. Greig assisted in planning the study, was the project director, and collected data. Dr. Wexler designed the study and participated in its oversight. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addington J, Saeedi H, Addington D. Facial affect recognition: a mediator between cognitive and social functioning in psychosis? Schizophrenia Research. 2006;85:142–150. doi: 10.1016/j.schres.2006.03.028. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders: DSM-IV. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Andreasen NC. Scale for the assessment of negative symptoms (SANS) University of Iowa; Iowa City, IA: 1984a. [Google Scholar]
- Andreasen NC. Scale for the assessment of positive symptoms (SAPS) University of Iowa; Iowa City, IA: 1984b. [Google Scholar]
- Bazin N, Brunet-Gouet E, Bourdet C, Kayser N, Falissard B, Hardy-Bayle MC, Passerieux C, Bazin N, Brunet-Gouet E, Bourdet C, Kayser N, Falissard B, Hardy-Bayle MC, Passerieux C. Quantitative assessment of attribution of intentions to others in schizophrenia using an ecological video-based task: a comparison with manic and depressed patients. Psychiatry Research. 2009;167:28–35. doi: 10.1016/j.psychres.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Bell M, Bryson G, Lysaker P. Positive and negative affect recognition in schizophrenia: a comparison with substance abuse and normal control subjects. Psychiatry Research. 1997 Nov 14;73(1–2):73–82. doi: 10.1016/s0165-1781(97)00111-x. [DOI] [PubMed] [Google Scholar]
- Bell MD. Manual for the Bell Object Relations Reality Testing Inventory. Western Psychological Services; Los Angeles, CA: 1995. [Google Scholar]
- Bell MD, Lysaker PH, Beam-Goulet JL, Milstein RM, Lindenmayer JP. Five- component model of schizophrenia: assessing the factorial invariance of the positive and negative syndrome scale. Psychiatry Research. 1994 Jun;199452(3):295–303. doi: 10.1016/0165-1781(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Bell MD, Tsang HW, Greig TC, Bryson GJ. Neurocognition, social cognition, perceived social discomfort, and vocational outcomes in schizophrenia. Schizophrenia Bulletin. 2009;35:738–747. doi: 10.1093/schbul/sbm169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellack AS, Blanchard JJ, Mueser KT. Cue availability and affect perception in schizophrenia. Schizophrenia Bulletin. 1996;22:535–544. doi: 10.1093/schbul/22.3.535. [DOI] [PubMed] [Google Scholar]
- Bentall RP, Rowse G, Shryane N, Kinderman P, Howard R, Blackwood N, Moore R, Corcoran R. The cognitive and affective structure of paranoid delusions: a transdiagnostic investigation of patients with schizophrenia spectrum disorders and depression. Archives of General Psychiatry. 2009;66:236–247. doi: 10.1001/archgenpsychiatry.2009.1. [DOI] [PubMed] [Google Scholar]
- Bora E, Gokcen S, Kayahan B, Veznedaroglu B. Deficits of social-cognitive and social-perceptual aspects of theory of mind in remitted patients with schizophrenia: effect of residual symptoms. Journal of Nervous and Mental Disease. 2008;196:95–99. doi: 10.1097/NMD.0b013e318162a9e1. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: meta- analysis. Schizophrenia Research. 2009;109:1–9. doi: 10.1016/j.schres.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Brekke J, Kay DD, Lee KS, Green MF. Biosocial pathways to functional outcome in schizophrenia. Schizophrenia Research. 2005;80:213–225. doi: 10.1016/j.schres.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Brekke JS, Hoe M, Long J, Green MF. How neurocognition and social cognition influence functional change during community-based psychosocial rehabilitation for individuals with schizophrenia. Schizophrenia Bulletin. 2007;33:1247–1256. doi: 10.1093/schbul/sbl072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune M, Brune M. “Theory of mind” in schizophrenia: a review of the literature. Schizophrenia Bulletin. 2005;31:21–42. doi: 10.1093/schbul/sbi002. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Nienow TM, Dinzeo TJ, Docherty NM. Attribution biases in schizophrenia: relationship to clinical and functional impairments. Psychopathology. 2009;42:40–46. doi: 10.1159/000173702. [DOI] [PubMed] [Google Scholar]
- Corcoran R, Mercer G, Frith CD. Schizophrenia, symptomatology and social inference: investigating “theory of mind” in people with schizophrenia. Schizophrenia Research. 1995 Sep;17(1):5–13. doi: 10.1016/0920-9964(95)00024-g. [DOI] [PubMed] [Google Scholar]
- Corcoran R, Mercer G, Frith CD. Schizophrenia, symptomatology and social inference: investigating “theory of mind” in people with schizophrenia. Schizophrenia Research. 1995b;17:5–13. doi: 10.1016/0920-9964(95)00024-g. [DOI] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophrenia Bulletin. 2006;32(Suppl 1):S44–63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Zurilla TJ, Maydeu-Olivares A. Conceptual and methodological issues in social problem-solving assessment. Behavior Therapy. 1995;26:409–432. [Google Scholar]
- Dickinson D, Bellack AS, Gold JM, Dickinson D, Bellack AS, Gold JM. Social/communication skills, cognition, and vocational functioning in schizophrenia. Schizophrenia Bulletin. 2007;33:1213–1220. doi: 10.1093/schbul/sbl067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J, Jackson HJ, Pattison PE. Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review.[erratum appears in Clin Psychol Rev. 2002 Nov;22(8):1267–85] Clinical Psychology Review. 2002;22:789–832. doi: 10.1016/s0272-7358(02)00130-7. [DOI] [PubMed] [Google Scholar]
- First MBSRL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders--Patient Edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Fiszdon JM, Bell MD, Fiszdon JM, Bell MD. Effects of presentation modality and valence on affect recognition performance in schizophrenia and healthy controls. Psychiatry Research. 2009;170:114–118. doi: 10.1016/j.psychres.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Garety PA, Freeman D. Cognitive approaches to delusions: a critical review of theories and evidence. British Journal of Clinical Psychology. 1999;38:113–154. doi: 10.1348/014466599162700. [DOI] [PubMed] [Google Scholar]
- Green MF, Penn DL, Bentall R, Carpenter WT, Gaebel W, Gur RC, Kring AM, Park S, Silverstein SM, Heinssen R, Green MF, Penn DL, Bentall R, Carpenter WT, Gaebel W, Gur RC, Kring AM, Park S, Silverstein SM, Heinssen R. Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophrenia Bulletin. 2008;34:1211–1220. doi: 10.1093/schbul/sbm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig TC, Bryson GJ, Bell MD. Theory of mind performance in schizophrenia: diagnostic, symptom, and neuropsychological correlates. Journal of Nervous & Mental Disease. 2004 Jan;2004192(1):12–8. doi: 10.1097/01.nmd.0000105995.67947.fc. [DOI] [PubMed] [Google Scholar]
- Heaton R. The Wisconsin Card Sorting Test Manual. Psychological Assessment Resources, Inc; Odessa, FL: 1981. [Google Scholar]
- Heider F, Simmel M. An experimental study of apparent behavior. American Journal of Psychology. 1944;57:243–259. [Google Scholar]
- Hooker C, Park S. Emotion processing and its relationship to social functioning in schizophrenia patients. Psychiatry Research. 2002;112:41–50. doi: 10.1016/s0165-1781(02)00177-4. [DOI] [PubMed] [Google Scholar]
- Horan WP, Kern RS, Green MF, Penn DL. Social Cognition Training for Individuals with Schizophrenia: Emerging Evidence. American Journal of Psychiatric Rehabilitation. 2008;11:205–252. [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kee KS, Green MF, Mintz J, Brekke JS. Is emotion processing a predictor of functional outcome in schizophrenia? Schizophrenia Bulletin. 2003;29:487–497. doi: 10.1093/oxfordjournals.schbul.a007021. [DOI] [PubMed] [Google Scholar]
- Klin A. Attributing social meaning to ambiguous visual stimuli in higher-functioning autism and Asperger syndrome: The Social Attribution Task. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2000;41:831–846. [PubMed] [Google Scholar]
- Klin A, Jones W, Klin A, Jones W. Attributing social and physical meaning to ambiguous visual displays in individuals with higher-functioning autism spectrum disorders. Brain & Cognition. 2006;61:40–53. doi: 10.1016/j.bandc.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Li S, Bell MD. BORRTI journal articles and annotated bibliography. Western Psychological Services; 2008. http://portal.wpspublish.com/portal/page?_pageid=53,70155&_dad=portal&_schema=PORTAL. [Google Scholar]
- Lysaker PH, Davis LW, Tsai J. Suspiciousness and low self-esteem as predictors of misattributions of anger in schizophrenia spectrum disorders. Psychiatry Research. 2009;166:125–131. doi: 10.1016/j.psychres.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Martin JA, Penn DL. Attributional style in schizophrenia: an investigation in outpatients with and without persecutory delusions. Schizophrenia Bulletin. 2002;28:131–141. doi: 10.1093/oxfordjournals.schbul.a006916. [DOI] [PubMed] [Google Scholar]
- Mayer JD, Salovey P, Caruso DR. Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT) Multi-Health Systems, Inc; Toronto, Ontario: 2002. [Google Scholar]
- Meyer MB, Kurtz MM. Elementary neurocognitive function, facial affect recognition and social-skills in schizophrenia. Schizophrenia Research. 2009;110:173–179. doi: 10.1016/j.schres.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueser KT, Doonan R, Penn DL, Blanchard JJ, Bellack AS, Nishith P, DeLeon J. Emotion recognition and social competence in chronic schizophrenia. Journal of Abnormal Psychology. 1996;105:271–275. doi: 10.1037//0021-843x.105.2.271. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF. Matrics Consensus Cognitive Battery Manual. The Regents of the University of California; Los Angeles, CA: 2006. [Google Scholar]
- Peer JE, Rothmann TL, Penrod RD, Penn DL, Spaulding WD. Social cognitive bias and neurocognitive deficit in paranoid symptoms: evidence for an interaction effect and changes during treatment. Schizophrenia Research. 2004;71:463–471. doi: 10.1016/j.schres.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Penn DL, Sanna LJ, Roberts DL. Social cognition in schizophrenia: an overview. Schizophrenia Bulletin. 2008;34:408–411. doi: 10.1093/schbul/sbn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn DL, Spaulding W, Reed D, Sullivan M. The relationship of social cognition to ward behavior in chronic schizophrenia. Schizophrenia Research. 1996;20:327–335. doi: 10.1016/0920-9964(96)00010-2. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Penn DL. Neurocognitive and social cognitive predictors of interpersonal skill in schizophrenia. Psychiatry Research. 2006;143:167–178. doi: 10.1016/j.psychres.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Silverstein SM. Information processing, social cognition, and psychiatric rehabilitation in schizophrenia.[comment] Psychiatry. 1997;60:327–340. doi: 10.1080/00332747.1997.11024810. [DOI] [PubMed] [Google Scholar]
- Sprong M, Schothorst P, Vos E, Hox J, van Engeland H. Theory of mind in schizophrenia: meta-analysis. British Journal of Psychiatry. 2007;191:5–13. doi: 10.1192/bjp.bp.107.035899. [DOI] [PubMed] [Google Scholar]
- Vauth R, Rusch N, Wirtz M, Corrigan PW. Does social cognition influence the relation between neurocognitive deficits and vocational functioning in schizophrenia? Psychiatry Research. 2004;128:155–165. doi: 10.1016/j.psychres.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler Memory Scale-Revised. Psychological Corporation; New York, NY: 1987. [Google Scholar]
- Wechsler D. WAIS-III Manual: Wechsler Adult Intelligence Scale-III. Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wexler BE, Zito W, Greig TC, Bell MD. Large verbal ability contribution to MSCEIT scores of social cognition in schizophrenia [abstract] Schizophrenia Bulletin. 2009;35:250. [Google Scholar]
- Yager JA, Ehmann TS, Yager JA, Ehmann TS. Untangling social function and social cognition: a review of concepts and measurement. Psychiatry. 2006;69:47–68. doi: 10.1521/psyc.2006.69.1.47. [DOI] [PubMed] [Google Scholar]