Abstract
Although model protocellular membranes consisting of monoacyl lipids are similar to membranes composed of contemporary diacyl lipids, they differ in at least one important aspect. Model protocellular membranes allow for the passage of polar solutes and thus can potentially support cell-to functions without the aid of transport machinery. The ability to transport polar molecules likely stems from increased lipid dynamics. Selectively permeable vesicle membranes composed of monoacyl lipids allow for many lifelike processes to emerge from a remarkably small set of molecules.
Monoacyl lipids—unlike the diacyl phospholipids bounding modern cells—are permeable to polar molecules. They may therefore have formed the membranes of primitive cells, which lacked transmembrane transporters.
Lipid bilayer membranes are an integral component of living cells, providing a permeability barrier that is essential for nutrient transport and energy production. It is reasonable to assume that a similar boundary structure would be required for the origin of cellular life (Szostak et al. 2001). Even though bilayer membranes are a cellular necessity, they also pose a significant obstacle to early cellular functions, the most obvious being that the permeability barrier would inhibit chemical exchange with the environment. Such an exchange is important not only for acquiring nutrient substrates for primitive metabolic processes, but also for the release of inhibitory side-products.
Contemporary cells circumvent the permeability problem by incorporating complex transmembrane protein machinery that provides specific transport capabilities. It is unlikely that Earth’s first cells assembled bilayer membranes together with specific membrane protein transporters. Rather, intermediate evolutionary steps must have existed in which simple lipid molecules provided many of the characteristics of contemporary membranes without relying on advanced protein machinery. What seems to have been necessary was the appearance of a simple membrane system capable of retaining and releasing specific molecules. In short, a protocell needed to be selectively permeable.
BACKGROUND
Membrane Structure
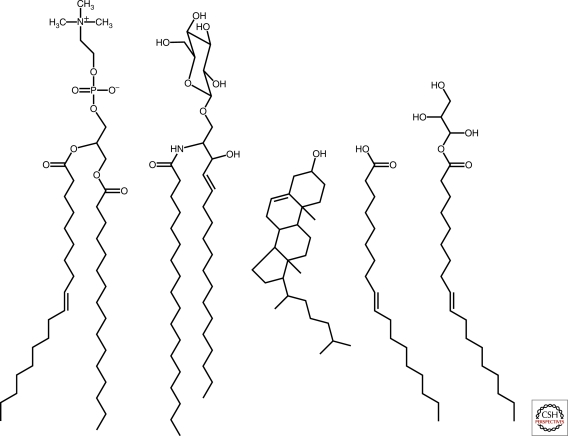
The structure of biological membranes has been extensively characterized, but extending this knowledge to protocellular membranes is problematic. Insight can be gained by comparing what is known of the structure and permeability of biological membranes with those derived from laboratory models of protocells. The lipid bilayers of present-day biological membranes consist primarily of diacyl lipids, such as glycerophospholipids and sphingolipids, in addition to other potential components including glycolipids (derivatives of either sphingo- or glycerophospholipids), sterols, and proteins (Fig. 1). A single bilayer membrane is approximately 5 nm thick (Wiener and White 1992), which corresponds to seven to nine amino acids of a β-strand or 20–25 amino acids of an α-helix (Nelson and Cox 2008). Within the membrane, lipids are arranged so that their hydrophilic head groups extend outward toward the bulk aqueous phase and their hydrophobic tails are buried in the nonpolar interior of the bilayer. The spatial organization of carbonyl ester dipole moments within the membrane produces a significant membrane dipole potential estimated to be +240 mV (Gawrisch et al. 1992; Peterson et al. 2002; Wang et al. 2006). The lipids that constitute biological membranes usually have hydrocarbon chains between 16 and 24 carbons long and have either zwitterionic or anionic head groups (Tanford 1980; Evans and Wennerstrom 1999). Unsaturated lipids have a cis double bond at carbon 9, which is the position within the hydrocarbon chain that optimally disrupts inter-acyl packing (Menger et al. 1988). The distribution of membrane lipids is asymmetric, with certain classes of lipids, e.g., glycolipids, predominating in one leaflet. Water molecules extensively interact with the hydrophilic exterior of the membrane (Gawrisch et al. 2007). The depth of water penetration extends past the head group and glycerol to the carbonyl groups, yielding a hydrophobic membrane interior of approximately 3 nm (Wiener and White 1992; Gawrisch et al. 2007). It should be emphasized that biological membranes also contain nonlipid components, with the mass of protein components often exceeding that of lipids (Singer 2004). In summary, for a solute to cross a lipid bilayer membrane of 5 nm, the solute must penetrate a 3-nm hydrophobic barrier.
Figure 1.
Lipid chemical structures. From left to right, phospholipid (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine), sphingolipid (glucosyl ceramide), sterol (cholesterol), fatty acid (oleate), and glycerol monoester of fatty acid (monoolein). Phospholipids, spingolipids, and sterols are common components of biological membranes, whereas fatty acids and glycerol monoesters of fatty acids are constituents of model protocellular membranes.
The prevalence of phosphate in lipid head groups is striking. With few exceptions (van Mooy et al. 2009), diacyl membrane lipids contain phosphate head groups, even though amphiphilic molecules that form bilayer membranes do not require the presence of phosphate (Israelachvili 1991; Evans and Wennerstrom 1999). Indeed, the importance of phosphate is a recurring theme in biology, with examples in lipids, nucleic acids (Benner and Hutter 2002), metabolism (Westheimer 1987; Deamer 1997), and cellular signaling (Tarrant and Cole 2009).
Prebiotic membrane composition and structure
The lipid composition of protocellular membranes is not known. However, inferences can be drawn from simulated prebiotic synthesis, geological and astronomical analyses, as well as data from constructed laboratory model systems. Thus far, interest has centered on fatty acid and fatty acid derivatives as plausible prebiotic membrane constituents. First, they can be synthesized under simulated prebiotic conditions via Fischer-Tropsch-type reactions (McCollom et al. 1999). Second, analysis of carbonaceous meteorites, such as the Murchison meteorite, has identified fatty acids as one of many complex organic molecules (Lawless and Yuen 1979). The latter should not necessarily be interpreted as evidence for seeding life on Earth through the extraterrestrial delivery of organic material. Rather, it shows that abiotic synthesis using molecules that were available on the prebiotic Earth can generate appreciable quantities of amphiphilic compounds such as fatty acids (Pizzarello 2006; Pizzarello 2007). Interestingly, organic extracts of the Murchison meteorite that contained a mixture of abiotically synthesized amphiphilc molecules self-assembled into vesicles in aqueous solution (Deamer 1985; Deamer and Pasley 1989).
Laboratory vesicle systems have shown that fatty acids alone can form bilayer membrane structures that are similar to the membranes composed of diacyl phospholipids (Gebicki and Hicks 1973; Gebicki and Hicks 1976; Hargreaves and Deamer 1978). From a structural perspective, the only difference is the presence of a single acyl chain in the interior, hydrophobic core of the membrane per hydrophilic head group. Other properties of membranes composed of fatty acids and phospholipids are similar. For example, both types of lipids generate similarly sized vesicles (Hargreaves and Deamer 1978) and require hydrocarbon chains of at least 10 carbons to form stable bilayers (Deamer 1997). Fatty acid and phospholipid membranes also are similar in thickness (Cistola et al. 1986), thermal stability (Mansy and Szostak 2008), and tensile strength (Chen et al. 2004). Finally, both kinds of membranes are capable of entrapping polymers (Walde et al. 1994) and retaining the activity of encapsulated enzymes (Walde et al. 1994; Chen et al. 2005). It should be noted that whereas meteorite samples show the presence of short, saturated fatty acids, laboratory model systems are often based on longer, unsaturated fatty acids. The data thus far show that many of the structural properties of fatty acid and phospholipid membranes are similar.
Lipid Dynamics
Membranes are highly fluid structures showing a wide range of lipid motions (Singer and Nicolson 1972; Gawrisch 2004). Within membranes, lipids can undergo trans-gauche isomerizations, rotate, wobble, laterally diffuse, and flip from one leaflet to another (Table 1). The dynamics of the carbons of a single lipid vary with respect to their position within the hydrocarbon chain, with greater dynamic motion observed toward the center of the membrane, i.e., near the terminal methyl groups, and less motility measured for the carbons near the surface of the membrane (McFarland and McConnell 1971). Lipid dynamics are influenced by acyl chain packing, head group interactions, and sterol content. For example, the presence of cholesterol can decrease lateral mobility by an order of magnitude (Rubenstein et al. 1979), decrease trans-gauche isomerization of the carbons near the head group, and increase the dynamics of terminal methyls for long acyl chain lipids (McIntosh 1978; Gawrisch 2004). Many of these smaller scale motions, such as trans-gauche isomerizations, are presumed to be similar between diacyl and monoacyl lipids, although differences likely exist.
Table 1.
Lipid dynamics within liquid-crystalline bilayer membranes.
| Motion type | Diacyl phospholipid | Monoacyl fatty acida | Reference |
|---|---|---|---|
| Trans-gauche isomerization | ps–ns | (Venable et al. 1993; Gawrisch 2004) | |
| Wobble | ns | (Klauda et al. 2008) | |
| Axial rotation | ns | (Klauda et al. 2008) | |
| Lateral diffusion | ns–ms | (Rubenstein et al. 1979; Gawrisch 2004; Kahya and Schwille 2006) | |
| Flip-flop | days | ms–s | (Kamp and Hamilton 1992; Chen and Szostak 2004b) |
| Escape | days | s | (Roseman and Thompson 1980; Fujikawa et al. 2005) |
aTrans-gauche isomerization, wobble, axial rotation, and lateral diffusion correlation times are presumed to be similar for fatty acid and phospholipid membranes.
Larger scale lipid motions, such as flip-flop, are influenced by acyl chain and head group compositions. Decreases in inter-acyl packing through unsaturation or branching increase lipid flip-flop rates (Armstrong et al. 2003; Chen and Szostak 2004a). Similarly, membranes in the more fluid, liquid-crystalline phase contain lipids that flip-flop faster than membranes in the gel phase. However, diacyl phospholipid flip-flop is greatest at the phase transition temperature in which packing defects presumably more strongly diminish acyl chain interactions (John et al. 2002). The lipid head group also influences flip-flop rates with highly polar head group containing lipids being incapable of flipping. Because fatty acids possess fewer acyl chains and a less polar head group than phospholipids, fatty acids flip-flop more quickly than phospholipids (Hamilton 2003; Chen and Szostak 2004b).
Lipid escape (exchange) dynamics represent an additional type of large-scale motion that is strongly influenced by hydrophobicity. Diacyl phospholipids have a large hydrophobic surface area that results in a substantial energetic barrier for the acyl chains of the lipid to move from the hydrophobic environment of the membrane’s interior to aqueous solution. Therefore, diacyl phospholipids do not readily exchange between vesicles or micelles and instead form kinetically trapped structures. The decreased hydrophobic surface area of monoacyl lipids poses a much lower energetic barrier for lipid escape, and so fatty acids rapidly equilibrate between vesicles and micelles. Therefore, fatty acids form highly dynamic aggregate structures that behave more like a system under thermodynamic rather than kinetic control (Luisi 2001). Aggregate systems containing mixtures of mono- and diacyl-lipids confirm this difference in dynamics (Fujikawa et al. 2005), which can be exploited for liposome generation by the detergent depletion method (Evans and Wennerstrom 1999). In short, monoacyl lipids, such as fatty acids, have higher escape and flip-flop dynamics than diacyl phospholipids, even though both monoacyl and diacyl lipids form structurally similar membranes.
RECENT RESULTS
Permeability Mechanisms
Although contemporary cells facilitate transport by using protein channels or carriers that provide less energetically costly paths for the solute to pass through the hydrophobic interior of the membrane, many small, neutral molecules such as water and carbon dioxide are able to cross the membrane without the aid of proteins (Inoue 1996). Permeability decreases with increasing molecular size, for instance, for solutes ranging in size from urea and glycerol up to glucose. The precise mechanism by which this occurs is not yet fully understood, as the route taken by the solute likely depends on a complex set of factors deriving from both the nature of the solute and the membrane. However, insight into the process has progressed considerably so that experimentally measured solute permeation rates can often be corroborated by mechanistically based computational modeling. Current permeability models take into account solute hydrophobicity, size, and shape along with membrane fluidity and packing density.
Solubility-Diffusion Model
Our current understanding of the barrier posed by lipid membranes is largely due to the work of Ernest Overton. His interpretation of permeability data is known as Overton’s rule and is simply a correlation between solute hydrophobicity, that is the solute’s ability to partition into the oily interior of the membrane, and permeation rates (Kleinzeller 1999). This remarkably simple correlation accurately predicts permeability trends for a large number of solutes and is incorporated into most currently used permeability models.
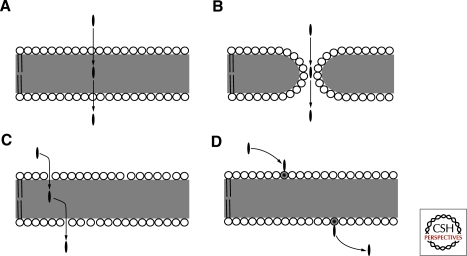
Overton’s rule forms the foundation of the solubility-diffusion permeability model (Fig. 2A). In essence, it is a combination of Overton’s hydrophobicity correlation with Fick’s first law of diffusion, which describes how molecules move down concentration gradients at a rate directly proportional to the magnitude of the gradient (Eisenberg and Crothers 1979). The mathematical expression of Fick’s first law is J =-D(dc/dx), in which J is the solute flux, D is the diffusion coefficient, c is the solute concentration, and x is the distance that the solute must traverse when it crosses the membrane. If the rate of diffusion through the interior of the membrane is taken to be the rate limiting step for solute flux through a membrane, then D represents the diffusion coefficient within the hydrophobic interior of the membrane and x becomes the hydrophobic thickness of the bilayer membrane (Paula and Deamer 1999). Also, because the total flux of a solute through a vesicle membrane is directly proportional to the surface area of the membrane, a term for the surface area (SA) of the membrane is added. Permeability is usually expressed as a permeability coefficient (P), rather than as solute flux, which more clearly shows the relationship between permeability and the properties of the solute and the membrane. P and J are related by P = −J/(c2−c1), in which c2−c1 represents the solute concentration gradient. Finally, to account for the ability of a molecule to dissolve in the hydrophobic region of the membrane, a partition coefficient (K) is included, which gives the more familiar form of the solubility-diffusion equation P = (K·D·SA)/x.
Figure 2.
Membrane transport mechanisms. (A) Solubility-diffusion model. (B) Transient pore model. (C) Head-group gated model. (D) Lipid flipping-carrier model. Circles represent lipid head groups and the gray area indicates the hydrophobic region occupied by lipid acyl chains. The black oval is the solute molecule.
This equation predicts a dependence on solute hydrophobicity, as more hydrophobic solutes can partition more effectively into the membrane, and a dependence on membrane thickness with thicker membranes having lower permeability coefficients. The model is consistent with a number of observations, working particularly well for neutral solutes like glycerol, urea, and ethanol up to hexoses. Membrane fluidity is taken into account by the diffusion term (D), because diffusion is dependent on solvent viscosity. Although the solubility-diffusion model appears well suited to describe a variety of solute permeability data, particularly for nonpolar molecules, the model does not take into consideration dynamic motion of the bilayer membrane structure (Paula et al. 1998).
Transient Pore Model
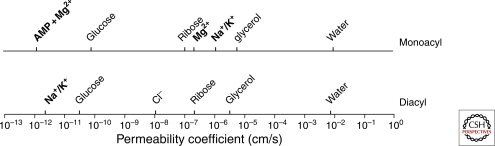
Although the elegantly simple solubility-diffusion model accurately predicts permeability trends for a large number of solutes, some inconsistencies between experimental data and theoretical modeling exist. For instance, ion permeability data are not consistent with the solubility-diffusion model. The major factor influencing ion permeation rates is their inability to partition into the nonpolar membrane phase, which is inhibited by the ion’s “self energy,” i.e., Born energy (Parsegian 1969). It is interesting that the encountered barrier is not the same for cations and anions. Because of the membrane’s intrinsic dipole potential, anions more readily cross the initial barrier of head groups than cations, resulting in greater anion permeability coefficients (Fig. 3).
Figure 3.
Comparison between solute permeability coefficients of liquid-crystalline diacyl phospholipid and monoacyl fatty acid membranes. Solutes in bold type permeate differently through both membranes. AMP permeation depends on the presence of Mg++. AMP and Mg++ permeability through diacyl phospholipid membranes does not occur within the time scale represented by the x-axis. Differences in permeability between monoacyl and diacyl lipid membranes are small for nonpolar solutes and large for polar solutes. (Data were compiled from Paula et al. 1996; Paula et al. 1998; Paula and Deamer 1999; Chen and Szostak 2004b; Chen et al. 2005; Sacerdote and Szostak 2005; Mansy et al. 2008.)
To account for this discrepancy, alternate models have been developed for ion permeation. One of these is known as the transient pore model (Fig. 2B), which invokes the opening of pores because of thermal fluctuations in membrane structure (Paula and Deamer 1999). If the transient pore is lined by lipid head groups or water molecules, then the Born energy barrier for ion permeation is removed, resulting in increased permeability coefficients (Paula et al. 1996). This alternate theory makes a variety of testable predictions that can be used in comparison with the solubility diffusion model. For instance, the creation of pores more strongly depends on membrane thickness because the required membrane thinning defects are more readily populated in membranes composed of lipids with short acyl chains (Paula et al. 1998). Permeability coefficients versus membrane thickness data for Na+ and K+ were consistent with a transient pore model rather than a solubility-diffusion model for phospholipids with short acyl chains, whereas acyl chains ≥18 carbons, showed permeation consistent with the solubility-diffusion model, particularly for anions (Paula et al. 1996).
Packing Defect Model
Packing defects have also been invoked as a mechanism by which solutes can pass through membranes. The differences, if any, are not clear as to what distinguishes transient pores from packing defects. Typically, packing defect mechanisms are used to describe the permeability of membranes composed of saturated lipids, whereas transient pores are typically used to describe processes occurring in membranes composed of lipids with at least one unsaturated acyl chain. Inconsistencies in permeability between these two types of membranes often have been observed (Xiang and Anderson 1998; Paula and Deamer 1999; Nagle et al. 2008). Nevertheless, packing defects are believed to exist for saturated lipid membranes at their phase transition temperature and result in dramatic increases in permeability (Nagle and Scott 1978; Jansen and Blume 1995). For example, even large ionic molecules, such as nucleotides, can traverse diacyl phospholipid membranes at the phase transition temperature of the membrane (Monnard and Deamer 2001; Cisse et al. 2007; Monnard et al. 2007).
Lipid Head Group-Gated Model
Despite the power of the earlier approaches in explaining solute permeability, inconsistencies continue to persist. For example, none of the models adequately take into account solute size and acyl chain ordering effects. This may in part be because of an overly simplified view of diffusion processes, particularly for solutes that diffuse through multiple phases. An insightful alternative perspective builds on concepts from the free volume model for diffusion in which solvent translational motions create cavities of free volume of sufficient size that can be occupied by an adjacent solute (Xiang and Anderson 1998). In other words, diffusion can be thought of as a statistical process of solute molecules finding nearby cavities to fill. This free volume approach can be adapted to better explain the processes of a solute passing through a barrier gated by lipid head groups. More specifically, solute passage through a membrane may depend on the probability of the solute finding free surface area to exploit (Xiang and Anderson 1998). The free surface area can be thought of as representing gaps within the hydrophilic head group region of the membrane (Fig. 2C). Two conclusions are obvious from such a perspective. First, the size and shape of the solute (described by its cross sectional area) influences its ability to find gaps, or free surface area, of sufficient size to allow for solute entry. Second, lipid packing density within the membrane similarly affects the size of free surface area and thus rates of permeation.
The head group gated model described earlier was combined with the solubility-diffusion model to give a model that takes into consideration both free surface area and solute size along with partitioning and diffusion through the membrane’s hydrophobic interior (Mathai et al. 2008; Nagle et al. 2008). This three-slab model describes a process in which solutes must find free surface area for entry before partitioning into and diffusing through an oily interior, and then finally emerging through another head group-gated passage into aqueous solution. The model is particularly satisfying as it can readily explain differences in diffusion arising from solute size and shape, whereas also maintaining a strong solubility-diffusion component consistent with Overton’s rule (Mathai et al. 2008; Nagle et al. 2008).
All of the models discussed thus far are based on data from diacyl phospholipid membranes. For nonpolar solutes, the model appears to fit data from both monoacyl and diacyl lipid membranes. Even subtle changes in apolar solute–water interactions (Wei and Pohorille 2009) can result in dramatic changes in permeability coefficients for both mono- and diacyl lipid membranes (Sacerdote and Szostak 2005). Conversely, ionic solute permeabilities differ greatly between these two types of membranes (Fig. 3).
Lipid Carrier Model
Ionic solutes appear to operate via a different permeability mechanism when encountering a membrane that contains monoacyl lipids. Initial insight into this process was obtained from diacyl phospholipid membranes containing small amounts of monoacyl fatty acids, which revealed dramatic increases in proton and monovalent cation permeabilities (Kamp and Hamilton 1992). The proposed mechanism differed from previous models by invoking lipid flipping (Fig. 2D). More specifically, neutralized fatty acids, either through protonation or association of monovalent cations with the fatty acid’s carboxylic acid head group, carry solute while flipping from one leaflet to the other before solute release (Kamp and Hamilton 1992). Similar studies with membranes composed solely of monoacyl fatty acids were consistent with this proposed carrier mechanism (Chen and Szostak 2004b).
The ability of monoacyl lipids to enhance the permeation rates of ionic solutes is not confined to alkali (Chen and Szostak 2004b) and alkaline Earth metals (Chen et al. 2005). Fatty acid membranes can similarly accommodate the passage of nucleotide mono- and diphosphates, if divalent cations are present to reduce the overall charge of the solute (Chen et al. 2005; Mansy et al. 2008). Although a mechanism for large, ionic, organic solute permeation has not been established, the data are consistent with a carrier-type process (Mansy et al. 2008).
Lipid Dynamics and Permeability
All of the described permeability mechanisms are dependent on lipid dynamics, such as membrane fluidity dynamics that facilitate diffusion through the hydrophobic region of the membrane or lipid dynamics at the surface of the membrane to create an entry space for solutes. The lipid dynamics that are exploited for solute passage depend on membrane composition and solute polarity. A well-documented example is the disparity between permeability data from saturated and unsaturated diacyl phospholipid membranes. It seems likely that such discrepancies reflect real differences in routes of solute passage and thus membrane dynamics. For example, the increased lipid flipping dynamics observed at the phase transition temperature of saturated diacyl phospholipid membranes (John et al. 2002) may facilitate the creation of hydrophilic pores (or packing defects lined with hydrophilic residues) that can be exploited for polar solute passage.
A revealing method to gain insight into the relationship between lipid dynamics and solute permeability is to compare solute permeabilities between monounsaturated diacyl phospholipids and fatty acid membranes. Many of the structural properties of the two membrane types are similar, but their associated lipid dynamics are significantly different, particularly those of lipid flip-flop and lipid escape (Table 1). Less polar molecules that are able to partition more effectively into the membrane permeate similarly through diacyl and monoacyl membranes. This suggests a similar dependence on the types of lipid dynamics, e.g., trans-gauche isomerizations, exploited for solute passage and in this case is consistent with a solubility-diffusion based mechanism. Conversely, highly polar molecules show much different behavior depending on the type of membrane encountered. Because polar molecules are less able to partition into the hydrophobic interior of the membrane, they must exploit alternate paths, and thus other available lipid dynamics, for solute passage. Unlike diacyl phospholipids, monoacyl fatty acids can rapidly flip-flop. It has been proposed previously that the flip-flop dynamics of fatty acid membranes may provide an alternate route for large, polar solute passage similar to the carrier mechanism proposed for monovalent cations (Mansy et al. 2008). Computational studies are consistent with monoacyl lipid dynamics providing lower Born energy paths for ion permeation (Wilson and Pohorille 1996). Although definitive proof of this mechanism is lacking, results so far are consistent with a link between the increased lipid dynamics of fatty acid membranes and their increased permeability characteristics.
Although the carrier mechanism appears different from typical biological mechanisms of solute transport, similarities between the fatty acid carrier mechanism and the mechanism used by membrane transport proteins exist. Membrane transport proteins function similarly to enzymes in lowering the activation energy barrier by providing polar side-chain interactions that substitute for the solute’s interactions with water. In effect, they are providing an alternate path for passage through the membrane that compensates for lost aqueous solvent interactions. This may not be much different than what occurs during the permeation of ionic solutes through fatty acid membranes. Solute interactions with carboxylate head groups may substitute for water interactions in the same way that protein-side chains substitute for hydration within a protein pore. Perhaps Earth’s first cells exploited membrane lipid compositions that functioned both as a solute barrier and simultaneously as a solute transporter.
CHALLENGES AND FUTURE RESEARCH DIRECTIONS
Implications of Selective Permeability for the Origin of Life
Despite the presumed difficulty in nutrient exchange across membranes, permeability data from laboratory models of protocells reveal that selective permeability is an inherent property of fatty acid membranes. Vesicles composed of fatty acids are able to retain large polymers and highly charged molecules, while simultaneously allowing for the diffusion of smaller, less polar solutes (Chen et al. 2006). Even subtle differences in partition coefficients can lead to large permeability differences that could potentially be exploited for early metabolic processes. For example, within a series of aldopentoses, ribose crosses monoacyl and diacyl lipid membranes approximately fivefold faster than diastereomers of equal molecular weight (Sacerdote and Szostak 2005). This remarkable kinetic selectivity apparently arises from less extensive solvent interactions for ribose than for other five-carbon sugars (Wei and Pohorille 2009) and is consistent with permeability models that incorporate Overton’s rule. Because the preferential acceleration of a few reaction rates can result in directional flux through a complex set of potential chemical reactions (Copley et al. 2007), the kinetic availability of ribose in protocellular structures could have significantly influenced the resulting encapsulated protometabolic network.
The ability of model protocells with membranes composed of monoacyl lipids to uptake nucleotides provides for a route to replicate nucleic acid genomes in the absence of proteins. Building on work from the Orgel laboratory (Lohrmann and Orgel 1976; Tohidi et al 1987), a sequence-general, template-directed, nonenzymatic nucleic acid replication method was developed by the Szostak laboratory (Schrum et al. 2009). Briefly, the method exploits nucleotides activated with an imidazole leaving group in place of the pyrophosphate and a 2′-amino nucleophile in place of the 3′-hydroxyl. Although nucleotides likely were not prebiotically activated in precisely this way, it seems probable that nucleotides were activated differently than they are now in contemporary cells (Lohrmann and Orgel 1976). Because fatty acid membranes allow for the passage of nucleotides (Chen et al. 2005; Mansy et al. 2008), the encapsulation of a nucleic acid template inside of monoacyl, fatty acid vesicles resulted in efficient template copying when 2′-amino, 5′-phosphorimidazolide nucleotides were added externally to the vesicles. The same conditions did not result in template copying inside of diacyl phospholipid vesicles because of the impermeability of the membrane (Mansy et al. 2008). These studies highlight the possibility of simple, cell-like structures composed of leaky (Deamer 2008), monoacyl lipid membranes to replicate its own genome by feeding off of the environment.
The permeability and selectivity of fatty acid vesicles suggest that the simplest prebiotic cell-like structures may have been heterotrophic, in the sense that growth was achieved by taking up nutrient compounds from the external solution, rather than by developing encapsulated metabolic networks to synthesize nutrients. Conversely, an autotrophic protocell would likely have required diacyl lipids to retain synthesized small molecules and thus would have additionally faced a series of transport problems that required greater complexity in the form of membrane transport proteins. Nevertheless, protocell research is still in its infancy and much more work is needed to define possible routes to the emergence of protocells.
Early Transporters
The arguments presented here show that nutrient acquisition can occur across lipid membranes without the aid of specialized proteins or RNA molecules. However, cell-like structures that depend on passive diffusion mechanisms are limited in the complexity that they can achieve. To fuel active metabolic processes, membrane transport must be rapid enough to replenish consumed nutrients. Although the total amount of solute flux across a membrane can be increased by increasing vesicle size, i.e., membrane surface area (r2 dependence), the accompanying changes in volume (r3 dependence) result in a net decrease in nutrient availability. This is a significant limitation because compartment size is directly related to the potential complexity of the system. In other words, small compartments can only hold a small number of molecules (Morowitz 1992), thus placing an upper limit on the number of possible encoded ribozymes, for example. Similarly, smaller compartments are more susceptible to fluctuations in molecular composition; even the addition of a single proton within a small vesicle results in significant pH changes (Morowitz 1992). Although these compartment size considerations assume active metabolic processes that may not be relevant to the early steps in the emergence of protocells, progression toward cell-like structures with increased complexity and metabolic activity likely would have selected for larger compartments and in turn active transport mechanisms.
If protocellular evolution resulted in progressively less permeable membrane compositions (Szathmary 2007), then the simultaneous development of transmembrane solute transporters would have been necessary. The simplest biological examples of transporters are short, antibiotic peptides that can mediate the passage of cations across otherwise impermeable phospholipid membranes. Such peptides often contain d- and l-amino acids, are nonribosomally synthesized, and often display remarkable solute specificity (Eisenberg 1998). Even simple hydrophobic polypeptides, such as poly-alanine, can increase proton permeation rates (Oliver and Deamer 1994). It is easy to envisage how short peptide sequences could have been exploited for solute passage by a protocell. However, a mechanism for the propagation of the resulting phenotype from one generation to another would be problematic in the absence of genetic material.
As evidence continues to grow suggesting that life originated in or passed through a RNA world, an important question is whether RNA is able to provide the membrane transport functionality provided by proteins and short peptides. Although RNA mediated transport across membranes appears unlikely because of the high polarity of the phosphodiester backbone of RNA molecules, RNA could modulate the properties of a membrane through interactions with the hydrophilic, solvent exposed membrane surfaces. Lipid head-group organization is an important determinant of membrane stability and fluidity, and for the ability of solutes to gain access to the membrane’s hydrophobic interior. Through in vitro selection experiments, RNA molecules have been identified that tightly associate with membrane surfaces and that may also partially penetrate the membrane (Khvorova et al. 1999; Janas and Yarus 2006). As some permeability mechanisms invoke transient pores and packing defects as routes for solute passage, it is plausible that RNA molecules that simply interact with membrane surfaces could influence the structure and dynamics of the membrane enough to effectively enhance solute permeation. In fact, a RNA chimera consisting of selected membrane and tryptophan binding motifs is able to facilitate tryptophan transport across diacyl phospholipid membranes (Janas and Yarus 2004). Although few examples exist of such RNA-membrane interactions, the ability of RNA to serve a wide range of functions in vitro and in contemporary cells suggests that facilitated solute transport may not have been impossibility during the origin of life (Janas et al. 2006).
Membrane Permeability Influences
Selective permeability influences processes beyond those of nutrient acquisition, including growth, shape, and division processes. Concentration gradients across vesicle membranes because of solute impermeability produce osmotic pressures that can drive chemical reactions, such as vesicle growth (Chen et al. 2004). A mixture of vesicles experiencing differing osmotic pressures arising from differences in RNA content, for example, can lead to the preferential growth of RNA containing vesicles at the expense of empty vesicles. In other words, osmotic gradients across monoacyl, fatty acid membranes create a competition for resources between model protocellular structures (Chen et al. 2004). Similarly, osmotic gradients across multilamellar membranes result in vesicle shape changes because of surface area–volume imbalances that predispose the vesicle to division during growth processes (Zhu and Szostak 2009). The requisite shape changes only occur in the presence of slowly permeable solutes, whereas multilamellar vesicle growth in the presence of highly permeable solutes does not result in vesicle shape changes (Zhu and Szostak 2009). Selectively permeable membranes endow vesicle systems with the lifelike properties of growth, competition, and division.
CONCLUSIONS
It appears that many of the desirable permeability properties of fatty acid membranes arise from the increased lipid dynamics of the vesicle system, whereas many of the early assumptions on the influences of membranes on protocellular evolution were based on less dynamic, diacyl phospholipid membranes. In addition to facilitating the selective absorption and release of molecular building blocks, the dynamic nature of monoacyl lipid membranes allow for growth (Hanczyc et al. 2003), competition (Chen et al. 2004), and division (Zhu and Szostak 2009). Importantly, many of these processes are coupled and so a complete cycle of growth, replication, and division appears achievable (Mansy and Szostak 2009). For example, the uptake of activated nucleotides can result in an increase in genomic content and thus an increase in osmotic pressures on the vesicle system that can be partially relieved by growth. Protocells incapable of genomic replication would not grow and would be selected against. Growing multilamellar vesicles with semipermeable membranes would then proceed through a series of shape changes resulting from surface area-volume imbalances and ultimately divide under mild agitation. Surprisingly, data thus far suggest that a complete replication cycle may only require four components, including two types of monoacyl lipids, activated nucleotides, and a nucleic acid template (Mansy and Szostak 2009).
Thus far a robust and complete cycle including both nucleic acid and compartment replication has not been shown, even though many of the necessary individual steps have been realized. Further efforts in developing nucleic acid replication mechanisms that properly coordinate strand separation, annealing, and copying with vesicle shape changes and division is needed (Mansy and Szostak 2009). Nearly every step in the growth and replication of protocellular structures is influenced by membrane permeability. Protocells with a fatty acid membrane can acquire polar nutrients without first evolving additional transport machinery. The demonstration of a complete cycle that can undergo Darwinian evolution will greatly deepen our understanding of the chemical and physical basis of life.
ACKNOWLEDGMENTS
I thank Cristina Del Bianco, members of CIBIO at the University of Trento, and the Szostak Laboratory for insightful discussions, the Editors for helpful comments, and the Armenise-Harvard foundation for support.
Footnotes
Editors: David Deamer and Jack W. Szostak
Additional Perspectives on The Origins of Life available at www.cshperspectives.org
REFERENCES
- Armstrong VT, Brzustowicz MR, Wassall SR, Jenski LJ, Stillwell W 2003. Rapid flip-flop in polyunsaturated (docosahexaenoate) phospholipid membranes. Arch Biochem Biophys 414: 74–82 [DOI] [PubMed] [Google Scholar]
- Benner SA, Hutter D 2002. Phosphates, DNA, and the search for nonterrean life: A second generation model for genetic molecules. Bioorg Chem 30: 62–80 [DOI] [PubMed] [Google Scholar]
- Chen IA, Szostak JW 2004a. A kinetic study of the growth of fatty acid vesicles. Biophys J 87: 988–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen IA, Szostak JW 2004b. Membrane growth can generate a transmembrane pH gradient in fatty acid vesicles. Proc Natl Acad Sci 101: 7965–7970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen IA, Roberts RW, Szostak JW 2004. The emergence of competition between model protocells. Science 305: 1474–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen IA, Salehi-Ashtiani K, Szostak JW 2005. RNA catalysis in model protocell vesicles. J Am Chem Soc 127: 13213–13219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen IA, Hanczyc MM, Sazani PL, Szostak JW 2006. Protocells: Genetic polymers inside membrane vesicles. in The RNA world (ed. Gesteland R.F., Cech T.R., Atkins J.F.), pp. 57–88 Cold Spring Harbor Laboratory Press, Cold Spring Harbor [Google Scholar]
- Cisse I, Okumus B, Joo C, Ha T 2007. Fueling protein-DNA interactions inside porous nanocontainers. Proc Natl Acad Sci 104: 12646–12650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cistola DP, Atkinson D, Hamilton JA, Small DM 1986. Phase behavior and bilayer properties of fatty acids: Hydrated 1:1 acid-soaps. Biochemistry 25: 2804–2812 [DOI] [PubMed] [Google Scholar]
- Copley SD, Smith E, Morowitz HJ 2007. The origin of the RNA world: Co-evolution of genes and metabolism. Bioorg Chem 35: 430–443 [DOI] [PubMed] [Google Scholar]
- Deamer DW 1985. Boundary structures are formed by organic components of the Murchison carbonaceous chondrite. Nature 317: 792–794 [Google Scholar]
- Deamer DW 1997. The first living systems: A bioenergetic perspective. Microbiol Mol Biol Rev 61: 239–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deamer DW 2008. Origins of life: How leaky were primitive cells? Nature 454: 37–38 [DOI] [PubMed] [Google Scholar]
- Deamer DW, Pasley RM 1989. Amphiphilic components of the Murchison carbonaceous chondrite: Surface properties and membrane formation. Orig Life Evol Biosphere 19: 21–38 [DOI] [PubMed] [Google Scholar]
- Eisenberg D, Crothers D 1979. Physical chemistry with applications to the life sciences The Benjamin/Cummings publishing company, Inc, Menlo Park [Google Scholar]
- Eisenberg E 1998. Ionic channels in biological membranes: Natural nanotubes. Acc Chem Res 31: 117–123 [Google Scholar]
- Evans DF, Wennerstrom H 1999. The colloidal domain: Where physics, chemistry, biology, and technology meet Wiley-VCH, New York [Google Scholar]
- Fujikawa SM, Chen IA, Szostak JW 2005. Shrink-wrap vesicles. Langmuir 21: 12124–12129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawrisch K 2004. The dynamics of membrane lipids. In The structure of biologial membranes (ed. Yeagle P.L.), pp. 147–172 CRC Press, Boca Raton [Google Scholar]
- Gawrisch K, Gaede HC, Mihailescu M, White SH 2007. Hydration of POPC bilayers studies by 1H-PFG-MAS-NOESY and neutron diffraction. Eur Biophys J 36: 281–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawrisch K, Ruston D, Zimmerberg J, Parsegian VA, Rand RP, Fuller N 1992. Membrane dipole potentials, hydration forces, and the ordering of water at membrane surfaces. Biophys J 61: 1213–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebicki JM, Hicks M 1973. Ufasomes are stable particles surrounded by unsaturated fatty acid membranes. Nature 243: 232–234 [DOI] [PubMed] [Google Scholar]
- Gebicki JM, Hicks M 1976. Preparation and properties of vesicles enclosed by fatty acid membranes. Chem Phys Lipids 16: 142–146 [DOI] [PubMed] [Google Scholar]
- Hamilton JA 2003. Fast flip-flop of cholesterol and fatty acids in membranes: Implications for membrane transport proteins. Curr Opin Lipidol 14: 263–271 [DOI] [PubMed] [Google Scholar]
- Hanczyc MM, Fujikawa SM, Szostsak JW 2003. Experimental models of primitive cellular compartments: Encapsulation, growth, and division. Science 302: 618–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves WR, Deamer DW 1978. Liposomes from ionic, single-chain amphiphiles. Biochemistry 17: 3759–3768 [DOI] [PubMed] [Google Scholar]
- Inoue T 1996. Interaction of surfactants with phospholipid vesicles. In Vesicles (Surfactant Science) Marcel Dekker, Inc, New York [Google Scholar]
- Israelachvili JN 1991. Intermolecular & surface forces Academic Press, London [Google Scholar]
- Janas T, Yarus M 2004. A membrane transporter for tryptophan composed of RNA. RNA 10: 1541–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janas T, Yarus M 2006. Specific RNA binding to ordered phospholipid bilayers. Nucleic Acids Res 34: 2128–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janas T, Janas T, Yarus M 2006. RNA, lipids, and membranes. In The RNA world (ed. Gesteland R.F., Cech T.R., Atkins J.F.), pp. 207–225 Cold Spring Harbor Laboratory Press, Cold Spring Harbor [Google Scholar]
- Jansen M, Blume A 1995. A comparative study of diffusive and osmotic water permeation across bilayers composed of phospholipids with different head groups and fatty acyl chains. Biophys J 68: 997–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John K, Schreiber S, Kubelt J, Herrmann A, Muller P 2002. Transbilayer movement of phospholipids at the main phase transition of lipid membranes: Implications for rapid flip-flop in biological membranes. Biophys J 83: 3315–3323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahya N, Schwille P 2006. How phospholipid-cholesterol interactions modulate lipid lateral diffusion, as revealed by fluorescence correlation spectroscopy. J Fluoresc 16: 671–678 [DOI] [PubMed] [Google Scholar]
- Kamp F, Hamilton JA 1992. pH gradients across phospholipid membranes caused by fast flip-flop of un-ionized fatty acids. Proc Natl Acad Sci 89: 11367–11370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khvorova A, Kwak YG, Tamkun M, Majerfeld I, Yarus M 1999. RNAs that bind and change the permeability of phospholipid membranes. Proc Natl Acad Sci 96: 10649–10654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauda JB, Roberts MF, Redfield AG, Brooks BR, Pastor RW 2008. Rotation of lipids in membranes: molecular dynamics simulation, 31P spin-lattice relaxation, and rigid-body dynamics. Biophys J 94: 3074–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinzeller A 1999. Charles Ernest Overton’s concept of a cell membrane. In Membrane permeability: 100 years since Ernest Overton (ed. Deamer D.W., Kleinzeller A., Fambrough D.M.), pp. 1–18 Academic Press, San Diego [Google Scholar]
- Lawless JG, Yuen GU 1979. Quantification of monocarboxylic acids in the Murchison carbonaceous meteorite. Nature 282: 396–398 [Google Scholar]
- Lohrmann R, Orgel LE 1976. Template-directed synthesis of high molecular weight polynucleotide analogues. Nature 261: 342–344 [DOI] [PubMed] [Google Scholar]
- Luisi PL 2001. Are micelles and vesicles chemical equilibrium systems? J Chem Ed 78: 380–384 [Google Scholar]
- Mansy SS, Szostak JW 2008. Thermostability of model protocell membranes. Proc Natl Acad Sci 105: 13351–13355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansy SS, Szostak JW 2009. Reconstructing the emergence of cellular life through the synthesis of model protocells. Cold Spring Harb Symp Quant Biol (in press) [DOI] [PubMed] [Google Scholar]
- Mansy SS, Schrum JP, Krishnamurthy M, Tobe S, Treco DA, Szostak JW 2008. Template-directed synthesis of a genetic polymer in a model protocell. Nature 454: 122–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathai JC, Tristram-Nagle S, Nagle JF, Zeidel ML 2008. Structural determinants of water permeability through the lipid membrane. J Gen Physiol 131: 69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollom TM, Ritter G, Simoneit BR 1999. Lipid synthesis under hydrothermal conditions by Fischer-Tropsch-type reactions. Orig Life Evol Biosph 29: 153–166 [DOI] [PubMed] [Google Scholar]
- McFarland BG, McConnell HM 1971. Bent fatty acid chains in lecithin bilayers. Proc Natl Acad Sci 68: 1274–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh TJ 1978. The effect of cholesterol on the structure of phosphatidylcholine bilayers. Biochim Biophys Acta 513: 43–58 [DOI] [PubMed] [Google Scholar]
- Menger FM, Wood MG, Zhou QZ, Hopkins HP, Fumero J 1988. Thermotropic properties of synthetic chain-substituted phosphatidylcholines: Effect of substituent size, polarity, number, and location on molecular packing. J Am Chem Soc 110: 6804–6810 [Google Scholar]
- Monnard PA, Deamer DW 2001. Nutrient uptake by protocells: A liposome model system. Orig Life Evol Biosph 31: 147–155 [DOI] [PubMed] [Google Scholar]
- Monnard PA, Luptak A, Deamer DW 2007. Models of primitive cellular life: Polymerases and templates in liposomes. Philos Trans R Soc Lond B Biol Sci 362: 1741–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morowitz HJ 1992. Beginnings of cellular life. Metabolism recapitulates biogenesis Yale University Press, New Haven [Google Scholar]
- Nagle JF, Scott HL Jr 1978. Lateral compressibility of lipid mono- and bilayers. Theory of membrane permeability. Biochim Biophys Acta 513: 236–243 [DOI] [PubMed] [Google Scholar]
- Nagle JF, Mathai JC, Zeidel ML, Tristram-Nagle S 2008. Theory of passive permeability through lipid bilayers. J Gen Physiol 131: 77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DL, Cox MM 2008. Lehninger: principles of biochemistry W. H. Freeman and Company, New York [Google Scholar]
- Oliver AE, Deamer DW 1994. α-helical hydrophobic polypeptides form proton-selective channels in lipid bilayers. Biophys J 66: 1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian A 1969. Energy of an ion crossing a low dielectric membrane: Solutions to four relevant electrostatic problems. Nature 221: 844–846 [DOI] [PubMed] [Google Scholar]
- Paula S, Deamer DW 1999. Membrane permeability barriers to ionic and polar solutes. In Membrane permeability: 100 years since Ernest Overton (ed. Deamer D.W., Kleinzeller A., Fambrough D.M.), pp. 77–95 Academic Press, San Diego [Google Scholar]
- Paula S, Volkov AG, Deamer DW 1998. Permeation of halide anions through phospholipid bilayers occurs by the solubility-diffusion mechanism. Biophys J 74: 319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula S, Volkov AG, Van Hoek AN, Haines TH, Deamer DW 1996. Permeation of protons, potassium ions, and small polar molecules through phospholipid bilayers as a function of membrane thickness. Biophys J 70: 339–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson U, Mannock DA, Lewis RN, Pohl P, McElhaney RN, Pohl EE 2002. Origin of membrane dipole potential: Contribution of the phospholipid fatty acid chains. Chem Phys Lipids 117: 19–27 [DOI] [PubMed] [Google Scholar]
- Pizzarello S 2006. The chemistry of life’s origin: A carbonaceous meteorite perspective. Acc Chem Res 39: 231–237 [DOI] [PubMed] [Google Scholar]
- Pizzarello S 2007. The chemistry that preceded life’s orgin: A study guide from meteorites. Chem Biodivers 4: 680–693 [DOI] [PubMed] [Google Scholar]
- Roseman MA, Thompson TE 1980. Mechanism of the spontaneous transfer of phospholipids between bilayers. Biochemistry 19: 439–444 [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Smith BA, McConnell HM 1979. Lateral diffusion in binary mixtures of cholesterol and phosphatidylcholines. Proc Natl Acad Sci 76: 15–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacerdote MG, Szostak JW 2005. Semipermeable lipid bilayers exhibit diastereoselectivity favoring ribose. Proc Natl Acad Sci 102: 6004–6008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrum JP, Ricardo A, Krishnamurthy M, Blain JC, Szostak JW 2009. Efficient and rapid template-directed nucleic acid copying using 2’-amino-2’,3’-dideoxyribonucleoside-5’-phosphorimidazolide monomers. J Am Chem Soc 131: 14560–14570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer SJ 2004. Some early history of membrane molecular biology. Annu Rev Physiol 66: 1–27 [DOI] [PubMed] [Google Scholar]
- Singer SJ, Nicolson GL 1972. The fluid mosaic model of the structure of cell membranes. Science 175: 720–731 [DOI] [PubMed] [Google Scholar]
- Szathmary E 2007. Coevolution of metabolic networks and membranes: the scenario of progressive sequestration. Philos Trans R Soc Lond B Biol Sci 362: 1781–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak JW, Bartel DP, Luisi PL 2001. Synthesizing life. Nature 409: 387–390 [DOI] [PubMed] [Google Scholar]
- Tanford C 1980. The hydrophobic effect: Formation of micelles and biological membranes John Wiley & Sons, New York [Google Scholar]
- Tarrant MK, Cole PA 2009. The chemical biology of protein phosphorylation. Ann Rev Biochem 78: 797–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohidi M, Zielinski WS, Chen CH, Orgel LE 1987. Oligomerization of 3’amino-3’ deoxyguanosine-5’ phosphorimidazolidate on a d(CpCpCpCpC) template. J Mol Evol 25: 97–99 [DOI] [PubMed] [Google Scholar]
- van Mooy BA, Fredricks HF, Pedler BE, Dyhrman ST, Karl ST, Noblizek M, Lomas MW, Mincer TJ, Moor LR, Moutin T, et al. 2009. Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature 458: 69–71 [DOI] [PubMed] [Google Scholar]
- Venable RM, Zhang Y, Hardy BJ, Pastor RW 1993. Molecular dynamics simulations of a lipid bilayer and of hexadecane: An investigation of membrane fluidity. Science 262: 223–226 [DOI] [PubMed] [Google Scholar]
- Walde P, Goto A, Monnard PA, Wessicken M, Luisi PL 1994. Oparin’s reactions revisited: Enzymatic synthesis of poly(adenylic acid) in micelles and self-reproducing vesicles. J Am Chem Soc 116: 7541–7547 [Google Scholar]
- Wang L, Bose PS, Sigworth FJ 2006. Using cryo-EM to measure the dipole potential of a lipid membrane. Proc Natl Acad Sci 103: 18528–18533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Pohorille A 2009. Permeation of membranes by ribose and its diastereomers. J Am Chem Soc 131: 10237–10245 [DOI] [PubMed] [Google Scholar]
- Westheimer FH 1987. Why nature chose phosphates. Science 235: 1173–1178 [DOI] [PubMed] [Google Scholar]
- Wiener MC, White SH 1992. Structure of a fluid dioleoylphosphatidycholine bilayer determined by joint refinement of x-ray and neutron diffraction data. III. Complete structure. Biophys J 61: 434–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Pohorille A 1996. Mechanism of unassisted ion transport across membrane bilayers. J Am Chem Soc 118: 6580–6587 [DOI] [PubMed] [Google Scholar]
- Xiang TX, Anderson BD 1998. Influence of chain ordering on the selectivity of dipalmitoylphosphatidylcholine bilayer membranes for permeant size and shape. Biophys J 75: 2658–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu TF, Szostak JW 2009. Coupled growth and division of model protocell membranes. J Am Chem Soc 131: 5705–5713 [DOI] [PMC free article] [PubMed] [Google Scholar]