Abstract
We examined the association between heart rate variability (HRV) and survival duration to evaluate the usefulness of HRV as a prognostic factor for hospice cancer patients. In terminally ill cancer patients who visited the Hospice clinic, we checked demographic data, Karnofsky performance scale (KPS), HRV, dyspnea, anorexia, as well as fasting blood glucose and total cholesterol. After following up their duration of survival, we examined meaningful prognostic factors for predicting life expectancy through the survival analysis. A total of 68 patients were included in final analysis. As KPS was lower, or when combined with dyspnea or anorexia, the survival duration was much shorter. HRV parameters except heart rate were all impaired in most patients. In particular, the group with mean heart rate of 100 or more beats per minute and the group with standard deviations of normal-to-normal R-R intervals (SDNN) of 21.3 ms (75 percentile) or less showed significantly shorter survival duration. The final multivariate analysis adjusting for age, gender, fasting blood glucose, and total cholesterol showed that KPS, dyspnea, anorexia, and SDNN were significant prognostic factors in survival duration. For the first time, we report that SDNN is a prognostic factor in terminal cancer patients.
Keywords: Terminal Care, Life Expectancy, Prognosis, Heart Rate Variability
INTRODUCTION
Cancer incidence rates and the number of cancer-related deaths continue to increase despite medical developments. In Korea, the number of deaths due to cancer went up from 51,449 in 1998 to 68,912 in 2008, and cancer has now become the leading cause of death in Korea (1). In particular, a high level of in-hospital mortality among terminal cancer patients is raising ethical issues as many patients die alone in hospitals, surrounded by medical equipment instead of family and friends, and the financial burden associated with increasing medical costs (2). Thus, more countries are adopting hospice and palliative care programs, and hospice services are being globally recognized as a means of improving quality of life and of reducing medical costs in terminal cancer patients (3-5). However, despite the advantages of hospice care, decision-making concerning eligibility for hospice care is problematic. In general, patients with less than six months to live with an underlying incurable disease are considered eligible for hospice care. However, in reality, as previous studies have reported, many predictions made by medical practitioners concerning time to death tend to be optimistic in terminal cancer patients (6, 7). As a result, many patients are not offered hospice care in a timely manner (8). Accordingly, the issue of accurately determining life span in terminal cancer patients is of great concern to patients, their caregivers, doctors, and policymakers.
Heart rate variability (HRV) is used in clinical research because fetal distress is preceded by alterations in the R-R interval. HRV reflects autonomic neuropathy in diabetic patients and predicts mortality in myocardial infarction patients (9-11). HRV also predicts mortality in cases of heart failure, cardiomyopathy, and renal failure; and HRV also predicts sudden death and cardiac death in apparently healthy individuals (12-16). Autonomic dysfunction is commonly associated with terminally ill cancer patients (17). Accordingly, we studied the relationship between HRV and survival duration to evaluate the usefulness of HRV as a prognostic factor for terminally ill cancer patients.
MATERIALS AND METHODS
Patients
Recruitment of potential subject was conducted from March 1, 2004, to August 31, 2006, among patients with terminal cancer. They were referred to the Department of Family Medicine at Korea University Guro-Hospital for hospice care after anticancer-therapy had proven ineffective. We consecutively enrolled patients who were eligible for HRV testing and agreed to participate in this study. Patients with arrhythmia or a cardiac disorder capable of affecting HRV parameters, such as paroxysmal supraventricular tachycardia, atrial fibrillation, recent myocardial infarction, or myocardial ischemia, were excluded. Patients who were unable to maintain a stable supine posture for 5 min were also excluded. Finally, a total of 70 patients were enrolled and we observed their survival duration until death. However, two patients were lost to follow up. Thus, 68 patients were included in the final analysis.
Data acquisition
Demographic variables were gender, age, primary cancer site, and previous treatment method. The Karnofsky performance status scale (KPS) and the presence of anorexia and dyspnea, which are parametric constituents of the palliative prognostic score (PaP), were also determined. In previous studies, fasting blood glucose (FBG) and total cholesterol were found to affect HRV (18-21). Therefore, we checked FBG and total cholesterol level, which were measured within one week before and after HRV test. After agreeing to participate in this study, the short term HRV was measured over five minutes using an HRV analyzer, BFM-5000 Plus (Medi-Core Co. Ltd., Seoul, Korea). By using time-domain analysis, mean heart rates, standard deviations of normal-to-normal R-R intervals (SDNN), and root mean square standard deviations of R-R intervals (RMSSD) were calculated. Frequency-domain analysis was applied to measure 5-min total power (TP), low frequency power (LFP), and high frequency power (HFP). HRV parameters were measured by the recommendations published by the Task Force of The European Society of Cardiology and The North American Society of Pacing and Electrophysiology in 1996 (22). The HRV test was explained to patients in a quiet, pleasant medical office and conducted over 5 min after applying four limb leads. Patients were advised not to smoke or consume caffeine for 2 hr prior to HRV testing, and tests were conducted more than 2 hr after a meal. Survival duration was defined from HRV testing until the date of death. This study was approved by the Institutional Review Board (IRB) of Korea University Guro Hospital (IRB No. GR0514-002).
Statistical analysis
Univariate analysis of log-rank test for categorical variables or Wald test for continuous variables were performed to identify variables that significantly related to survival duration. The relationship between HRV measures and the variables related to HRV, fasting blood glucose and total cholesterol, was examined using correlation analysis. Finally, Cox's proportional hazard regression model was used to determine the effects of dyspnea, anorexia, KPS, mean heart rates, and SDNN, which were significant in univariate analysis, on survival duration after adjusting for gender, age, fasting blood glucose, and total cholesterol. All tests were two-tailed with 5% level of significance. SAS version 9.1 (SAS Institute Cary, NC, USA) was used for statistical analysis.
RESULTS
Basic statistics and HRV
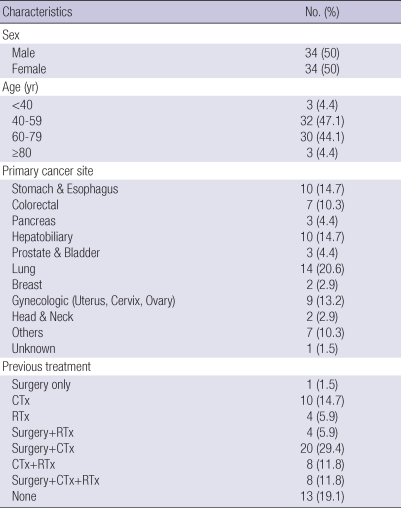
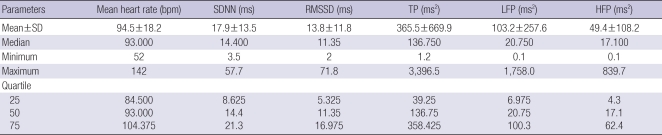
We analyzed 68 patients (34 men and 34 women) aged between 26 and 84 yr old. The most common primary cancer sites were: lung (20.6%), stomach & esophagus (14.7%), hepatobiliary (14.7%), gynecologic (13.2%), and colorectal (10.3%). Previous treatment types were (in descending order) surgery+chemotherapy (29.4%), no treatment (19.1%), chemotherapy (14.7%), surgery+ chemotherapy+radiotherapy (11.8%), and chemotherapy+radiotherapy (11.8%) (Table 1). The distribution of HRV parameters are described in Table 2. HRV levels were considerably lower than the general Korean population except mean heart rate (23).
Table 1.
Demographic characteristics of study population (N=68)
CTx, chemotherapy; RTx, radiotherapy.
Table 2.
Distribution of HRV parameters (N=68)
HRV, heart rate variability; SDNN, standard deviations of normal-to-normal R-R intervals; RMSSD, root mean square standard deviations of R-R intervals; TP, total power; LFP, low frequency power; HFP, high frequency power; bpm, beat per minute; ms, millisecond.
Clinical parameters and survival duration
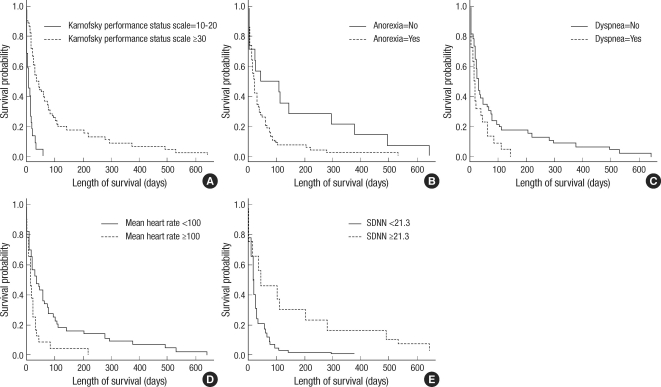
Gender, age, fasting blood glucose, and total cholesterol were independent of survival duration (P=0.584, 0.064, 0.421, 0.467, respectively). However, patients with higher KPS survived longer than those with lower scores (P<0.001). Patients with symptoms of dyspnea or anorexia had shorter survival durations than those without symptoms (P=0.037, 0.015, respectively). Fig. 1A-C showed Kaplan-Meier Survival curves of groups subdivided by these clinical parameters.
Fig. 1.
Kaplan-Meier survival curves (n=68). (A) Karnofsky performance status scale* (P<0.001). (B) Anorexia (P=0.015). (C) Dyspnea (P=0.037). (D) Mean heart rate (dichotomized by 100 bpm). (E) SDNN (dichotomized by quartile 3, 21.3 msec).
*KPS variable was grouped according to the reference level of palliative prognostic score.
Fasting blood glucose, total cholesterol, and HRV
Correlation analysis between HRV parameters and fasting blood glucose or total cholesterol showed that all of the HRV parameters except mean heart rate were significantly correlated with total cholesterol (for SDNN, correlation coefficients 0.43, P<0.001).
HRV and survival duration
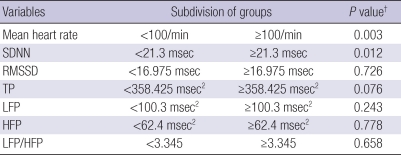
As in previous studies on the association between HRV and prognosis, patients were dichotomized by the highest quartile (75%) of each HRV parameter (11, 14-16). However, 100 beats per min (bpm) was adopted as the reference level for grouping by mean heart rate because it is generally accepted as the definition of tachycardia. Subsequently, significant differences in survival duration were found between patients with an SDNN of <21.3 ms or ≥21.3 ms and between patients with mean heart rate of <100 bpm or ≥100 bpm (Table 3). Fig. 1D, E showed Kaplan-Meier survival curves of patients dichotomized on mean heart rate and SDNN, respectively. For HRV parameters other than SDNN and mean heart rate, survival durations after dichotomization were not significantly different.
Table 3.
Univariate analysis of survival duration for dichotomized HRV parameters (N=68)
*HRV parameters were dichotomized about the highest quartile; †Log-rank test.
HRV, heart rate variability; SDNN, standard deviations of normal-to-normal R-R intervals; RMSSD, root mean square standard deviations of R-R intervals; TP, total power; LFP, low frequency power; HFP, high frequency power.
Multivariate regression analysis using Cox's proportional hazard model
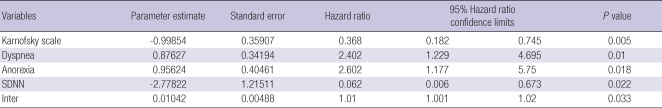
To identify factors associated with survival duration among all variables examined, we performed multivariate regression analysis using Cox's proportional hazard model. The final model included gender, age, fasting blood glucose, total cholesterol, KPS, dyspnea, anorexia, mean heart rate, and SDNN. In addition to these variables, we added an interaction variable (inter=Cholesterol×SDNN) to the final regression model because total cholesterol level was significantly correlated with SDNN, indicating a possible interaction. Finally, SDNN, KPS, the interaction variable, dyspnea, and anorexia were significant variables by Backward selection (Table 4).
Table 4.
Multivariate analysis using Cox's proportional hazard model to identify factors associated with survival duration (N=54)
SDNN, standard deviations of normal-to-normal R-R intervals; Inter, SDNN×total cholesterol.
DISCUSSION
When faced with the reality that anticancer treatment is no longer effective, the patient and his or her family as well as health professionals are faced with a number of difficult decisions; for example, breaking bad news about prognosis, complications of treatment associated with progressive cancer, ect. One of most difficult questions put to doctors by cancer patients and their families is likely to be patient's survival duration (24, 25). The question is very important to the patient or family members, because knowledge of time remaining might help a patient spend the time more effectively and meaningfully. Therefore, an accurate estimation of survival duration may help patients and their families resolve conflicts and better cope with emotional issues. Not surprisingly, such considerations have prompted many studies on predicting survival duration in terminal cancer patients.
The KPS is a common method to objectively quantify the ability to perform daily activities in cancer patients and is essential for predicting survival duration. We also found that KPS was a significant predictor of survival duration. Patients with difficulty to perform daily activities generally have only a short time to live. However, a good ability to perform daily activities does not necessarily mean longer survival (26). The palliative prognostic score (PaP) also proved a useful tool for survival prediction, and has been used to estimate 30 day survival probability based on doctors' clinical prediction, clinical manifestations (dyspnea, anorexia, KPS), and blood count (the number of white blood cells and lymphocyte fraction) (27, 28). In our study, dyspnea, anorexia, and KPS were all significant prognostic factors of survival duration. Fasting blood glucose and total cholesterol were checked due to their relationship with heart rate variability, but they didn't effect on survival duration in univariate analysis. On the other hand, relatively high correlations were found between total cholesterol and HRV parameters (except mean heart rate), implying interactions between these variables in multivariate analysis.
HRV tests are objective, non-invasive, and quantitative tools for assessing changes in cardiac autonomic nervous activity. HRV is calculated from variations in beat-to-beat (R-R) intervals, which are controlled by the parasympathetic and sympathetic nervous systems. These activities change according to internal and external stimuli if they are normal. However, HRV decreases if the autonomic nervous system is impaired (22). Along with traditional time-domain analysis, frequency-domain analysis based on power spectrum analysis, which began to attract attention in the early 1980s, has been used to measure HRV. In frequency-domain analysis, R-R heartbeat intervals are altered by breathing, baroreceptor reflex, renin-angiotensin-aldosterone system, vasomotor activity, and parasympathetic and sympathetic activities, the latter of which generates impulses of constant frequency. When determining R-R intervals associated with these specific impulses by power spectrum analysis, parasympathetic and sympathetic nervous activities can be classified and quantified (10, 22).
After HRV parameters were found to be a significant prognostic factor in patients with myocardial infarction, many studies were conducted to determine whether HRV parameters can predict mortality in other disorders (11, 13-16). We tested the usefulness of HRV parameters in terms of predicting survival duration in terminally ill cancer patients. Survival analyses were performed by dichotomizing patients using specific levels, namely the highest quartiles of HRV measures to conduct the Kaplan-Meier survival analysis. Mean heart rate and SDNN were significantly related to survival duration in univariate analysis (Table 3). Final multivariate regression analysis was performed using Cox's proportional hazard model by adjusting for gender, age, fasting blood glucose level and total cholesterol level, and considering KPS, dyspnea, anorexia, mean heart rate, and SDNN. There was high correlation between total cholesterol and SDNN, which could lead to an interaction. Therefore, we considered an interaction variable between total cholesterol and SDNN in the final regression model. Consequently, SDNN, KPS, dyspnea, anorexia, and the interaction variable were all significant variables (Table 4). These results suggest that HRV is impaired in most terminal cancer patients and SDNN could be useful to predict survival duration in terminal cancer patients. Heart rate is associated with survival duration in terminally ill cancer patients (29). We also verified a similar result in univariate analysis.
This study was conducted at a single medical institution in Korea and is therefore preliminary data for Korean hospice cancer patients. Furthermore, if all palliative prognostic score (PaP) variables including doctor's clinical prediction, white blood cell count, and lymphocyte fraction could be evaluated, we could discover more interesting facts as to whether HRV can improve the prediction power of PaP as prognostic factor.
To our knowledge, this is the first study to suggest that SDNN can play a role in survival prediction for terminal cancer patients. As the short-term HRV test is a simple, non-invasive, objective, and quantitative tool, it has advantages over other prognostic factors. Future work is needed to verify the usefulness and character of SDNN for predicting survival duration in terminally ill cancer patients.
Footnotes
This work was supported by a Korea University Grant (#K0618221).
References
- 1.Korea National Statistical Office. Annual report on the cause of Korean mortality in 2006. Seoul: Korea National Statistical Office; 2007. [Google Scholar]
- 2.Keam B, Oh DY, Lee SH, Kim DW, Kim MR, Im SA, Kim TY, Bang YJ, Heo DS. Aggressiveness of cancer-care near the end-of-life in Korea. Jpn J Clin Oncol. 2008;38:381–386. doi: 10.1093/jjco/hyn031. [DOI] [PubMed] [Google Scholar]
- 3.Mor V, Kidder D. Cost savings in hospice: final results of the National Hospice Study. Health Serv Res. 1985;20:407–422. [PMC free article] [PubMed] [Google Scholar]
- 4.Kidder D. The effects of hospice coverage on Medicare expenditures. Health Serv Res. 1992;27:195–217. [PMC free article] [PubMed] [Google Scholar]
- 5.Pyenson B, Connor S, Fitch K, Kinzbrunner B. Medicare cost in matched hospice and non-hospice cohorts. J Pain Symptom Manage. 2004;28:200–210. doi: 10.1016/j.jpainsymman.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Christakis NA, Lamont EB. Extent and determinants of error in doctors' prognoses in terminally ill patients: prospective cohort study. BMJ. 2000;320:469–472. doi: 10.1136/bmj.320.7233.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glare P, Virik K, Jones M, Hudson M, Eychmuller S, Simes J, Christakis N. A systematic review of physicians' survival predictions in terminally ill cancer patients. BMJ. 2003;327:195–198. doi: 10.1136/bmj.327.7408.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christakis NA, Escarce JJ. Survival of Medicare patients after enrollment in hospice programs. N Engl J Med. 1996;335:172–178. doi: 10.1056/NEJM199607183350306. [DOI] [PubMed] [Google Scholar]
- 9.Laederach-Hofmann K, Mussgay L, Winter A, Klinkenberg N, Ruddel H. Early autonomic dysfunction in patients with diabetes mellitus assessed by spectral analysis of heart rate and blood pressure variability. Clin Physiol. 1999;19:97–106. doi: 10.1046/j.1365-2281.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- 10.Risk M, Bril V, Broadbridge C, Cohen A. Heart rate variability measurement in diabetic neuropathy: review of methods. Diabetes Technol Ther. 2001;3:63–76. doi: 10.1089/152091501750220028. [DOI] [PubMed] [Google Scholar]
- 11.Balanescu S, Corlan AD, Dorobantu M, Gherasim L. Prognostic value of heart rate variability after acute myocardial infarction. Med Sci Monit. 2004;10:CR307–CR315. [PubMed] [Google Scholar]
- 12.Molgaard H, Sorensen KE, Bjerregaard P. Attenuated 24-h heart rate variability in apparently healthy subjects, subsequently suffering sudden cardiac death. Clin Auton Res. 1991;1:233–237. doi: 10.1007/BF01824992. [DOI] [PubMed] [Google Scholar]
- 13.Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, Ota M, Nagai K, Araki T, Satoh H, Ito S, Hisamichi S, Imai Y. Prognostic significance of blood pressure and heart rate variabilities: the Ohasama study. Hypertension. 2000;36:901–906. doi: 10.1161/01.hyp.36.5.901. [DOI] [PubMed] [Google Scholar]
- 14.Bilchick KC, Fetics B, Djoukeng R, Fisher SG, Fletcher RD, Singh SN, Nevo E, Berger RD. Prognostic value of heart rate variability in chronic congestive heart failure (Veterans Affairs' Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure) Am J Cardiol. 2002;90:24–28. doi: 10.1016/s0002-9149(02)02380-9. [DOI] [PubMed] [Google Scholar]
- 15.Reyners AK, Hazenberg BP, Reitsma WD, Smit AJ. Heart rate variability as a predictor of mortality in patients with AA and AL amyloidosis. Eur Heart J. 2002;23:157–161. doi: 10.1053/euhj.2001.2972. [DOI] [PubMed] [Google Scholar]
- 16.Fukuta H, Hayano J, Ishihara S, Sakata S, Mukai S, Ohte N, Ojika K, Yagi K, Matsumoto H, Sohmiya S, Kimura G. Prognostic value of heart rate variability in patients with end-stage renal disease on chronic haemodialysis. Nephrol Dial Transplant. 2003;18:318–325. doi: 10.1093/ndt/18.2.318. [DOI] [PubMed] [Google Scholar]
- 17.Walsh D, Nelson KA. Autonomic nervous system dysfunction in advanced cancer. Support Care Cancer. 2002;10:523–528. doi: 10.1007/s00520-002-0376-x. [DOI] [PubMed] [Google Scholar]
- 18.Danev S, Nikolova R, Kerekovska M, Svetoslavov S. Relationship between heart rate variability and hypercholesterolaemia. Cent Eur J Public Health. 1997;5:143–146. [PubMed] [Google Scholar]
- 19.Christensen JH, Toft E, Christensen MS, Schmidt EB. Heart rate variability and plasma lipids in men with and without ischaemic heart disease. Atherosclerosis. 1999;145:181–186. doi: 10.1016/s0021-9150(99)00052-0. [DOI] [PubMed] [Google Scholar]
- 20.Stein PK, Barzilay JI, Domitrovich PP, Chaves PM, Gottdiener JS, Heckbert SR, Kronmal RA. The relationship of heart rate and heart rate variability to non-diabetic fasting glucose levels and the metabolic syndrome: the Cardiovascular Health Study. Diabet Med. 2007;24:855–863. doi: 10.1111/j.1464-5491.2007.02163.x. [DOI] [PubMed] [Google Scholar]
- 21.Min KB, Min JY, Paek D, Cho SI. The impact of the components of metabolic syndrome on heart rate variability: using the NCEP-ATP III and IDF definitions. Pacing Clin Electrophysiol. 2008;31:584–591. doi: 10.1111/j.1540-8159.2008.01045.x. [DOI] [PubMed] [Google Scholar]
- 22.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 23.Park SB, Lee BC, Jeong KS. Standardized tests of heart rate variability for autonomic function tests in healthy Koreans. Int J Neurosci. 2007;117:1707–1717. doi: 10.1080/00207450601050097. [DOI] [PubMed] [Google Scholar]
- 24.Lamont EB, Christakis NA. Complexities in prognostication in advanced cancer: "to help them live their lives the way they want to". JAMA. 2003;290:98–104. doi: 10.1001/jama.290.1.98. [DOI] [PubMed] [Google Scholar]
- 25.Maltoni M, Caraceni A, Brunelli C, Broeckaert B, Christakis N, Eychmueller S, Glare P, Nabal M, Vigano A, Larkin P, De Conno F, Hanks G, Kaasa S. Prognostic factors in advanced cancer patients: evidence-based clinical recommendations--a study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol. 2005;23:6240–6248. doi: 10.1200/JCO.2005.06.866. [DOI] [PubMed] [Google Scholar]
- 26.den Daas N. Estimating length of survival in end-stage cancer: a review of the literature. J Pain Symptom Manage. 1995;10:548–555. doi: 10.1016/0885-3924(95)00103-6. [DOI] [PubMed] [Google Scholar]
- 27.Maltoni M, Nanni O, Pirovano M, Scarpi E, Indelli M, Martini C, Monti M, Arnoldi E, Piva L, Ravaioli A, Cruciani G, Labianca R, Amadori D. Successful validation of the palliative prognostic score in terminally ill cancer patients. Italian Multicenter Study Group on Palliative Care. J Pain Symptom Manage. 1999;17:240–247. doi: 10.1016/s0885-3924(98)00146-8. [DOI] [PubMed] [Google Scholar]
- 28.Pirovano M, Maltoni M, Nanni O, Marinari M, Indelli M, Zaninetta G, Petrella V, Barni S, Zecca E, Scarpi E, Labianca R, Amadori D, Luporini G. A new palliative prognostic score: a first step for the staging of terminally ill cancer patients. Italian Multicenter and Study Group on Palliative Care. J Pain Symptom Manage. 1999;17:231–239. doi: 10.1016/s0885-3924(98)00145-6. [DOI] [PubMed] [Google Scholar]
- 29.de Miguel Sanchez C, Elustondo SG, Estirado A, Sanchez FV, de la Rasilla Cooper CG, Romero AL, Otero A, Olmos LG. Palliative performance status, heart rate and respiratory rate as predictive factors of survival time in terminally ill cancer patients. J Pain Symptom Manage. 2006;31:485–492. doi: 10.1016/j.jpainsymman.2005.10.007. [DOI] [PubMed] [Google Scholar]