Abstract
Uncarboxylated osteocalcin (ucOC) is important in evaluating vitamin K status and it is inversely associated with bone mineral density (BMD). We studied the correlationship between ucOC and BMD in healthy Korean women. This study recruited 337 healthy women between ages 20-70 were recruited. Serum ucOC, calcium, alkaline phosphatase, body mass index (BMI), and BMD were measured and compared. Mean BMI was lowest (20.3±1.9 kg/m2) in the 20 yr old group and highest (24.8±2.6 kg/m2) in the 60 yr old group. Women age 20-70 yr old had ucOC inversely related to BMD independent of other factors that may influence BMD. Serum ucOC concentration and BMD of lumbar spine showed a significant inverse relationship. Serum mean alkaline phosphatase was lowest (122±30 IU/L) in the age 30 group and highest (190.3±55.8 IU/L) in the age 60 group. Serum ucOC was inversely associated with BMI, and positively associated with alkaline phosphatase. Uncarboxylated osteocalcin (ucOC) was inversely associated with spinal BMD in healthy Korean women. Serum mean ucOC was highest in the age 20 group, followed by age 50 group, which may indicate vitamin K insufficiency could be related to high bone turnover in these groups. These results suggest that vitamin K supplement may be considered to help both bone growth and bone loss during these periods.
Keywords: Undercarboxylated osteocalcin, Bone Density, Vitamin K, Korean Women
INTRODUCTION
Osteoporosis is the most frequently observed in bone metabolic diseases, which results in a higher risk of bone fracture (1). As the average life expectancy increases, the diagnosis of osteoporosis and the cost of the treatment have also increased (2). While the lifetime risk of fracture is 13 percent for males, it reaches 40 percent in females (1, 3).
Osteocalcin, a vitamin K-dependent protein that exists abundantly in bone, is synthesized by osteoblast in bones when bone stroma forms. A small amount of osteocalcin is released to the bloodstream; thus, blood osteocalcin concentration represents the activity of osteoblast and bone formation (4). In many clinical studies, the blood osteocalcin level has been used as an indicator for bone formation in various bone metabolic diseases (5, 6).
Recent studies have shown that vitamin K is necessary in γ-carboxylation where osteocalcin combines with minerals. Vitamin K acts as a coenzyme of γ-carboxylase, which converts glutamate in osteocalcin into γ-carboxyglutamate (Gla) (7). It is clear that vitamin K is an essential component in ossification (8, 9). Undercarboxylated osteocalcin (ucOC) has been used as hip fracture indicator, since it is related to vitamin K and the observed amount of ucOC increases in osteoporosis patients (6, 10). Many studies have shown that bone mineral density (BMD) and ucOC concentration are inversely related: ucOC concentration increases in female patients with hip fracture as they age (11). Therefore, ucOC is not only an indicator for ossification and osteolysis, but it is also an index of bone health in females.
Compared to other countries, there have been no studies published in Korea that measure the serum ucOC concentration. Thus, our research group has measured the ucOC concentration of healthy females ranging from 20 to 80 yr in age to confirm the relationship between serum ucOC concentration and BMD.
MATERIALS AND METHODS
Subjects
Among the women who visited the comprehensive medical testing center at a hospital in Seoul, 337 women between the ages of 20 to 80 yr were recruited from March 2007 to June 2008 after written informed consent. Patients who had been administered hormonal replacement therapy and drugs that may influence bone metabolism (bisphosphonate, calcitonin, steroid, phenytoin, carbamazepine, rifampicin etc.) were excluded from this study. Patients who had been administered vitamin K metabolism-related drugs such as heparin and warfarin, as well as patients with other metabolic bone diseases were also excluded. Institutional Review Board of CHA hospital approved this study (EKI-GLA-06-32).
Data collection and method of measurement
All patients' height and weight information was collected to compute the body mass index (BMI). All blood sampling was completed in the morning after 10 hours of fasting. Total cholesterol, triglyceride, high density lipoprotein cholesterol, and low density lipoprotein cholesterol were measured by enzymatic colorimetric assay (HITACHI 7600, Tokyo, Japan). BMD of spine and hip was measured by QDR 4500 (HOLOGIC, Waltham, USA). The serum ucOC concentration was assayed by an enzyme-linked immunosorbent assay (ELISA) using two monoclonal antibodies, an anti-OC antibody and a solid-phase anti-Glu 21, 24-OC antibody, with recombinant human ucOC (Biotechnology Research Laboratory, Takara Shuzo Co., Otsu, Shiga, Japan). The intra- and inter-assay coefficients of variation (CVs) were 7.3% and 9.7%, respectively, and the sensitivity of the assay was 0.5 ng/mL. The ucOC concentration was measured twice per visit and the average value was recorded.
Analysis
Mean and standard deviation of each variable measured from the patients was reported. Partial correlation computation was used to calculate the correlation coefficient between serum ucOC concentration and bone mineral density, and menopausal period, age, body weight and triglyceride have been incorporated to adjust the values. P values less than 0.05 were statistically significant; however, P values between 0.05 and 0.10 were regarded to be borderline significant. All statistical analysis was computed with SPSS 13 (SPSS, Chicago, IL, USA).
RESULTS
General characteristics of the study subjects
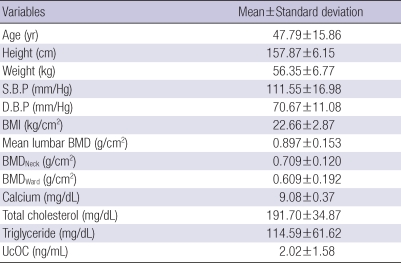
There were 337 patients between 20 to 80 yr of age and each age group had 60 patients 37 patients in the 70s. The average age, ucOC concentration, total cholesterol, triglyceride, calcium concentration and BMI were 47.79±15.86 yr old, 2.018±1.580 ng/mL, 191.70±34.87 mg/dL, 114.59±61.62 mg/dL, 9.08±0.37 mg/dL, and 22.66±2.87 kg/m2, respectively (Table 1).
Table 1.
Baseline characteristics of study subjects
Data are mean±standard deviation.
S.B.P, Systolic blood pressure; D.B.P, Diastolic blood pressure; BMI, Body mass index; UcOC, Undercarboxylated Osteocalcin; BMD, Bone Mineral density; BMDNeck, BMD of femur neck; BMDWard, BMD of femur ward.
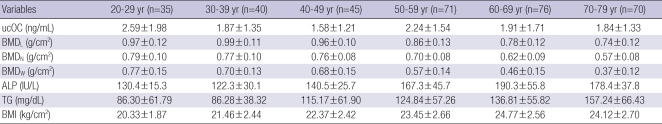
Serum ucOC, triglyceride, BMI and BMD of each age group
Serum ucOC was highest in the age 20s and decreased until the age 50s. However, it increased again in the age 50s, when menopause starts, and then continuously declined. Triglyceride and BMI increased and BMD decreased As age increased (Table 2).
Table 2.
Serum ucOC level, triglyceride and body mass index by age-group
Data are mean±standard deviation.
UcOC, undercarboxylated osteocalcin; BMI, body mass index; TG, triglyceride; BMD, bone mineral density; BMDL, lumbar bone mineral density; BMDN, bone mineral density of neck of hip bone; BMDW, bone mineral density of ward of hip bone.
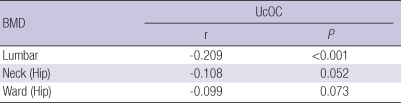
Serum ucOC level and BMD
Serum ucOC concentration and BMD of spine from L1 to L4 showed a significant inverse relationship (P<0.01). BMD of hip was borderline significant with r=-0.108, P=0.052 at the neck and r=-0.099, P=0.073 at the ward triangle. Serum ucOC concentration and triglyceride showed no significant correlation with r=0.047, P=0.394 (Table 3).
Table 3.
Correlation between serum ucOC level and BMD of spine and hip
Controlled for age, body weight, menopausal period and triglyceride.
r, correlation coefficient; BMD, bone mineral density.
DISCUSSION
Korean women at the age 20-70 yr old demonstrated an inverse relationship between ucOC and BMD independent of other factors that may influence BMD. Serum ucOC concentration and BMD of spine from L1 to L4 showed a significant inverse relationship. BMD of hip was inversely related with serum ucOC concentration with borderline significance. Serum mean alkaline phosphatase was lowest (122±30 IU/L) in the age 30 group and highest (190.3±55.8 IU/L) in the age 60 group. Serum ucOC was inversely associated with BMI and lumbar spine BMD, and positively associated with alkaline phosphatase. Serum mean ucOC was highest in the age 20 group, then the age 50 group, which may indicate vitamin K might be related to bone turnover in these age groups.
Vitamin K was discovered by Danish scientist, Henrik Dam in 1929. Coagulation was thought to be the only known function of vitamin K for decades; however, recent studies revealed the vital function of vitamin K in bone metabolism. Vitamin K deficiency is related to increased serum ucOC concentration, low BMD, and increased risk of bone fracture. Daily recommended requirements of vitamin K can be estimated based on the need for coagulation and most people can acquire sufficient amount of vitamin K from each meal. Nevertheless, the required amount for coagulation is smaller than that for bone metabolism (12).
Nutritional insufficiency of vitamin K, which is often observed at old ages, can be a risk factor for osteoporotic hip fracture. Many researchers have stated that calcium and vitamin D is effective in maintaining bone density and reducing bone fracture (13), in addition, recent researches have reported that vitamin K is important for osteoporosis and bone health (9, 14).
When vitamin K concentration in the body is low, insufficient amount of osteocalcin completes γ-carboxylation; consequently, ucOC concentration increases and the affinity to calcium to bone matrix decreases. Gla-residues of osteocalcin combine with calcium and require vitamin K to activate the reaction site. Therefore, measuring ucOC concentration is a more accurate method of monitoring vitamin K than prothrombin in the liver (15). Vitamin K taken from a regular meal cannot reach the full carboxylation of osteocalcin (16) and short term vitamin K insufficiency can increase the bone turnover rate (17). Many epidemiology researchers have reported that vitamin K is related to hip fracture, BMD and ultimately the healthy bone (18). Thus, vitamin K intake can reduce fracture from osteoporosis (9).
It has been reported that insufficient vitamin K in elderly patients will increase the risk of hip fracture (19). Weber et al. (20) reported that minute vitamin K insufficiency will cause osteoporosis. According to the results in this study, the ucOC concentration of each age group was 2.59 ng/mL, 1.87 ng/mL, 1.58 ng/mL, 2.24 ng/mL, 1.91 ng/mL, 1.84 ng/mL, marking age 20s (2.6 ng/mL) and age 50s (2.2 ng/mL) at the highest. In other words, a considerable amount of vitamin K is needed at the 20s, where bone formation is rapid, and the 50s, when the bone turnover rate increases at the early menopausal state. Nimptsch et al. have shown similar results of ucOC concentration for each age group (21).
Sokoll et al. (22) have reported parallel results that ucOC concentration decreases from age 20s to 50s, and increases again at age 50s to 60s and then levels off. Women who are able to be pregnant (ages 20 to 49) in Thailand showed an average ucOC level of 2.69 ng/mL, which has no significant difference from the 2.01 ng/mL of this study (23).
Yashui et al. (24) have reported a rapid increase of ucOC concentration after removing the ovaries, which simultaneously represents the increased ucOC concentration at the early menopausal period. Therefore, ucOC concentration is related with age (25), and it abruptly increases at the early menopausal state. Moreover, ucOC concentration is an accurate indicator of bone quality (26), thus, women whose ucOC concentration increases after menopause should closely monitor their bone turnover rate and BMD. In Yashui et al., the serum ucOC concentration of Japanese between 30 to 80 yr of age was positively related to age and the patients' BMI, and serum triglyceride level was lower at older ages. In our study, ucOC concentration decreased until age 50s and increased thereafter, and then continued to decrease. The BMI and serum triglyceride level of our study subjects were significantly higher at older ages. Consequently, the serum ucOC of Japanese study increased with age, but not in Koreans, because of their body fat and serum triglyceride level. Serum triglyceride is thought to be the main vehicle that carries vitamin K into the bloodstream. A recent study showed significantly lower ucOC concentration in menopausal women who received hormonal replacement therapy than menopausal women who did not (27). The hormonal replacement therapy increased the triglyceride and further increased vitamin K absorption, which decreased ucOC concentration (30).
Schurgers et al. (28) stated that triglyceride is a major vitamin K carrier, and other researches show a positive relationship between vitamin K concentration and the triglyceride amount in body (17). Our results have showed increased BMI and triglyceride concentration at older ages which is identical to the results of Chang et al. where the total cholesterol and triglyceride amount increases as age, time after menopause, and BMI increase (29). Therefore, weight gaining increases triglyceride and affects ucOC concentration. In addition, ucOC concentration varies with the seasons (summer being the lowest), as well as other daily living habits: smoking, activity level, diet of leafy green vegetables and dairy products (21). Nonetheless, the correlation between serum ucOC concentration and triglyceride has not been observed in our study.
The ucOC concentration and BMD of spine showed a statistically significant negative relationship, while BMD of hip was only borderline significant. However, Szulc et al. (11) have shown a definite negative relationship between ucOC concentration and hip BMD, and other studies confirmed a relationship between decreased bone density and serum ucOC concentration in menopausal women (30). Szulc et al. (11) states that the BMD of all bones shows a negative relationship with ucOC concentration. According to other researchers, ucOC concentration has shown to have a stronger relationship with quality of the bone than the density (14, 15), and thus, ucOC is an accurate indicator for hip fracture in aged patients (11, 13).
Consequently, vitamin K may become insufficient at the 20s, when bone turnover rate is the fastest, and the 50s, when menopause starts, as represented by high ucOC concentration. Vitamin K is closely related to the risk of fracture and increased bone formation; thus, vitamin K may be recommended for osteoporosis and bone fracture treatment.
Our study has some limitations that the collected patients did not represent the total population because they visited a particular hospital for health examinations, and only a small number of over 70-yr-old patients were able to participate in the study. We did not examine Vitamin K concentration. Also, other factors that can affect vitamin K metabolism such as smoking, alcohol, diet, exercise, and vitamin D absorption were not incorporated as a correction value. Although statistically strong correlation was not shown, it is still compelling, since we have measured ucOC concentrations and other influential factors to monitor the vitamin K condition of Korean women and suggested further research of osteoporosis and risk of fracture in elderly patients in respect to ucOC concentration.
In conclusion, ucOC was inversely associated with spinal BMD in healthy Korean women. There were two significant age groups when they had high serum ucOC concentrations at the 20s and at the 50s. This result suggest that vitamin K supplement may improve both bone growth and bone loss at these ages.
ACKNOWLEDGMENTS
Authors would like to acknowledge Pam Fry for her time and support writing this paper.
References
- 1.Kanis JA WHO Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. Osteoporosis Int. 1994;4:368–381. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 2.Chrischilles E, Shireman T, Wallace R. Costs and health effects of osteoporotic fracture. Bone. 1994;15:377–386. doi: 10.1016/8756-3282(94)90813-3. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz AV, Kelsey JL, Maggi S, Tuttleman M, Ho SG, Jonsson PV, Poor G, Sisson de Castro JA, Xu L, Matkin CC, Nelson LM, Heyse SP. International variation in the incidence of hip fractures: cross-national project on osteoporosis for the World Health Organization Program for Research on Aging. Osteoporosis Int. 1999;9:242–253. doi: 10.1007/s001980050144. [DOI] [PubMed] [Google Scholar]
- 4.Price PA. Vitamin K nutrition and postmenopausal osteoporosis. J Clin Invest. 1993;91:1268. doi: 10.1172/JCI116324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price PA, Parthemore JG, Deftos LJ, Nishimoto SK. New biochemical marker for bone metabolism: measurement by radioimmunoassay of bone gla-protein in the plasma of normal subjects and proteins with bone disease. J Clin Invest. 1980;66:878–883. doi: 10.1172/JCI109954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miura M. Biochemical markers of bone turnover New aspect An automated assay for measuring bone turnover markers. Clin Calcium. 2009;19:1160–1169. [PubMed] [Google Scholar]
- 7.Furie B, Bouchard BA, Furie BC. Vitamin K dependent biosynthesis of gamma-carboxyglutamic acid. Blood. 1999;93:1798–1808. [PubMed] [Google Scholar]
- 8.Vermeer C, Jie KS, Knapen MH. Role of vitamin K in the bone metabolism. Annu Rev Nutr. 1995;15:1–22. doi: 10.1146/annurev.nu.15.070195.000245. [DOI] [PubMed] [Google Scholar]
- 9.Haffa A, Krueger D, Bruner J, Engelke J, Gundberg C, Akhter M, Binkley N. Diet- or warfarin-induced vitamin K insufficiency elevates circulating undercarboxylated osteocalcin without altering skeletal status in growing female rats. J Bone Miner Res. 2000;15:872–878. doi: 10.1359/jbmr.2000.15.5.872. [DOI] [PubMed] [Google Scholar]
- 10.Price PA, Williamson MK, Lothringer JW. Origin of the vitamin K-dependent bone protein found in plasma and its clearance by kidney and bone. J Biol Chem. 1981;256:12760–12766. [PubMed] [Google Scholar]
- 11.Szulc P, Chapuy MC, Meunier PJ, Delmas PD. Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture in elderly women. J Clin Invest. 1993;91:1769–1774. doi: 10.1172/JCI116387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bügel S. Vitamin K and bone health in adult humans. In: Litwack G, editor. Vitamins and Hormones. 1st ed. vol 78. USA: Academic Press (Elsevier); 2008. pp. 393–416. [DOI] [PubMed] [Google Scholar]
- 13.Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med. 1992;327:1637–1642. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 14.Reid DM, Macdonald HM. Nutrition and bone: Is there more to it than just calcium and vitamin D? QJM. 2001;94:53–56. doi: 10.1093/qjmed/94.2.53. [DOI] [PubMed] [Google Scholar]
- 15.Furie B, Furie BC. Molecular basis of vitamin K-dependent gamma-carboxylation. Blood. 1990;75:1753–1762. [PubMed] [Google Scholar]
- 16.Binkley NC, Krueger DC, Kawahara TN, Engelke JA, Chappell RJ, Suttie JW. A high phylloquinone intake is required to achieve maximal osteocalcin γ-carboxylation. Am J Clin Nutr. 2002;76:1055–1060. doi: 10.1093/ajcn/76.5.1055. [DOI] [PubMed] [Google Scholar]
- 17.Booth SL, Lichtenstein AH, O'Brien-Morse M, McKeown NM, Wood RJ, Saltzman E, Gundberg CM. Effects of a hydrogenated form of vitamin K on bone formation and resorption. Am J Clin Nutr. 2001;74:783–790. doi: 10.1093/ajcn/74.6.783. [DOI] [PubMed] [Google Scholar]
- 18.Booth SL, Broe KE, Peterson JW, Cheng DM, Dawson-Hughes B, Gundberg CM, Cupples LA, Wilson PW, Kiel DP. Associations between vitamin K biochemical measures and bone mineral density in men and women. J Clin Endocrinol Metab. 2004;89:4904–4909. doi: 10.1210/jc.2003-031673. [DOI] [PubMed] [Google Scholar]
- 19.Hodges SJ, Akesson K, Vergnaud P, Obrant K, Delmas PD. Circulating levels of Vitamin K1 and K2 decreased in elderly women with hip fracture. J Bone Miner Res. 1993;8:1241–1245. doi: 10.1002/jbmr.5650081012. [DOI] [PubMed] [Google Scholar]
- 20.Weber P. The role of vitamins in the prevention of osteoporosis-a brief status report. Int J Vitam Nutr Res. 1999;69:194–197. doi: 10.1024/0300-9831.69.3.194. [DOI] [PubMed] [Google Scholar]
- 21.Nimptsch K, Hailer S, Rohrmann S, Gedrich K, Wolfram G, Linseisen J. Determinants and correlates of serum undercarboxylated osteocalcin. Ann Nutr Metab. 2007;51:563–570. doi: 10.1159/000114211. [DOI] [PubMed] [Google Scholar]
- 22.Sokoll LJ, Sadowski JA. Comparison of biochemical indexes for assessing vitamin K nutritional status in a healthy adult population. Am J Clin Nutr. 1996;63:566–573. doi: 10.1093/ajcn/63.4.566. [DOI] [PubMed] [Google Scholar]
- 23.Bunyaratavej N, Soontrapa S, Rojanasthin S, Kitimanon N, Lektrakul S. Level of undercarboxylated osteocalcin in reproductive Thai females. J Med Assoc Thai. 2005;1(Suppl 5):S37–S39. [PubMed] [Google Scholar]
- 24.Yasui T, Uemura H, Tomita J, Miyatani Y, Yamada M, Kuwahara A, Matsuzaki T, Maegawa M, Miura M, Irahara M. Change in serum undercarboxylated osteocalcin concentration in bilaterally oophorectomized women. Maturitas. 2007;56:288–296. doi: 10.1016/j.maturitas.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Epstein S, Poser J, McClintock R, Johnston CC, Jr, Bryce G, Hui S. Differences in serum bone GLA protein with age and sex. Lancet. 1984;1:307–310. doi: 10.1016/s0140-6736(84)90360-x. [DOI] [PubMed] [Google Scholar]
- 26.Liu G, Peacock M. Age-related changes in serum undercarboxylated osteocalcin and its relationship with bone density, bone quality, and hip fracture. Calcified Tissue Int. 1998;62:286–289. doi: 10.1007/s002239900432. [DOI] [PubMed] [Google Scholar]
- 27.Yasui T, Uemura H, Umino Y, Yamada M, Kuwahara A, Matsuzaki T, Maegawa M, Furumoto H, Miura M, Irahara M. Undercarboxylated osteocalcin concentration in postmenopausal women receiving hormone therapy daily and on alternate days. Menopause. 2006;13:314–322. doi: 10.1097/01.gme.0000177908.40257.cf. [DOI] [PubMed] [Google Scholar]
- 28.Schurgers LJ, Vermeer C. Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochim Biophys Acta. 2002;1570:27–32. doi: 10.1016/s0304-4165(02)00147-2. [DOI] [PubMed] [Google Scholar]
- 29.Kim SY, Oh HJ, Chang SY. Comparison of bone mineral density and lipid profiles in pre and postmenopausal women. J Korean Acad Fam Med. 1997;18:910–917. [Google Scholar]
- 30.Knapen MH, Nieuwenhuijzen-Kruseman AC, Wouters RS, Vermeer C. Correlation of serum osteocalcin fractions with bone mineral density in women during the first 10 years after menopause. Calcif Tissue Int. 1998;63:375–379. doi: 10.1007/s002239900543. [DOI] [PubMed] [Google Scholar]