Abstract
Purpose
To evaluate the effectiveness of laser photocoagulation as adjuvant therapy in the treatment of large macular holes.
Methods
A randomized clinical trial. Thirty-one eyes from 29 subjects with idiopathic macular holes of diameters larger than 400 µm were randomized into a laser group and a control group. All eyes underwent vitrectomy with peeling of the internal limiting membrane. Contrary to the control group eyes, the laser group eyes underwent laser photocoagulation at the center of the macular hole before vitrectomy. Visual acuity and anatomic outcomes assessed by optical coherence tomography (OCT) were analyzed 3 months after surgery.
Results
On postoperative OCT, closure of the macular hole was noted in 17 of the 18 (94.4%) laser group eyes and 10 of the 13 (76.9%) control group eyes. Hole closure without bare retinal pigment epithelium was observed in 16 eyes in the laser group and 6 eyes in the control group (P<0.05). The amount of improvement in logMAR visual acuity 3 months after surgery was 0.40±0.29 in the laser group, and 0.19±0.23 in the control group (P<0.05).
Conclusions
Laser photocoagulation constitutes a potent adjuvant therapy that may improve anatomical and visual outcomes of surgery for macular holes larger than 400 µm.
Keywords: Laser photocoagulation, Macular hole, Optical coherence tomography
The primary goal of surgery for macular holes is to induce visual improvement via the reappositioning of the detached or thickened rim of the neurosensory retina to the retinal pigment epithelium. The macular hole is sealed by formation of a glial plug, which reattaches the rim of the neurosensory retina around the edges of the hole.1-3 In order to improve the success rate of surgery, it is necessary to provide stimulus to the glial cells, a template for migration, and a scaffold for proliferation.4 Several adjuvants have been introduced in the hopes of improving the outcomes associated with macular hole surgery,5-8 but the effects remain somewhat controversial.9-11
Laser photocoagulation at the center of the macular hole, one of the adjuvant therapies for the treatment of macular holes, was introduced by Del Priore.12 The theoretical basis of this procedure is that the retinal pigment epithelium will produce cytokines after completion of laser photocoagulation which will promote the formation of a glial plug to close the macular hole.13-15 Several consecutive series have previously demonstrated that laser photocoagulation, when performed as an adjuvant therapy, is able to promote the closure of primary or recurrent macular holes and, as a result, improve visual outcomes.13,14,16-18 The above results suggest that laser photocoagulation might constitute a useful adjuvant therapy for the treatment of idiopathic macular holes. However, this benefit has not been confirmed in a controlled clinical trial.
In this randomized clinical trial, we attempted to evaluate the effectiveness of laser photocoagulation as an adjuvant therapy in the surgical treatment of macular holes with diameters larger than 400 µm.
Materials and Methods
1. Patient eligibility
This study was conducted between April 2004 and March 2005. A total of 31 idiopathic macular holes in 29 patients were included in this trial. All patients were of Asian extraction. Five males and 24 females were included. Patients were included in this study if they exhibited a full-thickness macular hole with a diameter larger than 400 µm, as assessed by optical coherence tomography (OCT). Eyes with any other ocular diseases, including macular degeneration, diabetic retinopathy, history of glaucoma, or intraocular pressure higher than 30 mmHg at baseline, were excluded from this study. Eyes with previous history of intraocular surgery, with the exception of cataract extraction, were also determined to be ineligible. Highly myopic eyes (>5 diopters) with macular holes and eyes with traumatic macular holes were excluded from this trial. This study was approved by the institutional review board. All patients provided informed consent after study protocol and surgical risks were explained.
2. Preoperative examination
For each studied eye, baseline data included the date of onset, objective refraction values, best-corrected visual acuity as measured by the Early Treatment Diabetic Retinopathy Study (ETDRS) charts,19 slit-lamp examination, applanation tonometry, biomicroscopic fundus examination, fundus photography, and optical coherence tomography (OCT3, Humphrey Instrument, San Leandro, Calif). Preoperative macular hole diameter was acquired by taking the average of the vertical and horizontal diameters, both of which were determined at the minimal extent of the hole on OCT examination.
3. Study groups and randomization
All eyes included in this trial were randomized into 2 groups: the laser group and the control group. Allocation into each group was dependent on the last digit of the subject's hospital identification number. Odd numbers were assigned to the laser group and even numbers to control group. The resultant randomization code was masked to all investigators or clinical observers, except the one who applied laser photocoagulation, until the completion of data collection. We determined there to be no differences between the two groups, except that laser photocoagulation at the center of macular hole was applied only to the eyes of the laser group. Best-corrected visual acuity with refraction on each visit and OCT were examined throughout the study period by masked independent observers.
Two patients exhibited bilateral macular holes. The eyes of each patient with bilateral macular holes were randomly allocated such that one eye had laser and other was a control eye.
4. Laser photocoagulation at the center of the macular hole
In the laser group, laser photocoagulation was performed before the vitrectomy, but on the same day. The procedure was performed with the patient under topical anesthesia using a fundus contact lens (TransEquator® lens, Volk Optical Inc., Ohio), slit-lamp delivery system, and an argon green wavelength laser (Novus Omni™; Coherent Inc., Palo Alto, Calif). Three burns of 100-µm size, 0.04~0.1 second duration, and 60~100 mW were applied directly to the pigment epithelium at the center of the macular hole. The endpoint of laser photocoagulation resulted in the appearance of subtle gray burns with a triangular arrangement.
5. Surgical procedures
Vitrectomies were performed by one surgeon (S.W.K), using the same surgical technique for all macular holes. The vitreous body and posterior hyaloid face were removed via standard three-port pars plana vitrectomy. When present, the epiretinal membrane around the hole was removed. The circular laminorrhexis of the retinal internal limiting membrane was successfully performed in all eyes of both groups. After complete fluid-air exchange, the air in the vitreous cavity was then replaced with a 14% perfluoropropane gas mixture.
Postoperative face-down positioning instructions were given, and the patients typically maintained this position for at least 2 weeks.
6. Postoperative evaluation
At 1, 4, and 8 weeks after vitrectomy, patients were encouraged to recieve ocular examinations. At the 3-month follow-up visit, all patients received complete ophthalmological examinations, which included evaluation of bestcorrected ETDRS visual acuity, biomicroscopic fundus examination, and OCT. Postoperative 3-month data were used for analysis.
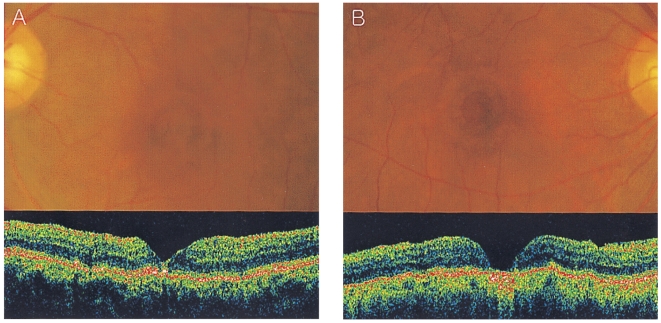
Assessment of anatomic results after surgery for macular hole depended on OCT images, rather than on biomicroscopy, in order to reduce inter-observer bias. Accordingly, our masked investigators determined closure of the macular hole, as well as the types of closure achieved from the OCT image, Macular hole closure was defined on OCT as the flattening of the entire circumference of the macular hole against the retinal pigment epithelium. Eyes which were judged to have achieved the closure of the macular hole were then classified into two groups: type 1 closure and type 2 closure.20 Type 1 closure on OCT examination indicates that the macular hole has been closed without bare retinal pigment epithelium (Fig. 1A). Type 2 closure indicates the presence of bare retinal pigment epithelium on OCT examination, although the entire rim of the macular hole is attached to the underlying retinal pigment epithelium with a flattening of the cuff (Fig. 1B). Reopening, in this study, was defined as the detection of an opened macular hole at any time after surgery for macular hole. Thus, primary failure and recurrent macular hole were lumped together under the term "reopening" for the purpose of analysis.
Fig. 1.
Postoperative funduscopic findings of a patient with bilateral macular holes. The preoperative hole diameter of the right and left eyes was 689.5 µm and 711.5 µm, respectively. The left eye, which was randomized into the laser group, showed type 1 closure 3 months after surgery (A). In contrast, the right eye in the control group had type 2 closure (B).
7. Statistical analysis
ETDRS visual acuity values were converted to logarithms of the minimum angle of resolution (logMAR) values, for the purpose of statistical analysis. Since the numbers of patients in each group was small and the data were not normally distributed, a Mann-Whitney test was utilized to compare best-corrected visual acuity between the 2 groups. Comparisons of postoperative anatomic results and complications between the 2 groups were conducted via Fisher's exact tests. Statistical analysis was conducted using SPSS software (version 12.0).
Results
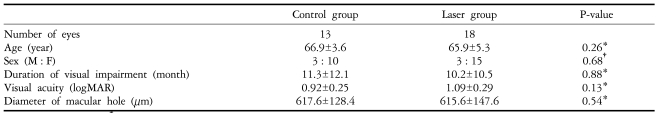
The mean age of the patients was 66.4 years (range 55 to 76 years). The laser group included 18 eyes and the control group consisted of 13 eyes. Tables 1 and 2 summarize the preoperative status and demographic characteristics of the patients in each group. All of the patients enrolled in this trial completed examinations 3 months after initial surgeries.
Table 1.
Summary of preoperative status of study groups
*Mann-Whitney test, †Fisher's exact test.
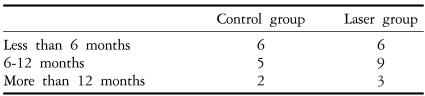
Table 2.
Duration of subjective visual impairment in study groups
Chi-square test, P>0.05.
1. Anatomical outcome
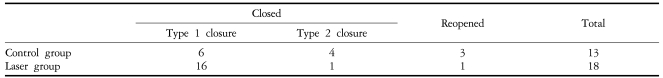
Table 3 summarizes anatomical outcomes after macular hole surgery. When assessed by OCT, the macular hole closure was achieved in 17 of the 18 eyes (94.4%) in the laser group and in 10 of the 13 eyes (76.9%) in the control group (P=0.28). Type 1 closure on OCT examination was observed in 16 eyes (88.8%) in the laser group and 6 eyes (46.2%) in the control group. The rates of type 1 closure was significantly higher in the laser group, with an odds ratio of 9.33 (P=0.02). Macular holes reopened in 3 of the control group eyes and in 1 laser group eye. All cases of reopening were managed by laser photocoagulation at the center of the macular hole, and fluid gas exchange with perfluoropropane gas. All 3 reopened control group macular holes closed after the additional intervention. The one reopened macular hole in the laser group, whose initial diameter was 961 µm, retained the reopened macular hole despite the additional procedure.
Table 3.
Anatomical results of macular hole surgery
Type 1 closure versus type 2 closure and reopened; Odds ratio: 9.33, P=0.02 (examined with Fisher's exact test).
Two patients exhibited bilateral macular holes. Type 1 closure was achieved postoperatively in both of the laser group eyes, whereas the fellow eyes in the control group consistently exhibited type 2 closure (Fig. 1).
2. Visual outcome
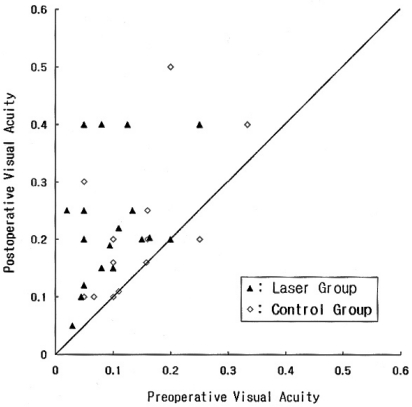
Preoperative logMAR visual acuity was 1.09±0.29 (mean ± standard deviation) in the laser group, and 0.92±0.25 in the control group (P=0.13). Postoperative logMAR visual acuity was 0.69±0.23 in the laser group and 0.73±0.23 in the control group (P=0.51). Preoperative visual acuity was poorer in the laser group rather than in the control group and so we compared the extent of visual improvement between the 2 groups. Changes in visual acuity in all of the individual cases enrolled in this study are shown in Figure 2. The amount of improvement in logMAR visual acuity 3 months after surgery was 0.40±0.29 in the laser group, which was significantly larger than that in the control group, 0.19±0.23 (P=0.03). The amount of improvement in logMAR visual acuity among the eyes which achieved macular hole closure was 0.52±0.32 in the laser group and 0.31±0.30 in the control group (P=0.09).
Fig. 2.
Scatterplot distribution of preoperative versus postoperative Snellen visual acuity of all study eyes. The diagonal line represents no change in visual acuity.
3. Complications
None of the patients experienced any intraoperative complication. Four eyes in each group underwent cataract surgery during or after macular hole surgery. Postoperative elevation in intraocular pressure to more than 30 mmHg was noted in one eye, and was controlled with medical therapy.
Discussion
This study excluded macular holes with diameters of less than 400 µm, as most of these exhibited the complete sealing of macular hole postoperatively, without the necessity for any adjuvant therapy.20-23
Most of all, this study demonstrated that laser photocoagulation promotes the completeness of macular hole closure, and induces a greater degree of postoperative visual improvement at 3 months. Because it was identified that type 1 closure was associated with larger postoperative visual improvement and less recurrence,20 type 1 closure should be considered as an anatomic endpoint for better prognosis. Hence, it is both encouraging and clinically significant, that laser photocoagulation, when used as an adjuvant therapy, promoted the rate of the type 1 closure.
One may raise the question whether type 2 closure in this study could be regarded as a form of closed macular hole. According to one review article,24 the traditional biomicroscopic criterion of macular hole closure has been the flattened edge of the macular hole, which corresponds to type 2 closure in this study. Compared with preoperative macular hole diameter, the diameter of foveal tissue defects in type 2 closure was always smaller on OCT examination. Type 2 closure also accompanied improved postoperative vision. Accordingly, type 2 closure should also be regarded as a type of anatomic success as in previous reports.
The anatomic success rate (76.9%) in the control group seems lower than in recent studies employing similar surgical techniques. We postulate that this difference might arise partly from the chronicity of the macular holes in our study. The mean durations of symptoms in both groups of our study was more than 10 months and the mean hole diameters were accordingly larger than 615 µm in both groups. Additionally, most of the previous reports did not determine the closure of macular holes by OCT but rather by biomicroscopy, which might also result in a different estimation of anatomic success from our study.
Some of the results in this study were noteworthy. First, after applying laser photocoagulation and fluid-gas exchange in cases of reopened macular holes, we achieved the closure of reopened macular holes in all 3 eyes which were initially included in the control group. Second, the laser-applied eyes of 2 patients with bilateral macular holes achieved the type 1 closure, but the fellow eyes included in the control group consistently did not. These two results imply that laser photocoagulation at the center of the macular hole is a potent tool for the improvement of anatomic success.
The preoperative diameter of the one reopened macular hole in the laser group was 961 µm, the largest observed in this study. This suggests that laser photocoagulation at the center of the hole may promote macular hole closure, and type 1 closure in particular, but has limitations in cases of extremely large macular holes.
There would be concerns regarding central scotoma induced by laser photocoagulation. According to previous studies on the changes in visual fields, which utilized scanning laser ophthalmoscopic microperimetry after the laser photocoagulation for recurrent macular holes, only clinically insignificant changes in visual field could be observed.16 The findings among the eyes which achieved anatomical success in our study indicated that the laser group eyes tended to show greater visual improvement than the control group eyes did (P=0.09). Therefore, laser photocoagulation at the center of the macular hole likely caused no serious problems in visual function, although we could not completely dismiss the possibility of a scotoma related to laser photocoagulation.
Some modifications from previous studies were made with regard to the application of laser photocoagulation in this study. Laser photocoagulation was performed with a slit lamp delivery system instead of an endolaser, which allowed us to focus the laser spot on a more exact location and to titrate laser power more predictably. In addition, we applied three spots of 100 µm-sized laser burn, rather than one larger laser spot, which helped us to prevent excessive retinal pigment epithelial damage at the center of the large laser spot.
Compared with other modes of adjuvant therapy, laser photocoagulation at the center of the macular hole has several advantages. First, the technique is not associated with any additional complications such as intraocular inflammation, which may result from the intraocular application of transforming growth factor-beta 2, autologous platelet concentrate, and tissue sealant.5-8 Secondly, this technique is quite easy to apply, as it only requires a fundus laser photocoagulator. Third, the visual and anatomic benefits associated with the technique have been consistently suggested by a host of authors.12-14
In conclusion, the results of this randomized trial suggest that laser photocoagulation at the center of macular holes causes no harm and may improve the anatomic and visual outcomes of surgery for idiopathic macular holes with diameters larger than 400 µm. Further studies with larger numbers of cases and longer-term follow-up are warranted.
References
- 1.Gass JDM. Idiopathic senile macular hole. Its early stages and pathogenesis. Arch Ophthalmol. 1988;106:629–639. doi: 10.1001/archopht.1988.01060130683026. [DOI] [PubMed] [Google Scholar]
- 2.Funata M, Wendel RT, de la Cruz Z, Green WR. Clinicopathologic study of bilateral macular holes treated with pars plana vitrectomy and gas tamponade. Retina. 1992;12:289–298. doi: 10.1097/00006982-199212040-00001. [DOI] [PubMed] [Google Scholar]
- 3.Madreperla SA, Geiger GL, Funata M, et al. Clinicopathologic correlation of a macular hole treated by cortical vitreous peeling and gas tamponade. Ophthalmology. 1994;101:682–686. doi: 10.1016/s0161-6420(94)31278-4. [DOI] [PubMed] [Google Scholar]
- 4.Smiddy WE, Feuer W, Cordahi G. Internal limiting membrane peeling in macular hole surgery. Ophthalmology. 2001;108:1471–1478. doi: 10.1016/s0161-6420(00)00645-x. [DOI] [PubMed] [Google Scholar]
- 5.Glaser BM, Michels RG, Kuppermann BD, et al. Transforming growth factor-beta2 for the treatment of full-thickness macular holes. A prospective randomized study. Ophthalmology. 1992;99:1162–1172. doi: 10.1016/s0161-6420(92)31837-8. [DOI] [PubMed] [Google Scholar]
- 6.Smiddy WE, Glaser BM, Thompson JT, et al. Transforming growth factor-beta 2 significantly enhances the ability to flatten the rim of subretinal fluid surrounding macular holes. Preliminary anatomic results of a multicenter prospective randomized study. Retina. 1993;13:296–301. [PubMed] [Google Scholar]
- 7.Paques M, Chastang C, Mathis A, et al. Effect of autologous platelet concentrate in surgery for idiopathic macular hole. Results of a multicenter, double-masked, randomized trial. Ophthalmology. 1999;106:932–938. doi: 10.1016/s0161-6420(99)00512-6. [DOI] [PubMed] [Google Scholar]
- 8.Liggett PE, Skolik DS, Horio B, et al. Human autologous serum for the treatment of full-thickness macular holes. Ophthalmology. 1995;102:1071–1076. doi: 10.1016/s0161-6420(95)30909-8. [DOI] [PubMed] [Google Scholar]
- 9.Thompson JT, Smiddy WE, Williams GA, et al. Comparison of recombinant transforming growth factor-beta-2 and placebo as an adjunctive agent for macular hole surgery. Ophthalmology. 1998;105:700–706. doi: 10.1016/S0161-6420(98)94027-1. [DOI] [PubMed] [Google Scholar]
- 10.Ezra E, Gregor ZJ For the macular hole study group. Surgery for idiopathic full-thickness macular hole. Two-year results of randomized clinical trial comparing natural history, vitrectomy, and vitrectomy plus autologous serum: Moorfields macular hole study group report No.1. Arch Ophathlmol. 2004;122:224–236. doi: 10.1001/archopht.122.2.224. [DOI] [PubMed] [Google Scholar]
- 11.Banker AS, Freeman WR, Azen SP, Lai MY Vitrectomy for Macular Hole Study Group. A multicentered clinical study of serum as adjuvant therapy for surgical treatment of macular holes. Arch Ophthalmol. 1999;117:1499–1502. doi: 10.1001/archopht.117.11.1499. [DOI] [PubMed] [Google Scholar]
- 12.Del Priore LV, Kaplan HJ, Bonham RD. Laser photocoagulation and fluid-gas exchange for recurrent macular hole. Retina. 1994;4:381–382. doi: 10.1097/00006982-199414040-00018. [DOI] [PubMed] [Google Scholar]
- 13.Ohana E, Blumenkranz MS. Treatment of reopened macular hole after vitrectomy by laser and outpatient fluid-gas exchange. Ophthalmology. 1998;105:1398–1403. doi: 10.1016/S0161-6420(98)98019-8. [DOI] [PubMed] [Google Scholar]
- 14.Min WK, Lee JH, Ham DI. Macular hole surgery in conjunction with endolaser photocoagulation. Am J Ophthalmol. 1999;127:306–311. doi: 10.1016/s0002-9394(98)00409-7. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto M, Yoshimura N, Honda Y. Increased production of transforming growth factor-beta 2 from cultured human retinal pigment epithelial cells by photocoagulation. Invest Ophthalmol Vis Sci. 1994;35:4245–4252. [PubMed] [Google Scholar]
- 16.Ikuno Y, Kamei M, Saito Y, et al. Photocoagulation and fluid-gas exchange to treat persistent macular holes after prior vitrectomy. A pilot study. Ophthalmology. 1998;105:1411–1418. doi: 10.1016/S0161-6420(98)98021-6. [DOI] [PubMed] [Google Scholar]
- 17.Johnson RN, McDonald HR, Schatz H, Ai E. Outpatient postoperative fluid-gas exchange after early failed vitrectomy surgery for macular hole. Ophthalmology. 1997;104:2009–2013. doi: 10.1016/s0161-6420(97)30064-5. [DOI] [PubMed] [Google Scholar]
- 18.Ikuno Y, Kamei M, Ohno T, et al. Photocoagulation and fluid-gas exchange for persistent macular hole. Retina. 1996;16:264–266. doi: 10.1097/00006982-199616030-00018. [DOI] [PubMed] [Google Scholar]
- 19.Ferris FL, III, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94:91–96. [PubMed] [Google Scholar]
- 20.Kang SW, Ahn K, Ham DI. Types of macular hole closure and their clinical implications. Br J Ophthalmol. 2003;87:1015–1019. doi: 10.1136/bjo.87.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ullrich S, Haritoglou C, Gass C, et al. Macular hole size as a prognostic factor in macular hole surgery. Br J Ophthalmol. 2002;86:390–393. doi: 10.1136/bjo.86.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amari F, Ohta K, Kojima H, Yoshimura N. Predicting visual outcome after macular hole surgery using scanning laser ophthalmoscope microperimetry. Br J Ophthalmol. 2001;85:96–98. doi: 10.1136/bjo.85.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ip MS, Baker BJ, Duker JS, et al. Anatomical outcomes of surgery for idiopathic macular hole as determined by optical coherence tomography. Arch Ophthalmol. 2002;120:29–35. doi: 10.1001/archopht.120.1.29. [DOI] [PubMed] [Google Scholar]
- 24.Benson WE, Cruickshanks KC, Fong DS, et al. Surgical management of macular holes: a report by the American Academy of Ophthalmology. Ophthalmology. 2001;108:1328–1335. doi: 10.1016/s0161-6420(01)00731-x. [DOI] [PubMed] [Google Scholar]