Abstract
Purpose
Oral squamous carcinoma (OSCC) cells exhibit resistance to chemotherapeutic agent-mediated apoptosis in the late stage of malignancy. Increased levels of heat shock proteins 70 (HSP70) in cancer cells are known to confer resistance to apoptosis. Since recent advances in the understanding of bacterial toxins have produced new strategies for the treatment of cancers, we investigated the effect of Pseudomonas aeruginosa exotoxin A (PEA) on HSP70 expression and induction of apoptosis in chemoresistant OSCC cell line (YD-9).
Materials and Methods
The apoptotic effect of PEA on chemoresistant YD-9 cells was confirmed by MTT, Hoechst and TUNEL stains, DNA electrophoresis, and Western blot analysis.
Results
While YD-9 cells showed high resistance to chemotherapeutic agents such as etoposide and 5-fluorouraci (5-FU), HSP70 antisense oligonucelotides sensitized chemoresistant YD-9 cells to etoposide and 5-FU. On the other hand, PEA significantly decreased the viability of YD-9 cells by deteriorating the HSP70-relating protecting system through inhibition of HSP70 expression and inducing apoptosis in YD-9 cells. Apoptotic manifestations were evidenced by changes in nuclear morphology, generation of DNA fragmentation, and activation of caspases. While p53, p21, and E2F-1 were upregulated, cdk2 and cyclin B were downregulated by PEA treatment, suggesting that PEA caused cell cycle arrest at the G2/M checkpoint.
Conclusion
Therefore, these results indicate that PEA reduced the chemoresistance through inhibition of HSP70 expression and also induced apoptosis in chemoresistant YD-9 cells.
Keywords: Pseudomonas aeruginosa exotoxin A, chemoresistance, oral squamous cell carcinoma cells, heat shock protein 70, apoptosis
INTRODUCTION
Human oral squamous cell carcinoma (OSCC) is the most frequent malignancy of the oral cavity. Treatment of OSCC has primarily relied on classical modalities such as encompassing surgery, radiation, and chemotherapy, or a combination of these methods. However, the five-year survival rate of this disease has remained in low levels (approximately 50%) during the past 30 years.1 In the late stage of malignancy, neoplastic cells show high resistance to cancer-therapy-mediated apoptosis.2,3 Chemoresistance of these cancer cells is the major impediment to successful chemotherapies in OSCC. Furthermore, if the OSCCs are removed surgically, many patients are reported to suffer from facial distortion. Therefore, inhibition of chemoresistance and selective removal of OSCC cells could be a promising strategy for the treatment of OSCC.
Tumor cells express several proteins that suppress apoptosis. Among them, the antiapoptotic members of the Bcl-2 protein family and members of the inhibitor of apoptosis protein family have been known to play a major role in chemoresistance.4-6 Recently, a group of stress proteins known as the heat shock proteins (HSP) has been recognized as a group of apoptosis inhibitors.7,8 HSP70, especially, is expressed in response to a wide variety of physiological and environmental stimulations including anticancer chemotherapy, thus allowing the cell to survive lethal conditions. In cancer cells, the expression of HSP70 is abnormally high, making the cells resistant to chemotherapy.8 This is because HSP70 protects cells from many cell damaging agents or environmental stressors. Inducible HSP70 is suggested to play multiple roles in cytoprotection against apoptosis. One study indicated that HSP70-mediated cytoprotection involved the inhibition of caspase activation and overexpression of HSP70 prevented stress-induced apoptosis by interfering with events both upstream and downstream of the stress-activated protein kinase/c-Jun N-terminal kinase signaling pathway, which might be associated with caspase activation.9 In addition, 5-fluorouraci (5-FU), the most commonly used drug in colon cancer therapy, is reported to induce HSP70 synthesis in a colon cancer cell line, thereby underlying colon cancer chemoresistance.10 Therefore, the inhibition of HSP70 has become a novel strategy of chemoresistant cancer therapy.
Bacterial toxins have emerged as powerful therapeutic agents with possible applications in treating cancer.11,12 Several bacterial toxins have been used in the form of an immunotoxin composed of antibodies linked to bacterial toxins,13,14 as well as in purified forms.15,16 Purified bacterial toxins such as shiga toxin, cholera toxin, and vacuolating cytotoxin exhibited apoptosis-inducing activity in cancer cells.16,17 Pseudomonas aeruginosa bacterium produces several extracellular products such as proteases, hemolysins, exotoxin A, exoenzyme S, elastase, and pyocyanin,18 among which PEA is known to be the most toxic factor secreted by Pseudomons aeruginosa. PEA enters into eukaryotic cells via receptor-mediated endocytosis, to be cleaved in the endocytic vesicles and translocated into the cytoplasm where it catalyzes the transfer of the ADP-ribosyl moiety of NAD+ to elongation factor 2 (EF-2). This inactivation of EF-2 in cells infected with Pseudomons aeruginosa is shown to cause inhibition of protein synthesis, leading to death of the host cell death.19 In many studies undertaken for cancer treatment, PEA has been employed in the form of immunotoxin.14,20 PEA-containing immunotoxin-induced apoptosis in breast cancer cells and leukemia cells.21,22 Although PEA-containing immunotoxin has been studied in various cancer cells, no previous reports have examined the effect of PEA on HSP70 activity in relation to chemoresistance of cancer cells. Therefore, in this work, we investigate the effect of PEA on HSP70 expression and chemoresistance of OSC cell line YD-9 cells. Here we demonstrate that PEA decreases the expression of HSP70, leading to a strong induction of apoptosis in chemoresistant YD-9 cells.
MATERIALS AND METHODS
Antibodies
Mouse monoclonal anti-human caspase-6 and Lamin A antibodies were from Upstate biotechnology (Upstate, Lake Placid, NY, USA). Mouse monoclonal anti-human caspase-3, -9 and DFF45, p53, p21, E2F-1, cdk1, cyclin B, and HSP70 were from Santa Cruz Biotechnology (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
Agents
PEA, dimethyl sulfoxide (DMSO), Hoechst33258, RNase A, proteinase K, aprotinin, leupeptin, PMSF, and thiazolyl blue tetrazolium bromide (MTT) were from Sigma Aldrich (Sigma Aldrich, St. Louis, MO, USA). TUNEL reaction mixture was from Roche Molecular Biochemicals (Roche Molecular Biochemicals, Mannheim, Germany). ECL Western blotting detection reagents were from Amersham International (Amersham Int., Buckinghamshire, UK).
Cell culture
The YD-9 cell line established from the OSCC patient was obtained from the Korean Cell Line Bank.23 The cells were cultured at 37℃ in a humidified atmosphere containing 5% CO2 in Dulbecco's modified Eagles medium: Nutrient Mixture F12 (DMEM/F12, 3 : 1) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1% glutamine, and 100 µg/mL penicillin/streptomycin.
Cell viability assay
The viability of cultured cells was estimated by an MTT assay. In the MTT assay, cells were placed in a 96-well plate and incubated for 24 hours. Then cells were treated with PEA at 0, 1.5, 7.5, 15, 30, and 60 nM for 24 hours. And then, the cells were treated with 1 mg/mL of MTT in the growth medium. Cells were incubated at 37℃ for 4 hours. The medium was aspirated and the formazan crystals, which are formed from MTT by NADH-generating dehydrogenases in metabolically active cells, were dissolved in 200 mL DMSO. Cell viability was evaluated in comparison to the control culture (taken as 100%) by measuring the intensity of the blue color (OD at 570 nm) by a multiwell reader (Quant, BioTek, Highland Park, VT, USA). Four independent experiments were undertaken and each experiment was conducted in triplicate.
Identification of proteins using LC-MS/MS
Protein spots of interest were excised and digested in the gel with a sequencing grade, modified trypsin. The separation and analysis of tryptic peptides were performed using reversed phase capillary HPLC directly coupled to a Finnigan LCQ ion trap mass spectrometer (LC-MS/MS). Both of a 0.075×20 mm trapping and a 0.075×130 mm resolving column were packed in-house with Vydac 218MS low trifluoroactic acid C18 beads (5 µm Vydac, Hesperia, CA, USA) and placed in-line. Peptides were bound and pre-concentrated in the trapping column using 5% (v/v) acetonitrile in 0.1% (v/v) formic acid. The eluting gradient was 5-80% (v/v) acetonitrile in 0.1% (v/v) formic acid for 50 min at a flow rate of 0.15 µL/min. Eluent from the capillary column was directly sprayed into the ion trap mass spectrometer. All the data were collected in centroid mode using a "triple play"; a full mass scan at a mass range of 3,952,000 da (m/z), determination of the ion charge states on zoom scan, and then an acquisition of MS/MS spectrum of the ion on a full MS/MS scan, whose collision energy was preset at its value of 55%. Sequences of uninterpreted MS/MS spectra were identified by correlating with peptide sequences obtained from the MSDB, OWL, or NCBI non-redundant protein database using Mascot software (www.matrixscience.com).
HSP70 antisense oligonucleotide treatment
2×105 cells were plated in OptiMEM I medium (Life Technologies, Inc., Gaitherburg, MD, USA). containing 5 µL of lipofectin (Invitrogen, Life Technologies, Inc., Carlsbad, California, USA) per mL of OptiMEM I medium per 100 nM oligonucleotide. 500 nM oligonucleotides (5'-CACCTTGCCGTGCTGGAA-3') were added to cells and incubated for 4 hours. Subsequently, the cells were washed and complete medium was added for 20 hours. The control sample was incubated for 4 hours in OptiMEM I and lipofectin, but not in oligonucleotides.
Hoechst staining
Cells treated with PEA for 24 hours and the cell suspension was centrifuged onto a clean fat-free glass slide with a cytocentrifuge. The samples were stained 4 µg/mL Hoechst 33258 at 37℃ for 30 min and fixed for 10 min in 4% paraformaldehyde.
TUNEL assay
To detect breakage of DNA in situ, the TUNEL assay was conducted with a TUNEL reaction mixture kit (Boehringer Mannheim, Germany). After PEA treatment, cells were washed twice with PBS, fixed in 4% paraformaldehyde for 30 min, applied with a permeabilisation solution for 5 min at 4℃, and washed again with PBS. This was followed by in situ end labeling according to the manufacturer's instructions.
DNA electrophoresis
Cells (2×106) were resuspended in 1.5 mL of lysis buffer [10 mM Tris (pH 7.5), 10 mM EDTA (pH 8.0), 10 mM NaCl and 0.5% SDS] into the added proteinase K (200 µg/mL). After samples were incubated overnight at 48℃, 200 µL of ice cold 5 M NaCl was added and the supernatant containing fragmented DNA was collected after centrifugation. The DNA was then precipitated overnight at - 20℃ in 50% isopropanol and RNase A-treated for 1 hour at 37℃. A loading buffer containing 100 mM EDTA, 0.5% SDS, 40% sucrose, and 0.05% bromophenol blue were added at 1 : 5 (v/v). Separation was achieved in 2% agarose gels in Tris-acetic acid/EDTA buffer (containing 0.5 µg/mL ethidium bromide) using 50 mA for 1.5 hour.
Western blot analysis
Cells (2×106) treated with PEA were washed twice with ice-cold PBS, resuspended in a 200 µL ice-cold solubilizing buffer [300 mM NaCl, 50 mM Tris-Cl (pH 7.6), 0.5% TritonX-100, 2 mM PMSF, 2 µL/mL aprotinin and 2 µL/mL leupeptin], and incubated at 4℃ for 30 min. The lysates were centrifuged at 14,000 revolutions per min for 15 min at 4℃. Protein concentrations of cell lysates were determined with a Bradford protein assay (BioRad, Richmond, CA, USA) and 50 µg of proteins were loaded onto 7.5-15% SDS/PAGE. The gels were transferred to the Nitrocellulose membrane and reacted with each antibody. Immunostaining with antibodies was performed using SuperSignal West Pico enhanced chemiluminescence substrate and detected with LAS-3000PLUS (Fuji Photo Film Company, Kanagawa, Japan).
RESULTS
PEA reduced the viability of YD-9 cells
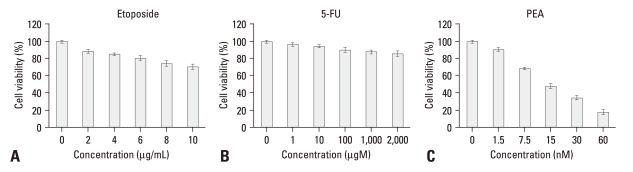
To investigate if PEA has a cytotoxic effect on YD-9 cells, an MTT assay was conducted. Viability of YD-9 cells did not significantly decrease after treatment with etoposide (2-10 µg/mL) and 5-FU (1-2,000 µM) at concentrations shown where many cancer cells are responsive. Furthermore, the proliferation of YD-9 cells was rarely affected by various concentrations of another antitumor agent genistein (data not shown). However, PEA at concentrations raging from 1.5 to 60 nM substantially reduced the viability of YD-9 cells (Fig. 1).
Fig. 1.
Effects of PEA treatment on the viability of chemoresistant YD-9 cells. (A) After 24 h incubation with etoposide (2 - 10 µg/mL) and (B) 5-FU (1 - 2,000 µM), cell viabilities were determined by MTT assay. YD-9 cell line showed resistance to etoposide and 5-FU, (C) whereas PEA significantly decreased the proliferation of YD-9 cells. Four independent assays were performed and data shown are the mean ± SD of the means obtained from triplicates of each assay.
PEA decreased HSP70 expression
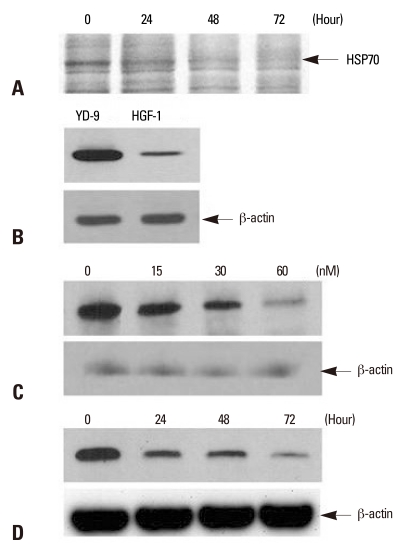
In an attempt to identify changes of protein expression by PEA treatment, identification of proteins using LC-MS/MS was performed. Among many differentially expressed proteins, HSP70 was identified and exhibited a significant decrease in response to PEA treatment (Fig. 2A). The level of HSP70 expression was compared by Western blotting on YD-9 and human normal gingival fibroblast (HGF-1). The result illustrated in Fig. 2B showed an increase in HSP70 expression in YD-9 cells. Densiometric analysis indicated that HSP70 expression was five times higher in YD-9 compared to what is obtained from HGF-1. Overexpression of HSP70 decreased in various concentrations of PEA after 24 hours incubation (Fig. 2C) and at various time points after incubation with 15 nM PEA (Fig. 2D).
Fig. 2.
Detection of HSP70 and the effect of PEA on HSP70 expression in YD-9 cells. (A) After incubation with15 nM PEA for indicated periods, identification of differentially expressed proteins using LC-MS/MS indicated HSP70. (B) Western blot for HSP70 from YD-9 and HGF-1 cells was performed. The expression level of HSP70 in YD-9 cells was higher than HGF-1 cells. (C) After incubation with indicated doses of PEA for 24 hours, Western blot analysis showed a decrease of HSP70 expression. (D) After incubation with 15 nM of PEA for indicated time periods, Western blot analysis showed a time-dependent continual decrease in HSP70 expression.
Inhibition of HSP70 by PEA decreased resistance to etoposide and 5-FU
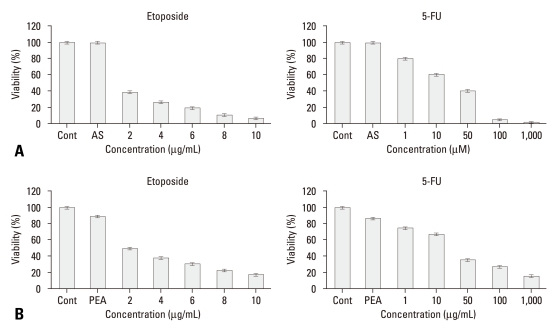
To examine if HSP70 plays any role in chemoresistance of YD-9 cells to anti-cancer drugs such as etoposide and 5-FU, HSP70 antisense oligonucleotide was administered to YD-9 cells. The treatment of HSP70 antisense oligonucleotide significantly increased the sensitivity of the cells to these anti-cancer drugs compared to anti-cancer drug treatment alone (Fig. 3A). We further confirmed the inhibitory effect of PEA on chemoresistance of YD-9 cells to etoposide and 5-FU. The treatment of YD-9 cells with etoposide and 5-FU, following incubation in 1.5 nM PEA for 24 hours, significantly decreased the viability of YD-9 cells compared to anti-cancer drug treatment alone (Fig. 3B).
Fig. 3.
Restoration of sensitivity to etoposide and 5-FU by inhibition of HSP70. (A) After incubation with HSP70 antisense oligonucleotide for 24 hours as described in the material and methods section, YD-9 cells were treated with various concentrations of etoposide and 5-FU. (B) After incubation with 15 nM PEA for 24 hours, YD-9 cells were treated with various concentrations of etoposide and 5-FU. Cell viabilities were determined by an MTT assay. Four independent assays were performed and data shown are the mean ± SD of the means obtained from triplicates of each assay.
Reduced viability of YD-9 cells was resulted from PEA induced apoptosis
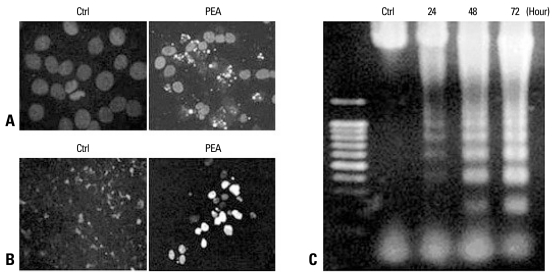
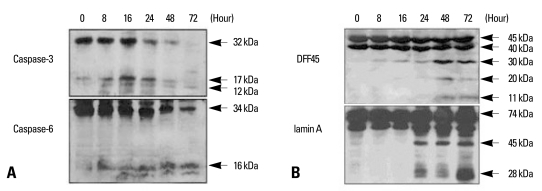
We examined whether the reduced viability of YD-9 cells was caused by apoptosis. Hoechst staining showed nuclear condensation and fragmentation in YD-9 cells after 24 hours treatment of 15 nM PEA (Fig. 4A). In the TUNEL assay, a large number of cells treated with 15 nM PEA for 24 hours exhibited a positive response (Fig. 4B). Cells treated with 15 nM PEA showed DNA degradation characteristics of an apoptotic ladder (Fig. 4C). To examine if the apoptosis was mediated by caspases, we carried out Western blotting experiments which indicated that PEA activated two effectors, caspases caspase-3 and -6. In addition to the degradation of caspase-3 and -6, cleaved products of each caspase were obtained (Fig. 5A). In the experiment to study the effects of PEA on caspase target proteins such as DFF45 and lamin A, the cleaved products of DFF45 (30 and 11 kDa) and lamin A (45 and 28 kDa) were observed (Fig. 5B).
Fig. 4.
Demonstration of apoptosis in YD-9 cells treated with 15 nM PEA. (A) Nuclear condensation and fragmentation were clearly shown at 24 hours after treatment with 15 nM PEA. (B) A TUNEL assay showed apoptotic cells in YD-9 cells treated with PEA. (C) DNA electrophoresis showed a DNA ladder in YD-9 cells treated with PEA.
Fig. 5.
Demonstration of PEA induced the cleavage of caspase-3, capase-6, DFF45, and lamin A. (A) Pro-form of caspase-3 (32 kDa) was degraded, and 17 kDa and 12 kDa cleaved forms were produced after PEA treatment. Caspase-6 pro-enzyme (34 kDa) was degraded, and 16 kDa cleaved forms were produced after PEA treatment. (B) Three cleaved products of DFF45 (30, 20, and 11 kDa) were demonstrated in YD-9 cells treated with PEA, which are evident in later time points (48 and 72 hours). Two cleaved products of lamin A (45 and 28 kDa) are demonstrated in YD-9 cells treated with PEA, which are shown at 24 hours after PEA treatment.
Cell cycle protein E2F-1 was involved in PEA induced-apoptosis
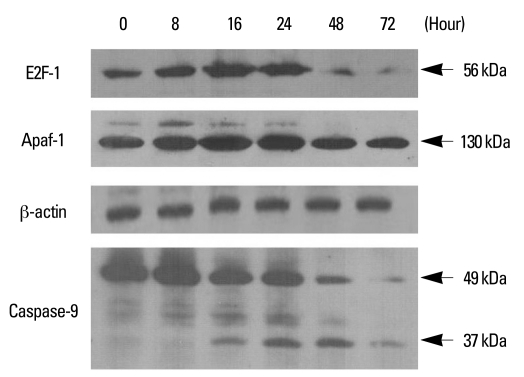
The transcription factor E2F-1 plays a role in apoptosis as well as cell cycle progression and enhances the expression of Apaf-1. Therefore, the changes in the expression of E2F-1, Apaf-1, and caspase-9 were investigated in YD-9 cells. As shown in Fig. 6, E2F-1 expression levels were markedly increased at 16 and 24 hours after PEA treatment and decreased thereafter. The expression of Apaf-1 increased at 16 and 24 hours after PEA treatment. These observations were accompanied by initial caspase-9 cleavage at 16 hours, with cleaved protein, reaching a peak concentration between 16 and 48 hours (Fig. 6).
Fig. 6.
Involvement of E2F-1, Apaf-1, and caspase-9 in PEA-induced apoptosis. YD-9 cells were cultured in the presence of 15 nM PEA for the indicated time, and whole cell lysates were subjected to Western blot analysis of E2F-1 (56 kDa), Apaf-1 (130 kDa), and caspase-9 (full-length, 49 kDa; cleaved, 37 kDa).
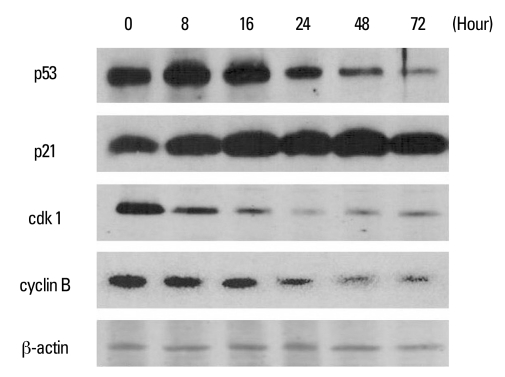
PEA treatment induced cell cycle arrest at G2/M checkpoint
We then investigated if PEA could modulate cell cycle regulatory proteins. We examined changes in protein levels using antibodies against key players in G2/M cell cycle regulation. Western blot analysis demonstrated that p53 increased at 8 and 16 hours after incubation with PEA, and then decreased. The expression of p21 increased after incubation with PEA, whereas the expression of cdk1 and cyclin B decreased in a time-dependent manner (Fig. 7).
Fig. 7.
Western blot analysis of cell cycle regulation proteins in YD-9 cells treated with 15 nM PEA. YD-9 cells were treated with 15 nM PEA for the indicated time. Total cellular lysate proteins were resolved by SDS-PASE, transferred to nitrocellulose membranes, and probed for p53, p21, cdk1, and cyclin B.
DISCUSSION
Chemoresistant tumor cells have acquired the ability to evade the action of multiple classes of anti-cancer drugs. One mechanism by which tumor cells survive in the presence of chemotherapy is the increase of anti-apoptotic activities. Because resistance to chemotherapy is a major failure in the treatment of OSCC, there is great interest in the search for new compounds that may destroy OSCC cells, which eventually can be incorporated into therapy for the treatment of OSCC. We have shown here that the purified form of PEA has a strong cytotoxic effect on chemoresistant YD-9 OSC cells through the induction of apoptosis.
Etoposide and 5-FU are known to inhibit the proliferation of various cancer cells.10,24 In particular, 5-FU has been widely used in the treatment of OSCC and is reported to induce apoptosis in OSC cells.25,26 However, etoposide and 5-FU at usual doses did not substantially inhibit the proliferation of YD-9 cells in this study. These results demonstrate that YD-9 cells are resistant to etoposide and 5-FU. Previous studies elucidated that PEA induces cellular death via apoptosis in normal cells including mouse hepatocyte27 and human mast cells.28 Because of its apoptosis-inducing activity, PEA in the form of immunotoxin has been widely used to kill target cells. However, very little is known about the effect of PEA on the chemoresistance of cancer. In this study, PEA at nanomolar concentrations strongly inhibited the growth of YD-9 cells dose-dependently, suggesting that PEA has a strong cytotoxic effect on chemoresistant cancer cells.
HSP70 is overexpressed in many cancer cells and is involved in antiapoptotic pathways by the inhibition of apoptotic signals.8,9 It was reported that the overexpression of HSP70 in U937 lymphoma cells clearly prevented caspase-3 activation, poly (ADP-ribose) polymerase cleavage, and DNA laddering.29 These antiapoptotic roles suggest that HSP70 may be involved in resistance to chemotherapeutic agents. In this study, HSP70 was weakly expressed in normal HGF-1 cells, whereas it was detected at a five-fold increase in chemoresistant YD-9 cells. PEA decreased highly expressed HSP70 in a dose- and time-dependent manner in these cells. To determine whether the decrease of HSP70 is related to resistance of YD-9 cells to 5-Fu and etoposide, we blocked HSP70 expression with HSP70 antisense oligonucleotides. We then observed that 5-FU or etoposide treatment significantly inhibited the proliferation of chemoresistant YD-9 cells at a low dose. Furthermore, after the low dose of PEA treatment, 5-FU or etoposide also significantly decreased the viability of YD-9 cells. These results suggest that PEA works through a HSP70-dependent mechanism, so that chemoresistant YD-9 cells become sensitive to anticancer drugs. Moreover, these findings are in agreement with a study showing that prolonged downregulation of HSP72 lead to severe suppression of the major survival pathways which may be responsible for enhanced sensitivity of prostate carcinoma cells to a variety of anticancer treatments.30
Recent studies have suggested that many therapeutic agents used against cancer, such as immunotherapy, chemotherapy, and irradiation, mediate their effects by induction of apoptosis of the cancer cells.31,32 Apoptosis is a regulated cell death process induced by signaling from diverse stimuli through specific cell surface receptors which trigger a cascade of intracellular molecules that initiate the cell death program.33,34 Caspases play an important role in the execution phase of apoptosis and are responsible for many of the biochemical and morphological changes associated with apoptosis.35 Caspases are known to cleave a number of proteins and the consequences of these cleavage events were suggested to be responsible for many of the phenotypic changes in the cell undergoing apoptosis.36,37 PEA-containing immunotoxin induced a loss of mitochondrial membrane potential and activation of the caspase cascade in MA-11 breast cancer cells.21 Here, the growth inhibitory effect of the PEA was demonstrated to be derived from induction of apoptosis. We presented the nuclear condensation in PEA-induced apoptosis by Hoechst staining. PEA treatment induced caspase-3 and -6 cleavages, and activation evidently resulted in cleavages of substrates such as DNA fragmentation factor 45 (DFF45) and lamin A.
E2F is a family of transcription factors that control G1/S transition of eukaryotic cells by regulating the expression of a large spectrum of genes required for DNA synthesis.38 Among E2F family members, E2F-1 has been shown to participate in apoptotic pathways by stimulating the accumulation of caspases through a direct transcriptional mechanism.39,40 The apoptosis-inducing ability of E2F-1 plays an important role in suppressing the expansion of proliferating cells, and thus provides a cancer-defense mechanism. Deregulation of E2F-1 activity contributes to enhanced proliferation and resistance to cytotoxic drugs in human melanoma cells.41 E2F-1 has been reported to activate caspase-9 in order to initiate the caspase cascade without mitochondrial damage; this activity of caspase-9 was caused by the up-regulation of Apaf-1.42,43 Furthermore, due to its contribution in selective killing of cancer cells by beta-lapachone,44 E2F-1 has been an attractive target as a new type of cancer therapeutics. In this study, increased protein levels of E2F-1 and Apaf-1 were observed at 16 and 24 hours after PEA treatment in YD-9 cells. Initial caspase-9 was cleaved at 16 hours after PEA treatment, suggesting that caspase-9 may be activated by the increase of Apaf-1 and E2F-1. We propose that PEA causes the activation of E2F-1 to provide a potent apoptotic signal in YD-9 cells.
Usually, p53 acts as an "emergency break" inducing either cell cycle arrest or apoptosis, protecting the genome from accumulating excess mutations. In response to stimuli, p53 is stabilized and activated by multiple mechanisms (including phosphorylation, dephosphorylation, and acetylation). Once activated, p53 either induces cell-cycle arrest at G1 or G2 by increasing the transcription of the p21 gene or initiate apoptosis.45 Several transcriptional targets of p53 including p21 can inhibit cdk1, thereby deficiency of activated cyclin B-cdk1 induces cell cycle arrest at the G2/M checkpoint. This study demonstrated that PEA treatment upregulated p53 and p21, and downregulated cdc2 and cyclin B, which suggests that PEA induces cell cycle arrest at the G2/M checkpoint and subsequent progress to apoptosis.
Taken together, PEA dimishes the HSP70-related protective system and thereby causes YD-9 cells to lose resistance to anti-cancer drugs. Furthermore, PEA has a strong apoptotic effect on chemoresistant YD-9 cells via the activation of caspases and the regulation of cell cycle genes. Therefore, an inhibitory effect of HSP70 and apoptotic effect of PEA suggests a potential use of this compound in the chemoresistant oral cancer cells.
ACKNOWLEDGEMENTS
This work was supported by a Korea Research Foundation grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2008-331-E00342).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.D'Silva NJ, Ward BB. Tissue biomarkers for diagnosis & management of oral squamous cell carcinoma. Alpha Omegan. 2007;100:182–189. doi: 10.1016/j.aodf.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Crowe DL, Sinha UK. p53 apoptotic response to DNA damage dependent on bcl2 but not bax in head and neck squamous cell carcinoma lines. Head Neck. 2006;28:15–23. doi: 10.1002/hed.20319. [DOI] [PubMed] [Google Scholar]
- 3.Tong D, Poot M, Hu D, Oda D. 5-Fluorouracil-induced apoptosis in cultured oral cancer cells. Oral Oncol. 2000;36:236–241. doi: 10.1016/s1368-8375(99)00079-2. [DOI] [PubMed] [Google Scholar]
- 4.Spierings D, McStay G, Saleh M, Bender C, Chipuk J, Maurer U, et al. Connected to death: the (unexpurgated) mitochondrial pathway of apoptosis. Science. 2005;310:66–67. doi: 10.1126/science.1117105. [DOI] [PubMed] [Google Scholar]
- 5.Beere HM, Green DR. Stress management - heat shock protein-70 and the regulation of apoptosis. Trends Cell Biol. 2001;11:6–10. doi: 10.1016/s0962-8924(00)01874-2. [DOI] [PubMed] [Google Scholar]
- 6.Rashmi R, Kumar S, Karunagaran D. Human colon cancer cells lacking Bax resist curcumin-induced apoptosis and Bax requirement is dispensable with ectopic expression of Smac or downregulation of Bcl-XL. Carcinogenesis. 2005;26:713–723. doi: 10.1093/carcin/bgi025. [DOI] [PubMed] [Google Scholar]
- 7.Sreedhar AS, Csermely P. Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy: a comprehensive review. Pharmacol Ther. 2004;101:227–257. doi: 10.1016/j.pharmthera.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- 9.Li M, Wang J, Jing J, Hua H, Luo T, Xu L, et al. Synergistic promotion of breast cancer cells death by targeting molecular chaperone GRP78 and heat shock protein 70. J Cell Mol Med. 2009;13:4540–4550. doi: 10.1111/j.1582-4934.2008.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grivicich I, Regner A, Zanoni C, Correa LP, Jotz GP, Henriques JA, et al. Hsp70 response to 5-fluorouracil treatment in human colon cancer cell lines. Int J Colorectal Dis. 2007;22:1201–1208. doi: 10.1007/s00384-007-0307-x. [DOI] [PubMed] [Google Scholar]
- 11.Bickels J, Kollender Y, Merinsky O, Meller I. Coley's toxin: historical perspective. Isr Med Assoc J. 2002;4:471–472. [PubMed] [Google Scholar]
- 12.Zacharski LR, Sukhatme VP. Coley's toxin revisited: immunotherapy or plasminogen activator therapy of cancer? J Thromb Haemost. 2005;3:424–427. doi: 10.1111/j.1538-7836.2005.01110.x. [DOI] [PubMed] [Google Scholar]
- 13.Alley SC, Zhang X, Okeley NM, Anderson M, Law CL, Senter PD, et al. The pharmacologic basis for antibody-auristatin conjugate activity. J Pharmacol Exp Ther. 2009;330:932–938. doi: 10.1124/jpet.109.155549. [DOI] [PubMed] [Google Scholar]
- 14.Pastan I. Immunotoxins containing Pseudomonas exotoxin A: a short history. Cancer Immunol Immunother. 2003;52:338–341. doi: 10.1007/s00262-002-0353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koo HM, VanBrocklin M, McWilliams MJ, Leppla SH, Duesbery NS, Woude GF. Apoptosis and melanogenesis in human melanoma cells induced by anthrax lethal factor inactivation of mitogen-activated protein kinase kinase. Proc Natl Acad Sci U S A. 2002;99:3052–3057. doi: 10.1073/pnas.052707699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SY, Cherla RP, Caliskan I, Tesh VL. Shiga toxin 1 induces apoptosis in the human myelogenous leukemia cell line THP-1 by a caspase-8-dependent, tumor necrosis factor receptor-independent mechanism. Infect Immun. 2005;73:5115–5126. doi: 10.1128/IAI.73.8.5115-5126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho SJ, Kang NS, Park SY, Kim BO, Rhee DK, Pyo S. Induction of apoptosis and expression of apoptosis related genes in human epithelial carcinoma cells by Helicobacter pylori VacA toxin. Toxicon. 2003;42:601–611. doi: 10.1016/j.toxicon.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Demir M, Cevahir N, Kaleli I, Yildirim U, Sahin R, Cevik Tepeli E. [Investigation of siderophore, total matrix protease and elastase activity in Pseudomonas aeruginosa isolates from lower respiratory tract and extra-respiratory tract samples.] Mikrobiyol Bul. 2008;42:197–208. [PubMed] [Google Scholar]
- 19.Marra M, Agostinelli E, Tempera G, Lombardi A, Meo G, Budillon A, et al. Anticancer drugs and hyperthermia enhance cytotoxicity induced by polyamine enzymatic oxidation products. Amino Acids. 2007;33:273–281. doi: 10.1007/s00726-007-0536-x. [DOI] [PubMed] [Google Scholar]
- 20.Kreitman RJ. Immunotoxins for targeted cancer therapy. AAPS J. 2006;8:E532–E551. doi: 10.1208/aapsj080363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersson Y, Juell S, Fodstad Ø. Downregulation of the antiapoptotic MCL-1 protein and apoptosis in MA-11 breast cancer cells induced by an anti-epidermal growth factor receptor-Pseudomonas exotoxin a immunotoxin. Int J Cancer. 2004;112:475–483. doi: 10.1002/ijc.20371. [DOI] [PubMed] [Google Scholar]
- 22.Decker T, Oelsner M, Kreitman RJ, Salvatore G, Wang QC, Pastan I, et al. Induction of caspase-dependent programmed cell death in B-cell chronic lymphocytic leukemia by anti-CD22 immunotoxins. Blood. 2004;103:2718–2726. doi: 10.1182/blood-2003-04-1317. [DOI] [PubMed] [Google Scholar]
- 23.Lee EJ, Kim J, Lee SA, Kim EJ, Chun YC, Ryu MH, et al. Characterization of newly established oral cancer cell lines derived from six squamous cell carcinoma and two mucoepidermoid carcinoma cells. Exp Mol Med. 2005;37:379–390. doi: 10.1038/emm.2005.48. [DOI] [PubMed] [Google Scholar]
- 24.Zhang P, Su DM, Liang M, Fu J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol Immunol. 2008;45:1470–1476. doi: 10.1016/j.molimm.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Azuma M, Harada K, Supriatno, Tamatani T, Motegi K, Ashida Y, et al. Potentiation of induction of apoptosis by sequential treatment with cisplatin followed by 5-fluorouracil in human oral cancer cells. Int J Oncol. 2004;24:1449–1455. [PubMed] [Google Scholar]
- 26.Hsu S, Singh B, Schuster G. Induction of apoptosis in oral cancer cells: agents and mechanisms for potential therapy and prevention. Oral Oncol. 2004;40:461–473. doi: 10.1016/j.oraloncology.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Schümann J, Angermüller S, Bang R, Lohoff M, Tiegs G. Acute hepatotoxicity of Pseudomonas aeruginosa exotoxin A in mice depends on T cells and TNF. J Immunol. 1998;161:5745–5754. [PubMed] [Google Scholar]
- 28.Jenkins CE, Swiatoniowski A, Issekutz AC, Lin TJ. Pseudomonas aeruginosa exotoxin A induces human mast cell apoptosis by a caspase-8 and -3-dependent mechanism. J Biol Chem. 2004;279:37201–37207. doi: 10.1074/jbc.M405594200. [DOI] [PubMed] [Google Scholar]
- 29.Li CY, Lee JS, Ko YG, Kim JI, Seo JS. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J Biol Chem. 2000;275:25665–25671. doi: 10.1074/jbc.M906383199. [DOI] [PubMed] [Google Scholar]
- 30.Gabai VL, Budagova KR, Sherman MY. Increased expression of the major heat shock protein Hsp72 in human prostate carcinoma cells is dispensable for their viability but confers resistance to a variety of anticancer agents. Oncogene. 2005;24:3328–3338. doi: 10.1038/sj.onc.1208495. [DOI] [PubMed] [Google Scholar]
- 31.Meyn RE, Milas L, Ang KK. The role of apoptosis in radiation oncology. Int J Radiat Biol. 2009;85:107–115. doi: 10.1080/09553000802662595. [DOI] [PubMed] [Google Scholar]
- 32.Moretto P, Hotte SJ. Targeting apoptosis: preclinical and early clinical experience with mapatumumab, an agonist monoclonal antibody targeting TRAIL-R1. Expert Opin Investig Drugs. 2009;18:311–325. doi: 10.1517/13543780902752463. [DOI] [PubMed] [Google Scholar]
- 33.Kim SY, Bae YS. Cell death and stress signaling in glycogen storage disease type I. Mol Cells. 2009 Sep 07; doi: 10.1007/s10059-009-0126-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Creagh EM, Martin SJ. Caspases: cellular demolition experts. Biochem Soc Trans. 2001;29:696–702. doi: 10.1042/0300-5127:0290696. [DOI] [PubMed] [Google Scholar]
- 35.Oancea M, Mazumder S, Crosby ME, Almasan A. Apoptosis assays. Methods Mol Med. 2006;129:279–290. doi: 10.1385/1-59745-213-0:279. [DOI] [PubMed] [Google Scholar]
- 36.Thant AA, Wu Y, Lee J, Mishra DK, Garcia H, Koeffler HP, et al. Role of caspases in 5-FU and selenium-induced growth inhibition of colorectal cancer cells. Anticancer Res. 2008;28:3579–3592. [PMC free article] [PubMed] [Google Scholar]
- 37.Wong SH, Santambrogio L, Strominger JL. Caspases and nitric oxide broadly regulate dendritic cell maturation and surface expression of class II MHC proteins. Proc Natl Acad Sci U S A. 2004;101:17783–17788. doi: 10.1073/pnas.0408229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harbour JW, Dean DC. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 2000;14:2393–2409. doi: 10.1101/gad.813200. [DOI] [PubMed] [Google Scholar]
- 39.Endo-Munoz L, Dahler A, Teakle N, Rickwood D, Hazar-Rethinam M, Abdul-Jabbar I, et al. E2F7 can regulate proliferation, differentiation, and apoptotic responses in human keratinocytes: implications for cutaneous squamous cell carcinoma formation. Cancer Res. 2009;69:1800–1808. doi: 10.1158/0008-5472.CAN-08-2725. [DOI] [PubMed] [Google Scholar]
- 40.Nahle Z, Polakoff J, Davuluri RV, McCurrach ME, Jacobson MD, Narita M, et al. Direct coupling of the cell cycle and cell death machinery by E2F. Nat Cell Biol. 2002;4:859–864. doi: 10.1038/ncb868. [DOI] [PubMed] [Google Scholar]
- 41.Halaban R, Cheng E, Smicun Y, Germino J. Deregulated E2F transcriptional activity in autonomously growing melanoma cells. J Exp Med. 2000;191:1005–1016. doi: 10.1084/jem.191.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furukawa Y, Nishimura N, Furukawa Y, Satoh M, Endo H, Iwase S, et al. Apaf-1 is a mediator of E2F-1-induced apoptosis. J Biol Chem. 2002;277:39760–39768. doi: 10.1074/jbc.M200805200. [DOI] [PubMed] [Google Scholar]
- 43.Stevens C, La Thangue NB. The emerging role of E2F-1 in the DNA damage response and checkpoint control. DNA Repair (Amst) 2004;3:1071–1079. doi: 10.1016/j.dnarep.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Sun X, LaMont JT, Pardee AB, Li CJ. Selective killing of cancer cells by beta -lapachone: direct checkpoint activation as a strategy against cancer. Proc Natl Acad Sci U S A. 2003;100:2674–2678. doi: 10.1073/pnas.0538044100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ismail IA, Kang KS, Lee HA, Kim JW, Sohn YK. Genistein-induced neuronal apoptosis and G2/M cell cycle arrest is associated with MDC1 up-regulation and PLK1 down-regulation. Eur J Pharmacol. 2007;575:12–20. doi: 10.1016/j.ejphar.2007.07.039. [DOI] [PubMed] [Google Scholar]