Abstract
MicroRNAs (miRNAs) are small non-coding RNAs that regulate messenger RNAs at the post-transcriptional level. They play an important role in the control of cell physiological functions, and their alterations have been related to cancer, where they can function as oncogenes or tumor suppressor genes. Recently, they have emerged as key regulators of "stemness", collaborating in the maintenance of pluripotency, control of self-renewal, and differen-tiation of stem cells. The miRNA pathway has been shown to be crucial in embryonic development and in embryonic stem (ES) cells, as shown by Dicer knockout analysis. Specific patterns of miRNAs have been reported to be expressed only in ES cells and in early phases of embryonic development. Moreover, many cancers present small populations of cells with stem cell characteristics, called cancer stem cells (CSCs). CSCs are responsible for relapse and treatment failure in many cancer patients, and the comparative analysis of expression patterns between ES cells and tumors can lead to the identification of a miRNA signature to define CSCs. Most of the key miRNAs identified to date in ES cells have been shown to play a role in tumor diagnosis or prognosis, and may well prove to be essential in cancer therapy in the foreseeable future.
Keywords: miRNA, microRNA, embryonic stem cell, cancer stem cell
INTRODUCTION
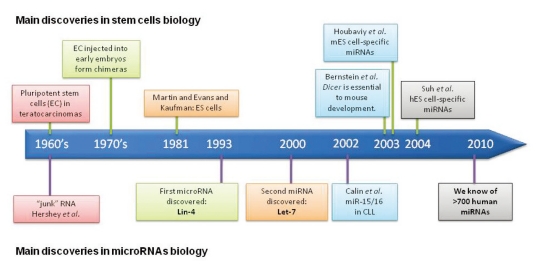
When a stem cell divides, it can produce a copy of itself as well as differentiated cell progeny. Stem cells allow the generation of embryonic tissues (embryonic stem cells) and the maintenance of adult tissue (adult stem cells). Stem cells play a key role in embryonic development. A complex organism derives from a single cell, the fertilized egg. During embryogenesis, cells are initially proliferative and pluripotent; they only gradually become restricted to different cell fates. The first evidence of pluripotent stem cells' existence in the embryo comes from the 1960-1970s, when it was seen that early mouse embryos transplanted to ectopic sites produced teratocarcinomas, tumors that contain a variety of differentiated cell types as well as embryonal carcinoma (EC) cells (undifferentiated cells).1 A tumor containing both EC cells and differentiated cells can be generated from one EC cell.2 In the 1970s, it was shown that when EC cells were injected back into early embryos, they could reveal their pluripotency and participate in the formation of chimeras.3,4 But it was not until 1981 that Martin5 and Evans and Kaufman6 discovered embryonic stem (ES) cells, permanent pluripotent cell lines which could be derived directly from an embryo at blastocyst stage.
ES cells may participate in normal embryonic development when they are injected into the blastocyst. In contrast, ES cells develop into tumors if injected in ectopic sites in adult mice. Moreover, the role of microenvironment in the growth control of embryonic stem cells has also been examined. When ES cells were injected in pregnant mouse uteri, no tumor formation was observed; in contrast, the ES cells injected into pseudopregnant uteri often developed into tumors, and those injected into non-pregnant uteri always developed into teratocarcinomas. These results showed that the pregnant-uterine microenvironment may participate in the control of ES cell growth.7 Now we know that stem cells are controlled by extrinsic signals from their regulatory niche and also by intrinsic factors like the hyperdynamic plasticity of chromatin proteins.8 In summary, ES cells can readily be shown to differentiate into essentially all cell phenotypes, whereas most isolates of adult stem cells have more limited potential for differentiation. Both retain the capacity to divide indefinitely, and this division must be finely regulated because few divisions can alter tissue homeostasis and many divisions can drive to cancer initiation. Many cancers present small populations of cells with stem cell characteristics, called cancer stem cells (CSCs). Recently, novel elements, microRNAs (miRNAs), have emerged as crucial regulators for proper stem cell maintenance and function (Fig. 1).
Fig. 1.
Timeline showing the main discoveries in stem cell biology and miRNA research. miRNA, microRNA.
miRNA characteristics
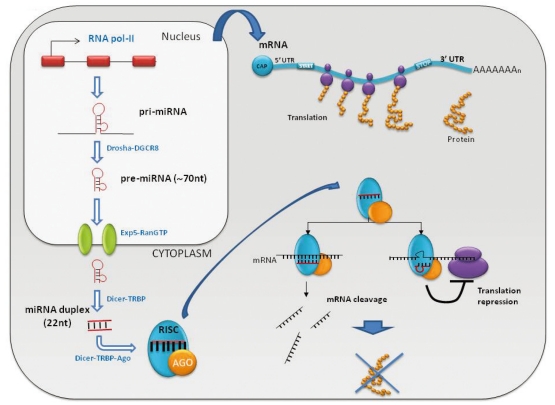
miRNAs are small non-coding RNAs (22-24 nucleotides in length) that negatively regulate messenger RNA translation to protein by binding to 3'UTRs of target messenger RNA.9 These molecules were described for the first time in 1993 by Victor Ambros and colleagues in Caenorhabditis elegans, and to date, more than 700 miRNAs have been identified in humans. They are encoded in the entire genome, including the exonic, intronic, and intergenic regions, but 90% are found in intronic regions.10 They are transcribed from these regions through RNA polymerase II action - first as large molecules, called pri-miRNAs, that can exceed 1 Kb and that could be a polycistronic transcript containing more than one miRNA. In the same nucleus they are processed by RNase complexes (Drosha and DGCR8) into -70 nucleotide fragments with stem-loop structures called pre-miRNAs. The pre-miRNA is exported to the cytoplasm by Exportin-5 and RAN is the official full name action. There it is further cleaved by Dicer action to generate a duplex molecule of -25 nucelotides in length. These duplex molecules bind a protein complex called the RNA induced silencing complex (RISC), where one of the two chains is selected, known as mature miRNA, to guide this complex to the target mRNA. In general, when a miRNA and a mRNA exhibit total complementarities, RISC is capable of degrading target mRNA, whereas if base pairing complementarity is incomplete, translational silencing of the target occurs. Through these mechanisms, miRNAs decrease translation of human genes (Fig. 2).9,10
Fig. 2.
miRNA biogenesis pathway.
Since their discovery, miRNAs have been shown as powerful key regulator elements, since a single miRNA can silence several hundreds of genes and one gene can be targeted by several miRNAs, producing different grades of repression.9 miRNAs target a large number of genes in relation to a great variety of biological processes, including regulation of proliferation and apoptosis and stem cell self-renewal and differentiation. Moreover, miRNA expression is regulated in a tissue- and developmental stage-specific manner.11-15
miRNA deregulation is thus related to an increasing number of diseases, including cancer. All the mechanisms that can alter the normal expression of a gene can affect the expression of a miRNA, including chromosomal translocations, amplifications, and deletions or mutations.16 High-throughput analyses have shown that miRNA expression is commonly deregulated in several types of cancer.17
miRNAs in stem cell regulation
ES cell regulation by miRNAs
Human ES cells are cell lines established from the explanted inner cell mass of human blastocysts. Experiments with Dicer and DGCR8 knockouts have provided evidence that miRNAs play a crucial role in development in general and specifically in ES cells. Dicer deletion is lethal in the early stages of development in Drosophila18 and mice.19 Dicer conditional knockouts showed defects in proliferation, miRNA maturation, and differentiation,20,21 and DGCR8 conditional knockout showed defects in proliferation and cell cycle progression,22 demonstrating that the miRNA pathway is closely related with the regulation of ES cells.
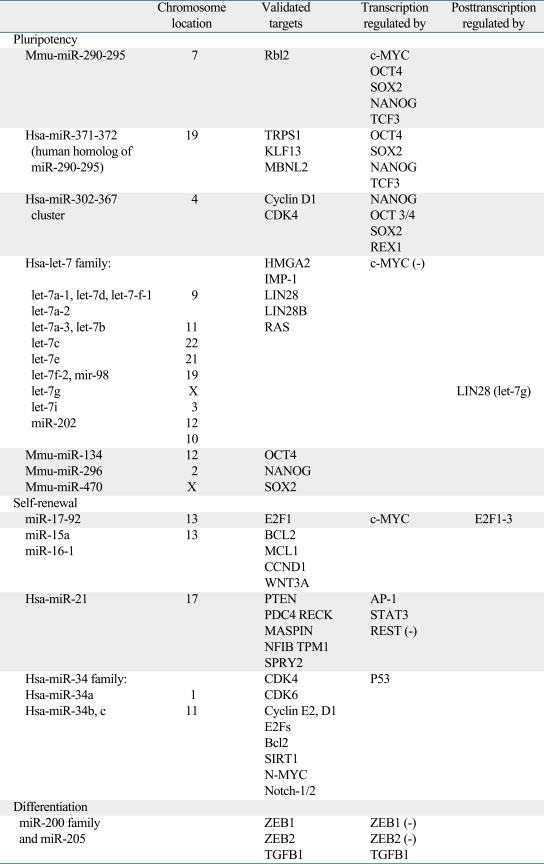
Houbaviy, et al.23 were the first to analyze ES cell-specific miRNAs. They identified a signature of overexpressed miRNAs in mouse ES cells that were repressed as they differentiated into embryoid bodies and were absent in the adult organ tissues. From the set of miRNAs identified, the authors distinguished three groups of miRNAs: miRNAs with ES cell-specific functions (the miR-290-295 cluster and miR-296); miRNAs also found in adult tissues, which could thus regulate general cell physiology (miR-15a, miR-16, miR-19b, miR-92, miR-93, miR-96, miR-130 and miR-130b); and miRNAs whose expression increases upon differentiation (miR-21 and miR-22).
One year later, Suh, et al.24 reported the first miRNA signature in human ES (hES) cells. As in the previous work, the miRNAs identified are related to each other in clusters (polycistronic primary transcripts). They identified 36 miRNAs and grouped them into four categories: ES cell-specific miRNAs (miR-200c, 368, 154*, 371, 372, 373*, 373); miRNAs found in both ES cells and their malignant counterpart the EC cells (miR-302a, 302a*, 302b, 302b*, 302c, 302c*, 302d and 367); miRNAs that are rare in ES cells but abundant in Hela and STO cell lines (let-7a, miR-301, 374, 21, 29b, 29); and miRNAs expressed in the majority of the cell lines tested (miR-16, 17-5p, 19b, 26a, 92, 103, 130a and 222).
ES cell identity can be formed by the transcription factors Nanog, OCT4, and Sox2.25-31 These genes can be regulated by miR-134, miR-296, and miR-470.32 Moreover, Marson, et al.33 report that these genes regulate the expression of miRNAs related to the pluripotency state by binding to the promoter region. The miR-302-367 cluster is one of the miRNAs regulated by these transcription factors and its expression is restricted to the ES cell compartment. This cluster regulates the cell cycle by promoting self-renewal and pluripotency.34
Analyses of the role of miRNAs in the differentiation of hES cell lines have identified two groups of miRNAs: those that are expressed in the undifferentiated state and are markers of pluripotency (miR-200c, 371, 372, 302a, 320d , 373, 302c, 21, 222, 296, 494, 367), and miRNAs that act to regulate the differentiation of cells into one of the different embryonic lineages (miR-17, 92 and 93, which are overexpressed in differentiated cells, and miR-154, 29a, 143, 29c, and let-7a, which are underexpressed in differentiated cells).35 In an analysis of miRNAs that change during hES cell differentiation into embryoid bodies, a set of 30 miRNAs was identified, some of which had previously been identified as let-7a.36 The majority of the miRNAs that characterize hES cells are expressed from chromosomes 19 and X.36
Other investigators examined the target related to hES cell-specific miRNAs, and identified TRPS1 and KLF13 transcription factors and MBNL2 a RNA binding protein as targets of miR-302d and miR-372.37 The same authors identified in these hES cell lines a set of miRNAs upregulated (miR-372, 302d, 367, 200c, 199a, 19a and 217) or downregulated (miR-19b, 221, 222, let-7b, and let-7c) by Activin A, an important gene that participates in the maintenance of pluripotency.38
Another work reported that the miR-302 cluster on chromosome 4 and the miR-520 cluster on chromosome 19 were highly expressed in undifferentiated hES cells. These miRNAs negatively correlate with target genes with chromatin structure modification function and play a role in the maintenance of chromatin structure.39
Epithelial-mesenchymal transition
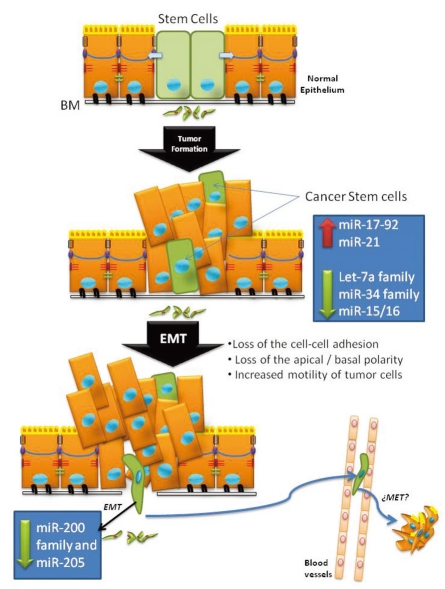
During embryogenesis, epithelial-mesenchymal transition (EMT) plays a role in the formation of various tissues and organs. EMT consists in the conversions of epithelial cells to mesenchymal cells; the reverse process is called mesenchymal to epithelial transition (MET). Epithelial cells establish close contacts with their neighbors and an apicobasal axis of polarity through the sequential arrangement of adherens junctions, desmosomes, and tight junctions. The epithelial cell layer maintains global communication through gap junctional complexes, and it remains separated from adjacent tissues by a basal lamina. Epithelia have the capacity to function as barriers or in absorption. Conversely, mesenchymal or stromal cells are loosely organized in a three-dimensional extracellular matrix and comprise connective tissues adjacent to epithelia. The conversion of epithelial cells to mesenchymal cells is fundamental for embryonic development and involves profound phenotypic changes that include the loss of cell-cell adhesion, the loss of cell polarity, and the acquisition of migratory and invasive properties.40 Most adult tissues arise from a series of EMT and MET. However, during one's adult life, only a certain subset of cells retains the ability to undergo EMT. Epithelial tumor cells often activate EMT, a latent embryonic program, to acquire tumoral properties, including enhanced motility and an increased invasiveness capacity.40
Recently, miRNAs have been related to the control of EMT. Gregory, et al.41 showed that the miR-200 family (miR-200a, miR-200b, miR-200c, miR-141 and miR-429) and miR-205 were key regulators of EMT. These miRNAs were markedly downregulated in cells that had undergone EMT in response to TGF-β, and their enforced expression was enough to prevent EMT. These miRNAs act through their targets ZEB1 and ZEB241 and TGF-β2,42 which are E-cadherin transcriptional repressors. These miRNAs are thus able to enforce the epithelial phenotype through post-transcriptional repression of these genes, allowing the expression of E-cadherin and of polarity factors which are integral in forming cell-cell junctions (Fig. 2). The miR-200 family and miR-205 have been reported to be expressed in an ephithelial-specific manner in the adult (in tissues that contain a high proportion of epithelial cells)43 and during embryogenesis.44-46 Recent findings have also connected the let-7 family with the miR-200 family in EMT and stem cell formation (reviewed in47).
MicroRNAs in CSCs
Many cancers have small populations of CSCs, cells with stem cell characteristics, which are a likely cause of relapse in cancer patients. The early phases of carcinogenesis resemble embryonic development, often involving the reexpression of embryonic mesenchymal genes. Undifferentiated stem cells display miRNA expression profiles reminiscent of cancer cells. Numerous known oncogenic miRNAs are expressed in early stages of development in undifferentiated cells; however, their expression decreases in differentiated tissue, whereas the opposite holds true for tumor suppressive miRNAs. In a previous study, we established what was to our knowledge the first connection between hES cells and tumor tissues through miRNA expression.48 We carried out a comparative study of miRNA expression in human colon mucosa in early embryonic stages (7-8 weeks) versus late embryonic stages (9-12 weeks). Likewise, we compared miRNA expression in normal colon tissue versus colorectal cancer stages I and II. Overlapping miRNA expression was detected between embryonic colonic mucosa and colorectal.48
To date, no specific profiles of miRNAs in CSCs have been described. However, alterations in stem cell miRNAs have been reported in some tumors, where they can play a role as tumor suppressors or oncogenes.
Let-7/miR-98 family
Let-7 was the first human miRNA to be discovered. This miRNA family is highly conserved in invertebrates and mammals and is required for the timing of cell fate determination in C. elegans.49,50 Many human let-7 genes map to regions altered or deleted in human tumors,16 indicating that these genes function as tumor suppressors. Let-7 plays a role in lung cancer progression, where it is downregulated and where the postoperative survival time in lung cancer patients directly correlated with let-7 expression levels.51,52 Let-7 may directly control cellular proliferation by negatively regulating the human RAS genes,53 known lung cancer oncogenes.
Moreover, let-7 is a marker for less advanced cancer and its expression inversely correlated with HMGA2, an early embryonic gene, but not with classical epithelial or mesenchymal markers, such as E-cadherin or vimentin, in SC1 and SC2 cells.54 The expression of let-7 and HMGA2 acts as prognostic markers in ovarian cancer.54 The loss of let-7 in cancer occurs early during the transformation process and results in reverse embryogenesis and dedifferentiation.55 Along these lines, we investigated whether there are similar patterns of the let-7 family miRNA expression in pseudoglandular human embryonic lungs and in human lung tumors and found they were downregulated both in embryonic lung tissue and in lung tumors. Our findings support the model of cancer as an alteration of normal development, as many miRNAs were similarly expressed in early human lung development and stage I-II of lung cancer development.56 Moreover, let-7 has been shown to be strikingly downregulated in mouse ES cells, where LIN28 is highly expressed but decreases during differentiation.33 These two factors (let-7 and LIN28) form a tight feed-back loop that is fundamental to maintaining the precise levels of each to maintain the "stemness", and so their dysregulation may lead to cancer.
miR-17-92 cluster
The miR-17-92 cluster (miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1 and miR-92-1) was one of the first recognized miRNAs with an oncogenic function; the overexpression of the miR-17-92 cluster is known to promote cell proliferation.57 miR-17-92 is transcriptionally activated by c-MYC, which in combination with Oct4, Nanog, Sox2, and Klf4, is necessary for the generation of induced pluripotent stem cells.58-63 The c-Myc-activated miR-17-92 could thus play a role in maintaining pluripotence and self-renewal in stem cells. In our study of colorectal,48 we established the first connection between hES cells and tumor tissue through miRNA expression.48 We carried out a comparative study of miRNA expression in colon mucosa in human embryos and colorectal cancer. We focused on the miR-17-92 cluster and specifically on miR-17-5p, which was overexpressed in an early embryonic colon and in stage I and II colorectal tumors. In situ hybridization confirmed the high level of expression of miR-17-5p in the crypt progenitor compartment in the normal tissue (stem cell compartment), in the epithelial embryonic tissue and in the tumor.48 In a similar study in lung cancer and human lung embryogenesis, we found similar results.56 These findings support the potential existence of a relationship between stem cells, cell differentiation, and cancer. Moreover, this miRNA cluster, located in chromosome 13q31, is amplified in a number of B-lymphomas57 and is overexpressed in multiple tumors, including lung, lymphoma, myeloid leukemias, hepatocellular carcinomas, and colorectal.64
Moreover, a paralog cluster of miR-17-92 - the miR-106a-363 cluster located in chromosome X - has been reported to have oncogenic properties. The miR-106a-363 cluster is overexpressed in 46% of human T-cell leukemias, leading to increased miR-92 and miR-19 levels.65
miR-302-367 cluster
This miRNA cluster plays an important role in the maintenance of pluripotency and quickly decreases after cell differentiation and proliferation. It is also expressed in human embryonic carcinoma cells. This miRNA cluster is encoded in chromosome 4 and is comprised of nine miRNAs: miR-302a, miR-302a*, miR-302b, miR-302b*, miR-302c, miR-302c*, miR-302d, miR-367, and miR-67* (polycistronic transcript). There is scarce evidence that this miRNA cluster could play a role in cancer. Recently, however, Lin, et al.66 reprogrammed human skin cancer cells into a pluripotent ES-cell-like state with ectopic expression of the miR-302 cluster. Moreover, some members of the cluster are found expressed in certain acute immature leukemia cell lines but not in normal somatic hematopoietic cells.67
miR-34 family
Some miRNAs have been implicated in cell cycle control related to p53, a crucial tumor supressor that responds to cellular stress. Through its DNA binding domain, the p53 protein activates the expression of the miR-34 family in response to DNA damage or oncogenic stress, leading to apoptosis or cell cycle arrest.68-70 The conserved miR-34 family is composed of three miRNAs: miR-34a, miR-34b, and miR-34c. There are two miR-34 loci, one encoding miR-34a (chromosome 1) and the other encoding both miR-34b and miR-34c (chromosome 11). The promoter regions of both loci contain a p53-binding site.68 The miR-34 family has been shown to form part of the p53 network,68,71 and its expression is directly induced by p53 in response to DNA damage or oncogenic stress. Moreover, the promoter region of the MIRN34A gene contains CpG islands, and aberrant CpG methylation that reduces miR-34a expression levels has been reported in multiple types of cancer.72 The high frequency of miR-34 silencing in tumors suggests that the miR-34 family acts as a tumor suppressor gene, indicating a potential role as a prognostic marker. Recently, our group identified miR-34a as a prognostic marker of relapse in NSCLC patients; patients with both p53 mutations and low miR-34a levels had the highest probability of relapse.69 Moreover, miR-34a has been shown to play a role in chemotherapy resistance in CLL.73
The miR-34 family has been related to the Notch pathway, an important pathway during normal development where it acts to regulate many cellular processes. miR-34a targets several mRNAs, such as SIRT1, Bcl-2, N-myc, and Cyclin D1, and has recently been reported to target Notch-1, Notch-2, and CDK6. In pancreatic cancer, stem cells have high levels of Notch-1/2 and loss of miR-34, suggesting that miR-34 is involved in the regulation of pancreatic CSCs via regulation of the Notch pathway.74
miR-15, miR-16
miR-15 and miR-16 were two of the first indentified miRNAs with a tumor suppressor function. These miRNAs are encoded in 13q14, a region often implicated in cancer, and they are frequently deleted and/or downregulated in chronic lymphocytic leukemia (CLL).75 Expression of these miRNAs inhibits cell proliferation, promotes apoptosis, and suppresses tumorigenicity both in vitro and in vivo (reviewed in76) by targeting multiple oncogenes, including BCL2, MCL1, CCND1, and WNT3A. Downregulation of these miRNAs has been reported in CLL,75 pituitary adenomas,77 prostate carcinoma,78 NSCLC,79 and ovarian cancer.80
These miRNAs play a key role in CSC regulation and in tumor cells, where they help to control self-renewal through cell cycle control.81 Moreover, in ovarian cancer, these miRNAs regulate Bmi-1. Bmi-1 plays a key role in regulating the proliferative activity of normal stem and progenitor cells.82 It is also indispensable for the self-renewal of neural83 and hematopoietic stem cells.84 Oncogenic activation of Bmi-1 is found in a wide variety of epithelial malignancies, including ovarian cancer, where it correlates with the downregulation of miR-15/16.81
Recently, miR-16 has been linked to the p53 pathway. Suzuki, et al.85 have shown that p53 interacts with proteins of the Drosha complex to promote processing of a subset of miRNAs, including miR-16-1. We have analyzed the prognostic implications of miR-16 levels in NSCLC patients and found that miR-16 works as a marker for disease-free and overall survival marker in these patients (unpublished data).
miR-21
miR-21 is encoded in chromosome 17q23 and is overexpressed in a large number of tumors, including breast cancer, Hodgkin lymphoma,86 leukemia, glioma, head and neck cholangiocarcinoma,87 hepatocellular carcinoma,88 colorectal,48,89 cervical, ovarian, and pancreatic cancer.64 In these tumors, miR-21 promotes proliferation by inhibiting apoptosis and promotes invasion by acting on different targets: PDC4, PTEN, RECK, MASPIN, NFIB, TPM1, or SPRY2 (reviewed in90). In contrast, relatively low expression of miR-21 was observed in the HL-60 (promyelocytic leukemia) and the K562 (chronic myelogenous leukemia) cell lines and in a prostatic adenocarcinoma cell line. The transcription of miR-21 has been shown to be activated by AP-191 and STAT3,92 and the overexpression of miR-21 in multiple types of cancers may reflect an elevated AP-1or STAT3 activity. In contrast to its high expression in tumors, miR-21 expression was low in ES cells, where miRNAs play a complex role in self-renewal/proliferation, since REST, a gene essential for pluripotency and self-renewal, binds to the miR-21 promoter region, and represses its expression.93
miR-200 family and miR-205
Recent evidence suggests that cells that undergo EMT acquire stem cell-like properties.94,95 Moreover, it has been showed that ES cells can adopt a mesenchymal phenotype without losing their pluripotency.96,97 Loss of the miR-200 family seems to be a late-stage event in epithelial tumor progression, occurring in de-differentiated tumor cells with a greater metastatic capacity via EMT (Fig. 3).The miR-200 family and miR-205 have been reported to be dysregulated in specific cancers. The miR-200 family is down-regulated in benign liver tumors98 and upregulated in ovarian99 and cholangyocyte100 tumors. Our group has observed overexpression of the miR-200 family in colorectal cancer (non-published data). miR-205 is downregulated in breast cancer101 and esophageal tumors87 and upregulated in ovarian99 and bladder tumors.102 The miR-200 family and miR-205 are also downregulated in poorly differentiated tumors and mesenchymal cancer cell lines.
Fig. 3.
EMT in tumors.
Microvesicles
Microvesicles (MVs) are plasma membrane-derived vesicles (from -30 nm to 1 µm), originally considered inert cellular debris, released into the extracellular environment by a variety of cell types, including ES cells and tumor cells. Originally characterized from platelets,103 MVs are a normal constituent of human plasma, where they play an important role in maintaining hematostasis. MVs contain a variety of elements, including proteins, lipids, and RNA, and recently it has been shown that they are enriched in miRNAs.104 MVs play a role in stem cell-microenvironment communication, where MVs account for the transfer of genetic information between the microenvironment and stem cells, thus modulating the stem-phenotype.105 Recently, MVs have been isolated from ES cells. These ES cell microvesicles (ESMVs) were capable of reprogramming hematopoietic progenitors.106 Moreover, ES cells produce microvesicles which contain abundant miRNAs.107 Tumor cells can also release MVs, which can be detected in the plasma of cancer patients,108 where they may function as prognostic markers. In ovarian cancer, eight miRNAs (miR-21, miR-141, miR-200a, miR-200c, miR-200b, miR-203, miR-205, and miR-214) previously shown to be diagnostic were found more highly expressed in MVs from advanced ovarian cancer patients than in patients with benign ovarian diseases; moreover, these miRNAs were not present in normal controls.109 These miRNAs could be useful as diagnostic biomarkers in tumors like NSCLC.109 The detection of miRNAs in plasma/serum MVs in cancer patients opens a new window for tumor screening and therapeutic approaches.
CONCLUSION
miRNAs have emerged as key regulators of "stemness", collaborating in the maintenance of pluripotency, control of self-renewal, and differentiation of stem cells. Recently, several breakthroughs have increased our knowledge of the role of miRNAs in stem cells. Firstly, miRNAs expressed specifically in ES cells have been identified. Secondly, recent findings indicate that cancer may arise from stem cells (CSCs) and that many cell signaling pathways essential for normal development are also involved in cancer initiation and progression. Moreover, overlapping expression patterns of miRNAs have been identified in embryonic and cancer cells. Taken together, these findings provide hints that a strong link exists between embryonic cells and cancer cells. Thirdly, miRNAs have been linked to the main pathways of stem cells, where some miRNAs regulate or are regulated by stem cell-genes such as Nanog, Oct4, or Sox2. Finally, although miRNAs are not at the top of the stem cell hierarchy, they are crucial intermediate regulators, and the ectopic expression of certain miRNAs, such as the miR-302-367 cluster, can induce pluripotency. As we increase our knowledge of miRNAs in ES cells, we will be able to identify new targets in the fight against tumors. CSCs are responsible for relapse and treatment failure in many cancer patients, and the comparative analysis of expression patterns between ES cells and tumors can lead to the identification of a miRNA signature to define CSCs. Most of the key miRNAs identified to date in ES cells have been shown to play a role in tumor diagnosis or prognosis and may well prove to be essential in cancer therapy in the foreseeable future.
Table 1.
Main microRNAs Related to Pluripotency, Self-Renewal and Differentiation in Stem Cells
ACKNOWLEDGEMENTS
This work was supported by Fondo de Investigaciones Sanitarias de la Seguridad Social [FIS-PI09/00547].
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Stevens LC. Embryonic potency of embryoid bodies derived from a transplantable testicular teratoma of the mouse. Dev Biol. 1960;2:285–297. doi: 10.1016/0012-1606(60)90010-5. [DOI] [PubMed] [Google Scholar]
- 2.Kleinsmith LJ, Pierce GB., Jr Multipotentiality of single embryonal carcinoma cells. Cancer Res. 1964;24:1544–1551. [PubMed] [Google Scholar]
- 3.Brinster RL. The effect of cells transferred into the mouse blastocyst on subsequent development. J Exp Med. 1974;140:1049–1056. doi: 10.1084/jem.140.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mintz B, Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci U S A. 1975;72:3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 7.Monzo M, de Anta JM, Peris B, Ruano D. Growth control of embryonic stem cells injected into mouse uterus on fifth day of pregnancy. Int J Cancer. 1994;56:387–392. doi: 10.1002/ijc.2910560317. [DOI] [PubMed] [Google Scholar]
- 8.Meshorer E, Yellajoshula D, George E, Scambler PJ, Brown DT, Misteli T. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev Cell. 2006;10:105–116. doi: 10.1016/j.devcel.2005.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 11.Aravin AA, Lagos-Quintana M, Yalcin A, Zavolan M, Marks D, Snyder B, et al. The small RNA profile during Drosophila melanogaster development. Dev Cell. 2003;5:337–350. doi: 10.1016/s1534-5807(03)00228-4. [DOI] [PubMed] [Google Scholar]
- 12.Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS. A microRNA array reveals extensive regulation of microRNAs during brain development. RNA. 2003;9:1274–1281. doi: 10.1261/rna.5980303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 14.Pasquinelli AE, Ruvkun G. Control of developmental timing by micrornas and their targets. Annu Rev Cell Dev Biol. 2002;18:495–513. doi: 10.1146/annurev.cellbio.18.012502.105832. [DOI] [PubMed] [Google Scholar]
- 15.Sempere LF, Sokol NS, Dubrovsky EB, Berger EM, Ambros V. Temporal regulation of microRNA expression in Drosophila melanogaster mediated by hormonal signals and broad-Complex gene activity. Dev Biol. 2003;259:9–18. doi: 10.1016/s0012-1606(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 16.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jay C, Nemunaitis J, Chen P, Fulgham P, Tong AW. miRNA profiling for diagnosis and prognosis of human cancer. DNA Cell Biol. 2007;26:293–300. doi: 10.1089/dna.2006.0554. [DOI] [PubMed] [Google Scholar]
- 18.Hatfield SD, Shcherbata HR, Fischer KA, Nakahara K, Carthew RW, Ruohola-Baker H. Stem cell division is regulated by the microRNA pathway. Nature. 2005;435:974–978. doi: 10.1038/nature03816. [DOI] [PubMed] [Google Scholar]
- 19.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, et al. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 20.Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, et al. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005;19:489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murchison EP, Partridge JF, Tam OH, Cheloufi S, Hannon GJ. Characterization of Dicer-deficient murine embryonic stem cells. Proc Natl Acad Sci U S A. 2005;102:12135–12140. doi: 10.1073/pnas.0505479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Medvid R, Melton C, Jaenisch R, Blelloch R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat Genet. 2007;39:380–385. doi: 10.1038/ng1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houbaviy HB, Murray MF, Sharp PA. Embryonic stem cell-specific MicroRNAs. Dev Cell. 2003;5:351–358. doi: 10.1016/s1534-5807(03)00227-2. [DOI] [PubMed] [Google Scholar]
- 24.Suh MR, Lee Y, Kim JY, Kim SK, Moon SH, Lee JY, et al. Human embryonic stem cells express a unique set of microRNAs. Dev Biol. 2004;270:488–498. doi: 10.1016/j.ydbio.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 25.Babaie Y, Herwig R, Greber B, Brink TC, Wruck W, Groth D, et al. Analysis of Oct4-dependent transcriptional networks regulating self-renewal and pluripotency in human embryonic stem cells. Stem Cells. 2007;25:500–510. doi: 10.1634/stemcells.2006-0426. [DOI] [PubMed] [Google Scholar]
- 26.Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 27.Moorthy PP, Kumar AA, Devaraj H. Expression of the Gas7 gene and Oct4 in embryonic stem cells of mice. Stem Cells Dev. 2005;14:664–670. doi: 10.1089/scd.2005.14.664. [DOI] [PubMed] [Google Scholar]
- 28.Mossman AK, Sourris K, Ng E, Stanley EG, Elefanty AG. Mixl1 and oct4 proteins are transiently co-expressed in differentiating mouse and human embryonic stem cells. Stem Cells Dev. 2005;14:656–663. doi: 10.1089/scd.2005.14.656. [DOI] [PubMed] [Google Scholar]
- 29.Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 30.Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005;26:495–502. doi: 10.1093/carcin/bgh321. [DOI] [PubMed] [Google Scholar]
- 31.Velkey JM, O'Shea KS. Oct4 RNA interference induces trophectoderm differentiation in mouse embryonic stem cells. Genesis. 2003;37:18–24. doi: 10.1002/gene.10218. [DOI] [PubMed] [Google Scholar]
- 32.Tay Y, Zhang J, Thomson AM, Lim B, Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. 2008;455:1124–1128. doi: 10.1038/nature07299. [DOI] [PubMed] [Google Scholar]
- 33.Marson A, Levine SS, Cole MF, Frampton GM, Brambrink T, Johnstone S, et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134:521–533. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barroso-del Jesus A, Lucena-Aguilar G, Menendez P. The miR-302-367 cluster as a potential stemness regulator in ESCs. Cell Cycle. 2009;8:394–398. doi: 10.4161/cc.8.3.7554. [DOI] [PubMed] [Google Scholar]
- 35.Lakshmipathy U, Love B, Goff LA, Jörnsten R, Graichen R, Hart RP, et al. MicroRNA expression pattern of undifferentiated and differentiated human embryonic stem cells. Stem Cells Dev. 2007;16:1003–1016. doi: 10.1089/scd.2007.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao H, Yang CS, Rana TM. Evolutionary emergence of microRNAs in human embryonic stem cells. PLoS One. 2008;3:e2820. doi: 10.1371/journal.pone.0002820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li SS, Yu SL, Kao LP, Tsai ZY, Singh S, Chen BZ, et al. Target identification of microRNAs expressed highly in human embryonic stem cells. J Cell Biochem. 2009;106:1020–1030. doi: 10.1002/jcb.22084. [DOI] [PubMed] [Google Scholar]
- 38.Tsai ZY, Singh S, Yu SL, Kao LP, Chen BZ, Ho BC, et al. Identification of microRNAs regulated by activin A in human embryonic stem cells. J Cell Biochem. 2010;109:93–102. doi: 10.1002/jcb.22385. [DOI] [PubMed] [Google Scholar]
- 39.Ren J, Jin P, Wang E, Marincola FM, Stroncek DF. MicroRNA and gene expression patterns in the differentiation of human embryonic stem cells. J Transl Med. 2009;7:20. doi: 10.1186/1479-5876-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 42.Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wienholds E, Kloosterman WP, Miska E, Alvarez-Saavedra E, Berezikov E, de Bruijn E, et al. MicroRNA expression in zebrafish embryonic development. Science. 2005;309:310–311. doi: 10.1126/science.1114519. [DOI] [PubMed] [Google Scholar]
- 45.Choi PS, Zakhary L, Choi WY, Caron S, varez-Saavedra E, Miska EA, et al. Members of the miRNA-200 family regulate olfactory neurogenesis. Neuron. 2008;57:41–55. doi: 10.1016/j.neuron.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yi R, O'Carroll D, Pasolli HA, Zhang Z, Dietrich FS, Tarakhovsky A, et al. Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nat Genet. 2006;38:356–362. doi: 10.1038/ng1744. [DOI] [PubMed] [Google Scholar]
- 47.Peter ME. Let-7 and miR-200 microRNAs: guardians against pluripotency and cancer progression. Cell Cycle. 2009;8:843–852. doi: 10.4161/cc.8.6.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monzo M, Navarro A, Bandres E, Artells R, Moreno I, Gel B, et al. Overlapping expression of microRNAs in human embryonic colon and colorectal cancer. Cell Res. 2008;18:823–833. doi: 10.1038/cr.2008.81. [DOI] [PubMed] [Google Scholar]
- 49.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 50.Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408:86–89. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- 51.Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 52.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 53.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 54.Shell S, Park SM, Radjabi AR, Schickel R, Kistner EO, Jewell DA, et al. Let-7 expression defines two differentiation stages of cancer. Proc Natl Acad Sci U S A. 2007;104:11400–11405. doi: 10.1073/pnas.0704372104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park SM, Shell S, Radjabi AR, Schickel R, Feig C, Boyerinas B, et al. Let-7 prevents early cancer progression by suppressing expression of the embryonic gene HMGA2. Cell Cycle. 2007;6:2585–2590. doi: 10.4161/cc.6.21.4845. [DOI] [PubMed] [Google Scholar]
- 56.Navarro A, Marrades RM, Viñolas N, Quera A, Agustí C, Huerta A, et al. MicroRNAs expressed during lung cancer development are expressed in human pseudoglandular lung embryogenesis. Oncology. 2009;76:162–169. doi: 10.1159/000201569. [DOI] [PubMed] [Google Scholar]
- 57.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 59.Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 60.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 61.Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 62.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 63.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 64.Medina PP, Slack FJ. microRNAs and cancer: an overview. Cell Cycle. 2008;7:2485–2492. doi: 10.4161/cc.7.16.6453. [DOI] [PubMed] [Google Scholar]
- 65.Landais S, Landry S, Legault P, Rassart E. Oncogenic potential of the miR-106-363 cluster and its implication in human T-cell leukemia. Cancer Res. 2007;67:5699–5707. doi: 10.1158/0008-5472.CAN-06-4478. [DOI] [PubMed] [Google Scholar]
- 66.Lin SL, Chang DC, Chang-Lin S, Lin CH, Wu DT, Chen DT, et al. Mir-302 reprograms human skin cancer cells into a pluripotent ES-cell-like state. RNA. 2008;14:2115–2124. doi: 10.1261/rna.1162708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu J, Wang F, Yang GH, Wang FL, Ma YN, Du ZW, et al. Human microRNA clusters: genomic organization and expression profile in leukemia cell lines. Biochem Biophys Res Commun. 2006;349:59–68. doi: 10.1016/j.bbrc.2006.07.207. [DOI] [PubMed] [Google Scholar]
- 68.He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gallardo E, Navarro A, Viñolas N, Marrades RM, Diaz T, Gel B, et al. miR-34a as a prognostic marker of relapse in surgically resected non-small-cell lung cancer. Carcinogenesis. 2009;30:1903–1909. doi: 10.1093/carcin/bgp219. [DOI] [PubMed] [Google Scholar]
- 70.Hermeking H. p53 enters the microRNA world. Cancer Cell. 2007;12:414–418. doi: 10.1016/j.ccr.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 71.Yamakuchi M, Lowenstein CJ. MiR-34, SIRT1 and p53: the feedback loop. Cell Cycle. 2009;8:712–715. doi: 10.4161/cc.8.5.7753. [DOI] [PubMed] [Google Scholar]
- 72.Lodygin D, Tarasov V, Epanchintsev A, Berking C, Knyazeva T, Körner H, et al. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle. 2008;7:2591–2600. doi: 10.4161/cc.7.16.6533. [DOI] [PubMed] [Google Scholar]
- 73.Zenz T, Mohr J, Eldering E, Kater AP, Bühler A, Kienle D, et al. miR-34a as part of the resistance network in chronic lymphocytic leukemia. Blood. 2009;113:3801–3808. doi: 10.1182/blood-2008-08-172254. [DOI] [PubMed] [Google Scholar]
- 74.Ji Q, Hao X, Zhang M, Tang W, Yang M, Li L, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aqeilan RI, Calin GA, Croce CM. miR-15a and miR-16-1 in cancer: discovery, function and future perspectives. Cell Death Differ. 2010;17:215–220. doi: 10.1038/cdd.2009.69. [DOI] [PubMed] [Google Scholar]
- 77.Bottoni A, Piccin D, Tagliati F, Luchin A, Zatelli MC, degli Uberti EC. miR-15a and miR-16-1 down-regulation in pituitary adenomas. J Cell Physiol. 2005;204:280–285. doi: 10.1002/jcp.20282. [DOI] [PubMed] [Google Scholar]
- 78.Bonci D, Coppola V, Musumeci M, Addario A, Giuffrida R, Memeo L, et al. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med. 2008;14:1271–1277. doi: 10.1038/nm.1880. [DOI] [PubMed] [Google Scholar]
- 79.Bandi N, Zbinden S, Gugger M, Arnold M, Kocher V, Hasan L, et al. miR-15a and miR-16 are implicated in cell cycle regulation in a Rb-dependent manner and are frequently deleted or down-regulated in non-small cell lung cancer. Cancer Res. 2009;69:5553–5559. doi: 10.1158/0008-5472.CAN-08-4277. [DOI] [PubMed] [Google Scholar]
- 80.Bhattacharya R, Nicoloso M, Arvizo R, Wang E, Cortez A, Rossi S, et al. MiR-15a and MiR-16 control Bmi-1 expression in ovarian cancer. Cancer Res. 2009;69:9090–9095. doi: 10.1158/0008-5472.CAN-09-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Q, Fu H, Sun F, Zhang H, Tie Y, Zhu J, et al. miR-16 family induces cell cycle arrest by regulating multiple cell cycle genes. Nucleic Acids Res. 2008;36:5391–5404. doi: 10.1093/nar/gkn522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- 83.Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raaphorst FM. Self-renewal of hematopoietic and leukemic stem cells: a central role for the Polycomb-group gene Bmi-1. Trends Immunol. 2003;24:522–524. doi: 10.1016/s1471-4906(03)00241-2. [DOI] [PubMed] [Google Scholar]
- 85.Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, Miyazono K. Modulation of microRNA processing by p53. Nature. 2009;460:529–533. doi: 10.1038/nature08199. [DOI] [PubMed] [Google Scholar]
- 86.Navarro A, Gaya A, Martinez A, Urbano-Ispizua A, Pons A, Balagué O, et al. MicroRNA expression profiling in classic Hodgkin lymphoma. Blood. 2008;111:2825–2832. doi: 10.1182/blood-2007-06-096784. [DOI] [PubMed] [Google Scholar]
- 87.Feber A, Xi L, Luketich JD, Pennathur A, Landreneau RJ, Wu M, et al. MicroRNA expression profiles of esophageal cancer. J Thorac Cardiovasc Surg. 2008;135:255–260. doi: 10.1016/j.jtcvs.2007.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jiang J, Gusev Y, Aderca I, Mettler TA, Nagorney DM, Brackett DJ, et al. Association of MicroRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin Cancer Res. 2008;14:419–427. doi: 10.1158/1078-0432.CCR-07-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bandrés E, Cubedo E, Agirre X, Malumbres R, Zárate R, Ramirez N, et al. Identification by Real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumoral tissues. Mol Cancer. 2006;5:29. doi: 10.1186/1476-4598-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. J Cell Mol Med. 2009;13:39–53. doi: 10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fujita S, Ito T, Mizutani T, Minoguchi S, Yamamichi N, Sakurai K, et al. miR-21 Gene expression triggered by AP-1 is sustained through a double-negative feedback mechanism. J Mol Biol. 2008;378:492–504. doi: 10.1016/j.jmb.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 92.Löffler D, Brocke-Heidrich K, Pfeifer G, Stocsits C, Hackermüller J, Kretzschmar AK, et al. Interleukin-6 dependent survival of multiple myeloma cells involves the Stat3-mediated induction of microRNA-21 through a highly conserved enhancer. Blood. 2007;110:1330–1333. doi: 10.1182/blood-2007-03-081133. [DOI] [PubMed] [Google Scholar]
- 93.Singh SK, Kagalwala MN, Parker-Thornburg J, Adams H, Majumder S. REST maintains self-renewal and pluripotency of embryonic stem cells. Nature. 2008;453:223–227. doi: 10.1038/nature06863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morel AP, Liévre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eastham AM, Spencer H, Soncin F, Ritson S, Merry CL, Stern PL, et al. Epithelial-mesenchymal transition events during human embryonic stem cell differentiation. Cancer Res. 2007;67:11254–11262. doi: 10.1158/0008-5472.CAN-07-2253. [DOI] [PubMed] [Google Scholar]
- 97.Ullmann U, In't Veld P, Gilles C, Sermon K, De Rycke M, Van de Velde H, et al. Epithelial-mesenchymal transition process in human embryonic stem cells cultured in feeder-free conditions. Mol Hum Reprod. 2007;13:21–32. doi: 10.1093/molehr/gal091. [DOI] [PubMed] [Google Scholar]
- 98.Ladeiro Y, Couchy G, Balabaud C, Bioulac-Sage P, Pelletier L, Rebouissou S, et al. MicroRNA profiling in hepatocellular tumors is associated with clinical features and oncogene/tumor suppressor gene mutations. Hepatology. 2008;47:1955–1963. doi: 10.1002/hep.22256. [DOI] [PubMed] [Google Scholar]
- 99.Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67:8699–8707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- 100.Meng F, Henson R, Lang M, Wehbe H, Maheshwari S, Mendell JT, et al. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology. 2006;130:2113–2129. doi: 10.1053/j.gastro.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 101.Sempere LF, Christensen M, Silahtaroglu A, Bak M, Heath CV, Schwartz G, et al. Altered MicroRNA expression confined to specific epithelial cell subpopulations in breast cancer. Cancer Res. 2007;67:11612–11620. doi: 10.1158/0008-5472.CAN-07-5019. [DOI] [PubMed] [Google Scholar]
- 102.Gottardo F, Liu CG, Ferracin M, Calin GA, Fassan M, Bassi P, et al. Micro-RNA profiling in kidney and bladder cancers. Urol Oncol. 2007;25:387–392. doi: 10.1016/j.urolonc.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 103.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 104.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 105.Deregibus MC, Tetta C, Camussi G. The dynamic stem cell micro-environment is orchestrated by microvesicle-mediated transfer of genetic information. Histol Histopathol. 2010;25:397–404. doi: 10.14670/HH-25.397. [DOI] [PubMed] [Google Scholar]
- 106.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 107.Yuan A, Farber EL, Rapoport AL, Tejada D, Deniskin R, Akhmedov NB, et al. Transfer of microRNAs by embryonic stem cell microvesicles. PLoS One. 2009;4:e4722. doi: 10.1371/journal.pone.0004722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 109.Rabinowits G, Gercel-Taylor C, Day JM, Taylor DD, Kloecker GH. Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer. 2009;10:42–46. doi: 10.3816/CLC.2009.n.006. [DOI] [PubMed] [Google Scholar]