Abstract
Purpose
Several signaling pathways have been shown to regulate the lineage commitment and terminal differentiation of bone marrow stromal cells (BMSCs). Bone morphogenetic protein (BMP) signaling has important effects on the process of skeletogenesis. In the present study, we tested the role of bone morphogenetic protein receptor (BMPR) in the osteogenic differentiation of rat bone marrow stromal cells in osteogenic medium (OM) with or without BMP-2.
Materials and Methods
BMSCs were harvested from rats and cultured in OM containing dexamethasone, β-glycerophosphate, and ascorbic acid, with or without BMP-2 in order to induce osteogenic differentiation. The alkaline phosphatase (ALP) activity assay and von kossa staining were used to assess the osteogenic differentiation of the BMSCs. BMPR mRNA expression was assessed using reverse transcription-polymerase chain reaction (RT-PCR).
Results
The BMSCs that underwent osteogenic differentiation in OM showed a higher level of ALP activity and matrix mineralization. BMP-2 alone induced a low level of ALP activity and matrix mineralization in BMSCs, but enhanced the osteogenic differentiation of BMSCs when combined with OM. The OM significantly induced the expression of type IA receptor of BMPR (BMPRIA) and type II receptor of BMPR (BMPRII) in BMSCs after three days of stimulation, while BMP-2 significantly induced BMPRIA and BMPRII in BMSCs after nine or six days of stimulation, respectively.
Conclusion
BMSCs commit to osteoblastic differentiation in OM, which is enhanced by BMP-2. In addition, BMP signaling through BMPRIA and BMPRII regulates the osteogenic differentiation of rat BMSCs in OM with or without BMP-2.
Keywords: Bone marrow stromal cells, osteogenesis, differentiation, bone morphogenetic protein receptor
INTRODUCTION
Successful outcomes for bone fracture repair, alveolar ridge augmentation, dental implants, and craniofacial surgery require formation of new bone. Efficient bone formation in adults relies on the recruitment of osteoblast precursors to the site, followed by osteoblast maturation, matrix deposition, and mineralization. A multitude of studies have shown that bone marrow stromal stem cells (BMSCs) have the potential to differentiate into osteogenic lineages by the addition of various induction factors to their growth medium.1-4 Dexamethasone, ascorbic acid, and β-glycerophosphate are the most popular induction factors.1,2 Other agents, such as bone morphogenetic protein-2 (BMP-2), are also known to play an important role in the bone healing process and in enhancing therapeutic efficacy.5-7
Several signaling pathways have been shown to regulate the lineage commitment and terminal differentiation of BMSCs. It is important to understand the molecular mechanism of BMSC differentiation, as this knowledge could aid in our understanding of the pathogenesis of skeletal diseases and may lead to the development of strategies for regenerative medicine.8 BMP signaling is initiated by the binding of extracellular BMPs to heterodimeric BMP receptors (BMPR), resulting in BMPR type II receptor (BMPRII)-mediated activation of the BMPR type I receptor (BMPRI), which, in turn, causes the phos-phorylation and activation of intracellular Smad signaling molecules.9 Accumulating evidence suggests that specific signaling through BMPR type IA receptors (BMPRIA) has important effects on the process of skeletogenesis both in vitro and in vivo.10-14 Kaps, et al.12 found that BMPR-IA is responsible for the initiation of the osteogenic, as well as the chondrogenic, development in mesenchymal progenitors C3H10T1/2. Furthermore, BMPR-IA mRNA was highly expressed in the BMP-induced bone forming tissues in a study performed by Takeda, et al.14
In order to determine the role of BMPR in the osteogenic differentiation of BMSCs, BMSCs were harvested from rats and were cultured in osteogenic medium containing dexamethasone, β-glycerophosphate, and ascorbic acid, with or without BMP-2, in order to induce osteogenic differentiation. The alkaline phosphatase activity assay and von kossa staining were used to assess the differentiation of the BMSCs. BMPR mRNA expression was assessed using reverse transcription-polymerase chain reaction (RT-PCR).
MATERIALS AND METHODS
Bone marrow stromal cell isolation
BMSCs were harvested from the femurs and tibias of 6-week-old male Wistar rats, using protocols approved by the Ethics Committee for Animal Experiments of Sun Yatsen University. Briefly, bones were aseptically excised from the hind limbs of the rats. The bone marrow was then flushed from the shaft and spun down at 800 rpm for 5 min. The pellet was resuspended in fresh control medium (CM) and then seeded into dishes. On the third day after seeding, the cells were rinsed with phosphate buffer saline (PBS) (pH 7.2-7.4), and adherent cells were replated. These first passage cultures were used for all experiments and cultured in four different media conditions, including CM, 10 ng/mL BMP-2 (Peprotech, Rocky Hill, NJ, USA) (according to the concentration reported by Weston, et al.15), osteogenic medium (OM), or OM with BMP-2. The medium was changed every two days. CM consisted of αMEM supplemented with 10% fetal calf serum (Hyclone, Logan, UT, USA), 100 U/mL penicillin, 100 U/mL streptomycin, and 100 U/mL amphotericin B (Sigma, St. Louis, MO, USA). OM was identical to CM, but was supplemented with 10 mM β-glycerophosphate, 50 mg/mL L-ascorbic acid, and 0.1 µM dexamethasone (Sigma, St. Louis, MO, USA) (according to the concentration reported by Deliloglu-Gurhan, et al.16). We added osteogenic supplements to marrow-derived cells early in the culture, a time which had been shown to not inhibit proliferation and to greatly enhance the osteoblastic phenotype of cells in a rat model.17
Proliferation assay
To determine proliferation, BMSCs were plated at 2,000 cells per well (6-well) in triplicate and treated as indicated above. The medium was changed every two days. Cells were counted every other day using a Coulter counter (BeckmanCoulter, Hialeah, FL, USA). The number of cells was determined after staining with trypan blue to exclude dead cells.
Alkaline phosphatase (ALP) activity assay
Alkaline phosphatase (ALP) assays were performed using a Sigma-Aldrich diagnostic kit. BMSCs were cultured as indicated above, and cells were harvested at three, six, and nine days by trypsinization and lysed with a radio-immune precipitation assay (RIPA) lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The cell lysates were then mixed with an assay mixture containing p-nitrophenyl phosphate and incubated at 37℃ for 30 min, at which time the reaction was stopped by the addition of 0.4 M NaOH. After incubation, the amount of p-nitrophenol released by the reaction was measured with a spectrophotometer at 410 nm. All values were normalized against the cell number. Histochemical assays of ALP (Kaplow assay) were performed after nine days of culture. Briefly, cells were washed with PBS (pH 7.4), dried, and fixed in ice-cold methanol at room temperature for 30 sec. Fixed cells were incubated for 10 min at 37℃ in ALP incubating medium and then counterstained in hematoxylin for 5 min.
Von kossa staining
Mineralization of the extracellular matrices was demonstrated at 21 days of culture by the von kossa technique. Briefly, cell layers were washed with PBS, fixed in cold methanol, immersed in a solution of 2% AgNO3, and exposed to bright sunlight for 30 min. A black color, indicating the presence of phosphate, was visualized by developing the samples in a 5% sodium thiosulfate bath for 10 min.
Isolation of total RNA and RT-PCR analysis
It has been previously reported that BMSCs differentiate seven days after OM.16 Therefore, we detected BMP receptors by RT-PCR at three, six, and nine days after OM stimulation. Treated cells were harvested, and total RNA was extracted using an RNeasy mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. RT-PCR was performed using one-step RT-PCR assays (Qiagen). Specific primers for detecting mRNA transcripts of the BMPRIA, BMPRII, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes are as shown here:
BMPRIA
Forward: 5'-gtattgtcgccatgatcgtct-3'; Reverse: 5'-cttctccata ccggcctttac-3'
BMPRII
Forward: 5'-agaaatcaaaaggggacatcaat-3'; Reverse: 5'-cat aaggcgactatcaaaacagc-3'
GAPDH
Forward: 5'-tgctgagtatgtcgtggagtct-3'; Reverse: 5'-acagtct tctgagtggcagtga-3'
RT-PCR products were resolved by agarose gel electrophoresis. Signal intensity was quantified by image-analysis computer software (NIH Image J, NY, USA). Transcripts were normalized to GAPDH transcript levels, and the relative mRNA level was depicted as the ratio of the density of the detected genes to GAPDH at the same time point.
Statistical analysis
In all cases, experiments were replicated in triplicate. Data were reported as mean ± standard deviation (SD). Statistical analyses were performed using a one-way ANOVA (analysis of variance). A p value < 0.05 was considered statistically significant.
RESULTS
Proliferation of BMSC in osteogenic medium with or without BMP-2
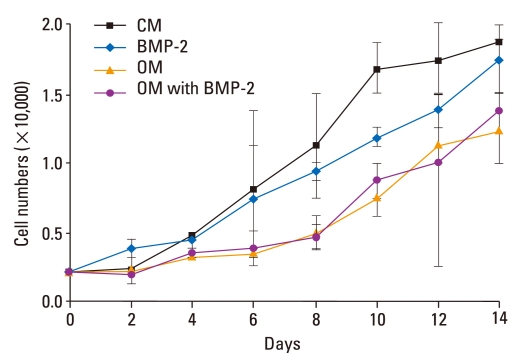
The proliferation assay was performed using four different media conditions, including CM, BMP-2, OM, and OM + BMP-2. Cell numbers increased six- to ten-fold under all four conditions after two weeks of culture. Although cell numbers for these four groups were not significantly different (p > 0.05), the osteogenic medium with or without BMP-2 slightly inhibited the proliferation of BMSCs (Fig. 1).
Fig. 1.
Proliferation of BMSCs in osteogenic medium with or without BMP-2. Proliferation profiles of BMSCs cultured for 14 d in the presence of OM with or without BMP-2 were obtained. Although the cell numbers in these four groups were not significantly different (p > 0.05), cell growth was slightly inhibited in the presence of OM with or without BMP-2. CM, control medium; OM, osteogenic medium; BMSCs, bone marrow stromal cells; BMP-2, bone morphogenetic protein-2.
ALP activity of BMSC in osteogenic medium with or without BMP-2
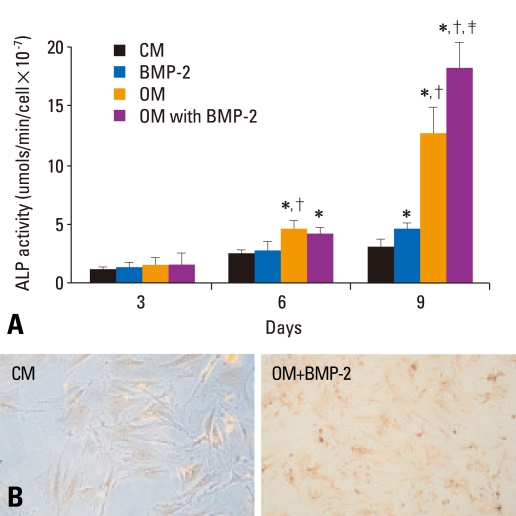
Because a high level of ALP activity is considered a hallmark of the osteogenic phenotype, we evaluated the ALP activity of rat BMSCs cultures. As expected, OM-stimulated ALP activity was time-dependent. ALP activity in the OM group was significantly higher than that of the CM group after more than six days of treatment (p < 0.05) (Fig. 2A). Cells grown in OM showed a more than 11-fold increase in ALP activity as compared to cells grown in CM after nine days of OM stimulation. Although BMP-2 induced the expression of ALP in BMSCs, a significant difference between BMP-2 and CM was not found until 9 days after the initial stimulation (p < 0.05). The level of ALP was higher after treatment with the osteogenic medium with BMP-2 than without BMP-2, but the difference was significant only after 9 days of stimulation (p < 0.05).
Fig. 2.
ALP activity of BMSCs in osteogenic medium with or without BMP-2. (A) BMSCs were treated with CM or OM with or without BMP-2. ALP activity (mean ± SD) was determined on days 3, 6, and 9. *Compared with CM at the same time point, p < 0.05. †Compared with BMP-2 at the same time point, p < 0.05. ‡Compared with OM at the same time point, p < 0.05. (B) A visible red-brown precipitate indicates ALP activity in enzyme histochemistry. The activity of cellular ALP was higher after the BMSCs were cultured in OM with or without BMP-2 as compared to CM (×200). CM, control medium; OM, osteogenic medium; ALP, alkaline phosphatase; BMSCs, bone marrow stromal cells; BMP-2, bone morphogenetic protein-2.
ALP activity produced a visible red-brown precipitate when examined by enzymatic histochemistry (Fig. 2B). The ALP activity of BMSCs was more pronounced in cells cultured in OM with or without BMP-2 as compared to the CM group.
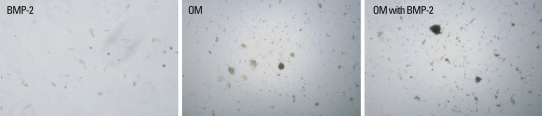
Matrix mineralization of BMSCs in osteogenic medium with or without BMP-2
BMSCs were examined for their ability to undergo matrix mineralization when cultured in OM with or without BMP-2 using von kossa staining (Fig. 3). BMSCs showed a high level of matrix mineralization when cultured in the presence of OM with or without BMP-2. BMSCs did not show any matrix mineralization when cultured in CM, and a low level of matrix mineralization was found in the presence of BMP-2.
Fig. 3.
Matrix mineralization of BMSCs in osteogenic medium with or without BMP-2. Von kossa staining of BMSCs was performed. BMSCs showed a high level of matrix mineralization when cultured in the presence of OM with or without BMP-2. No matrix mineralization was observed in cells cultured in the control medium. A low level of matrix mineralization was found in cells cultured in the presence of BMP-2 (×100). BMP-2, bone morphogenetic protein-2; OM, osteogenic medium; BMSCs, bone marrow stromal cells.
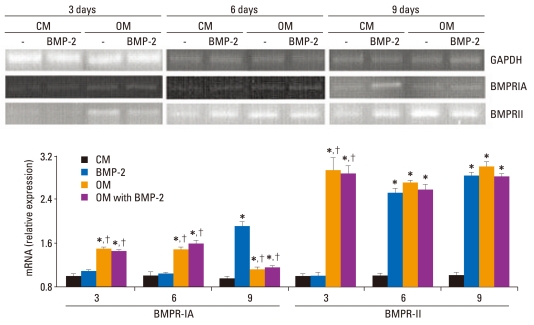
mRNA expression of the BMP receptor during BMSC differentiation
Because BMP signaling has been shown to promote the osteoblastic differentiation of BMSCs, BMP receptors were assessed by RT-PCR during BMSC differentiation. As shown in Fig. 4, the expression levels of BMPR-IA in OM with or without BMP-2 were significantly higher than CM or BMP-2 at three or six days of stimulation (p < 0.05). No difference was found between the CM and BMP-2 groups. Nine days after stimulation, the level of BMPRIA mRNA was continuously induced in OM with or without BMP-2 (p < 0.05). The expression of BMPRIA significantly increased in the BMP-2 groups as compared to the other three groups at nine days of stimulation (p < 0.05).
Fig. 4.
mRNA expression of the BMP receptor in BMSCs. BMSCs were treated with CM or OM with or without BMP-2. mRNA was extracted from cells on day 3, 6, and 9 and analyzed by RT-PCR. GADPH mRNA was used as an internal control to normalize the amount of RNA. The relative expression level of mRNA was depicted as the ratio of the density of mRNA to GADPH mRNA at the same time point. The results correspond to a representative of three experiments. RT-PCR analysis revealed that the osteogenic medium significantly induced BMPR-IA and BMPRII expression in BMSCs after three days of stimulation, while BMP-2 significantly induced BMPRIA and BMPRII in BMSCs after nine or six days of stimulation, respectively. *Compared with CM at the same time point, p < 0.05. †Compared with BMP-2 at the same time point, p < 0.05. CM, control medium; OM, osteogenic medium; BMP-2, bone morphogenetic protein-2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; BMPRIA, type IA receptor of BMPR; BMPRII, type II receptor of BMPR; RT-PCR, reverse transcription-polymerase chain reaction; BMSCs, bone marrow stromal cells.
BMPRII mRNA expression in BMSCs was significantly induced in OM with or without BMP-2 as compared to CM after more than three days of treatment. Moreover, after more than six days of treatment, BMP-2 also significantly induced the expression of BMPRII as compared to CM (p < 0.05).
DISCUSSION
Bone marrow-derived cells can be encouraged to follow one of several possible lineages through the addition of various induction factors to their growth medium. Thus, variations in medium components have differing effects in inducing osteogenic differentiation of mesenchymal stem cells derived from bone marrow. Osteoblastic differentiation is characterized by ALP expression and matrix mineralization. The importance of ALP in bone formation resides in its ability to regulate the mineralization of the bone matrix.18,19 ALP serves as a useful marker of early osteogenesis and usually increases by the end of the first week of BMSC culture.20 Previous studies have proven that media containing dexamethasone, ascorbic acid, and β-glycerophosphate has an effect on the osteogenic differentiation of BMSCs.16,17,21 In our study, we also found that OM-stimulated ALP activity was time-dependent and significantly higher than that of the CM group by the end of the first week of culture. An 11-fold increase in ALP activity was found at nine days of OM stimulation as compared to CM stimulation. Similar to other reports,4,17 cells grown in a control medium also produced a slightly ALP activity, which may be related to cellular differentiation occurring in the control medium. The ability of BMSCs to undergo matrix mineralization can be detected by von kossa staining. In our study, BMSCs showed a high level of matrix mineralization when cultured in the presence of OM, while BMSCs did not show any matrix mineralization when cultured in CM.
Many studies have demonstrated that osteogenic differentiation is regulated by a complex network of multiple BMPs and that BMP-2 was a central regulator in this network.22 Previous studies5,6,23,24 indicated that BMSCs treated with BMP-2 or transfected with BMP-2 cDNA will commit to osteogenic differentiation. Nishii, et al.7 found that BMP-2-stimulated ALP activity was dose- and timedependent in TBR31-2 cells (a bone marrow stromal cell line). Similar to the Hu, et al.25 study, which found that ALP activity in MSCs cultured with BMP-2 for 14 and 28 days elevated two- to five-fold, we found that BMP-2 induced the expression of ALP in BMSCs and that ALP activity was elevated 1.5-fold after nine days of stimulation. Although ALP activity and matrix mineralization was lower in BMP-2-induced cells than that of OM induced cells, we found that the level of ALP activity and matrix mineralization was augmented after the combination of BMP-2 with the osteogenic medium. Taken together, these results suggest that the osteogenic medium has a strong effect on committing BMSCs to osteoblastic differentiation. BMP-2-induced BMSC differentiation was weak and slow, but BMP-2 was able to enhance BMSC differentiation when combined with the osteogenic medium.
BMP signaling in BMSC differentiation and proliferation has been investigated in many studies.26-28 Previous studies29,30 have suggested that BMP signaling through BMPRIA promotes adipogenesis of mouse adipose-derived adult stromal cells. Skillington, et al.26 provided evidence that BMPRIA and BMPRIB, in combination with BMPRII, exert similar effects on the osteoblast differentiation of BMSCs.26 However, many other researchers sug- gested that BMPRIA is responsible for the initiation of the osteogenic differentiation.12,13,31 In our study, we found that the osteogenic medium with or without BMP-2 significantly induced the expression of BMPRIA and BMPRII at the early stage of differentiation (after three days of stimulation), while BMP-2 induced the expression of these two receptors in later stages. The expression of BMPRIA and BMPRII was significantly activated by BMP-2 at nine or six days of stimulation, respectively. This result may explain why BMP-2 weakly and slowly induced ALP activity and matrix mineralization compared to the osteogenic medium. From our data, we can presume that the osteogenic medium and BMP-2 induced the expression of BMPRII and subsequently BMPRIA, resulting in the initiation of BMP signaling and the osteogenic differentiation of BMSCs.
In conclusion, we demonstrated that BMSCs commit to osteoblastic differentiation in the osteogenic medium, and this event is enhanced by BMP-2. BMP signaling through BMPRIA and BMPRII regulates the osteogenic differentiation of rat bone marrow stromal cells in the osteogenic medium with or without BMP-2.
ACKNOWLEDGEMENTS
This work was supported by grants from the Science and Technique Project of Guangdong Province, No. 2008 B080701002 and No. 2005A30801003.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 2.Cheng SL, Zhang SF, Avioli LV. Expression of bone matrix proteins during dexamethasone-induced mineralization of human bone marrow stromal cells. J Cell Biochem. 1996;61:182–193. doi: 10.1002/(sici)1097-4644(19960501)61:2<182::aid-jcb3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 3.Heckman JD, Ehler W, Brooks BP, Aufdemorte TB, Lohmann CH, Morgan T, et al. Bone morphogenetic protein but not transforming growth factor-beta enhances bone formation in canine diaphyseal nonunions implanted with a biodegradable composite polymer. J Bone Joint Surg Am. 1999;81:1717–1729. doi: 10.2106/00004623-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Bi LX, Simmons DJ, Mainous E. Expression of BMP-2 by rat bone marrow stromal cells in culture. Calcif Tissue Int. 1999;64:63–68. doi: 10.1007/s002239900580. [DOI] [PubMed] [Google Scholar]
- 5.Seol YJ, Kim KH, Park YJ, Lee YM, Ku Y, Rhyu IC, et al. Osteogenic effects of bone-morphogenetic-protein-2 plasmid gene transfer. Biotechnol Appl Biochem. 2008;49:85–96. doi: 10.1042/BA20060247. [DOI] [PubMed] [Google Scholar]
- 6.Hsu WK, Sugiyama O, Park SH, Conduah A, Feeley BT, Liu NQ, et al. Lentiviral-mediated BMP-2 gene transfer enhances healing of segmental femoral defects in rats. Bone. 2007;40:931–938. doi: 10.1016/j.bone.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 7.Nishii N, Arai M, Yanai N, Togari A, Nakabayashi T. Effect of bone morphogenetic protein-2 (BMP-2) or troglitazone, as an inducer of osteogenic cells or adipocytes, on differentiation of a bone marrow mesenchymal progenitor cell line established from temperature-sensitive (ts) simian virus (SV) 40 T-antigen gene transgenic mice. Biol Pharm Bull. 2009;32:10–17. doi: 10.1248/bpb.32.10. [DOI] [PubMed] [Google Scholar]
- 8.Kang Q, Song WX, Luo Q, Tang N, Luo J, Luo X, et al. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev. 2008;18:545–559. doi: 10.1089/scd.2008.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massagué J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashique AM, Fu K, Richman JM. Signalling via type IA and type IB bone morphogenetic protein receptors (BMPR) regulates intramembranous bone formation, chondrogenesis and feather formation in the chicken embryo. Int J Dev Biol. 2002;46:243–253. doi: 10.1387/ijdb.011535. [DOI] [PubMed] [Google Scholar]
- 11.Mishina Y, Starbuck MW, Gentile MA, Fukuda T, Kasparcova V, Seedor JG, et al. Bone morphogenetic protein type IA receptor signaling regulates postnatal osteoblast function and bone remodeling. J Biol Chem. 2004;279:27560–27566. doi: 10.1074/jbc.M404222200. [DOI] [PubMed] [Google Scholar]
- 12.Kaps C, Hoffmann A, Zilberman Y, Pelled G, Häupl T, Sittinger M, et al. Distinct roles of BMP receptors Type IA and IB in osteo-/chondrogenic differentiation in mesenchymal progenitors (C3H10T1/2) Biofactors. 2004;20:71–84. doi: 10.1002/biof.5520200202. [DOI] [PubMed] [Google Scholar]
- 13.Fujii M, Takeda K, Imamura T, Aoki H, Sampath TK, Enomoto S, et al. Roles of bone morphogenetic protein type I receptors and Smad proteins in osteoblast and chondroblast differentiation. Mol Biol Cell. 1999;10:3801–3813. doi: 10.1091/mbc.10.11.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeda K, Oida S, Ichijo H, Iimura T, Maruoka Y, Amagasa T, et al. Molecular cloning of rat bone morphogenetic protein (BMP) type IA receptor and its expression during ectopic bone formation induced by BMP. Biochem Biophys Res Commun. 1994;204:203–209. doi: 10.1006/bbrc.1994.2445. [DOI] [PubMed] [Google Scholar]
- 15.Weston AD, Rosen V, Chandraratna RA, Underhill TM. Regulation of skeletal progenitor differentiation by the BMP and retinoid signaling pathways. J Cell Biol. 2000;148:679–690. doi: 10.1083/jcb.148.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deliloglu-Gurhan SI, Vatansever HS, Ozdal-Kurt F, Tuglu I. Characterization of osteoblasts derived from bone marrow stromal cells in a modified cell culture system. Acta Histochem. 2006;108:49–57. doi: 10.1016/j.acthis.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Peter SJ, Liang CR, Kim DJ, Widmer MS, Mikos AG. Osteoblastic phenotype of rat marrow stromal cells cultured in the presence of dexamethasone, beta-glycerolphosphate, and L-ascorbic acid. J Cell Biochem. 1998;71:55–62. doi: 10.1002/(sici)1097-4644(19981001)71:1<55::aid-jcb6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, et al. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A. 2002;99:9445–9449. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson HC, Sipe JB, Hessle L, Dhanyamraju R, Atti E, Camacho NP, et al. Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am J Pathol. 2004;164:841–847. doi: 10.1016/s0002-9440(10)63172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner EF, Karsenty G. Genetic control of skeletal development. Curr Opin Genet Dev. 2001;11:527–532. doi: 10.1016/s0959-437x(00)00228-8. [DOI] [PubMed] [Google Scholar]
- 21.Lee CI, Kohn DB, Ekert JE, Tarantal AF. Morphological analysis and lentiviral transduction of fetal monkey bone marrow-derived mesenchymal stem cells. Mol Ther. 2004;9:112–123. doi: 10.1016/j.ymthe.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Edgar CM, Chakravarthy V, Barnes G, Kakar S, Gerstenfeld LC, Einhorn TA. Autogenous regulation of a network of bone morphogenetic proteins (BMPs) mediates the osteogenic differentiation in murine marrow stromal cells. Bone. 2007;40:1389–1398. doi: 10.1016/j.bone.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osyczka AM, Diefenderfer DL, Bhargave G, Leboy PS. Different effects of BMP-2 on marrow stromal cells from human and rat bone. Cells Tissues Organs. 2004;176:109–119. doi: 10.1159/000075032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osyczka AM, Leboy PS. Bone morphogenetic protein regulation of early osteoblast genes in human marrow stromal cells is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase signaling. Endocrinology. 2005;146:3428–3437. doi: 10.1210/en.2005-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu Z, Peel SA, Ho SK, Sándor GK, Clokie CM. Role of bovine bone morphogenetic proteins in bone matrix protein and osteoblast-related gene expression during rat bone marrow stromal cell differentiation. J Craniofac Surg. 2005;16:1006–1014. doi: 10.1097/01.scs.0000170449.72040.ee. [DOI] [PubMed] [Google Scholar]
- 26.Skillington J, Choy L, Derynck R. Bone morphogenetic protein and retinoic acid signaling cooperate to induce osteoblast differentiation of preadipocytes. J Cell Biol. 2002;159:135–146. doi: 10.1083/jcb.200204060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yanagisawa J, Yanagi Y, Masuhiro Y, Suzawa M, Watanabe M, Kashiwagi K, et al. Convergence of transforming growth factor-beta and vitamin D signaling pathways on SMAD transcriptional coactivators. Science. 1999;283:1317–1321. doi: 10.1126/science.283.5406.1317. [DOI] [PubMed] [Google Scholar]
- 28.Helvering LM, Sharp RL, Ou X, Geiser AG. Regulation of the promoters for the human bone morphogenetic protein 2 and 4 genes. Gene. 2000;256:123–138. doi: 10.1016/s0378-1119(00)00364-4. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura R, Kato Y, Chen D, Harris SE, Mundy GR, Yoneda T. Smad5 and DPC4 are key molecules in mediating BMP-2-induced osteoblastic differentiation of the pluripotent mesenchymal precursor cell line C2C12. J Biol Chem. 1998;273:1872–1879. doi: 10.1074/jbc.273.4.1872. [DOI] [PubMed] [Google Scholar]
- 30.Wan DC, Shi YY, Nacamuli RP, Quarto N, Lyons KM, Longaker MT. Osteogenic differentiation of mouse adipose-derived adult stromal cells requires retinoic acid and bone morphogenetic protein receptor type IB signaling. Proc Natl Acad Sci U S A. 2006;103:12335–12340. doi: 10.1073/pnas.0604849103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeh LC, Unda R, Lee JC. Osteogenic protein-1 differentially regulates the mRNA expression of bone morphogenetic proteins and their receptors in primary cultures of osteoblasts. J Cell Physiol. 2000;185:87–97. doi: 10.1002/1097-4652(200010)185:1<87::AID-JCP8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]