Abstract
Purpose
The purpose of this study is to set guidelines for the management of renal angiomyolipoma (AML), clinical prognosis according to tumor size, in association with tuberous sclerosis complex (TSC), multiplicity, radiographic finding, and treatment modality.
Materials and Methods
Between March 1998 and October 2008, 129 out of 254 patients with AML who underwent surgical intervention or angioembolization were enrolled. Diagnosis of AML was determined by the presence of a low attenuated component on CT imaging or by pathological confirmation. Indications of treatment were intractable pain, hematuria, suspicion of malignancy, large tumor size, spontaneous rupture, and radiographically equivocal tumors in which a differential diagnosis was needed to rule out malignancy. Parameters including age, sex, tumor size, multiplicity, radiographic characteristics, association with TSC, and treatment modality were reviewed.
Results
Age at presentation was 50.6 years and mean tumor size was 3.5 cm. Presentation symptoms were flank pain, hematuria, spontaneous rupture, and fatigue. 97 (75.2%) patients were incidentally discovered. 100 (77.5%) were females. 68 (52.7%) underwent nephron-sparing surgery (NSS), 35 (27.1%) radical nephrectomy, and 26 (20.2%) angioembolization. TSC was accompanied in 12 (9.3%) patients. No patient developed renal function impairment during the mean follow-up period of 64.8 months. Patients with TSC presented at a younger age, along with larger, bilateral, and multiple lesions.
Conclusion
Significant differences in clinical manifestations and treatment outcomes were noted in respect to tumor characteristics, association with TSC, and treatment modality. Considering the benign nature of AML, these parameters ought to be considered when deciding upon active surveillance or prophylactic intervention.
Keywords: Angiomyolipoma, tuberous sclerosis, kidney
INTRODUCTION
Angiomyolipoma (AML) is a relatively rare benign tumor of the kidney consisting of fat, blood vessels, and smooth muscle. According to previous literature, AML is predominant in women and approximately 20% occurs in patients with tuberous sclerosis complex (TSC), in which 80% is bilateral or multiple.1-3 TSC is an autosomal disease entity with various features such as mental retardation, adenoma sebaceum, seizure, and renal manifestations. Renal manifestations include AML, benign cyst, and renal cell carcinoma (RCC), which is the leading cause of morbidity and mortality in these patients.4 Although clinically-insignificant AMLs can be safely observed, intervention is recommended for intractable pain, large size, suspicion of malignancy, and risk of life-threatening hemorrhages.5-7 Given the benign nature of AML, renal preserving treatment modalities such as nephron-sparing surgery (NSS) or selective renal artery angioembolization are preferred.6 To set a strategy for AML management, clinical prognosis according to tumor size, association with TSC, multiplicity, radiologic finding, and treatment modality were investigated.
MATERIALS AND METHODS
Patient demographics
Upon approval from the Yonsei University Health System Institutional Review Board, complete data were available for 254 patients diagnosed of renal AML between March 1998 and October 2008. Among these patients, 129 patients who have been treated by surgery or angioembolization were identified. The diagnosis of AML was determined either by the presence of a low attenuated component on CT imaging or by pathological confirmation through NSS or radical nephrectomy. The cutoff value of -10 Hounsfield units was used to differentiate AMLs between classic and fat poor. TSC was suspected in patients with facial angiofibromas, mental retardation, seizure, or pulmonary lymphangiomyomatosis, and were categorized as definite when positive for TSC1 and TSC2 loss of heterozygosity.
Criteria standards
All 129 patients had complete charting records along with triphasic CT scan imaging. Parameters including age, sex, tumor size, multiplicity, radiologic characteristics, association with TSC, and treatment modalities were retrospectively reviewed. Tumor size was identified as the greatest dimension recorded on preoperative CT scan. The growth of lesions was estimated with the first and last imaging study. Indications of treatment were intractable pain, hematuria, suspicion of malignancy, large tumor size, spontaneous rupture, and radiographically equivocal tumors in which a differential diagnosis was needed to rule out malignancy. NSS or radical nephrectomy was chosen as the surgical modality. NSS was performed for tumors < 4 cm, tumors in a solitary kidney, multiple or bilateral tumors, or in compromised renal function patients whose renal parenchyma resection should be minimized. Radical nephrectomy was performed for tumors ≥ 4 cm or complex renal tumors including completely endophytic or hilar tumors within 5 mm from hilar vessels.
Statistical analysis
Student's t-test and Pearson's chi-square test were used for analysis of continuous and categorical variables, respectively. All tests were two sided, and p values < 0.05 were considered statistically significant. The Statistical Package for Social Sciences software, version 12 (SPSS Inc, Chicago, IL, USA) was used for statistical analysis.
RESULTS
Patient demographics
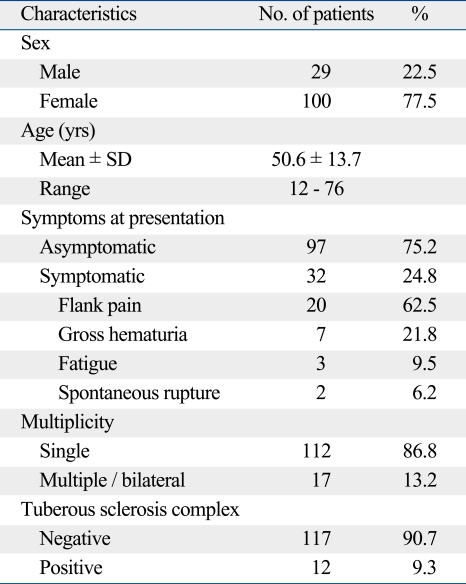
Age at presentation was 50.6 ± 13.7 years; 100 were females and 29 were males. The prevalence of AML was highest in the fifth decade (n = 41, 31.8%). TSC was present in 12 patients whose age at presentation was 30.1 ± 12.1 years. The lesion was incidentally discovered in 97 patients and was revealed due to symptoms in 32 patients. Chief complaints included flank pain, hematuria, fatigue, and shock due to spontaneous rupture. 112 had single lesions on imaging whereas 17 and 11 patients had multiple and bilateral lesions, respectively (Table 1).
Table 1.
Patient Characteristics
Comparison between sporadic and TSC associated AML
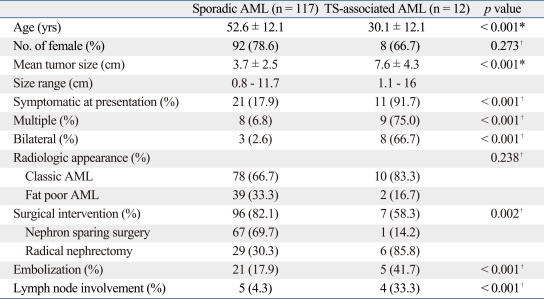
The differences of clinical spectrum in between sporadic and TSC associated AMLs were investigated. Patients with TSC associated AMLs were significantly younger at presentation, had a larger tumor, had more multiple and bilateral lesions, and were mostly symptomatic at presentation (p < 0.001). However, there were no significant differences of gender distribution between the two groups (p = 0.273). Surgical treatment tended towards a significantly higher rate in sporadic AMLs, which were equivocal with malignancy. Due to the preference of radical surgery in large-sized tumors, more sporadic AMLs were treated with NSS while more TSC-associated AMLs were managed with radical nephrectomy. A significant shift was found towards angioembolization in patients with TSC-association (p < 0.001). An interesting group included 4 TSC-associated patients with tumors ≥ 8 cm, in which 1 and 3 patients received angioembolization and radical nephrectomy due to spontaneous rupture, respectively. However a radical nephrectomy was eventually performed in the patient who previously underwent angioembolization because of intractable control of hemorrhages. With respect to lymph node involvement, preoperative CT scans revealed a significantly higher rate of lymph node enlargement in TSC-associated AMLs (p < 0.001) (Table 2).
Table 2.
Differences of Patient Characteristics between Sporadic and Tuberous Sclerosis (TS)-Associated AML
AML, angiomyolipoma.
Data presented as mean ± standard deviation or numbers.
*Student's t-test.
†Pearson's chi-square test.
Characteristics according to tumor size
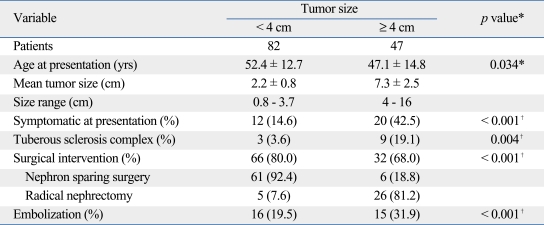
To determine the potential difference in clinical outcomes according to tumor size, the subsets were analyzed by a cutoff size of 4 cm. Tumors ≥ 4 cm were more commonly symptomatic, associated with TSC, and occurred in younger patients. 82 patients with tumors < 4 cm were mostly treated by NSS (n = 61, 92.4%). Among these patients, 3 underwent staged NSS for bilateral tumors and 1 underwent NSS for resection of two ipsilateral tumors. Radical nephrectomy was inevitable in 5 patients due to completely endophytic or hilar tumors located adjacent to the major branches of the renal vessels. 47 patients with tumors ≥ 4 cm were mostly treated by radical nephrectomy (n = 26, 81.2%). Our series of AML revealed a trend for NSS in small sized tumors, while large tumors tended to be managed by radical nephrectomy, angioembolization, or both (p < 0.001) (Table 3).
Table 3.
Differences of Patient Characteristics According to Tumor Si
Data presented as mean ± standard deviation or numbers.
*Student's t-test.
†Pearson's chi-square test.
Comparison between single and multiple AMLs
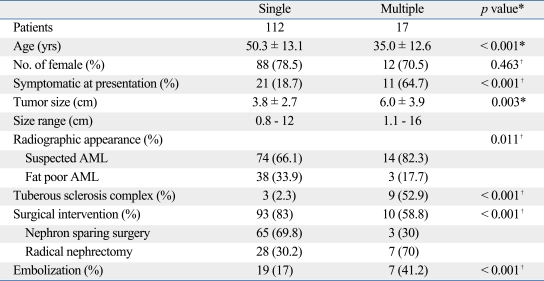
Several features differentiated patients with single AMLs from those with multiple AMLs including age, presence of symptoms, tumor size, and association with TSC. Patients with multiple AML were significantly younger, more symptomatic, larger, and were more associated with TSC. Moreover, radiographically-diagnosed AMLs composed 66.1% and 82.3% of single and multiple AMLs, respectively. With respect to treatment modality, surgical intervention including NSS or radical nephrectomy was more commonly performed in single AMLs while angioembolization was preferred in multiple AMLs (p < 0.001) (Table 4).
Table 4.
Differences of Patient Characteristics According to Tumor Multiplicity
AML, angiomyolipoma.
Data presented as mean ± standard deviation or numbers.
*Student's t-test.
†Pearson's chi-square test.
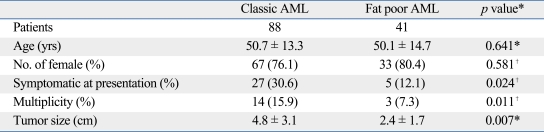
Comparison between radiographic subtypes: classic and fat poor
Surgical treatment was performed in 62 patients with radiographic characteristics indicating AML. All patients with fat poor AMLs who underwent prophylactic surgery on suspicion of malignancy were finally diagnosed with pathological AMLs. Although classic AMLs tended to be more symptomatic, multiple, and larger compared to fat poor AMLs, there were no significant differences in respect to age and gender distribution (Table 5).
Table 5.
Differences of Patient Characteristics between Classic and Fat Poor AML
AML, angiomyolipoma.
Data presented as mean ± standard deviation or numbers.
*Student's t-test.
†Pearson's chi-square test.
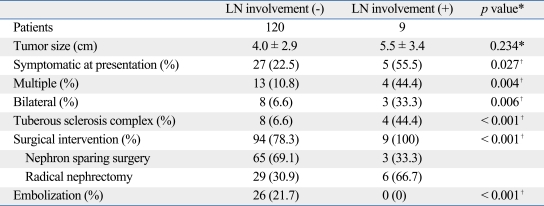
Characteristics according to lymph node involvement
Characteristics of AMLs according to presence of radiographic lymph node enlargement were analyzed. Among the 9 patients who received a lymphadenectomy, the lymph nodes of 3 patients were pathologically proven to be AML. AMLs with radiographic lymph node enlargement tended to be more symptomatic, multiple, bilateral, and more commonly accompanied by TSC. There were no statistically significant differences according to tumor size.
Clinical outcomes
The mean follow-up period was 64.8 months (range, 11-97 months). Of the 129 patients who received treatment of any kind, surgery and angioembolization were performed in 103 (79.8%) and in 26 (20.2%) patients, respectively. Despite repeated NSS or angioembolization, renal units retained adequate function to maintain serum creatinine at the normal range in all patients during the follow-up period. Although 122 patients (94.5%) remained asymptomatic during the follow-up, 7 (5.5%) patients necessitated additional treatment. 5 patients (3.8%) were refractory to angioembolization and tumor lesions grew at an average rate of 2.3 cm/year. These patients were followed by NSS and angioembolization in 2 and 3 patients, respectively. In 1 patient, angioembolization failed to reduce the size of a spontaneously-ruptured tumor at the 3 month follow-up and re-embolization was thus performed. In 1 female patient who underwent NSS for a 6 cm-sized TSC-associated AML, a radical nephrectomy was performed after the 23 month follow-up due to the spontaneous rupture of a recurred tumor in the ipsilateral kidney. The average tumor size of the 7 patients who required such secondary treatment during the follow-up was 9.9 cm, of which 4 (57.1%) were TSC-associated patients.
DISCUSSION
AMLs are recognized as hamartomatous lesions composed of heterogenous tissue components including blood vessels, smooth muscles, and fat. Renal AMLs occupy 1-3% of renal tumors and occur in 2 clinical spectrums: sporadic and those associated with TSC.8 TSC is an autosomal disease entity with various characteristics such as mental retardation, adenoma sebaceum, seizure, renal manifestations, and loss of heterozygosity of TSC1 and TSC2 genes.4,9 Although AML is uncommon among the general population, previous studies has shown that 20% of patients with TSC are known to develop AML while on the other hand, more than 50% of AMLs are associated with TSC.7 In this rare setting, tumors tend to be larger, develop earlier in life, and are more often multiple or bilateral.3
Our study demonstrated a relatively low prevalence rate for TSC-associated AMLs (9.3%) compared with 20% reported by Lendvay and Marshall.10 However, significant differences in the clinical spectrum of TSC-association according to age at presentation, tumor size, symptom, and multiplicity correlated with those of previous studies.7 TSC-associated AMLs tended to be more symptomatic, multiple, and bilateral. Therefore, active surveillance is strongly recommended for asymptomatic tumors < 4 cm, while angioembolization or surgical interventions with maximal parenchymal preservation including partial nephrectomy, enucleation, or wedge resection should be an alternative option for symptomatic tumors ≥ 4 cm. We reported a relatively large average tumor size for TSC-associated AML patients (7.6 cm, range, 1.1-16 cm) compared with 6.6 cm reported by Harabayashi, et al.11 Our experience of 4 TSC-associated patients with tumors ≥ 8 cm who finally ended up with receiving a radical nephrectomy demonstrated that, although angioembolization could be a primary option in controlling existing or impending hemorrhages in spontaneous ruptured AMLs, a prophylactic radical nephrectomy is relatively recommended for tumors ≥ 8 cm or those refractory to angioembolization.
Among TSC-associated AMLs, epitheliod components were pathologically observed in 9 patients. These tumors are thought to originate from common progenitor perivascular epitheloid cells, recognized as members of the perivascular epithelioid clear cell tumors (PEComas).12 Unlike classic AMLs, PEComas are distinctively characterized by local recurrence, lymph node involvement, and metastasis, thus demonstrating a substantial diagnostic challenge in differentiation with RCC.12-14 Brecher, et al.15 reported their results with AML with lymph node involvement, that no recurrence was noted during the follow-up and that it represents a multicentric growth pattern rather than metastasis. In addition, prognosis was considered to depend on remaining renal function rather than lymph node involvement.15 However, our study included one 16 year-old female patient who was an exception to this thesis. She presented with a 7 cm solid fat-containing parenchymal mass with multiple lymph node involvement along with facial adenoma sebaceum and multiple pulmonary lymphangioleiomatosis. Although NSS was performed due to her young age and disease trait, a radical nephrectomy was eventually performed after the 23 month follow-up due to a spontaneous rupture of the recurred tumor in the ipsilateral kidney. Although the patient remained asymptomatic for a follow-up period of 49 months, angioembolization or a radical nephrectomy is currently being considered for a 16 cm-sized recurred tumor on the contralateral kidney.
With the advancement of noninvasive radiological imaging techniques, the detection of fat poor AMLs has recently surged in line with increased detection of incidental and asymptomatic small sized renal tumors.16 Although the diagnosis of AML is typically based upon presence of fat component on CT scans, many AMLs with a scant fat component may evade radiographic diagnosis.17,18 Fergany, et al.19 described that solid renal tumors ought to be primarily considered as RCCs and surgical intervention are performed, due to the fact that solid tumors originating from renal parenchyma are mostly malignant and that 85-90% are RCCs.20 McCullough, et al.21 also recommended surgical treatment for tumors that are undistinguishable from RCC or those that contain calcification, a feature implying malignancy. Also, Lemaitre, et al.17 stated that 14% of AMLs demonstrate low fat content on radiographic imaging. However, of 129 AMLs in our series, 41 (31.7%) had radiographic features prompting surgical management based on clinical suspicion of RCC; all 41 cases were surgically managed based on the previously established principle.
Through our retrospective review of clinical outcomes of AML, we noticed a treatment trend based on tumor characteristics along with its associated symptoms. Although surgical intervention such as NSS or radical nephrectomy was the mainstay in the management of most renal tumors, our study demonstrated an surging number of angioembolization in patients with TSC-association. Several factors led to this conservative approach in recent years. Repeated nephrectomy reduces management options for the remaining kidney, subsequently mandating only a conservative approach. Regardless, characteristics of TSC-associated AMLs such as large size and multiplicity have limited the role of NSS. Angioembolization and NSS for renal AMLs both offer long-term renal function preservation with low complication rates. However, to better define the advantages of these two modalities a prospective head-to-head, comparisons will be needed.7,22,23
In conclusion, significant differences in clinical manifestations and treatment outcomes of AML were noted according to tumor size, association with TSC, multiplicity, radiologic characteristics, and treatment modalities. However, most patients treated for AML remained symptom-free with preservation of renal function. The fact that smal-sized AMLs with scant fat components and those associated with TSC may evade radiographic diagnosis is of clinical significance. Therefore, additional areas of research need to address the role of conventional imaging in identifying a fat component that would better differentiate AML from malignancy.
Table 6.
Differences of Patient Characteristics According to Presence of Lymph Nodes (LN)
Data presented as mean ± standard deviation or numbers.
*Student's t-test.
†Pearson's chi-square test.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A084120).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Hadley DA, Bryant LJ, Ruckle HC. Conservative treatment of renal angiomyolipomas in patients with tuberous sclerosis. Clin Nephrol. 2006;65:22–27. doi: 10.5414/cnp65022. [DOI] [PubMed] [Google Scholar]
- 2.Bae SK, Lee SW, Jung S, Song KS, Kim GA, Jeon JB, et al. A case of bilateral renal angiomyolipoma associated with tuberous sclerosis. Korean J Nephrol. 1997;16:793–796. [Google Scholar]
- 3.Kim JW, Lee TW, Kim MJ, Oh MM, Bae JH, Park HS, et al. Spontaneous rupture of renal angiomyolipoma in a female tuberous sclerosis patient with pulmonary lymphangioleiomyomatosis. Korean J Urol. 2007;48:344–347. [Google Scholar]
- 4.Danforth TL, Lane BR, Novick AC. Conservative management of giant symptomatic angiomyolipomas in patients with the tuberous sclerosis complex. BJU Int. 2007;100:794–797. doi: 10.1111/j.1464-410X.2007.07059.x. [DOI] [PubMed] [Google Scholar]
- 5.Ha HK, Seo HK, Chung MK. Radiologic characteristics of renal angiomyolipoma with minimal fat. Korean J Urol. 2004;45:163–167. [Google Scholar]
- 6.Boorjian SA, Frank I, Inman B, Lohse CM, Cheville JC, Leibovich BC, et al. The role of partial nephrectomy for the management of sporadic renal angiomyolipoma. Urology. 2007;70:1064–1068. doi: 10.1016/j.urology.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 7.Nelson CP, Sanda MG. Contemporary diagnosis and management of renal angiomyolipoma. J Urol. 2002;168:1315–1325. doi: 10.1016/S0022-5347(05)64440-0. [DOI] [PubMed] [Google Scholar]
- 8.Steiner MS, Goldman SM, Fishman EK, Marshall FF. The natural history of renal angiomyolipoma. J Urol. 1993;150:1782–1786. doi: 10.1016/s0022-5347(17)35895-0. [DOI] [PubMed] [Google Scholar]
- 9.Seyam RM, Bissada NK, Kattan SA, Mokhtar AA, Aslam M, Fahmy WE, et al. Changing trends in presentation, diagnosis and management of renal angiomyolipoma: comparison of sporadic and tuberous sclerosis complex-associated forms. Urology. 2008;72:1077–1082. doi: 10.1016/j.urology.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 10.Lendvay TS, Marshall FF. The tuberous sclerosis complex and its highly variable manifestations. J Urol. 2003;169:1635–1642. doi: 10.1097/01.ju.0000058253.40352.60. [DOI] [PubMed] [Google Scholar]
- 11.Harabayashi T, Shinohara N, Katano H, Nonomura K, Shimizu T, Koyanagi T. Management of renal angiomyolipomas associated with tuberous sclerosis complex. J Urol. 2004;171:102–105. doi: 10.1097/01.ju.0000100100.36354.61. [DOI] [PubMed] [Google Scholar]
- 12.Bahrami A, Schwartz MR, Ayala AG, Goldfarb RA, Brady JR, Takei H, et al. Concurrent angiomyolipoma and two oncocytomas in the same kidney. Ann Diagn Pathol. 2007;11:132–136. doi: 10.1016/j.anndiagpath.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Kim HM, Park JM, Yang SW, In YH, Kim MG, Yang WJ, et al. Renal Angiomyolipoma Partially Containing Epithelioid Component. Korean J Urol. 2007;48:655–658. [Google Scholar]
- 14.Lane BR, Aydin H, Danforth TL, Zhou M, Remer EM, Novick AC, et al. Clinical correlates of renal angiomyolipoma subtypes in 209 patients: classic, fat poor, tuberous sclerosis associated and epithelioid. J Urol. 2008;180:836–843. doi: 10.1016/j.juro.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 15.Brecher ME, Gill WB, Straus FH., 2nd Angiomyolipoma with regional lymph node involvement and long-term follow-up study. Hum Pathol. 1986;17:962–963. doi: 10.1016/s0046-8177(86)80647-5. [DOI] [PubMed] [Google Scholar]
- 16.Hafron J, Fogarty JD, Hoenig DM, Li M, Berkenblit R, Ghavamian R. Imaging characteristics of minimal fat renal angiomyolipoma with histologic correlations. Urology. 2005;66:1155–1159. doi: 10.1016/j.urology.2005.06.119. [DOI] [PubMed] [Google Scholar]
- 17.Lemaitre L, Claudon M, Dubrulle F, Mazeman E. Imaging of angiomyolipomas. Semin Ultrasound CT MR. 1997;18:100–114. doi: 10.1016/s0887-2171(97)90054-8. [DOI] [PubMed] [Google Scholar]
- 18.Choi D, Oh BR, Ryu SB, Park YI, Choi C. A case of angiomyolipoma without demonstrable fat component. Korean J Urol. 1998;39:1143–1147. [Google Scholar]
- 19.Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol. 2000;163:442–445. [PubMed] [Google Scholar]
- 20.Kim SI, Choi YD, Kim SJ, Chung BH, Seong DH, Kim CI, et al. A multi-institutional study on histopathological characteristics of surgically treated renal tumors: the importance of tumor size. Yonsei Med J. 2008;49:639–646. doi: 10.3349/ymj.2008.49.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCullough DL, Scott R, Jr, Seybold HM. Renal angiomyolipoma (hamartoma): review of the literature and report of 7 cases. J Urol. 1971;105:32–44. doi: 10.1016/s0022-5347(17)61455-1. [DOI] [PubMed] [Google Scholar]
- 22.Myong NH, Park BJ. Malignant glioma arising at the site of an excised cerebellar hemangioblastoma after irradiation in a von Hippel-Lindau disease patient. Yonsei Med J. 2009;50:576–581. doi: 10.3349/ymj.2009.50.4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho KS, Choi YD, Kim SJ, Kim CI, Chung BH, Seong do H, et al. A comprehensive prognostic stratification for patients with metastatic renal clear cell carcinoma. Yonsei Med J. 2008;49:451–458. doi: 10.3349/ymj.2008.49.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]