Abstract
This unit contains a protocol describing the isolation of brain mitochondria by using discontinuous Percoll gradient centrifugation. The Percoll density gradient centrifugation separates synaptosomes, myelin, and free non-synaptic mitochondria released from cells during tissue homogenization into individual fractions. Mitochondria entrapped in synaptosomes (synaptic mitochondria) can be liberated using nitrogen cavitation and then further purified by Percoll gradient centrifugation. These methods yield mitochondria that exhibit good respiratory coupling and high respiratory rates.
Keywords: brain, synaptosomes, non-synaptic, mitochondria, Percoll
Introduction
It is commonly believed that one of the underlying factors in neurological disease is mitochondrial impairment (for review see (Fiskum et al., 2000; Kristal et al., 2004; Kristian, 2004; Beal, 2005; Sullivan et al., 2005; Stavrovskaya and Kristal, 2005). While the significance of mitochondrial involvement in cell death is well established, the underlying mechanisms remain unclear. Research aimed at studying the role of mitochondrial dysfunction in cell death and development of neuroprotective strategies based on preserving mitochondrial functions preferentially utilizes mitochondria isolated from the CNS.
Initial approaches for obtaining enriched mitochondrial fractions from brain tissue used a simple procedure based on differential centrifugation (see Graham, 2001). However, this approach removed only nuclei, undisrupted cells and the cytosolic fraction from the tissue homogenate. Additionally, the mitochondrial fractions were heavily contaminated with synaptosomes and myelin (for review see Graham, 2001). Removal of these contaminants required gradient centrifugation using a density media. Sucrose gradient centrifugation had been applied to purify the mitochondrial fraction. However, this procedure exposed the mitochondria to markedly hypertonic conditions, resulting in poor preservation of their metabolic properties (Clark and Nicklas, 1970). Therefore, to obtain more metabolically active mitochondria, osmotically inactive compounds were later used in the isolation procedures. Ficoll was used to develop a procedure for isolating brain mitochondria and separating them into synaptic and non-synaptic fractions (Clark and Nicklas, 1970; Lai and Clark, 1976). These techniques allowed isolation of mitochondria with relatively good purity (90 to 95 % mitochondria), with acceptable metabolic functions and respiratory properties.
In addition to Ficoll, another osmotically inactive compound, Percoll, was used to develop a suitable isolation technique for separating free, non-synaptic mitochondria and synaptosomes from brain homogenates (see (Dunkley et al., 1986; Harrison et al., 1988; Sims, 1990; Zaidan and Sims, 1994; for review see Sims and Anderson, 2008). The Percoll gradient procedure yields pure non-synaptic mitochondria and also synaptosomes. Following synaptosomal disruption, synaptic mitochondria can be purified. The use of Percoll has several advantages over Ficoll. First, using the Percoll-based procedure, the isolation is relatively rapid when compared to Ficoll gradient techniques. Thus, one can isolate non-synaptic mitochondria from brain homogenates within 90 min. (Sims, 1990). The shorter isolation time yields mitochondria with better-preserved respiratory functions. Second, isotonic conditions are maintained throughout the procedure important in preserving mitochondrial functional and morphological integrity. Third, the procedure does not require an ultracentrifuge, but uses a fixed angle rotor in a medium- or high-speed centrifuge. Fourth, the procedure not only removes myelin and separates synaptosomes from non-synaptosomal mitochondria, but synaptic plasma membranes are also removed thereby allowing the investigator to study a highly purified population of synaptic mitochondria.
Percoll gradient centrifugation can be used to isolate both non-synaptic and synaptic mitochondria from whole forebrain (Naga et al., 2007; Hazelton et al., 2009). In this unit we describe both protocols. Using a single rat forebrain, one can isolate a sufficient amount of both non-synaptic and synaptic mitochondria to carry out several functional assays. However, it should be stressed that due to the multi-cellular origin of mitochondria isolated from brain tissue, the mitochondria are heterogeneous and one needs to exercise caution when interpreting the results.
A detailed protocol to isolate non-synaptic mitochondria from brain using Percoll gradient centrifugation is described by Sims and Anderson (2008). Neil Sims' laboratory pioneered the discontinuous Percoll gradient centrifugation technique and developed several modifications of this procedure dependent on the amount of brain tissue used for fractionation (see Sims and Anderson, 2008). Many laboratories, including ours, have adopted this approach with minor modifications (Friberg et al., 1999; Brustovetsky and Dubinsky, 2000; Kristian et al., 2000; Brown et al., 2006; Panov et al., 2002; Chinopoulos et al., 2003). A modified discontinuous Percoll gradient protocol has also been used to isolate mitochondria from spinal cord (Morota et al., 2007). In addition to the protocol for the isolation of non-synaptic mitochondria from brain that is similar to the Sims and Anderson method (Sims and Anderson, 2008), we describe a protocol for the isolation of mitochondria from synaptosomes.
Strategic planning
Before decapitation, centrifugation tubes, aliquots of isolation medium (IM) and a beaker with IM for cooling the brain tissue should be kept on ice. Furthermore, prepare correspondingly marked 1.5 ml microfuge tubes for synaptic and non-synaptic mitochondrial samples.
Basic Protocol
Isolation of non-synaptic and synaptic mitochondria from brain by using Percoll gradient centrifugation
Introduction
Percoll is a density gradient medium designed to separate cells, sub-cellular particles and viruses under gentle, physiological (isotonic with physiological pH) conditions. It is supplied as a sterile colloidal suspension comprised of silica particles, 15–30 nm in diameter, coated with polyvinylpyrrolidone (PVP).
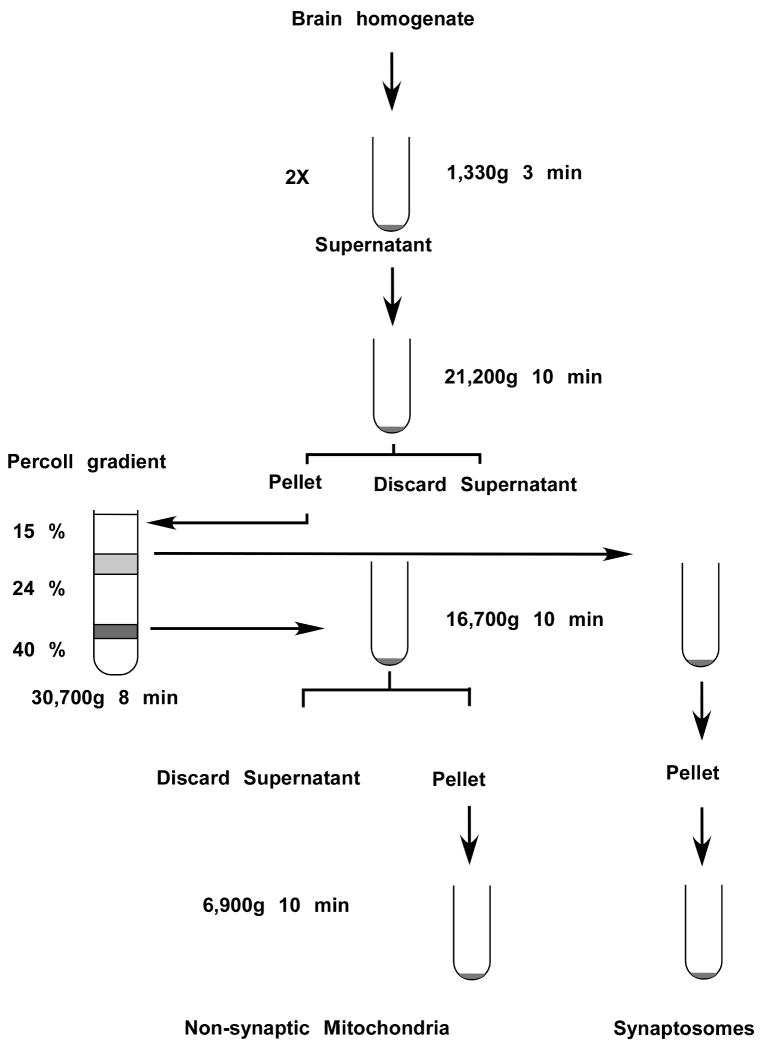
The Percoll gradient centrifugation technique separates suspensions of organelles and cell debris from brain homogenate according to their density. When a suspension of particles is centrifuged, the sedimentation rate of the particles is proportional to the force applied. Mitochondria are separated from brain tissue homogenate utilizing density separation. Each particle will sediment to the equilibrium position in the gradient where the gradient density is equal to the density of the particle. The Percoll gradient is designed so that mitochondria, synaptosomes and myelin separate into different fractions. These fractions are then collected and further processed to obtain highly purified and metabolically and morphologically intact non-synaptic and synaptic mitochondria. The schematic diagram of the whole procedure is shown in Figure 1.
Figure 1.
Flow schema of the separation procedure for mitochondria and synaptosomes from brain tissue. Following homogenization of brain tissue and low speed centrifugation the crude mitochondrial sample is fractionated into non-synaptic mitochondria and synaptosomes by Percoll gradient centrifugation.
Materials
Rodents
One rat, usually adult male Sprague-Dawley, or Fisher 344 rats (200-300 g) are used in our laboratory. We also use wild type and transgenic mice (strain C57Bl/6; 20-25 g). The combined brain tissue from two mouse forebrains can be processed according to the protocol described for one rat forebrain. Since the procedure requires the use of animals, it must be first approved by the Institutional Animal Care and Use Committee (IACUC).
Isolation medium (IM, see Reagents and Solutions)
Isolation medium with no EGTA (IM-EGTA)
Percoll (GE Healthcare, cat. No. 17089101)
Bovine serum albumin (BSA), fatty acid free (10mg/ml) (see Reagents and Solutions)
Small animal guillotine (Harvard apparatus, cat. No. PY8 73-1918)
Decapicones (Braintree Scientific, Model DC-200)
Beebee bone scissors (FST, cat. No. 16044-10)
Medium straight-edged scissors (FST, cat. No. 14002-14)
Glass vessel Teflon pestle Potter-Elvehjem Homogenizer (30 ml) (Colonial Scientific cat. No. 358049)
Kimwipes tissue (Kimtech Science)
Disposable centrifuge tubes, polypropylene 50 ml (Fisher Scientific, cat. No. 055396)
Single-use disposable Pasteur pipettes
Polycarbonate 10 ml centrifuge tubes for the JA-21 rotor (16 mm × 76 mm; Beckman Coulter cat. No. 355630)
Beckman Coulter (J2-MC) high-speed refrigerated centrifuge with fixed angle rotor JA 21 or equivalent high-speed centrifuge and rotor that holds 10 ml centrifuge tubes.
Refrigerated Eppendorf microfuge Model 5415R (Fisher Scientific cat. No. 0540105)
Cell disruption vessel (45 ml) (Parr Instrument Cat. No. 4639)
Nitrogen tank
Brain removal and tissue homogenization
-
Place an aliquot of IM (50ml) in a disposable centrifuge tube, a 50 ml glass beaker with 30 ml of IM, and centrifugation tubes and glass homogenizer with 10 ml of IM on ice. Place the rat into the decapicone and hold the animal by closing the decapicone at the root of the tail. Place the animal head into the guillotine so it will be decapitated just behind the ears.
To minimize the stress and possible pain to the animal the guillotine blades need to be sharp and the decapitation done with resolute and swift movement of the blade with no hesitation. This procedure should be carried out by experienced animal technician.
To remove the brain, retract the scalp to reveal the skull. Make an incision in the bone along the midline by placing the blade of the bone scissors into the spinal canal and cut the bone towards the eyes. Make sure that the inserted blade does not damage the brain by leaning it against the inside of the skull while cutting.
Make lateral horizontal incisions into the bone at the level of the ear on both sides by placing the blade of the bone scissors into the spinal canal and cutting the bone towards the eyes.
-
Use a 12-inch long nickel stainless spatula to apply a lateral force on the bone at the midline incision to reveal each hemisphere. Once the bones on both sides are retracted far enough exposing the whole brain, scoop out the brain from the skull by sliding the spatula between the surface of the brain and the skull then carefully remove the brain. Sometimes it is necessary to cut the meninges with fine scissors if they have not been disrupted during the skull retraction.
The brain should be removed and place into cold IM ideally within 30 sec after decapitation. If this process takes more than one minute the mitochondrial properties will be significantly deteriorated.
Place the brain into ice-cold IM and remove the cerebellum from the forebrain. Discard the cerebellum, brain stem and underlying midbrain structures.
Transfer the forebrain into 10 ml of fresh ice-cold IM in a beaker.
Chop the tissue into small pieces using straight scissors. Add 20 ml of IM, swirl the beaker and place it on ice for about 30 sec to allow the tissue pieces to settle on the bottom of the container.
-
Decant the IM and add 10 ml of fresh IM to the tissue pieces. Transfer the tissue suspension into the homogenizer (30 ml volume). Homogenize the tissue on ice using 8 to 10 up and down strokes while rotating the Teflon pestle with your fingers. More conveniently, place the pestle into a drill and spin it while performing strokes at about 400 rpm.
The up and down strokes with the pestle must be performed gently. Particularly during the up stroke, one should avoid creating negative pressure under the pestle by lifting it too fast so that a gap is observed between the homogenate and the pestle.
Tissue subfractionation
Transfer the homogenate into two 10 ml polycarbonate centrifuge tubes (16 mm × 76 mm) with a plastic transparent pipette. Make sure that there is an equal amount of homogenate in both tubes.
Centrifuge the homogenate at 1,300g (4000 rpm using the JA 21 rotor) at 4°C for 3 min.
Carefully transfer the supernatant into new tubes and place it on ice. Avoid collecting the fluffy, loose material from the top of the pellet.
Add 5 ml of IM to the pellet in each tube and gently re-suspend the pellet with an adjustable 5 ml volumetric pipette.
Centrifuge this homogenate at 1,300g at 4°C for 3 min. Decant the supernatant and combine it with the supernatant collected from the previous centrifugation.
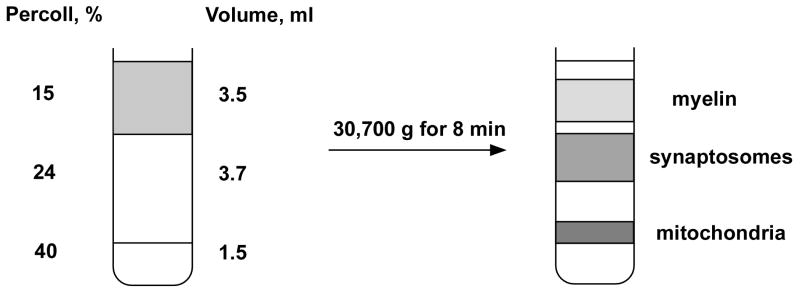
Centrifuge the pooled supernatant at 21,000g (16,000 rpm with this rotor) at 4°C for 10 min. During this period prepare the lower two layers of the Percoll gradient (Figure 2).
Add 3.7 ml of 24 % Percoll into a 10 ml polycarbonate centrifuge tube. Into a separate tube add 1.5 ml of 40 % Percoll.
-
Collect the 1.5 ml of 40 % Percoll using a disposable plastic pipette. Insert the pipette into the 24 % Percoll so the tip touches the bottom of the tube. Slowly introduce the 40 % Percoll solution to the bottom of the tube creating a discontinuous gradient of 24 % Percoll on top of the 40 % Percoll solution. Prepare two tubes with Percoll gradients per one whole rat forebrain.
The addition of the 40 % Percoll solution should be sufficiently slow to form a sharp interface between the two different Percoll layers. The tip of the disposable pipette should be leaning against the bottom wall of the centrifugation tube and not held vertically but rather under about 75°.
Decant the supernatant from the previous centrifugation step and discard it. Re-suspend the pellet in 3.5 ml of 15 % Percoll. Using a glass stirring rod, detach the pellet from the bottom of the tube and re-suspend the material using a disposable pipette.
-
Layer this material slowly above the 24 % Percoll with a disposable pipette.
Make sure that there is a sharp interface between the 24 % Percoll and the Percoll with the resuspended tissue. Begin introduction of the top layer by leaning the tip of the pipette against the tube wall close to the surface of the 24 % Percoll then slowly add the resuspended material on top of this layer.
-
Centrifuge at 30,700g (19,000 rpm for the JA 21 rotor) at 4° C for 8 min using slow acceleration (45 seconds from 0 to 500 rpm followed by normal acceleration) and deceleration (no brakes). This centrifugation should redistribute the tissue material into three major bands (Figure 2).
The slower acceleration and deceleration speeds are used to avoid disturbance of the Percoll gradient.
Remove the material accumulated at the top of the gradient, which mostly contains myelin.
Using an adjustable 1 ml volumetric pipette, collect the material banding near the interface of the upper two layers of the gradient and transfer it into a 10 ml polycarbonate tube. This contains mainly synaptosomes.
-
Collect the Percoll solution containing material accumulating at the interface between the 40 % and the 24 % Percoll solution that is enriched by non-synaptic mitochondria and add this suspension into a separate 10 ml polycarbonate tube.
Collect all the material within this band to maximize the mitochondrial yield.
-
Add 2 ml of IM to the collected synaptosomal fraction and 6 ml of IM to the non-synaptic mitochondria.
The isolation procedure of synaptic mitochondria from synaptosomes is described in the Support protocol (see below).
Centrifuge the resuspended non-synaptic fraction at 16,700g (14,000 rpm for the JA 21 rotor) at 4° C for 10 min. The mitochondria will be located at the bottom of the tube as a loose pellet. Carefully remove the supernatant leaving the bottom loose pellet undisturbed. Add 0.5 ml of 10 mg/ml fatty-acid-free BSA into each tube and gently stir the mixture. Then add 4.5 ml of the IM.
Centrifuge at 6,900g (9,000 rpm for the JA 21 rotor) at 4°C for 10 min. This will generate a firm pellet. Decant the supernatant and remove any remaining solutions from the wall of the centrifuge tube. Add 0.1 ml of the isolation medium lacking EGTA (IM-EGTA) to each tube and gently resuspend the pellet. Combine the mitochondrial suspension from all tubes into one conical 1.5 ml microfuge tube.
For functional assays e.g., measurement of mitochondrial respiration or calcium uptake capacity, keep the mitochondria on ice. Mitochondrial functional integrity is usually not significantly compromised for up to 2-3 hours after the preparation. To perform metabolic and enzymatic assays or for western blot analyses, mitochondrial samples can be aliquoted, and stored at − 80°C for several months following addition of protease inhibitors.
Figure 2.
Schematic illustration of the Percoll gradient and the distribution of brain homogenate fractions following centrifugation.
Support Protocol
Isolation of synaptic mitochondria from synaptosomes
Introduction
Synaptosomes are formed during brain tissue homogenization when the synaptic boutons are “pinched off” and resealed, trapping mitochondria localized at the synapses inside the synaptosome (Clark and Nicklas, 1970). Since only neurons form synapses, these mitochondria are neuron-specific and represent a relatively homogenous subpopulation of neuronal mitochondria. To isolate synaptic mitochondria from synaptosomes, several methods have been developed to disrupt the synaptosomal membranes such as the use of digitonin, osmotic lysis, or nitrogen cavitation (Booth and Clark, 1979; Lai and Clark, 1976; Brown et al., 2004; Hazelton et al., 2009). Digitonin disrupts the cellular membrane by binding to cholesterol making cholesterol-rich membranes fragile (Elias et al., 1978; Rosenthal et al., 1987). Since inner mitochondrial membranes contain little or no cholesterol (Colbeau et al., 1971) and the outer mitochondrial membranes contain much less cholesterol than the plasma membrane (Colbeau et al. 1971), the mitochondria are less susceptible to the digitonin effect. Thus, the respiratory functions are particularly well preserved (Elias et al., 1978; Booth and Clark 1979). However, the digitonin technique can alter the properties of the outer mitochondrial membrane (Brustovetsky et al., 2002). Similarly, utilizing hypo-osmotic shock can also adversely affect mitochondrial functions.
A method that applies high-pressure nitrogen decompression to disrupt the synaptic membrane (nitrogen cavitation) induces the least structural and functional damage to mitochondria (Brown et al., 2004; Naga et al., 2007; Hazelton et al., 2009). This approach was utilized to isolate the total mitochondrial fraction (both synaptic and non-synaptic mitochondria) from brain tissue homogenate (Brown et al., 2004). We have also used this technique to release mitochondria from primary cultures of neurons or astrocytes (Kristian et al., 2006).
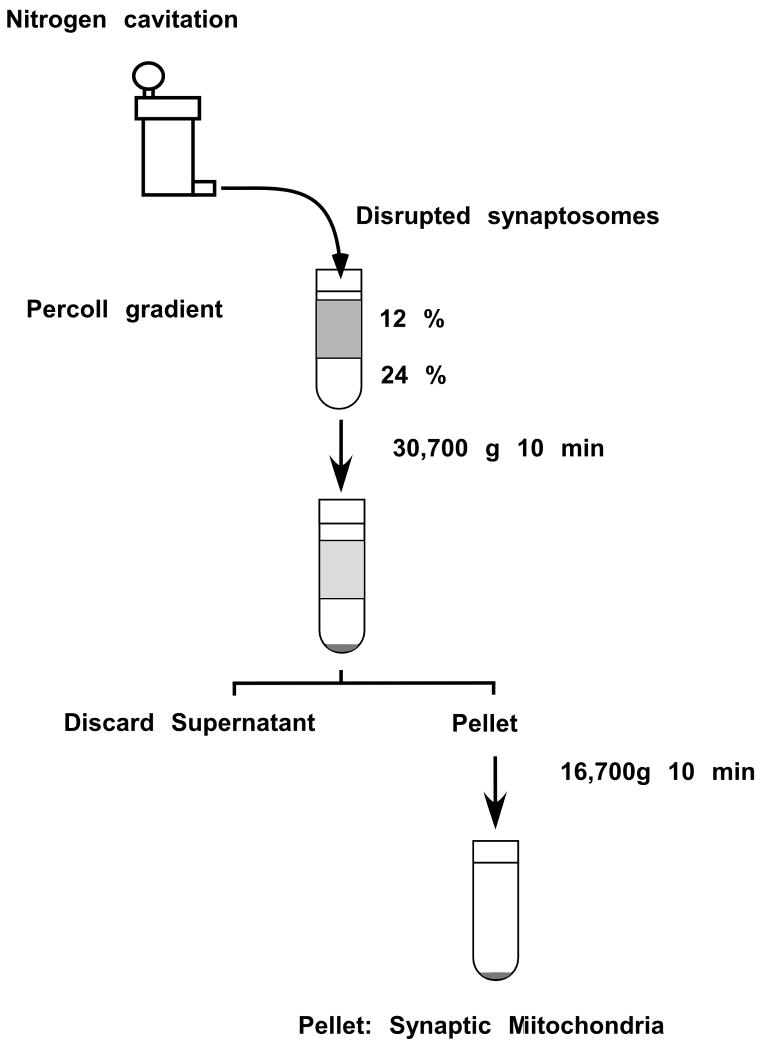
Add 2 ml of IM to the material collected at step 14 (synaptosomes). This will yield a suspension of synaptosomes in about 12 % Percoll solution. Combine the samples from both tubes. Pre-cool the nitrogen cavitation vessel by keeping it on ice with a stir bar inside. Transfer the synaptosomal suspension into the vessel and attach the nitrogen tank.
-
Slowly increase the pressure inside the vessel to 1,500 psi by letting the nitrogen gas enter the vessel from the tank. Wait for 15 minutes while stirring the suspension inside the vessel by magnetic stirrer. Then collect the material through the outflow tubing connected to the valve localized at the bottom of the cavitation chamber (Figure 3).
The pressure inside the vessel will decrease as the nitrogen enters the solution. After 15 min, the pressure between the liquid and gas compartment within the vessel equilibrates at around 900 psi. To facilitate this process, the solution inside the vessel should be stirred. Depressurization of the suspension from 900 psi to normal atmospheric pressure causes bubble formation within the suspended material. Bubbles that expand within the synaptosomes disrupt their membranes releasing mitochondria. The density of bubbles is related to the amount of nitrogen dissolved in the solution, which is directly dependent on the pressure at which the chamber is equilibrated.
Add into two 10 ml polycarbonate centrifugation tubes 4 ml of 24 % Percoll per tube.
Layer the collected material from the nitrogen cavitation vessel onto the 24 % Percoll.
Centrifuge at 30,700g (19,000 rpm for the JA 21 rotor) at 4°C for 10 min using slow acceleration (45 seconds from 0 to 500 rpm followed by normal acceleration) and deceleration (No brakes).
Remove the majority of the supernatant leaving the loose pellet containing free synaptic mitochondria in the tubes. Combine the synaptic mitochondria from the two tubes, add 6 ml of IM and gently mix the suspension.
Centrifuge at 16,700g (14,000 rpm for the JA 21 rotor) at 4°C for 10 min. The mitochondria form a loose pellet on the bottom of the tube. Carefully remove the supernatant, add 0.5 ml of 10 mg/ml fatty-acid-free BSA, gently stir then add 4.5 ml of IM.
Centrifuge at 6,900g (9,000 rpm for the JA 21 rotor) at 4°C for 10 min. This generates a firm pellet of synaptic mitochondria. Decant the supernatant and remove the residual isolation medium from the wall of the tubes, then add 50 μl of IM without EGTA.
-
For functional assays, keep the mitochondria on ice. For metabolic and enzymatic assays, or for western blots add protease inhibitors cocktail to the mitochondrial sample and store at -80 °C.
Mitochondria best preserve their respiration function when kept concentrated and on ice maintaining the protein concentration above 25 mg of mitochondrial protein per ml. When mitochondria are kept on ice at this protein concentration, their functional integrity is not affected significantly for up to 2-3 hours.
Figure 3.
Flow schema of the protocol for the isolation of synaptic mitochondria from synaptosomes by using Nitrogen cavitation. The numbers on the upper tube represent Percoll solutions concentrations used to create the gradient.
Reagents and solutions
Use deionized, distilled water in all recipes and protocol steps.
Bovine serum albumin (BSA) solution
Fatty-acid-free BSA 10 mg in one ml of water.
Store 1 ml aliquots at -20 °C for up to 6 months.
Isolation medium (IM), pH 7.4
225 mM sucrose
75 mM mannitol
1 mM EGTA
5 mM Hepes
Adjust pH to 7.4 with Tris-base at 4°C. Isolation medium with no EGTA (IM-EGTA) has the same composition but the EGTA is not added.
Store up to one month at 4°C.
100% Percoll solution
Prepare
225 mM sucrose
75 mM mannitol
1 mM EGTA
5 mM Hepes
in Percoll.
Adjust pH to 7.4 with HCl at 4°C.
Store up to one month at 4°C.
Protocol for preparation of solutions with different Percoll concentrations is given in Table 1.
Table 1. Percoll solutions required for the isolation procedure (50 ml total volume).
| a/ Using 100% Percoll as described in Reagents and Solutions | ||
|---|---|---|
| Final Percoll Concentration (vol/vol) | 100 % Percoll ml | Isolation medium ml |
| 40% | 20 | 30 |
| 24% | 12 | 38 |
| 15% | 7.5 | 42.5 |
| b/ Using 50% Percoll as described below | ||
|---|---|---|
| Final Percoll Concentration (vol/vol) | 50 % Percoll (ml) | Isolation medium (ml) |
| 40% | 40 | 10 |
| 24% | 24 | 26 |
| 15% | 15 | 35 |
Store up to one month at 4°C.
Prepare the 100% Percoll solution according to the protocol in the paragraph Reagents and Solutions
An alternative approach to preparing the different concentrations of Percoll solutions is to prepare isolation medium with double the concentration of each component then mix it with Percoll in a 1:1 (vol:vol) ratio. This will give a 50 % Percoll solution. The more diluted Percoll solutions can then be prepared by mixing this 50% Percoll with standard isolation medium in the appropriate ratios (see Table 1b/).
All reagents are purchased from Sigma with the highest purity grade.
Commentary
Background Information
Isolation of non-synaptic mitochondria
The major advantage of Percoll over alternative media such as sucrose and Ficoll is its viscosity, which allows more rapid sedimentation and the use of a lower centrifugation force. A second advantage over the sucrose-based procedure is that isotonicity can be maintained throughout the whole procedure. Percoll gradient centrifugation gives a relatively pure mitochondrial sample with little contamination from synaptosomes and myelin (Sims, 1990, Sims and Anderson, 2008).
Isolation of synaptic mitochondria
The protocol for isolating synaptic mitochondria utilizes the synaptic fraction that can be collected from the Percoll gradient used to isolate non-synaptic mitochondria. Different modifications of the Percoll gradient can be used to purify synaptosomes from brain homogenates (Dunkley et al., 1986; Harrison et al., 1988; Taupin et al., 1994), which are then further processed to isolate the synaptic mitochondria. For example, a discontinuous Percoll gradient composed of four different Percoll density layers is utilized to prepare hippocampal mossy fiber synaptosomes directly from the post-nuclear pellet (Taupin et al., 1994). When the 4-step discontinuous Percoll gradient is used, 5 major sub-cellular fractions are obtained after centrifugation. These fractions contained synaptosomes having different diameters and mitochondrial content (Dunkley et al., 1988). Each synaptosomal fraction also showed different degrees of contamination with non-synaptic mitochondria (au: if the goal is to isolate mitochondria, how can there be mitochondrial contamination--- or, do you mean: different degrees of contamination by non-mitochondrial elements/organelles). By using a Ficoll-sucrose discontinuous gradient, two synaptic fractions were identified, suggesting two subpopulations of synaptic mitochondria (Lai et al., 1977).
Critical Parameters and Troubleshooting
To isolate highly pure mitochondria with high respiratory activity it is important that:
During the entire isolation procedure all solutions are kept ice-cold.
Make sure that the isolation medium and Percoll solutions have the correct osmolarity and pH at 4°C.
Brain tissue removal from the skull after decapitation needs to be performed within one minute and immediately immersed into ice-cold isolation medium.
During homogenization, do not force the pestle movement in up and down directions. Rather, rotating the pestle with fingers or by electric drill during vertical movement reduces friction making the brain tissue pieces homogenization more efficient. Do not over-homogenize the tissue.
Make sure that there is a sharp interface between the different density Percoll solutions when preparing the discontinuous gradient.
When resuspending the mitochondrial pellet, do not scrape off the pellet from centrifuge tube.
The preparation of a discontinuous gradient requires considerable practice to achieve sharp borders between the layers of Percoll with different concentrations. To minimize the mixing of the different density Percoll solutions, one can use an infusion pump to slowly and continuously layer the solutions on top of the solution present in the centrifugation tube. This procedure however takes a considerably longer time requiring advance preparation of the Percoll gradient before the isolation procedure begins.
Anticipated Results
This method produces non-synaptic mitochondria that are metabolically active, exhibit good respiratory coupling, good purity, and have minimal synaptosomal and myelin contamination. Total mitochondrial protein for the non-synaptic mitochondria from one whole forebrain is about 2.5 mg protein. The typical yield of synaptic mitochondria is up to 1.0 mg protein. The synaptic mitochondrial fraction exhibits somewhat higher contamination by synaptosomes. Respiratory properties are expressed as rates of oxygen consumption per minute per mg of mitochondrial proteins. Mitochondria are incubated in the presence of NAD- or FAD- linked substrates and the oxygen consumption measured using Clark-type oxygen electrodes. Using the described protocol in the presence of malate and glutamate as the metabolic substrate, the ADP-stimulated respiration rates (state 3 respiration) of non-synaptic and synaptic mitochondria isolated from 3 month old rat brains are shown in the Table 2.
Table 2.
Respiratory rates of non-synaptic and synaptic mitochondria isolated from 3 month old rat brains assessed with 5 mM glutamate and 5 mM malate (nmol O2/min/mg protein)
| Mitochondria | state 3 | state 4 | Respiratory control ratio (RCR) |
|---|---|---|---|
| Non-synaptic | 243 ± 14 | 38 ± 7 | 6.6 ± 1.2 |
| Synaptic | 144 ± 30 | 22 ± 5.6 | 6.7 ± 1.4 |
RCR = state 3 / state 4. Values are expressed as the mean ± SEM (n=4). The oxygen consumption rates were determined at 37°C.
The state 3 respiratory activity (oxygen consumption rate in the presence of ADP) of non-synaptic mitochondria is similar to that reported by others using a Percoll discontinuous gradient (Sims, 1990; Brustovetsky et al., 2003; Brown et al., 2004; Sims and Anderson, 2008). However, the state 3 rates of both non-synaptic and synaptic mitochondria isolated using discontinuous Ficoll gradients are significantly slower (Lai et al., 1977). In our hands, the respiratory control RCR of both non-synaptic and synaptic mitochondria was not significantly different. The RCR is commonly used as an indicator of mitochondrial membrane integrity. Less leaky mitochondrial membranes will give slower oxygen consumption rates in state 4 when no ATP is generated that is then reflected in a higher RCR. Therefore RCR values lower than 4 represent problems either with the isolation procedure or the solutions used.
Time Considerations
Initial preparation of solution aliquots and setup before beginning the isolation procedure: 10-15 min. Removal of the brain and brain tissue homogenization takes 10 min. Tissue fractionation including gradient preparation during the first three centrifugation periods 60 – 70 min. Thus, the whole procedure takes about 90 min. The purification of synaptic mitochondria from synaptosomes takes about 45 min. However, the procedure can be carried out in parallel with the non-synaptic mitochondrial isolation after the synaptosomes and non-synaptic mitochondrial fractions are separated.
Literature Cited
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- Booth RF, Clark JB. A method for the rapid separation of soluble and particulate components of rat brain synaptosomes. FEBS Lett. 1979;107:387–392. doi: 10.1016/0014-5793(79)80414-7. [DOI] [PubMed] [Google Scholar]
- Brown MR, Sullivan PG, Dorenbos KA, Modafferi EA, Geddes JW, Steward O. Nitrogen disruption of synaptoneurosomes: an alternative method to isolate brain mitochondria. J Neurosci Methods. 2004;137:299–303. doi: 10.1016/j.jneumeth.2004.02.028. [DOI] [PubMed] [Google Scholar]; This paper describes the protocol that uses the nitrogen cavitation to isolate the whole mitochondrial population (non-synaptic and synaptic mitochondria) from brain homogenate.
- Brown MR, Sullivan PG, Geddes JW. Synaptic mitochondria are more susceptible to Ca2+overload than nonsynaptic mitochondria. J Biol Chem. 2006;281:11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- Brustovetsky N, Brustovetsky T, Purl KJ, Capano M, Crompton M, Dubinsky JM. Increased susceptibility of striatal mitochondria to calcium-induced permeability transition. J Neurosci. 2003;23:4858–4867. doi: 10.1523/JNEUROSCI.23-12-04858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustovetsky N, Dubinsky JM. Limitations of cyclosporin A inhibition of the permeability transition in CNS mitochondria. J Neurosci. 2000;20:8229–8237. doi: 10.1523/JNEUROSCI.20-22-08229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustovetsky N, Jemmerson R, Dubinsky JM. Calcium-induced Cytochrome c release from rat brain mitochondria is altered by digitonin. Neurosci Lett. 2002;332:91–94. doi: 10.1016/s0304-3940(02)00948-5. [DOI] [PubMed] [Google Scholar]
- Chinopoulos C, Starkov AA, Fiskum G. Cyclosporin A-insensitive permeability transition in brain mitochondria: inhibition by 2-aminoethoxydiphenyl borate. J Biol Chem. 2003;278:27382–27389. doi: 10.1074/jbc.M303808200. [DOI] [PubMed] [Google Scholar]
- Clark JB, Nicklas WJ. The metabolism of rat brain mitochondria. Preparation and characterization. J Biol Chem. 1970;245:4724–4731. [PubMed] [Google Scholar]
- Colbeau A, Nachbaur J, Vignais PM. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim Biophys Acta. 1971;249:462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Jarvie PE, Heath JW, Kidd GJ, Rostas JA. A rapid method for isolation of synaptosomes on Percoll gradients. Brain Res. 1986;372:115–129. doi: 10.1016/0006-8993(86)91464-2. [DOI] [PubMed] [Google Scholar]
- Elias PM, Goerke J, Friend DS, Brown BE. Freeze-fracture identification of sterol-digitonin complexes in cell and liposome membranes. J Cell Biol. 1978;78:577–596. doi: 10.1083/jcb.78.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskum G. Mitochondrial participation in ischemic and traumatic neural cell death. J Neurotrauma. 2000;17:843–855. doi: 10.1089/neu.2000.17.843. [DOI] [PubMed] [Google Scholar]
- Fiskum G. Mechanisms of neuronal death and neuroprotection. J Neurosurg Anesthesiol. 2004;16:108–110. doi: 10.1097/00008506-200401000-00025. [DOI] [PubMed] [Google Scholar]
- Friberg H, Connern C, Halestrap AP, Wieloch T. Differences in the activation of the mitochondrial permeability transition among brain regions in the rat correlate with selective vulnerability. J Neurochem. 1999;72:2488–2497. doi: 10.1046/j.1471-4159.1999.0722488.x. [DOI] [PubMed] [Google Scholar]
- Graham JM. Purification of a crude mitochondrial fraction by density-gradient centrifugation. Curr Protoc Cell Biol. 2001;Chapter 3(Unit 3):4. doi: 10.1002/0471143030.cb0304s04. [DOI] [PubMed] [Google Scholar]; Describes the principles of gradient centrifugation and use of variety of density media that can be use to prepare pure fractions of mitochondria.
- Harrison SM, Jarvie PE, Dunkley PR. A rapid Percoll gradient procedure for isolation of synaptosomes directly from an S1 fraction: viability of subcellular fractions. Brain Res. 1988;441:72–80. doi: 10.1016/0006-8993(88)91384-4. [DOI] [PubMed] [Google Scholar]
- Hazelton JL, Petrasheuskaya M, Fiskum G, Kristian T. Cyclophilin D is expressed predominantly in mitochondria of gamma-aminobutyric acidergic interneurons. J Neurosci Res. 2009;87:1250–1259. doi: 10.1002/jnr.21921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristal BS, Stavrovskaya IG, Narayanan MV, Krasnikov BF, Brown AM, Beal MF, Friedlander RM. The mitochondrial permeability transition as a target for neuroprotection. J Bioenerg Biomembr. 2004;36:309–312. doi: 10.1023/B:JOBB.0000041759.35731.70. [DOI] [PubMed] [Google Scholar]
- Kristian T. Metabolic stages, mitochondria and calcium in hypoxic/ischemic brain damage. Cell Calcium. 2004;36:221–233. doi: 10.1016/j.ceca.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Kristian T, Gertsch J, Bates TE, Siesjo BK. Characteristics of the calcium-triggered mitochondrial permeability transition in nonsynaptic brain mitochondria: effect of cyclosporin A and ubiquinone O. J Neurochem. 2000;74:1999–2009. doi: 10.1046/j.1471-4159.2000.0741999.x. [DOI] [PubMed] [Google Scholar]
- Lai JC, Clark JB. Preparation and properties of mitochondria derived from synaptosomes. Biochem J. 1976;154:423–432. doi: 10.1042/bj1540423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JC, Walsh JM, Dennis SC, Clark JB. Synaptic and non-synaptic mitochondria from rat brain: isolation and characterization. J Neurochem. 1977;28:625–631. doi: 10.1111/j.1471-4159.1977.tb10434.x. [DOI] [PubMed] [Google Scholar]
- Morota S, Hansson MJ, Ishii N, Kudo Y, Elmer E, Uchino H. Spinal cord mitochondria display lower calcium retention capacity compared with brain mitochondria without inherent differences in sensitivity to cyclophilin D inhibition. J Neurochem. 2007;103:2066–2076. doi: 10.1111/j.1471-4159.2007.04912.x. [DOI] [PubMed] [Google Scholar]
- Naga KS, G P, Geddes JW. High cyclophilin D content of synaptic mitochondria results in increased vulnerability to permeability transition. Journal of Neuroscience. 2007;27:7469–7475. doi: 10.1523/JNEUROSCI.0646-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT. Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nat Neurosci. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- Rosenthal RE, Hamud F, Fiskum G, Varghese PJ, Sharpe S. Cerebral ischemia and reperfusion: prevention of brain mitochondrial injury by lidoflazine. J Cereb Blood Flow Metab. 1987;7:752–758. doi: 10.1038/jcbfm.1987.130. [DOI] [PubMed] [Google Scholar]
- Sims NR. Rapid isolation of metabolically active mitochondria from rat brain and subregions using Percoll density gradient centrifugation. J Neurochem. 1990;55:698–707. doi: 10.1111/j.1471-4159.1990.tb04189.x. [DOI] [PubMed] [Google Scholar]
- Sims NR, Anderson MF. Isolation of mitochondria from rat brain using Percoll density gradient centrifugation. Nat Protoc. 2008;3:1228–1239. doi: 10.1038/nprot.2008.105. [DOI] [PubMed] [Google Scholar]; This paper offers a detail protocols for isolating mitochondria from brain tissues of different sample sizes by using a modifications of Percoll gradient centrifugation.
- Stavrovskaya IG, Kristal BS. The powerhouse takes control of the cell: is the mitochondrial permeability transition a viable therapeutic target against neuronal dysfunction and death? Free Radic Biol Med. 2005;38:687–697. doi: 10.1016/j.freeradbiomed.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Rabchevsky AG, Waldmeier PC, Springer JE. Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death? J Neurosci Res. 2005;79:231–239. doi: 10.1002/jnr.20292. [DOI] [PubMed] [Google Scholar]
- Taupin P, Ben-Ari Y, Roisin MP. Subcellular fractionation on Percoll gradient of mossy fiber synaptosomes: evoked release of glutamate, GABA, aspartate and glutamate decarboxylase activity in control and degranulated rat hippocampus. Brain Res. 1994;644:313–321. doi: 10.1016/0006-8993(94)91695-0. [DOI] [PubMed] [Google Scholar]
- Zaidan E, Sims NR. The calcium content of mitochondria from brain subregions following short-term forebrain ischemia and recirculation in the rat. J Neurochem. 1994;63:1812–1819. doi: 10.1046/j.1471-4159.1994.63051812.x. [DOI] [PubMed] [Google Scholar]