Abstract
The Wnt/β-catenin pathway is implicated in the pathogenesis of hepatocellular cancer (HCC). We have developed a transgenic mouse (TG) in FVB strain that overexpresses Ser45-mutated-β-catenin in hepatocytes to study the effects on liver regeneration and cancer. In the two independent TG lines, adult mice show elevated β-catenin at hepatocyte membrane with no increase in Wnt pathway targets cyclin-D1 or glutamine synthetase. However, TG hepatocytes upon culture exhibit a 2-fold increase in thymidine incorporation at day 5 (D5) when compared to hepatocytes from wild-type FVB mice (WT). When subjected to partial hepatectomy (PH), dramatic increases in the number of hepatocytes in S-phase are evident in TG at 40 and WT at 72 hours. Coincident with the earlier onset of proliferation, we observe nuclear translocation of β-catenin along with increase in total and nuclear cyclin-D1 protein at 40 hours in TG livers. To test if stimulation of β-catenin induces regeneration, we utilized hydrodynamic delivery of Wnt-1 naked DNA to control mice, which prompted an increase in Wnt-1, β-catenin and known targets-GS and cyclin-D1, along with a concomitant increase in cell proliferation. β-Catenin overexpressing TG mice, when followed up to 12 months showed no signs of spontaneous tumorigenesis. However, intra-peritoneal delivery of diethylnitrosamine, a known carcinogen (DEN) induced HCC at 6 months in TG mice only. Tumors in TG livers showed upregulation of β-catenin, cyclin-D1 and unique genetic aberrations while other canonical targets were unremarkable. In conclusion, β-catenin overexpression offers growth advantage during liver regeneration. Also, while no spontaneous HCC is evident, β-catenin overexpression makes TG mice susceptible to DEN-induced HCC.
Keywords: Wnt signaling, liver development, liver cancer, hepatectomy, cyclin-D1
Wnt/β-catenin signaling is evolutionarily well-conserved pathway and important in liver health and repair (1). In adult liver, β-catenin signaling is essentially quiescent with active-β-catenin restricted to hepatocytes in centrizonal area where it regulates expression of genes such as glutamine synthetase (GS) and others involved in xenobiotic metabolism (2). In other hepatocytes, β-catenin steady state is achieved by phosphorylation at key serine/threonine residues and subsequent degradation, and is predominantly localized to membrane to mediate cell-cell adhesion by forming a bridge between E-cadherin and actin cytoskeleton (3).
Activation of β-catenin signaling during liver regeneration has been reported in rats and mice (4–7). While a positive regulator in the activity of normal liver growth, aberrant activation of the Wnt/β-catenin pathway is implicated in hepatocarcinogenesis, although exact mechanism remains elusive. A significant subset of hepatocellular cancers (HCC) and hepatoblastomas display Wnt pathway activation due to various mechanisms including mutations in CTNNB1, the gene encoding for β-catenin (8, 9). Missense point mutations observed in tumors affect key phosphorylation sites involved in β-catenin degradation, with serine-45 being commonly altered (10). We sought to overexpress serine-45-mutated CTNNB1 in the liver to characterize these animals for hepatic growth.
Here, we report the generation and characterization of serine-45 mutated-β-catenin overexpressing transgenic mouse, which exhibit hepatocyte growth advantage both in culture and after partial hepatectomy (PH). We also demonstrate that in vivo induction of β-catenin signaling through hydrodynamic delivery of Wnt-1 naked DNA in normal mice increases hepatocyte proliferation. We also report accelerated chemical-induced hepatic tumorigenesis in transgenic mice, which may elucidate mechanisms of β-catenin-dependent hepatocarcinogenesis.
EXPERIMENTAL PROCEDURES
Mutagenesis and Animals
Using the GeneTailor™ site-directed mutagenesis kit, Serine-45 in CTNNB1 was mutated to aspartic acid (S45D), alanine (S45A), and phenylalanine (S45F), which were previously shown to prevent ubiquitination of β-catenin (11). These constructs, along with CTNNB1 exon-3 deletion mutant (Δ-nt:282–630), were tested for β-catenin activity by Topflash assay in HEK293 cells (12). cDNA containing S45D was inserted into BamHI sites of an albumin promoter/enhancer-driven expression vector to generate transgenic mice as described previously, albeit in FVB background (13). Two independent founder lines were identified and based on comparable β-catenin expression only one line was expanded for analysis. The transgenic line was maintained as homozygous and henceforth referred to as TG mice. Only male mice were used for all experiments and age-matched wild-type FVB mice (WT) served as controls. All animal studies were performed in strict accordance with Institutional and NIH guidelines.
Surgery
3-month old male FVB WT and TG mice were subjected to partial PH and sacrificed at 10 hours (H), 20H, 40H, 72H, 5 days (D5), D9, D14, 1 month (M) and 3M (n=3–5) as described elsewhere (7). While peak proliferation in FVB mice is evident at 72H after PH, early and late times were included to assess full impact of transgene on regeneration (14). Livers were processed for paraffin embedding and protein isolation (7).
Primary hepatocyte culture
Hepatocytes isolated from 3-month old WT and TG mouse livers (n=3) were cultured in media containing either 10% FBS or insulin/transferrin/selenium (ITS; 1g/L), dexamethasone (10−7M), HGF (40ng/ml), and epidermal growth factor (EGF; 25ng/ml), as described previously (15).
Hydrodynamic DNA delivery
Wnt-1 (Upstate Biotech) or pcDNA3 control plasmid were administered via hydrodynamic tail vein injection to CD-1 mice weighing around 18 grams at 1µg/gm of body weight, as described previously (16). 18H after injection mice were subjected to PH and sacrificed at 30H after PH, before the expected hepatocyte proliferation.
DEN-induced carcinogenesis
15D-old TG and WT mice were administered an intraperitoneal (IP) injection of DEN (5mg/kg) and sacrificed at 6 or 9 months. Livers were processed for histology, and lesions characterized as dysplastic foci, hepatic adenoma, or HCC, based on tumor size, thickness of hepatic plate and presence of mitotic figures by a pathologist (T.W.).
Immunoprecipitation (IP) and Western Blot (WB) Analysis
Whole-cell lysates from WT and TG livers at specific ages were prepared as previously described (7). Nuclear and cytoplasmic extracts were prepared using NE-PER Extraction kit (Fisher Scientific). Proteins were subjected to WB analysis and probed with antibodies (supplementary methods) (7). Whole-cell lysates were used to immunoprecipitate β-catenin or E-cadherin to assess association with E-cadherin and β-catenin, respectively as described elsewhere (4).
Immunohistochemistry (IHC)
H&E and IHC were performed as described elsewhere (7). Primary antibodies used are listed in supplementary methods.
Cell Growth and Viability Assays
A [3H]thymidine uptake assay and cell viability was assessed as previously published (12, 15).
Gene Array Analysis
6-month old TG and WT livers (n=3/group) after DEN exposure were utilized for RNA extraction and Affymetrix gene array analysis as described previously (7, 13).
Statistical Analysis
All experiments were performed three or more times and representative data is presented. Autoradiographs were scanned and analyzed for densitometry using the ImageJ software. Mean integrated optical density, proliferation and viability assays were compared for significance by Student T test (Kaleidagraph, Synergy Software) and P value of less than 0.05 was considered significant (*).
RESULTS
Generation of Serine-45 mutant β-catenin TG mice
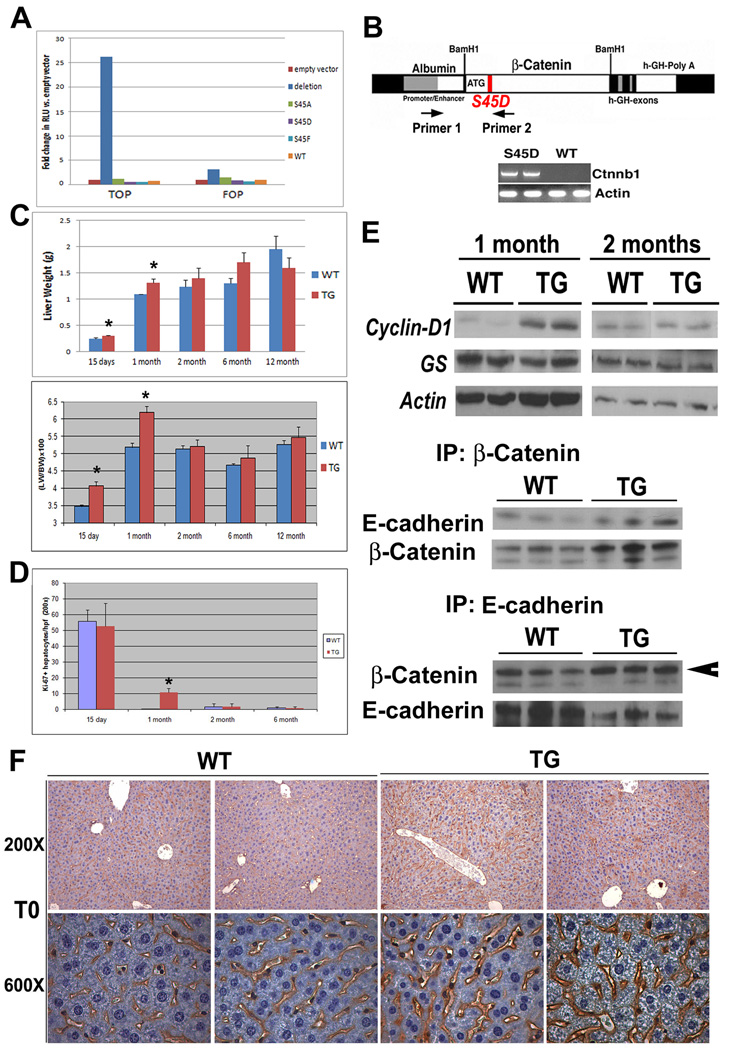
Previously, it was shown that mutation of the four residues (Serine-33, 37, 45 and Threonine-41) to either alanine (A) or aspartic acid (D) resulted in decreased ubiqutin tagging of β-catenin (13). S45A, S45D, S45F or active-β-catenin mutant (Δ-nt:282–630), were transfected in HEK293 cells along with TOPflash or FOPflash reporters. Transcriptional activity in response to the deletion mutant was increased 25-fold above empty vector alone (p=0.000435), while all other mutants did not enhance reporter activity (Fig. 1a). Despite these observations, and based on reported point mutations affecting this site in HCC, we generated liver-specific TG mice by placing S45D-β-catenin gene under the transcriptional control of albumin promoter/enhancer as discussed in methods (Fig. 1b).
Figure 1. Creation and characterization of TG mice. (*p<0.05).
(A) Topflash reporter assay showing transcriptional response in HEK293 cells following transfection with various β-catenin mutant expression vectors.
(B) Full length human Ctnnb1 containing a point mutation (S45D) was inserted downstream of an albumin promoter/enhancer and injected into FVB mouse eggs to create transgenic mice. Genomic DNA was subjected to PCR using indicated primers (X and Y) whose position is indicated. Representative PCR show the presence of transgene in TG mice and not WT mice.
(C) Liver weight in TG mice and age-matched WT (n≥3) (upper panel). Liver weight to body weight ratio (LW/BW) in TG and age-matched WT (n≥3) (lower panel).
(D) Increase in the number of Ki-67 positive hepatocytes in TG liver at 1 month compared to WT.
(E) WB showing expression levels of total cyclin-D1 and GS in TG and WT liver at 1 and 2 months. An increase in cyclin-D1 is observed in TG at 1 month. Actin was used as a loading control. IP studies from three representative livers shows enhanced association of β-catenin and E-cadherin in 6 months old TG livers as compared to WT.
(F) IHC for β-catenin reveals excess membranous localization in TG.
Transient in-vivo liver growth advantage in TG mice
Age-matched TG and WT mice were compared for liver weight and liver weight/body weight ratios (LW/BW). Significant increase in LW was evident in 15D (p=0.03) and 1M old TG mice (p=0.02) as compared to WT (Fig. 1c). Concomitant increases in LW/BW were also observed at 15D (p= 0.035) and 30D (p=0.0004) in TG mice. Interestingly, no significant differences in these parameters were evident in 2 months or older WT and TG mice.
Significant increase in basal cell proliferation was evident in 1M-old TG livers (p<0.0001) as assessed by Ki-67 IHC (Fig. 1d). WT and TG mice both show high proliferation at 15D, as postnatal hepatic growth is normally rampant and hence may be masking transgene–induced cell proliferation (Fig. 1e). Comparable cell proliferation was observed in 2M and older WT and TG livers.
To examine basis of enhanced cell proliferation, we compared the expression of cyclin-D1 in livers from 1M- and 2M- old animals. Increased levels of cyclin-D1 were evident in TG livers at 1M only (Fig. 1f). GS, another target of β-catenin, was unchanged in TG and WT livers at either time (Fig. 1e)
Since cyclin-D1 was not elevated at 2M, we hypothesized that excess β-catenin in TG livers might be associating with its membrane partners. Indeed, association of β-catenin and E-cadherin by coprecipitation was greater in 2M or older TG livers (shown at 6 months, Fig. 1e). Similarly, while it was more intense in TG, β-catenin localized to the hepatocyte membrane in both WT and TG at 2M (Fig. 1f).
Taken together, the above findings indicate that TG mice show temporal β-catenin activation during early postnatal development, followed by adaptive changes other than ubiquitin-mediated degradation, which prevent excessive β-catenin activation through its membranous sequestration.
Increased growth of TG hepatocytes in culture
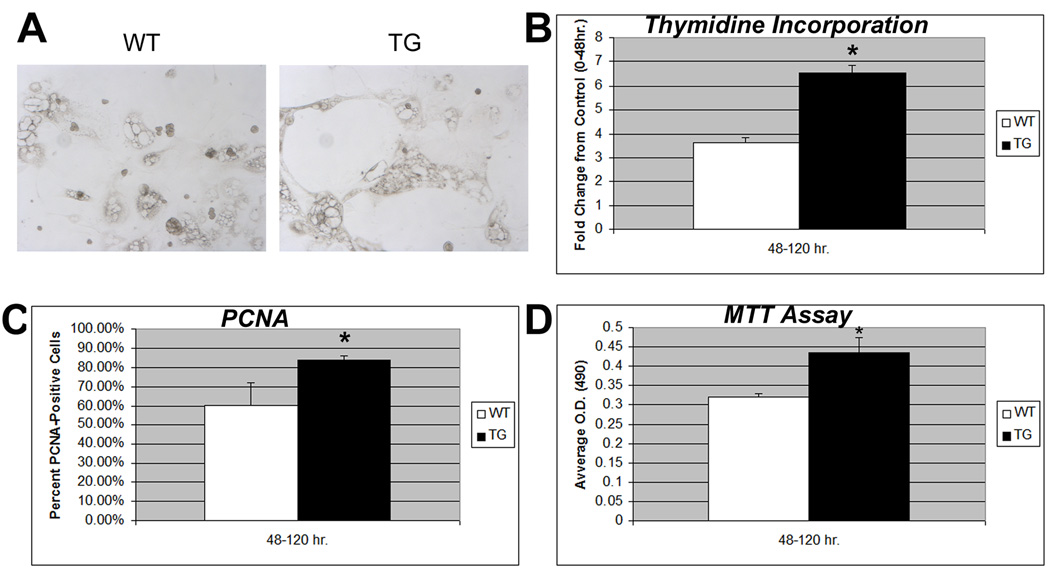
We next investigated growth and morphology of WT and TG hepatocytes in primary cultures from 3M-old animals. The hepatocytes appeared morphologically indistinguishable at 48H of culture. At 120H, TG hepatocytes appeared more flattened, spread out and healthy as compared to WT suggesting more robust growth (Fig. 2a). TG hepatocytes exhibited around 2-fold (p<0.05) increase in thymidine incorporation over WT after 120H of culture (Fig. 2b). TG hepatocytes showed a 27% and significant (p<0.01) gain in PCNA positivity at 120H over 0–40H cultures, while no increase in PCNA-positivity was evident in WT hepatocytes at corresponding times (Fig. 2c). MTT assay also showed significantly greater viability in hepatocytes from TG as compared to WT mice (p<0.05) at 120H (Fig. 2d). Thus, TG hepatocytes exhibit in vitro growth advantages over WT hepatocytes.
Figure 2. TG hepatocytes have a growth advantage over WT cells in culture. (*p<0.05).
(A) WT and TG hepatocytes in culture are morphologically distinct at 120H.
(B) Increased [3H] thymidine uptake by TG hepatocytes as compared to WT cells. The counts were normalized to values at 40H after culture for each group and presented as fold change.
(C) While numbers of PCNA-positive hepatocytes remained constant between 40H and 120H in WT hepatocyte culture, a net and significant gain in numbers of PCNA-positive cells was evident between 40H and 120H in the TG hepatocytes.
(D) A significant increase in cell viability as determined by MTT assay was observed in TG versus WT hepatocytes at 120 hours after culture.
β-Catenin overexpression provides a regenerative advantage to TG mice over WT after PH
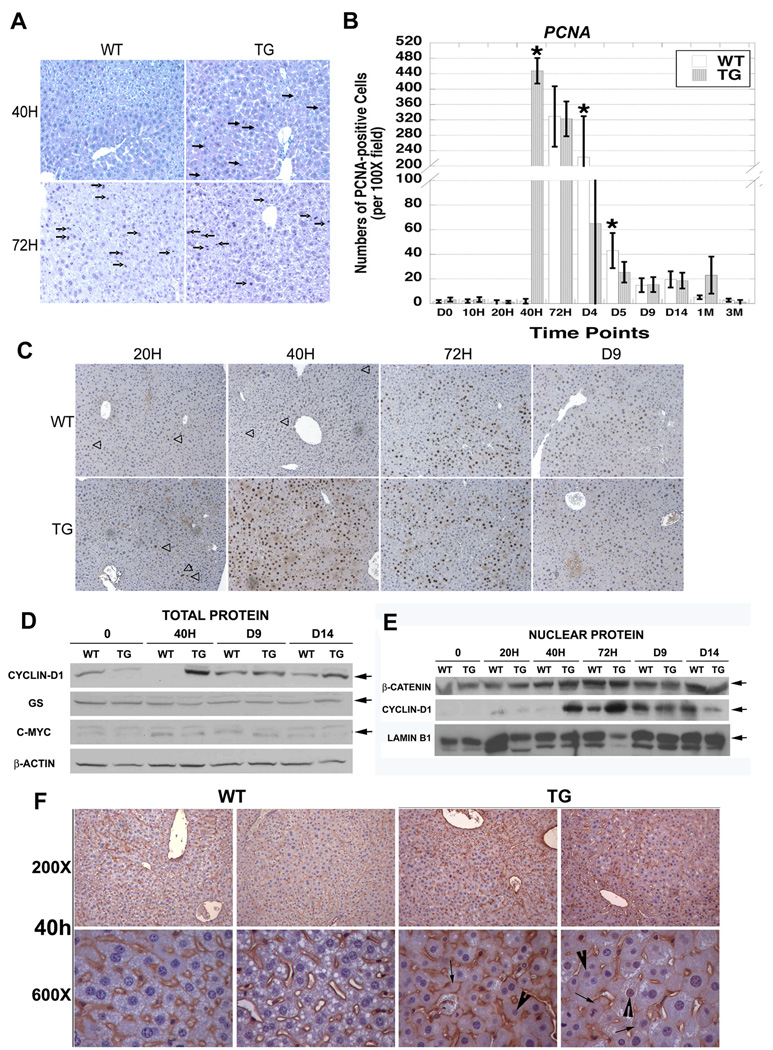
WT and TG mice were subjected to PH. Histology of WT livers at 40H exhibited less than 1 mitotic cell while TG livers showed around 9 mitotic hepatocytes per 20× field (Fig. 3a). At 72H, WT livers displayed 11–12 and TG livers around 19–20 mitotic cells per 20× field.
Figure 3. Accelerated liver regeneration in TG mice after PH. (*p<0.05).
(A) At 40H, WT livers are quiescent; however, TG livers display several cells in mitosis (arrow). Both WT and TG livers have several mitotic figures (arrow) at 72H.
(B) Quantification of the number of PCNA-positive cells in representative 10× fields (n=5) from three TG and WT livers (p<0.001).
(C) PCNA staining at 20H is low and approximately the same in both WT and TG samples; however, at 40H TG livers show increased PCNA-positive hepatocytes in S-phase, while WT livers are PCNA-negative. WT livers show several PCNA-positive hepatocytes at 72H post-PH.
(D) Increase in Cyclin-D1 in TG livers at 40H after PH is seen in whole cell extracts while levels of GS and c-myc remain unchanged. Actin is a loading control.
(E) Nuclear extracts from regenerating livers show an increase in both nuclear β-catenin and Cyclin-D1 that begins at 40H in TG and 72H in WT livers. Lamin B1 verifies equal loading.
(F) After PH, at 40H, a clear increase in cytoplasmic (arrow) and nuclear (arrowhead) staining of β-catenin is observed in the TG and not in WT livers.
IHC for PCNA was performed to address the mechanism of enhanced mitosis in TG livers after PH. A significant increase in PCNA-positive cells representing hepatocytes in S-phase was evident at 40H in TG (448 +/−33.2), and not in WT (1.9 +/− 1.9) livers (p<0.001) (Fig. 3b, c). At 72H, both WT and TG livers displayed comparable numbers of PCNA-positive hepatocytes (329 +/− 78.6 vs. 323 +/− 45.3, respectively). At D4 and D5, the numbers of PCNA-positive cells were greater in WT than TG (p<0.05). At all later times, few PCNA-positive cells were evident in WT or TG livers. Thus, β-catenin overexpression leads to earlier, robust but transient increase in hepatocyte proliferation after PH.
β-Catenin activation in TG mice imparts regenerative advantage over WT after PH
To determine the role of β-catenin in notable regenerative advantage in the TG livers, we assayed for β-catenin and its targets. An increase in nuclear β-catenin was evident at 40H in TG and 72H in both WT and TG livers (Fig. 3e) Akin to these observations, Cyclin-D1, which controls G1/S transition in cell cycle, was increased in total and nuclear lysates at 40H in TG and at 72H in WT and TG livers (Fig. 3d and 3e). Level of other β-catenin targets such as, GS and c-Myc, were unchanged (Fig. 3d). β-Catenin redistribution at 40H as assessed by IHC showed it at the hepatocyte membrane in WT, but additionally in the cytoplasm and nuclei in TG (Fig. 3f). In summary, the regenerative advantage in TG mice after PH is due to early β-catenin activation and Cyclin-D1 upregulation.
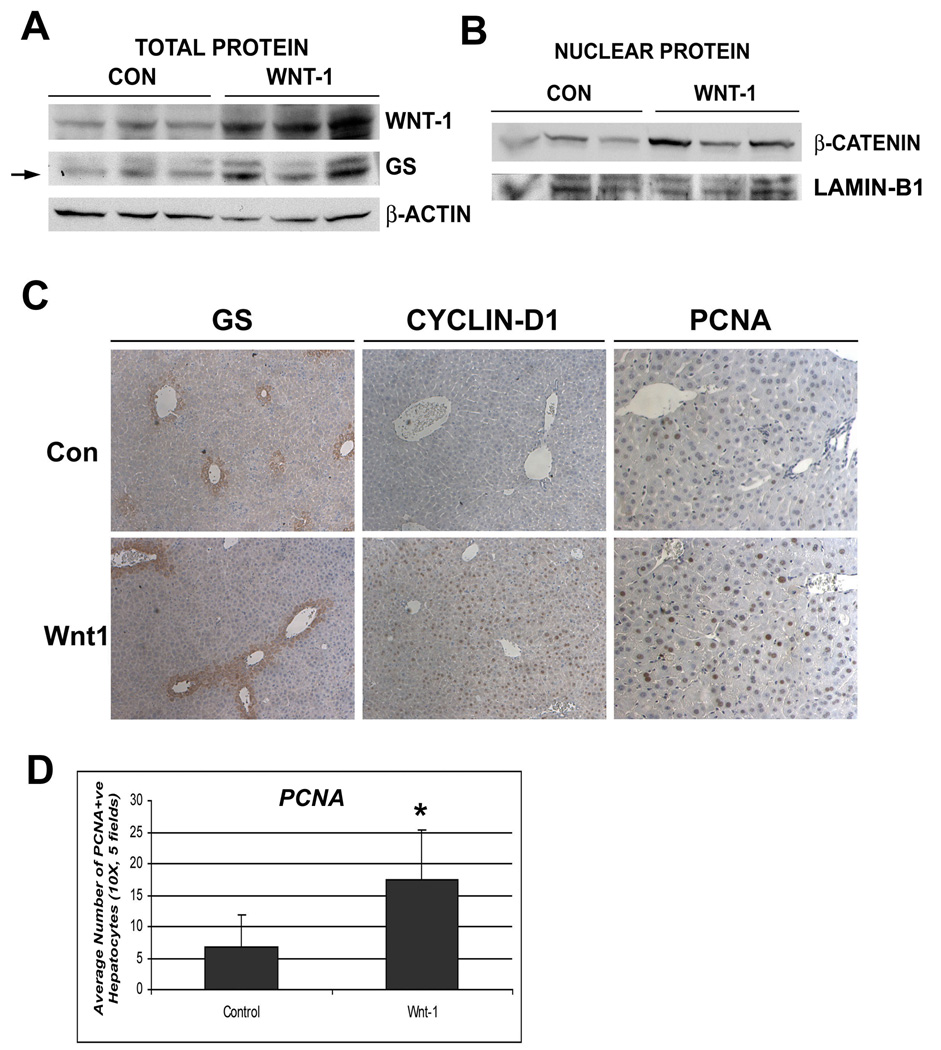
Hydrodynamic injection of Wnt-1 naked DNA induces β-catenin activation and regeneration in wild-type mice
In order to determine if temporal activation of Wnt/β-catenin signaling will stimulate proliferation during regeneration, we administered pCMV-Wnt-1 plasmid or control plasmid in control mice followed by PH and liver isolation after 30H, which precedes the expected hepatocyte proliferation after PH (17). Wnt-1 was increased at 30H after PH in Wnt-1-injected group only (Fig. 4a). A concurrent increase in nuclear β-catenin and cyclin-D1, and total GS, was observed by WB and IHC at 30H (Fig. 4a, b, c).
Figure 4. Hydrodynamic delivery of Wnt-1 plasmid and not pcDNA3 through tail vein induces Wnt/β-catenin activation in the liver. (*p<0.05).
(A) WB showing increased protein levels of Wnt-1 and GS in the Wnt-1 injected group vs. controls at 30H after PH
(B) WB shows increased nuclear β-catenin in Wnt-1 injected livers vs. pcDNA3-injected controls at 30H after PH.
(C) GS and Cyclin-D1 are increased in Wnt-1 injected animals at 30H after PH, as shown by IHC. A concomitant increase in PCNA staining is evident in the livers of Wnt-1 injected animals vs. control at 30H after PH.
(D) Quantification of the number of PCNA-positive cells in five representative 10× fields from Wnt-1 or pcDNA3-injected livers (n=3, p<0.05).
To determine the effect of Wnt-1 gene delivery on cell proliferation, we examined livers for PCNA by IHC. Almost 3-fold increase in numbers of PCNA-positive hepatocytes was evident at 30H in Wnt-injected group over controls (p<0.05) (Fig. 4c, d). Thus, these data support the positive role of Wnt-1/β-catenin activation in liver regeneration through enhancement of proliferation.
Accelerated hepatic tumorigenesis in TG mice following exposure to DEN
Age matched TG and WT mice were followed up to 12 months, but no spontaneous tumors were evident, despite the presence of mutant-β-catenin in TG livers. We next investigated the susceptibility of TG and WT mice to DEN-induced carcinogenesis as described in methods. We observed multiple liver tumors in the form of dysplastic foci, hepatic adenomas and HCC in TG mice at 6M and 9M and in WT at 9M after DEN exposure (Table 1). The preponderance and stage of tumors was clearly higher in TG livers. While only 2 out of 8 WT mice exhibited tumors with histological attributes of HCC, 70% OF TG mice showed HCC at 9 months (Table 1). Thus, overexpression of β-catenin accelerates tumorigenesis and progression to HCC following DEN exposure.
TABLE 1.
Hepatic tumors in WT and TG mice and 6 and 9 months after DEN exposure
| WT | TG | |
|---|---|---|
| 6 months post-DEN | ||
| Tumor Incidence | 0/8 (0%) | 5/5 (100%) |
| Dysplastic Foci | 0/8 (0%) | 5/5 (100%) |
| Hepatic Adenoma | 0/8 (0%) | 2/5 (40%) |
| HCC | 0/8 (0%) | 3/5 (60%) |
| 9 months post-DEN | ||
| Tumor Incidence | 4/8 (50%) | 6/7 (86%) |
| Dysplastic Foci | 3/8 (38%) | 4/7 (57%) |
| Hepatic Adenoma | 4/8 (50%) | 6/7 (86%) |
| HCC | 2/8 (25%) | 5/7 (71%) |
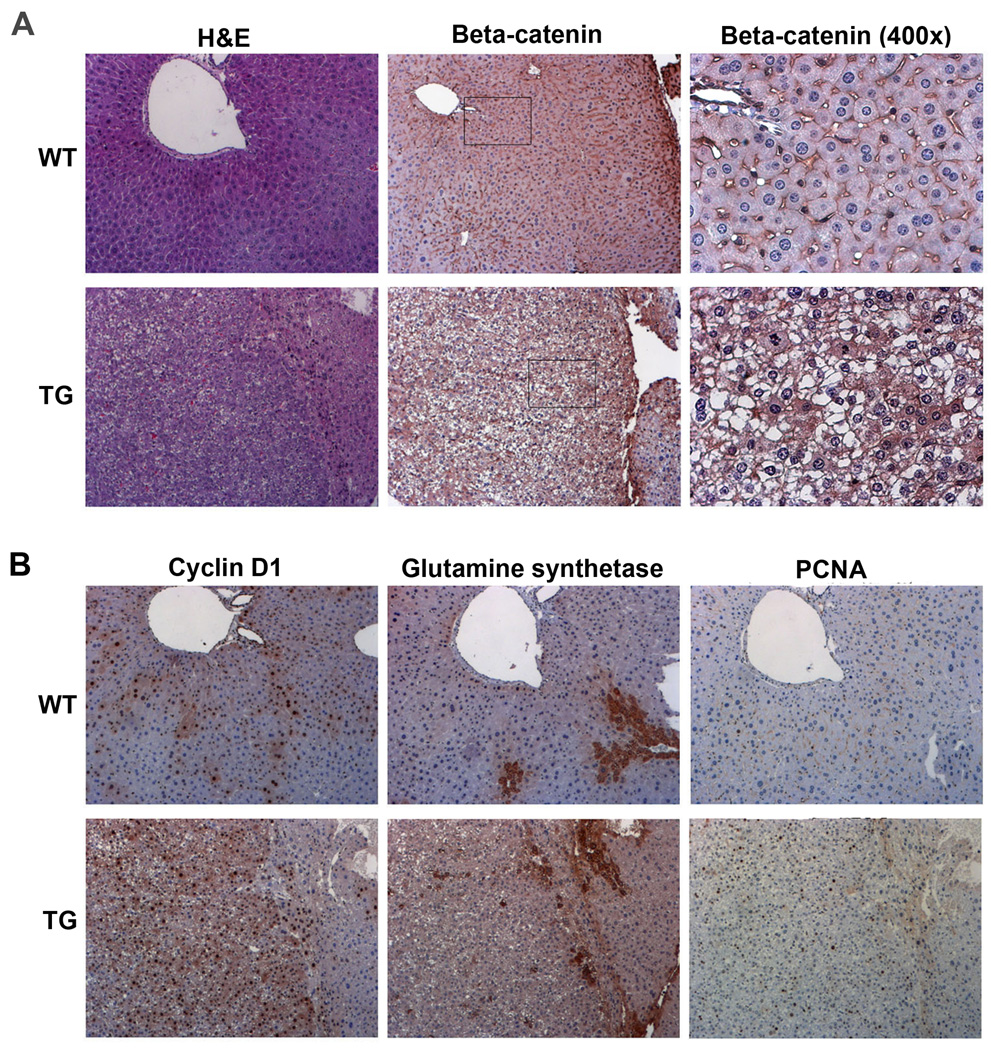
Tumors in TG mice show β-catenin activation
To examine state of Wnt signaling in TG liver tumors, we performed IHC for β-catenin and downstream targets of the pathway. Cytoplasmic and nuclear localization of β-catenin was observed in tumors in TG mice at 6 and 9 months after DEN exposure (Fig. 5a and 6a). This coincided with increased cyclin-D1 in TG tumors (Fig. 5b and 6b). Interestingly, GS was not dramatically different in the two groups at anytime. TG tumors were also notably PCNA-positive (Fig. 5b and 6b). As mentioned previously, WT livers show no tumors at 6 months after DEN exposure but at 9 months 50% of WT bear hepatic tumors (Table 1). Most tumors observed exhibited membranous β-catenin and a few cyclin-D1-positive cells (Fig. 6a and 6b). However minority of tumors in WT do exhibit nuclear/cytoplasmic β-catenin and many cyclin-D1- and PCNA-positive cells, although GS was unremarkable (Fig. 6c). Thus, at 9 months following DEN exposure, TG mice continue to show predominant hepatic tumors with β-catenin activation, while WT livers exhibit admix of β-catenin-active and inactive tumors.
Figure 5. Activation of Wnt pathway in TG tumors at 6 months after exposure to DEN. (All images at 100×, unless indicated).
(A) H&E staining shows tumor formation in TG and not WT at 6 months after exposure to DEN. Consecutive sections show IHC for β-catenin, seen localizing to cytoplasm and nuclei in TG tumors while mostly membranous localization is evident in WT.
(B) IHC for cyclin-D1, GS and PCNA in consecutive sections from TG and WT livers at 6-months after DEN-exposure. Increased cyclin-D1 is evident in TG tumor, while GS shows typical peri-central localization as compared to WT. Increase PCNA-positive cells are evident in TG tumors only.
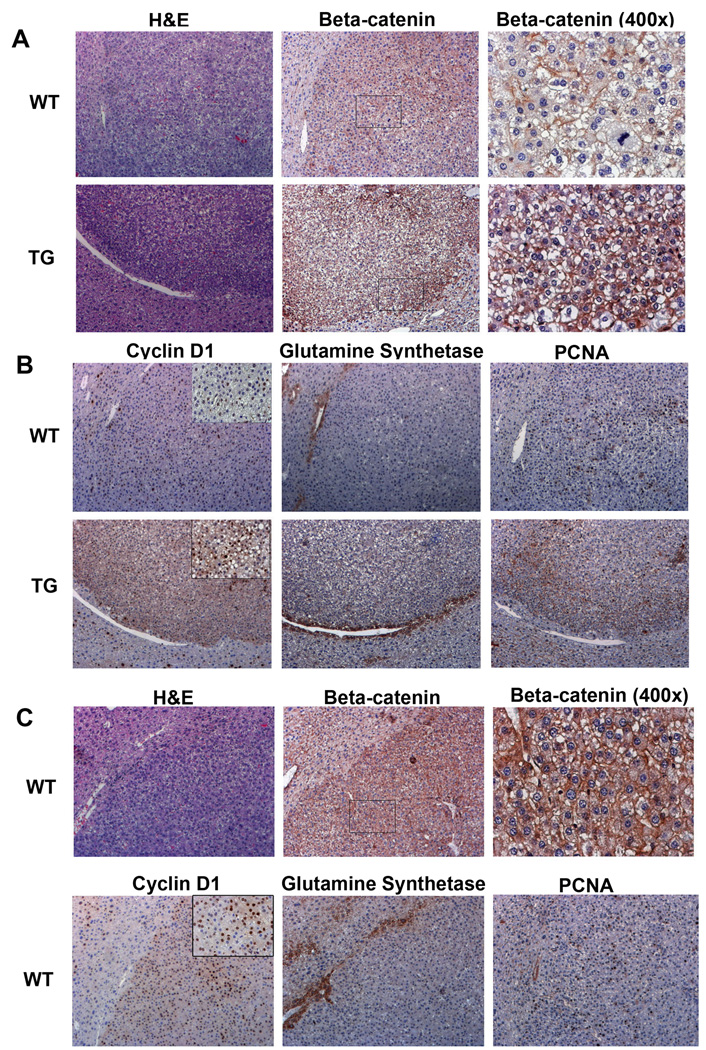
Figure 6. Activation of Wnt pathway in TG tumors at 9 months after exposure to DEN. (All images at 100×, unless indicated).
(A) H&E staining shows tumor formation in both WT and TG mouse liver 9 months after DEN exposure. Consecutive sections show IHC for β-catenin, which localizes predominantly to membrane in WT tumors and in addition in cytoplasm and nuclei in TG tumors.
(B) IHC on consecutive sections show greater numbers of cyclin-D1 and PCNA-positive cells in TG tumors as compared to WT, while GS localization continued to be pericentral and unaltered in tumors. Inset for cyclin-D1 is at 400× magnification.
(C)IHC for β-catenin and cyclin-D1 show activation of Wnt pathway in a minority of WT tumors as shown in a representative group. Consecutive sections show histology by H&E and nuclear/cytoplasmic β-catenin and increased numbers of cyclin-D1 and PCNA-positive cells by IHC. These tumors were negative for GS, which showed peri-central staining. Inset for cyclin-D1 is at 400× magnification.
To examine what other pathways may be involved in early tumorigenesis in TG mice, we performed gene array analysis on liver samples from WT and TG mice at 6 months after DEN exposure (Table 2). We report several select genes that were differentially up or down regulated in the TG livers when compared to WT liver and might further our understanding of the mechanisms by which β-catenin might be inducing tumorigenesis.
TABLE 2.
List of select genes that are up or down regulated in TG versus WT at 6 months after DEN-exposure.
| Upregulated Genes | Fold Change |
Downregulated Genes | Fold Change |
|---|---|---|---|
| Lipocalin 2 | 12.47 | Carbonic Anhydrase 3 | 0.11 |
| Calgranulin A | 5.18 | ATP5b | 0.16 |
| Cyp2b13 | 4.30 | Apolipoprotein M | 0.17 |
| Igfbp5 | 3.47 | Slc10a1 | 0.23 |
| Calgranulin B | 3.21 | Slc25a11 | 0.24 |
| FGF22 | 2.97 | Syndecan1 | 0.24 |
| Foxp1 | 2.92 | IGFBP4 | 0.25 |
| Sfrp1 | 2.90 | FABP5 | 0.26 |
| Calcyclin | 2.76 | FGF1 | 0.28 |
| Metallothionen 1 | 2.65 | Thioredoxin 2 | 0.28 |
| Jun oncogene | 2.60 | Transferrin | 0.32 |
| Metallothionien 2 | 2.53 | Cdc42 | 0.33 |
| Gro1oncogene | 2.53 | Cyclin I | 0.35 |
| Peroxiredoxin 1 | 2.39 | Hsp68 | 0.36 |
| Cyp2b9 | 2.22 | RhoA | 0.38 |
| Ribosomal protein L3 | 2.21 | Jak1 | 0.42 |
| Vimentin | 2.21 | Cathepsin H | 0.43 |
| Serum amyloid A2 | 2.19 | Hsp60 | 0.43 |
| PPAR gamma coactivator- 1beta protein |
2.17 | EIF4e | 0.44 |
| S100 calcium binding protein A10 |
2.14 | LKB1 | 0.51 |
DISCUSSION
β-Catenin is the crucial downstream effector of canonical Wnt pathway, and its activation is essential in liver development and regeneration (1). Mutations in CTNNB1 that render its protein stable, such as mutations affecting serine-45, have been implicated commonly in HCC (18). Both non-phosphorylatable and phospho-mimetic mutations affecting serine-45 along with others in exon-3 have been shown to retard degradation of β-catenin protein (11). It is relevant to note that none of the mutations affecting serine-45 alone (S45D, S45A or S45F) sufficiently enhanced β-catenin activation. We generated hepatocyte-specific TG mice overexpressing S45D-β-catenin mutant and characterized them for hepatocyte proliferation, liver regeneration and hepatocarcinogenesis.
It was intriguing to note a lack of any overt phenotype or any detectable abnormalities in metabolism, zonation or persistent proliferation in TG mice overexpressing S45D-β-catenin. The modest increase in LW/BW ratio at 1-month was transient and secondary to increased cyclin-D1 expression and proliferation. By 2-months, the TG livers had adapted and successfully monitored excessive β-catenin by sequestering it at the hepatocyte membrane, which was observed in complex with E-cadherin. This unique means of turning “off” β-catenin signaling is under investigation and identification of molecules, which promote such sequestration, will have novel therapeutic implications in the treatment of β-catenin-active tumors in the liver and elsewhere. Indeed, membranous localization of dephosphorylated β-catenin in response to Wnt signaling has been suggested as an alternate output of Wnt signaling (19).
Despite successful sequestration of β-catenin at the hepatocyte membrane TG hepatocytes show a growth advantage both in vitro and in vivo. This indicates that when suitable signals are provided, the excess β-catenin from its membranous pool is available for transactivation of target genes. Indeed in primary cultures, the growth advantage of TG hepatocytes is due to increased availability of β-catenin from the membrane due to presence of growth factors such as HGF and EGF in the culture media (20). Similar growth advantages of TG hepatocytes were also visible in vivo after PH.
The positive role of β-catenin in liver regeneration has been identified in rats, mice, zebrafish and humans (4, 6, 7, 21, 22). We identify enhanced regeneration after PH in TG mice, where hepatocyte proliferation was earlier and robust, albeit the regeneration process was completed unequivocally and without any adverse consequences. The regeneration advantage was due to enhanced cyclin-D1 expression, which is necessary for G1 to S phase transition (23). While cyclin-D1 as the target of β-catenin signaling is known for quite sometime (24, 25), its true relationship to β-catenin in the liver has been controversial with precedence given to targets such as GS (26). However, several studies support a key role of β-catenin in regulating cyclin-D1 in the liver especially during proliferation. In prenatal and postnatal liver development cyclin-D1 levels closely follow β-catenin activity in normal and β-catenin-conditional knockouts (27, 28). Similarly, β-catenin regulates cyclin-D1 expression during regeneration after sublethal doses of acetaminophen (21). Conditional hepatic disruption of β-catenin compromises cyclin-D1 expression during liver regeneration (5, 7). Intriguingly, TOPGAL reporter mice failed to elicit β-catenin activation following hepatectomy (5). It is conceivable that the unknown site of integration for the transgene in TOPGAL mice may be subject to developmental regulation keeping it permissive during development and rendering it non-permissive in adults. Thus, it is of relevance to emphasize that genetic targets of β-catenin signaling are tissue-and context–dependent and while cyclin-D1 may be subject to Wnt-independent regulation, β-catenin is a chief regulator of cyclin-D1 and, in turn, of cell proliferation in liver.
Our data also suggests that there may be an opportunity to stimulate regeneration from a therapeutic standpoint, through Wnt/β-catenin activation. Transfection of Wnt-1 plasmid was previously shown to induce nuclear localization of β-catenin in NIH3T3 and mammary epithelial cells (29, 30). Rat fibroblasts infected with Wnt-1 adenovirus or retrovirus showed proliferation in absence of serum, which was mediated in part by β-catenin activation (31, 32). In our study, hydrodynamic delivery of Wnt-1 naked DNA led to β-catenin activation, increased cyclin-D1 expression and high hepatocyte proliferation after PH. Thus, our report for the first time provides evidence that exogenous canonical Wnt activation might be useful to augment liver regeneration.
β-Catenin is implicated in development of clinical and preclinical liver tumors due to mutations in CTNNB1 or its regulators, which are evident in subset of hepatic adenomas, HCC, hepatoblastoma, and hepatic tumors in DEN-exposed mice (33). Intriguingly, no study to date has shown spontaneous HCC in TG mice overexpressing wild-type or constitutively active β-catenin (13, 34, 35). Interestingly the animal model that succumbs to spontaneous HCC is the conditional APC deletion, however APC deletions are not reported in HCC patients (36, 37). APC has since been shown to have direct DNA-binding functions through which it inhibits cell proliferation (38). Similarly, deletion of exon-3, while not reported in HCC, when used to generate transgenic mice, lacked spontaneous tumorigenesis, however, when combined with H-ras mutation, induced hepatic tumors (35, 39). Akin to these observations, spontaneous HCC was undetectable in our TG mice. In light of the observed membranous sequestration of β-catenin, the phenotype or lack thereof, in TG mice is not surprising. Nonetheless, as observed in primary cultures or after PH, DEN exposure also accelerated tumorigenesis in TG mice. Thus, it appears that mutation in CTNNB1 alone is insufficient to cause spontaneous HCC and suggests the requirement of a “second hit”, such as chemical induction in tumorigenesis.
The tumors in TG livers showed nuclear β-catenin, cyclin-D1 and increased proliferation, while GS was mostly unaltered. It was recently reported that hepatoblastomas harboring β-catenin mutations could be segregated into two subsets (8). A more mature subset of hepatoblastoma showed upregulation of target genes, which are commonly associated with zonality, i.e. GS. A second subset showed upregulation of progenitor-associated targets, such as c-myc and hepatoblast marker, α-fetoprotein. It will also be interesting to explore the possibility of HCC harboring CTNNB1 mutations to demonstrate heterogeneity in target gene expression based on extent of differentiation. It should be noted that heterogeneity in target gene expression based on mechanism of β-catenin activation is already evident in HCC (40, 41).
How β-catenin activation induces HCC remains elusive. The current model might allow elucidation of such mechanisms. We utilized gene array approach to compare genetic changes in livers of DEN-exposed 6M-old TG and WT mice. Several genes associated with HCC were upregulated, including insulin-like growth factor binding protein 5 (IGFBP5) and JUN oncogene (42, 43), and both have been shown to be regulated by β-catenin (44, 45). (53). We also observed downregulation of various genes of relevance in tumorigenesis. LKB1 expression was downregulated and its loss in exon-3-deleted β-catenin overexpressing mice, accelerates HCC development (46). Cyclin I expression was decreased and is a negative cell cycle regulator (47). Additional studies will be necessary to validate these findings as a mechanism of β-catenin driven hepatocarcinogenesis.
Supplementary Material
ACKNOWLEDGEMENT
The authors would like to acknowledge Dr. Tong Wu (T.W.) for his assistance with the histology of hepatic tumors in our preclinical study.
Grant Support: This study was funded by NIH grants 1R01DK62277 and 1R01CA124414 to SPSM, NIH grant 1F30DK083235 to MDT, and by Rango’s Fund for the Enhancement of Pathology Research.
Abbreviations
- TG
transgenic
- WT
wild-type
- PH
partial hepatectomy
- HGF
hepatocyte growth factor
- EGF
epidermal growth factor
- PCNA
proliferating cell nuclear antigen
- IOD
integrated optical density
- GS
glutamine synthetase
- HCC
hepatocellular cancer
REFERENCES
- 1.Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45:1298–1305. doi: 10.1002/hep.21651. [DOI] [PubMed] [Google Scholar]
- 2.Benhamouche S, Decaens T, Godard C, Chambrey R, Rickman DS, Moinard C, Vasseur-Cognet M, et al. Apc tumor suppressor gene is the "zonation-keeper" of mouse liver. Dev Cell. 2006;10:759–770. doi: 10.1016/j.devcel.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Orsulic S, Huber O, Aberle H, Arnold S, Kemler R. E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J Cell Sci. 1999;112(Pt 8):1237–1245. doi: 10.1242/jcs.112.8.1237. [DOI] [PubMed] [Google Scholar]
- 4.Monga SP, Pediaditakis P, Mule K, Stolz DB, Michalopoulos GK. Changes in WNT/beta-catenin pathway during regulated growth in rat liver regeneration. Hepatology. 2001;33:1098–1109. doi: 10.1053/jhep.2001.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sekine S, Gutierrez PJ, Lan BY, Feng S, Hebrok M. Liver-specific loss of beta-catenin results in delayed hepatocyte proliferation after partial hepatectomy. Hepatology. 2007;45:361–368. doi: 10.1002/hep.21523. [DOI] [PubMed] [Google Scholar]
- 6.Sodhi D, Micsenyi A, Bowen WC, Monga DK, Talavera JC, Monga SP. Morpholino oligonucleotide-triggered beta-catenin knockdown compromises normal liver regeneration. J Hepatol. 2005;43:132–141. doi: 10.1016/j.jhep.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 8.Cairo S, Armengol C, De Reynies A, Wei Y, Thomas E, Renard CA, Goga A, et al. Hepatic stem-like phenotype and interplay of Wnt/beta-catenin and Myc signaling in aggressive childhood liver cancer. Cancer Cell. 2008;14:471–484. doi: 10.1016/j.ccr.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 9.de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci U S A. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 11.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. Embo J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng G, Apte U, Cieply B, Singh S, Monga SP. siRNA-mediated beta-catenin knockdown in human hepatoma cells results in decreased growth and survival. Neoplasia. 2007;9:951–959. doi: 10.1593/neo.07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan X, Apte U, Micsenyi A, Kotsagrelos E, Luo JH, Ranganathan S, Monga DK, et al. Epidermal growth factor receptor: a novel target of the Wnt/beta-catenin pathway in liver. Gastroenterology. 2005;129:285–302. doi: 10.1053/j.gastro.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ku NO, Michie S, Resurreccion EZ, Broome RL, Omary MB. Keratin binding to 14-3-3 proteins modulates keratin filaments and hepatocyte mitotic progression. Proc Natl Acad Sci U S A. 2002;99:4373–4378. doi: 10.1073/pnas.072624299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nejak-Bowen KN, Zeng G, Tan X, Cieply B, Monga SP. Beta-catenin regulates vitamin C biosynthesis and cell survival in murine liver. J Biol Chem. 2009;284:28115–28127. doi: 10.1074/jbc.M109.047258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, Chen S, Huang L, Michalopoulos GK, Liu Y. Sustained expression of naked plasmid DNA encoding hepatocyte growth factor in mice promotes liver and overall body growth. Hepatology. 2001;33:848–859. doi: 10.1053/jhep.2001.23438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Quail E, Hung NJ, Tan Y, Ye H, Costa RH. Increased levels of forkhead box M1B transcription factor in transgenic mouse hepatocytes prevent age-related proliferation defects in regenerating liver. Proc Natl Acad Sci U S A. 2001;98:11468–11473. doi: 10.1073/pnas.201360898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Legoix P, Bluteau O, Bayer J, Perret C, Balabaud C, Belghiti J, Franco D, et al. Beta-catenin mutations in hepatocellular carcinoma correlate with a low rate of loss of heterozygosity. Oncogene. 1999;18:4044–4046. doi: 10.1038/sj.onc.1202800. [DOI] [PubMed] [Google Scholar]
- 19.Hendriksen J, Jansen M, Brown CM, van der Velde H, van Ham M, Galjart N, Offerhaus GJ, et al. Plasma membrane recruitment of dephosphorylated beta-catenin upon activation of the Wnt pathway. J Cell Sci. 2008;121:1793–1802. doi: 10.1242/jcs.025536. [DOI] [PubMed] [Google Scholar]
- 20.Block GD, Locker J, Bowen WC, Petersen BE, Katyal S, Strom SC, Riley T, et al. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J Cell Biol. 1996;132:1133–1149. doi: 10.1083/jcb.132.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apte U, Singh S, Zeng G, Cieply B, Virji MA, Wu T, Monga SP. Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. Am J Pathol. 2009 175;:1056–1065. doi: 10.2353/ajpath.2009.080976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goessling W, North TE, Lord AM, Ceol C, Lee S, Weidinger G, Bourque C, et al. APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development. Dev Biol. 2008;320:161–174. doi: 10.1016/j.ydbio.2008.05.526. [DOI] [PubMed] [Google Scholar]
- 23.Albrecht JH, Rieland BM, Nelsen CJ, Ahonen CL. Regulation of G(1) cyclin-dependent kinases in the liver: role of nuclear localization and p27 sequestration. Am J Physiol. 1999;277:G1207–G1216. doi: 10.1152/ajpgi.1999.277.6.G1207. [DOI] [PubMed] [Google Scholar]
- 24.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 26.Bioulac-Sage P, Rebouissou S, Thomas C, Blanc JF, Saric J, Sa Cunha A, Rullier A, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46:740–748. doi: 10.1002/hep.21743. [DOI] [PubMed] [Google Scholar]
- 27.Apte U, Zeng G, Thompson MD, Muller P, Micsenyi A, Cieply B, Kaestner KH, et al. beta-Catenin is critical for early postnatal liver growth. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1578–G1585. doi: 10.1152/ajpgi.00359.2006. [DOI] [PubMed] [Google Scholar]
- 28.Tan X, Yuan Y, Zeng G, Apte U, Thompson MD, Cieply B, Stolz DB, et al. Beta-catenin deletion in hepatoblasts disrupts hepatic morphogenesis and survival during mouse development. Hepatology. 2008;47:1667–1679. doi: 10.1002/hep.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu SC, Galceran J, Grosschedl R. Modulation of transcriptional regulation by LEF-1 in response to Wnt-1 signaling and association with beta-catenin. Mol Cell Biol. 1998;18:4807–4818. doi: 10.1128/mcb.18.8.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papkoff J, Aikawa M. WNT-1 and HGF regulate GSK3 beta activity and beta-catenin signaling in mammary epithelial cells. Biochem Biophys Res Commun. 1998;247:851–858. doi: 10.1006/bbrc.1998.8888. [DOI] [PubMed] [Google Scholar]
- 31.Young CS, Kitamura M, Hardy S, Kitajewski J. Wnt-1 induces growth, cytosolic beta-catenin, and Tcf/Lef transcriptional activation in Rat-1 fibroblasts. Mol Cell Biol. 1998;18:2474–2485. doi: 10.1128/mcb.18.5.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young CS, Masckauchan TN, Kitajewski J. Beta-catenin/Tcf activation partially mimics the transforming activity of Wnt-1 in Rat-1 fibroblasts. Differentiation. 2003;71:477–485. doi: 10.1046/j.1432-0436.2003.7108002.x. [DOI] [PubMed] [Google Scholar]
- 33.Monga SP. Role of Wnt/beta-catenin signaling in liver metabolism and cancer. Int J Biochem Cell Biol. 2009 doi: 10.1016/j.biocel.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cadoret A, Ovejero C, Saadi-Kheddouci S, Souil E, Fabre M, Romagnolo B, Kahn A, et al. Hepatomegaly in transgenic mice expressing an oncogenic form of beta-catenin. Cancer Res. 2001;61:3245–3249. [PubMed] [Google Scholar]
- 35.Harada N, Miyoshi H, Murai N, Oshima H, Tamai Y, Oshima M, Taketo MM. Lack of tumorigenesis in the mouse liver after adenovirus-mediated expression of a dominant stable mutant of beta-catenin. Cancer Res. 2002;62:1971–1977. [PubMed] [Google Scholar]
- 36.Colnot S, Decaens T, Niwa-Kawakita M, Godard C, Hamard G, Kahn A, Giovannini M, et al. Liver-targeted disruption of Apc in mice activates beta-catenin signaling and leads to hepatocellular carcinomas. Proc Natl Acad Sci U S A. 2004;101:17216–17221. doi: 10.1073/pnas.0404761101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haramis AP, Hurlstone A, van der Velden Y, Begthel H, van den Born M, Offerhaus GJ, Clevers HC. Adenomatous polyposis coli-deficient zebrafish are susceptible to digestive tract neoplasia. EMBO Rep. 2006;7:444–449. doi: 10.1038/sj.embor.7400638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qian J, Sarnaik AA, Bonney TM, Keirsey J, Combs KA, Steigerwald K, Acharya S, et al. The APC tumor suppressor inhibits DNA replication by directly binding to DNA via its carboxyl terminus. Gastroenterology. 2008;135:152–162. doi: 10.1053/j.gastro.2008.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harada N, Oshima H, Katoh M, Tamai Y, Oshima M, Taketo MM. Hepatocarcinogenesis in mice with beta-catenin and Ha-ras gene mutations. Cancer Res. 2004;64:48–54. doi: 10.1158/0008-5472.can-03-2123. [DOI] [PubMed] [Google Scholar]
- 40.Cieply B, Zeng G, Proverbs-Singh T, Geller DA, Monga SP. Unique phenotype of hepatocellular cancers with exon-3 mutations in beta-catenin gene. Hepatology. 2009;49:821–831. doi: 10.1002/hep.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoshida Y, Nijman SM, Kobayashi M, Chan JA, Brunet JP, Chiang DY, Villanueva A, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69:7385–7392. doi: 10.1158/0008-5472.CAN-09-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eferl R, Ricci R, Kenner L, Zenz R, David JP, Rath M, Wagner EF. Liver tumor development. c-Jun antagonizes the proapoptotic activity of p53. Cell. 2003;112:181–192. doi: 10.1016/s0092-8674(03)00042-4. [DOI] [PubMed] [Google Scholar]
- 43.Umemura A, Itoh Y, Itoh K, Yamaguchi K, Nakajima T, Higashitsuji H, Onoue H, et al. Association of gankyrin protein expression with early clinical stages and insulin-like growth factor-binding protein 5 expression in human hepatocellular carcinoma. Hepatology. 2008;47:493–502. doi: 10.1002/hep.22027. [DOI] [PubMed] [Google Scholar]
- 44.Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, Bodmer WF, et al. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci U S A. 1999;96:1603–1608. doi: 10.1073/pnas.96.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reichling T, Goss KH, Carson DJ, Holdcraft RW, Ley-Ebert C, Witte D, Aronow BJ, et al. Transcriptional profiles of intestinal tumors in Apc(Min) mice are unique from those of embryonic intestine and identify novel gene targets dysregulated in human colorectal tumors. Cancer Res. 2005;65:166–176. [PubMed] [Google Scholar]
- 46.Miyoshi H, Deguchi A, Nakau M, Kojima Y, Mori A, Oshima M, Aoki M, et al. Hepatocellular carcinoma development induced by conditional beta-catenin activation in Lkb1 mice. Cancer Sci. 2009 doi: 10.1111/j.1349-7006.2009.01284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Tang MK, Cai DQ, Li M, Wong WM, Chow PH, Lee KK. Cyclin I and p53 are differentially expressed during the terminal differentiation of the postnatal mouse heart. Proteomics. 2007;7:23–32. doi: 10.1002/pmic.200600456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.