Abstract
BACKGROUND
An inability to inhibit behaviors once they become maladaptive is a component of several psychiatric illnesses and the medial prefrontal cortex (mPFC) was identified as a potential mediator of behavioral inhibition. The current study tested if the mPFC is involved in inhibition of sexual behavior when associated with aversive outcomes.
METHODS
Using male rats, effects of lesions of the infralimbic (IL) and prelimbic (PL) areas of the mPFC on expression of sexual behavior and ability to inhibit mating were tested using a paradigm of copulation-contingent aversion.
RESULTS
mPFC lesions did not alter expression of sexual behavior. In contrast, mPFC lesions completely blocked the acquisition of sex-aversion conditioning and lesioned animals continued to mate, in contrast to the robust behavioral inhibition towards copulation in mPFC intact males, resulting in only 22% of intact males continuing to mate. However, rats with mPFC lesions were capable of forming a conditioned place preference to sexual reward and conditioned place aversion for lithium chloride, suggesting that these lesions did not alter associative learning or sensitivity for lithium chloride.
DISCUSSION
The current study indicates that animals with mPFC lesions are likely capable of forming the associations with aversive outcomes of their behavior, but lack the ability to suppress seeking of sexual reward in the face of aversive consequences. These data may contribute to a better understanding of a common pathology underlying impulse control disorders as compulsive sexual behavior has a high prevalence of comorbidity with psychiatric disorders and Parkinson’s Disease.
Keywords: Prefrontal Cortex, Inhibition, Mating, Conditioned, Reward, Aversion, Addiction, Sex
INTRODUCTION
The medial prefrontal cortex (mPFC) is involved in many higher order functions of the mammalian nervous system including the regulation of emotional arousal, anxiety-like behaviors, as well as behavioral flexibility and decision making (1–5). Reward-based decision making is thought to be controlled by a neuronal circuit comprised of the mPFC, amygdala, and striatum (6) in which the mPFC acts as a “top-down” controller of this process (7,8). A central feature of reward-based decision making is the ability to track “response-outcome” relationships over time (9). In this way, when consequences associated with a behavioral action become unfavorable, the frequency of these actions decreases. This results in a positive behavioral adaptation, and this response is dependent on intact mPFC function (8, 10). An inability to alter behavioral actions once they lead to adverse consequence is a symptom common to a variety of addictive disorders (11–15).
Rodent male sexual behavior is a natural reward-based behavior in which response-outcome relationships are monitored to achieve the goal of copulation (16). However, male rats abstain from copulating when sexual behavior is paired with the aversive stimulus lithium chloride (LiCl; 17, 18). mPFC activity has been correlated with male sexual behavior in rodents (19–25) and humans (26). However, the exact role of the mPFC in sexual behavior remains unclear. The goal of the present study was to characterize the effects of mPFC lesions on the expression of sexual behavior, and on the acquisition of behavioral inhibition towards sexual behavior in rats using a model of copulation-contingent aversion. Lesions included the infralimbic (IL) and prelimbic (PL) nuclei of the mPFC, as these subregions have been shown to project to brain areas involved in the regulation of sexual behavior (20). Results from this study show that intact mPFC function is not required for normal expression of sexual behavior. Instead, the results support the hypothesis that mPFC regulates the execution of behavioral inhibition towards sexual behavior once this behavior is associated with aversive outcomes.
MATERIALS AND METHODS
Animals
Adult male (250–260 grams) Sprague Dawley rats obtained from Harlan labs (Indianapolis) were housed individually in an artificially lighted room on a reversed light/dark cycle (12:12 h, lights off at 10 AM) at a temperature of 72°F. Food and water were available at all times. Ovariectomized, estrogen (s.c. silastic capsule with 5% 17-beta-estradiol benzoate) and progesterone (s.c. injection 500 μg in 0.1 ml of sesame oil) primed female Sprague Dawley rats (210–225 grams) were used in all mating tests, which began four hours after the onset of the dark period and behavior was conducted in a rectangular Plexiglas test cage (60×45×50 cm) under dim red illumination. All procedures were approved by the Animal Care and Use Committee of the University of Cincinnati, University of Western Ontario Animal Care Committee, and conformed to NIH and CCAC guidelines involving vertebrate animals in research.
Lesion Surgery
Animals were anesthetized with a 1-ml/kg dose (87 mg/kg Ketamine and 13 mg/kg Xylazine). Animals were placed in a stereotaxic apparatus (Kopf instruments, Tujunga, CA USA), an incision was made to expose the skull, and holes were drilled above the injection sites using a dremmel drill (Dremmel, USA). Ibotenic acid (0.25μl, 2% in PBS) was infused bilaterally using two injections at different dorsoventral coordinates, each over a 1.5 minute period using a 5μl Hamilton syringe at the following coordinates relative to Bregma (with skull leveled horizontally): For PL and IL lesions: AP= 2.9, ML= 0.6, DV= −5.0 and −2.5. Sham lesions were performed using the same methods, but using vehicle (PBS) injections. All animals were allowed to recover for 7–10 days prior to behavioral testing.
Design
Expression of sexual behavior
PL and IL lesions were performed in animals that were sexually naïve prior to surgery. After recovery, the animals were allowed to mate once a week until display of one ejaculation, for a total of four consecutive weeks following surgery. Differences in sexual parameters (i.e. latencies to mount, intromission, ejaculation, and numbers of mounts and intromissions) within each experiment were analyzed using a one-way ANOVA with lesion surgery as a factor. Post hoc comparisons were conducted using Fishers PLSD tests, all with 5% significance levels.
Elevated Plus Maze Experiments
Animals with lesions or sham treatment were tested on the elevated plus maze (EPM). This test was performed five weeks following surgery and one week following the last mating session. The EPM was made out of clear Plexiglas and consisted of four arms of equal length extending from a center arena that formed the shape of a plus sign. Two arms of the maze were open to the external environment and the other two arms of the maze were enclosed by dark sidings (40cm high) that stretched along the entire length of the arm. Borders between the middle area and the arms were defined by white stripes on the arms located 12cm from the middle of the maze. EPM tests were conducted under dim illumination, 1–4 hours after onset of the dark period. Differences between sham and lesioned animals were determined using student t-tests with 5% significance level.
Conditioned Sex Aversion
Male rats were subjected to three mating sessions to gain sexual experience prior to lesion or sham surgery. Animals that displayed an ejaculation during at least two out of three pre-surgery mating tests were included in this study and randomly divided over four experimental groups: Sham-LiCl, Lesion-LiCl, Sham-Saline, and Lesion-Saline. Lesion or sham surgeries were performed 3 days after the last training session. Animals were allowed to recover for one week after the surgeries before conditioning sessions began. During the conditioning sessions, half of the sham and lesioned males received LiCl immediately following mating (Sham-LiCl and Lesion-LiCl), while the other half of the sham and lesioned males served as controls and received saline immediately following mating (Sham-Saline and Lesion-Saline). On conditioning day 1, animals were allowed to mate to one ejaculation and were injected within one minute following ejaculation with a 20ml/kg dose of either 0.15M LiCl or saline and then placed back into their home cages. In the morning on conditioning day 2, all males were weighed and saline conditioned animals were given a 20ml/kg dose of 0.15M LiCl, while LiCl conditioned animals were injected with an equivalent dose of saline. This paradigm was repeated during twenty consecutive days totaling ten complete conditioning sessions. Parameters of sexual behavior were recorded during each trial. Differences in percentages of animals that displayed mounts and intromissions, or ejaculations were analyzed for each trial using Chi-Square analysis with a 5% significance level. Since no differences were detected between Sham-Saline and Lesion-Saline groups in any parameter, these two groups were combined for statistical analysis (n=9) and were compared with either the Lesion-LiCl or the Sham-LiCl group.
Conditioned Place Preference
Sexually naïve animals underwent lesion surgery as described above and were allowed to recover for one week prior to behavioral testing. All behavioral testing started 4 hours after onset of the dark period. The conditioned place preference apparatus was partitioned into three chambers with a neutral center chamber. One side of the chamber had white walls and a grid flooring, while the other side was black with stainless steel rods as flooring, the center chamber was grey with Plexiglas flooring (Med Associates, St. Albans, VT). First, a pre-test was performed to establish a natural preference for each individual before the conditioning began, all animals were placed into the center chamber with free access to all chambers for fifteen minutes and the total time spent in each chamber was recorded. On the next day, i.e. conditioning day 1, males mated to one ejaculation in their home cage upon which they were immediately placed into the initially non-preferred chamber for thirty minutes without access to the other chambers or were placed into their initially preferred chamber for thirty minutes without prior sexual behavior. On the second conditioning day, males received the opposite treatment. This conditioning paradigm was repeated once more. On the next day, a post-test was conducted that was procedurally identical to the pre-test. Two separate values were used to determine if mPFC lesioned animals formed a conditioned place preference to sex. The first score was the difference score, defined as the difference between the time spent in the initially preferred chamber and the time spent in the initially non-preferred chamber. The preference score was defined as the time spent in the initially non-preferred chamber divided by the time spent in the initially non-preferred chamber plus the time spent in the initially preferred chamber. The preference and difference scores were compared for each animal between pre-test and post test using paired student t-tests with 5% significance levels. Previous studies have demonstrated that mating results in robust conditioned place preference using this paradigm, and that control treatments do not result in changes in preference (27–29).
Conditioned Place Aversion
Sexually naïve animals underwent lesion or sham surgery as described above and were allowed to recover for one week prior to behavioral testing. All behavioral testing started 4 hours after onset of the light period. Using the CPP apparatus described above, LiCl or saline injections were paired with the initially preferred or non-preferred chamber respectively during two conditioning trials in a counter balanced manner. Pre- and post tests were conducted and data analyzed as described above using paired student t-tests with 5% significance levels.
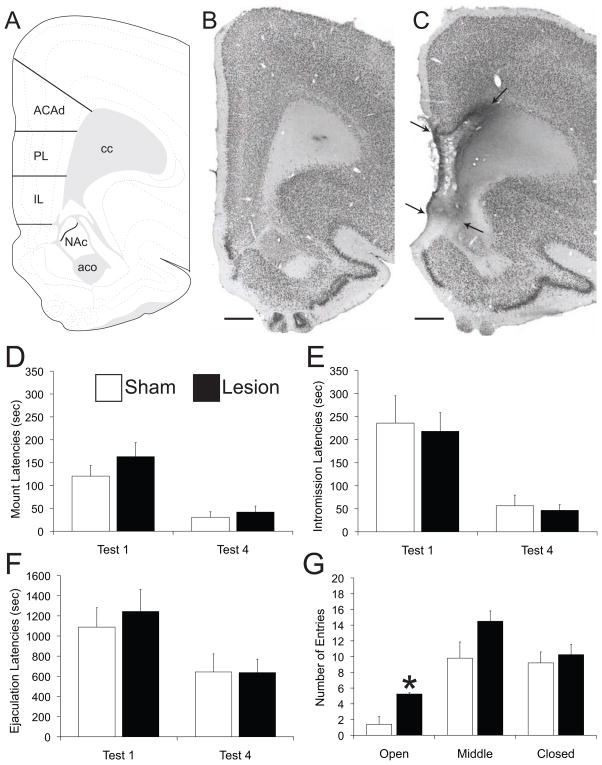
Lesion Verification
For lesion verification animals were perfused transcardially with 4% paraformaldehyde and brains were sectioned (coronally). Sections were and immunoprocessed for neuronal marker NeuN using a primary antiserum in incubation solution recognizing NeuN (monoclonal anti-NeuN antiserum; 1:10,000; Chemicon) and standard immunoperoxidase methods (19). The location and size of the ibotenic lesions was determined by analyzing the area in adjacent mPFC sections devoid of NeuN neuron staining. Lesions of the mPFC typically spanned a distance from AP +4.85 to +1.70 relative to bregma (Figure 1A–C). Lesions were considered complete if 100% of the IL and 80 % of the PL was destroyed, and only animals with complete lesions were included in statistical analyses (Sex behavior experiment, lesion n=11, sham n=12; EPM experiment, lesion n=5, sham n=4; conditioned sex aversion experiment, sham-saline n=4, sham-LiCl n=9, lesion-saline n=5, lesion-LiCl n=12; conditioned place preference experiment, lesion n=5; conditioned place aversion experiment, sham n=12, lesion n=9).
Figure 1.
A) Schematic drawing of coronal section through the mPFC illustrating the general location of all lesions (45). B–C) Images of coronal section stained for NeuN of representative sham (B) and lesion (C) animal. Arrows indicate the location of the lesion, while no damage was detected in sham animals. Scale bar indicates 600μm. IL/PL lesions did not affect latencies to mount (D), intromission (E), or ejaculation (F) in sexually naïve rats during the first mating test (test 1; p values ranged 0.27–0.81) or after sexual experience was gained (test 4; p values ranged 0.32–0.97). G) Total number of entries in open arms, closed arms, and middle portion of the elevated plus maze in sham or PL/IL lesioned male rats. * indicates significant difference between sham and lesion group (p=0.011). Abbreviations: ACAd: Anterior cingulated area, dorsal part, aco: anterior commissure, cc: corpus callosum, IL: infralimbic area, NAc: nucleus accumbens, PL: prelimbic area.
RESULTS
Sexual Behavior
PL/IL lesions did not affect any sexual parameter tested in males that were sexually naïve prior to surgery (Figure 1D–F). In agreement, no effects of PL/IL lesions on sexual behavior were detected in the sexually experienced males included in the conditioned sex aversion experiment, during the first trial, hence prior to pairing of LiCl with sexual behavior (Table 1). Hence, PL/IL lesions did not affect sexual behavior independent of sexual experience.
Table 1.
Latencies (in seconds) to mount (M), intromission (IM), and ejaculation (Ej) in sham (n=13) and PL/IL lesion males (n=16) during the first mating trial of the conditioned aversion paradigm. PL/IL lesions did not affect any parameter of sexual behavior (p-values ranged 0.51–0.93).
| M Latencies | IM Latencies | Ej Latencies | |
|---|---|---|---|
| Sham | 24.2 ± 8.3 | 47.1 ± 14.9 | 476.3 ± 54.2 |
| PL/IL Lesion | 24.8 ± 10.5 | 41.9 ± 10.0 | 467.7 ± 77.7 |
Elevated Plus Maze
In agreement with prior reports (27–29), male rats with mPFC lesions males displayed more entries into the open arms of the EPM compared to controls (Figure 1G), suggesting that mPFC function is critical for situations that require risk assessment.
Conditioned sex aversion
Effects of LiCl conditioning on sexual behavior
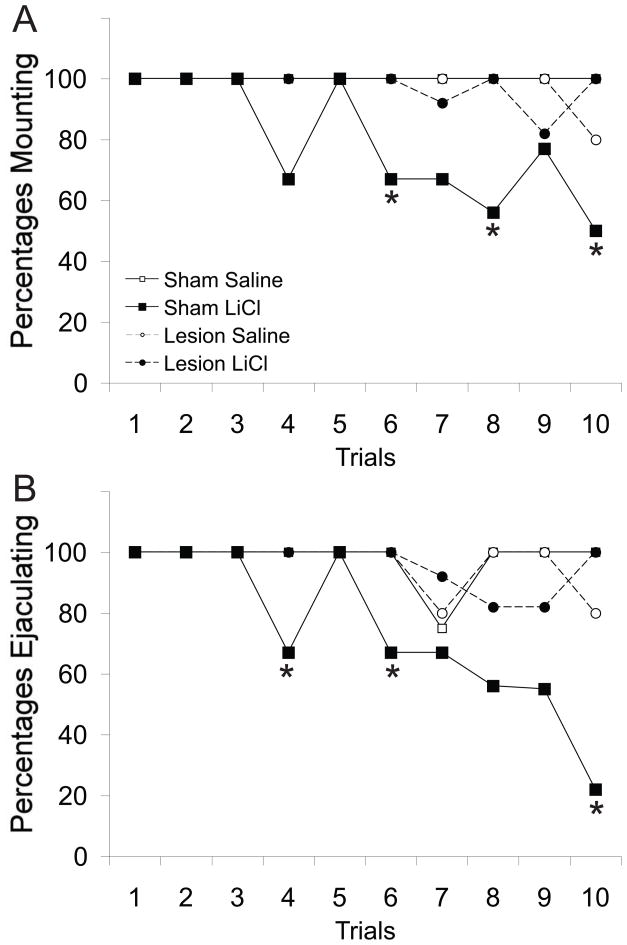
LiCl conditioning resulted in a significant reduction of the percentages of sham males that displayed mounts, intromissions, or ejaculation compared to sham saline controls (Figure 2A–B). However, mPFC lesions completely blocked the inhibition caused by LiCl conditioning. Chi-square analysis revealed significant differences between groups detected in the percentages of animals that displayed mounts (Figure 2A), intromissions (not shown; data identical to Figure 2A), or ejaculations (Figure 2B). Specifically, percentages of males that displayed mounts, intromissions, or ejaculation were significantly lower in the Sham-LiCl group compared to Saline-treated control animals (Sham and Lesion), indicating a disruptive effect of LiCl conditioning on copulation in Sham animals. In contrast, no effect of LiCl conditioning was observed in Lesion-LiCl males (Figures 2A–B). Thus, mPFC function is critical for the acquisition of conditioned inhibition of sexual behavior. However, it is possible that PL/IL lesions attenuate associative learning associated with sexual reward, thus in a separate study effects of PL/IL lesions on acquisition of a conditioned place preference for sexual reward was tested.
Figure 2.
A) Percentage of animals that displayed mounts or B) ejaculated during the copulation contingent aversion procedure expressed across all 10 trials in sham or PL/IL lesioned male rats. * indicates significant difference (p<0.05) between sham LiCl group and the saline-treated control groups. In A, trial 6: p=0.024; 8: p=0.024; and 10: p=<0.0001. In B, trial 4: p=0.024; 6: p=0.024, and 10: p<0.0001 relative to saline-treated animals. Although differences between groups in percentages of animals that displayed mounts or ejaculations during trials 7 (A), 8 (B), and 9 (B) failed to reach significance, trends towards significance were observed (p<0.092);
Conditioned Place Preference and Aversion
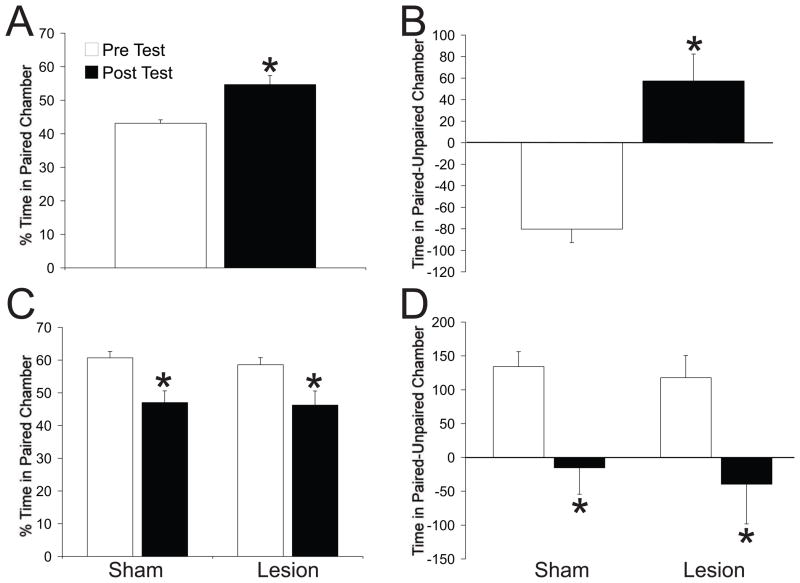
Rats with mPFC lesions displayed normal associative learning of contextual cues paired with sexual reward, as indicated by an increased difference score and preference score during the post-test (Figure 3A–B). Moreover, lesions did not affect associative learning of contextual cues with LiCl-induced malaise, indicated by significant decreases in difference and preference scores during the post test (Figure 3C–D).
Figure 3.
C) Preference score calculated as percentage of total time spent in the paired chamber during pretest and posttest in PL/IL lesioned rats. * = p=0.01 compared to pretest. D) Difference score calculated as time (seconds) in paired chamber minus time in unpaired chamber during pretest and posttest in mPFC lesioned rats. * = p=0.007 compared to pretest. E) Preference scores in sham and mPFC lesioned rats. * = p=0.006 and 0.002 resp compared to pretest. F) Difference score (seconds) in sham and mPFC lesioned rats. * = p=0.001 and 0.007 resp compared to pretest.
DISCUSSION
In this study, we report that lesions of the IL and PL regions of the mPFC do not affect the expression of sexual behavior, nor the acquisition of a conditioned place preference to sexual reward. Instead, lesions prevent the acquisition of conditioned sex-aversion. These results provide functional evidence for the hypothesis that the ability to make adaptive behavioral alterations is regulated by the IL and PL subregions of the mPFC.
Previous data from our laboratory indicated that mPFC neurons are activated during sexual behavior in male rats (20). However, the mPFC lesioned rats in this study are indistinguishable from sham control rats in any of the analyzed parameters of sexual behavior. In agreement with prior reports (30, 32) mPFC lesions did produce anxiolytic effects as assessed by performance on the elevated plus maze, indicating that our lesioning protocol was effective. Therefore, the current results suggest activation of the IL and PL subdivisions within the mPFC during sexual behavior is not necessary for normal expression of sexual behavior. In contrast, a previous study by Agmo and coworkers demonstrated that lesions of the anterior cingulate area (ACA) increased mount and intromission latencies and reduced the percentage of males that copulated (25). Therefore, it is possible that the ACA plays a role in the performance of sexual behavior, while IL and PL regions mediate inhibition of behavior once associated with aversive outcomes.
Although mPFC lesions have been reported to disrupt various forms of memory consolidation (33, 34), the effects of mPFC lesions on behavioral inhibition reported here cannot be ascribed to learning deficits. In a separate set of experiments mPFC lesioned males were tested for the ability to establish a conditioned place preference to sex behavior. Reward-related associative learning remained intact in mPFC lesioned animals as these males were able to form a conditioned place preference to a sexual reward paired chamber. This finding is in agreement with previous studies examining the role of the PL or complete mPFC for the acquisition of psychostimulant induced CPP (35, 36), Moreover, associative learning for the aversive stimulus LiCl was not affected by mPFC lesions, consistent with previous reports that PFC lesions did not prevent the acquisition of conditioned taste aversion (34). Collectively these data suggest that the previously observed activation of the PL/IL subdivisions within the mPFC (20) are not necessary for the acquisition of reward related associative learning, however are necessary for the proper utilization of this information as it relates to the execution of behavioral control. This notion is in agreement with the current contention that intact IL function is necessary to survey and act upon inhibitory and excitatory inputs which convey information about the reward-aversion contingencies (37). Furthermore, animals with PL (35) or IL (8, 37, 38) lesions display normal extinction learning despite an inability to utilize this information to make goal directed decisions.
In conclusion, the current study indicates that animals with mPFC lesions are likely capable of forming the associations with aversive outcomes of their behavior, but lack the ability to suppress seeking of sexual reward in the face of aversive consequences. In humans sexual arousal is a complex experience whereby the processing of cognitive-emotional information serves to determine if the hedonic properties of a particular stimulus are sufficient to act as a sexual incentive (39). The current data suggest that mPFC dysfunction may contribute to sexual risk taking or to compulsive seeking of sexual behavior. Moreover, mPFC dysfunction has been associated with several psychiatric disorders (13, 40) suggesting that dysfunction of the mPFC may be an underlying pathology that is shared with the other disorders and that compulsive sexual behavior may be associated with other disorders. Indeed, in humans, hypersexuality or compulsive sexual behavior has been reported to have a high prevalence of comorbidity with psychiatric conditions (including substance abuse, anxiety, and mood disorders) (41), and approximately10% prevalence in Parkinson’s Disease together with compulsive buying, gambling, and eating (42–44).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huang H, Ghosh P, van den Pol A. Prefrontal Cortex–Projecting Glutamatergic Thalamic Paraventricular Nucleus-Excited by Hypocretin: A Feedforward Circuit That May Enhance Cognitive Arousal. J Neurophysiol. 2005;95:1656–1668. doi: 10.1152/jn.00927.2005. [DOI] [PubMed] [Google Scholar]
- 2.Floresco SB, Braaksma D, Phillips AG. Thalamic-cortical-striatal circuitry subserves working memory during delayed responding on a radial arm maze. J Neurosci. 1999;24:11061–11071. doi: 10.1523/JNEUROSCI.19-24-11061.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christakou A, Robbins TW, Everitt B. Prefrontal Cortical–Ventral Striatal Interactions Involved in Affective Modulation of Attentional Performance: Implications for Corticostriatal Circuit Function. J Neurosci. 2004;4:773–780. doi: 10.1523/JNEUROSCI.0949-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wall P, Flinn J, Messier C. Infralimbic muscarinic M1 receptors modulate anxiety-like behaviour and spontaneous working memory in mice. Psychopharmacology. 2001;155:58–68. doi: 10.1007/s002130000671. [DOI] [PubMed] [Google Scholar]
- 5.Marsh ABK, Vythilingam M, Busis S, Blair R. Response options and expectations of reward in decision-making: The differential roles of dorsal and rostral anterior cingulate cortex. NeuroImage. 2007;35:979–988. doi: 10.1016/j.neuroimage.2006.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers R, Ramanani N, Mackay C, Wilson J, Jezzard P, Carter C, Smith SM. Distinct Portions of Anterior Cingulate Cortex and Medial Prefrontal Cortex Are Activated by Reward Processing in Separable Phases of Decision-Making Cognition. Biol Psychiatry. 2004:55. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 8.Quirk G, Russo GK, Barron J, Lebron K. The Role of Ventromedial Prefrontal Cortex in the Recovery of Extinguished Fear. J Neurosci. 2000;16:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickinson A. Actions and habits: the development of behavioural autonomy. Philos Trans R Soc Lond Ser B Biol Sci. 1985;308:67–78. [Google Scholar]
- 10.Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nat Neurosci. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- 11.Dalley J, Cardinal R, Robbins T. Prefrontal executive and cognitive functions in rodents:neural and neurochemical substrates. Neuroscience and Biobehavioral Reviews. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 13.Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- 14.Reuter JRT, Rose M, Hand I, Glascher J, Buchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nature Neuroscience. 2005;8:147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- 15.Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- 16.Pfaus JG, Kippin TE, Centeno S. Conditioning and sexual behavior: a review. Horm Behav. 2001;2:291–321. doi: 10.1006/hbeh.2001.1686. [DOI] [PubMed] [Google Scholar]
- 17.Agmo A. Copulation-contingent aversive conditioning and sexual incentive motivation in male rats: evidence for a two-stage process of sexual behavior. Physiol Behav. 2002;77:425–435. doi: 10.1016/s0031-9384(02)00874-0. [DOI] [PubMed] [Google Scholar]
- 18.Peters RH. Learned aversions to copulatory behavior in male rats. Behav Neurosci. 1983;97:140–145. doi: 10.1037//0735-7044.97.1.140. [DOI] [PubMed] [Google Scholar]
- 19.Balfour ME, Yu L, Coolen LM. Sexual behavior and sex-associated environmental cues activate the mesolimbic system in male rats. Neuropsychopharmacology. 2004;29:718–730. doi: 10.1038/sj.npp.1300350. [DOI] [PubMed] [Google Scholar]
- 20.Balfour ME, Brown JL, Yu L, Coolen LM. Potential contributions of efferents from medial prefrontal cortex to neural activation following sexual behavior in the male rat. Neuroscience. 2006;137:1259–1276. doi: 10.1016/j.neuroscience.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Gonzalez M, Guevara A, Morali G, Cervantes M. Subcortical Multiple Unit Activity Changes During Rat Male Sexual Behavior. Physiology and Behavior. 1997;61(2):285–291. doi: 10.1016/s0031-9384(96)00367-8. [DOI] [PubMed] [Google Scholar]
- 22.Hendricks SE, Scheetz HA. Interaction of hypothalamic structures in the mediation of male sexual behavior. Physiol Behav. 1973;10:711–716. doi: 10.1016/0031-9384(73)90150-9. [DOI] [PubMed] [Google Scholar]
- 23.Pfaus JG, Phillips AG. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behav Neurosci. 1991;105:727–743. doi: 10.1037//0735-7044.105.5.727. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Guasti A, Omana-Zapata I, Lujan M, Condes-Lara M. Actions of sciatic nerve ligature on sexual behavior of sexually experienced and inexperienced male rats: effects of frontal pole decortication. Physiol Behav. 1994;55:577–581. doi: 10.1016/0031-9384(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 25.Agmo A, Villalpando A, Picker Z, Fernandez H. Lesions of the medial prefrontal cortex and sexual behavior in the male rat. Brain Res. 1995;696:177–186. doi: 10.1016/0006-8993(95)00852-h. [DOI] [PubMed] [Google Scholar]
- 26.Karama S, Lecours AR, Leroux J, Bourgouin P, Beaudoin G, Joubert S, Beauregard M. Areas of Brain Activation in Males and Females During Viewing of Erotic Film Excerpts. Human Brain Mapping. 2002;16:1–13. doi: 10.1002/hbm.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tenk CM, Wilson H, Zhang Q, Pitchers KK, Coolen LM. Sexual reward in male rats: effects of sexual experience on conditioned place preferences associated with ejaculation and intromissions. Horm Behav. 2009;55:93–7. doi: 10.1016/j.yhbeh.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pitchers KK, Balfour ME, Lehman MN, Richtand NM, Yu L, Coolen LM. Neuroplasticity in the mesolimbic system induced by natural reward and subsequent reward abstinence. Biol Psych. 2009 doi: 10.1016/j.biopsych.2009.09.036. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webb IC, Baltazar RM, Wang X, Pitchers KK, Coolen LM, Lehman MN. Diurnal variations in natural and drug reward, mesolimbic tyrosine hydroxylase, and clock gene expression in the male rat. J Biol Rhythms. 2009 doi: 10.1177/0748730409346657. In Press. [DOI] [PubMed] [Google Scholar]
- 30.Shah AA, Treit D. Excitotoxic lesions of the medial prefrontal cortex attenuate fear responses in the elevated-plus maze, social interaction and shock probe burying tests. Brain Res. 2003;969:183–194. doi: 10.1016/s0006-8993(03)02299-6. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan RM, Gratton A. Behavioral effects of excitotoxic lesions of ventral medial prefrontal cortex in the rat are hemisphere-dependent. Brain Res. 2002a;927:69–79. doi: 10.1016/s0006-8993(01)03328-5. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology. 2002b;27:99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- 33.Franklin T, Druhan JP. Involvement of the Nucleus Accumbens and Medial Prefrontal Cortex in the Expression of Conditioned Hyperactivity to a Cocaine-Associated Environment in Rats. Neuropsychopharmacology. 2000;23:633–644. doi: 10.1016/S0893-133X(00)00162-7. [DOI] [PubMed] [Google Scholar]
- 34.Hernadi I, Karadi Z, Vigh J, Petyko Z, Egyed R, Berta B, Lenard L. Alterations of conditioned taste aversion after microiontophoretically applied neurotoxins in the medial prefrontal cortex of the rat. Brain Res Bull. 2000;53:751–758. doi: 10.1016/s0361-9230(00)00361-0. [DOI] [PubMed] [Google Scholar]
- 35.Zavala A, Weber S, Rice H, Alleweireldt A, Neisewander JL. Role of the prelimbic subregion of the medial prefrontal cortex in acquisition, extinction, and reinstatement of cocaine-conditioned place preference. Brain Research. 2003;990:157–164. doi: 10.1016/s0006-8993(03)03452-8. [DOI] [PubMed] [Google Scholar]
- 36.Tzschentke TM, Schmidt W. Functional heterogeneity of the rat medial prefrontal cortex: effects of discrete subarea-specific lesions on drug-induced conditioned place preference and behavioural sensitization. Eur J Neurosci. 1999;11:4099–4109. doi: 10.1046/j.1460-9568.1999.00834.x. [DOI] [PubMed] [Google Scholar]
- 37.Rhodes SE, Killcross AS. Lesions of rat infralimbic cortex result in disrupted retardation but normal summation test performance following training on a Pavlovian conditioned inhibition procedure. Eur J Neurosci. 2007;9:2654–2660. doi: 10.1111/j.1460-9568.2007.05855.x. [DOI] [PubMed] [Google Scholar]
- 38.Rhodes SE, Killcross S. Lesions of rat infralimbic cortex enhance recovery and reinstatement of an appetitive Pavlovian response. Learn Mem. 2004;5:611–616. doi: 10.1101/lm.79704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stoleru S, Gregoire MC, Gerard D, Decety J, Lafarge E, Cinotti L, Lavenne F, Le Bars D, Vernet-Maury E, Rada H, Collet C, Mazoyer B, Forest MG, Magnin F, Spira A, Comar D. Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch Sex Behav. 1999;28:1–21. doi: 10.1023/a:1018733420467. [DOI] [PubMed] [Google Scholar]
- 40.Taylor SF, Liberzon I, Decker LR, Koeppe RA. A functional anatomic study of emotion in schizophrenia. Schizophrenia Res. 2002;58:159–172. doi: 10.1016/s0920-9964(01)00403-0. [DOI] [PubMed] [Google Scholar]
- 41.Bancroft J. Sex behavior that is “out of control”: a theoretical conceptual approach. Psychiatric Clinics of North America. 2008;31(4):593–601. doi: 10.1016/j.psc.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Weintraub MD. Dopamine and impulse control disorders in Parkinson’s disease. Annals Neurol. 2008;64:S93–100. doi: 10.1002/ana.21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Isaias IU, et al. The relationship between impulsivity and impulse control disorders in Parkinson’s disease. Movement Disorders. 2008;23:411–415. doi: 10.1002/mds.21872. [DOI] [PubMed] [Google Scholar]
- 44.Wolters EC. Parkinson’s disease-related disorders in the impulse-compulsive spectrum. J Neurol. 2008;255:48–56. doi: 10.1007/s00415-008-5010-5. [DOI] [PubMed] [Google Scholar]
- 45.Swanson LW. Brain Maps: Structure of the Rat Brain. Elsevier; Amsterdam: 1998. [Google Scholar]