Abstract
To maximize reproductive success, organisms restrict breeding to optimal times of day or year, when internal physiology and external environmental conditions are suitable for parent and offspring survival. To appropriately coordinate reproductive activity, internal and external standing is communicated to the hypothalamo-pituitary-gonadal (HPG) axis via a coordinated balance of stimulatory and inhibitory neurochemical systems. The cumulative balance of these mediators ultimately drives the pattern of gonadotropin-releasing hormone (GnRH) secretion, a neurohormone that stimulates pituitary gonadotropin secretion. Until 2000, a complementary inhibitor of pituitary gonadotropin secretion had not been identified. At this time, Tsutsui and colleagues uncovered a novel, avian hypothalamic peptide capable of inhibiting gonadotropin secretion in cultured quail pituitary cells. We later examined the presence and functional role for the mammalian ortholog of GnIH, RFamide-related peptide (RFRP-3), in mammals, and confirmed a conserved role for this peptide across several rodent species. To date, a similar distribution and functional role for RFRP-3 have been observed across all mammals investigated, including humans. This overview summarizes the role that RFRP-3 plays in mammals and considers the implications and opportunities for further study by those interested in reproductive physiology and the neural control of sexual behavior and motivation.
Keywords: photoperiod, ovulation, gonad, luteinizing, gonadotropin
Successful reproduction requires that the hypothalamo-pituitary-gonadal (HPG) axis be maintained within finely tuned operating limits while considering the internal state and external environment an organism occupies. To ensure that reproductive effort will not compromise parent or offspring survival given prevailing conditions, the state of the reproductive system is optimized by a multitude of positive and negative regulators. These neurochemical systems converge on the gonadotropin-releasing hormone (GnRH) system to regulate its activity both transiently (e.g., reproductive suppression during acute stress) and for more extended durations (e.g., over winter in temperate climes). GnRH acts on the anterior pituitary to control release of the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) that, in turn, regulate steroidogenesis and gametogenesis, respectively. Before the discovery of a gonadotropin-inhibitory hormone (GnIH) in the brain of quail by Tsutsui and colleagues (1), a neurochemical directly and negatively regulating pituitary gonadotropin secretion was not known. This discovery motivated the search for GnIH homologues and related peptides within the same superfamily, across taxa. This review will focus on the discovery and characterization of the GnIH ortholog in mammals and the roles they serve in mediating physiology and behavior.
Discovery of RFamide-Related Peptides
The identification of a cardioexcitatory neuropeptide containing the C-terminal Phe-Met-Arg-Phe-NH2 (FMRFamide) in the ganglia of the bivalve mollusk, Macrocallista nimbosa (2), inevitably motivated the exploration and discovery of a host of peptides in the same class that markedly regulate neuroendocrine functioning. Following the work of Price and Greenberg, antibodies to FMRFamide peptides were applied as tools to identify structurally similar peptides across phyla. Although these antibodies recognized cells in the CNS of several species, the identity of these labeled peptides remained unknown. In 1983, the first vertebrate RFamide peptide, Leu-Pro-Leu-Arg-Phe-NH2 (LPLRFamide), was identified in chicken (3). Injections of this peptide increased arterial blood pressure and altered brain stem neuronal firing activity is rats (4, 5) These data provided the first evidence for vertebrate expression of RFamide peptides (Arg-Phe C-terminal motif) and indicated a potential modulatory role in mammalian brain and periphery.
In 2000, Tsutsui and colleagues identified a novel hypothalamo-hypophysial RFamide peptide that rapidly and dose-dependently inhibited gonadotropin release from cultured quail pituitaries (1). Based on these initial findings, the peptide was named gonadotropin-inhibiting hormone (GnIH). In avian species, GnIH neurons are found only in the paraventricular nucleus of the hypothalamus (PVN) with extensive fibers projecting rostrally to the ventral paleostriatum, lateral and medial septum, preoptic area and caudally to the median eminence, optic tectum and brainstem (6, 7). The receptor for GnIH, a novel G-protein-coupled (GPR) receptor (GPR147) was later cloned and characterized in Japanese quail (8). In vivo, GnIH injections result in a decrease in luteinizing hormone (LH) (9) and rapid suppression of female sexual behavior in avian species, the latter finding suggesting a potential neuromodulatory role for this neurochemical (10). GnIH fibers form close contacts with GnRH-I neurons in the POA in birds and GnRH-II neurons in the midbrain, suggesting modulation of the reproductive axis at the level of the brain (7, 11). GnIH neurons also project to the median eminence to directly regulate pituitary gonadatropin secretion and potentially terminal release of GnRH (7, 11). These findings laid the foundation for exploring whether or not a conserved functional role for GnIH is seen across taxa (see Tsutsui et al. in this issue).
The Mammalian Ortholog of GnIH
The presence of mammalian cDNAs that encode novel RFamide peptides structurally similar to GnIH were first uncovered through a gene database search (12). The cDNAs of human and bovine encoded three peptides that were termed RFamide-related peptide-1, -2 and -3 (RFRP-1, -2 and -3). It was later determined that the mammalian RFRP gene encodes only two RFamide peptides, RFRP-1 and -3, and the amino acid sequence thought to encode the C-terminus of RFRP-2 was, instead, included at the N-terminus of RFRP-3 (13–17). Initial evidence for RFamide peptides as endocrine mediators was suggested by the observation that intracerebroventricular (i.c.v.) injections of RFRP-1 promoted prolactin secretion (12). Subsequently, RFRP-1 and -3 were purified from bovine (13, 17) and rat hypothalami (15), although the role of this latter peptide in neuroendocrine regulation and its potential control of motivated behavior remained to be explored.
Following the discovery of RFamide peptides in mammalian brain and a potential role of RFRP-1 in prolactin regulation (13–16), we sought to determine whether or not RFRP-3 serves a conserved role as a gonadotropin inhibitory hormone in rodents. Our initial investigation focused on rats (Rattus norvegicus), mice (Mus musculus), and Syrian hamsters (Mesocricetus auratus) to determine the generality of our findings across rodent species. In all three rodents, immunohistochemical analysis revealed that RFRP-3-immunoreactive (ir) cell bodies were concentrated in the dorsomedial nucleus of the hypothalamus (DMH) with no other brain regions showing evidence of cell body staining (Figure 1) (18). To provide converging evidence that our antibody was labeling RFRP and not a related RFamide peptide, we cloned the RFRP precursor polypeptide in Syrian hamsters and labeled hamster brains for RFRP-3 mRNA using in situ hybridization (18). Cells expressing RFRP-3 mRNA were confined to the DMH and localized in a pattern identical to RFRP-3-ir labeling. As in birds, RFRP-3 fibers and terminal fields were omnipresent in midline brain regions that concentrate GnRH neurons and fibers, with the medial septum, diagonal band of Broca, preoptic area, and anterior hypothalamus all being major targets for RFRP-3. There were no differences between the sexes in the number of RFRP-3-ir cells labeled or the fiber distribution pattern (18). Given this projection pattern, we examined whether or not RFRP-3-ir cells project upon GnRH perikarya as a mechanism of direct regulation. A marked percentage of GnRH cells (>40%) received projections from RFRP-3-ir fibers suggesting the potential for direct inhibitory control. In our initial investigation, fiber terminals were sparse in the outer layer of the median eminence (18). This finding is consistent another study using direct immunohistochemisty, where RFRP-3 fibers were not detected in the outer layer of the median eminence (19). However, in our subsequent analysis using an optimized, immunohistochemical protocol and biotinylated tyramide amplification revealed more pronounced fibers terminating in the median eminence, with RFRP-3 receptor, GPR147, expression in the pituitary (20).
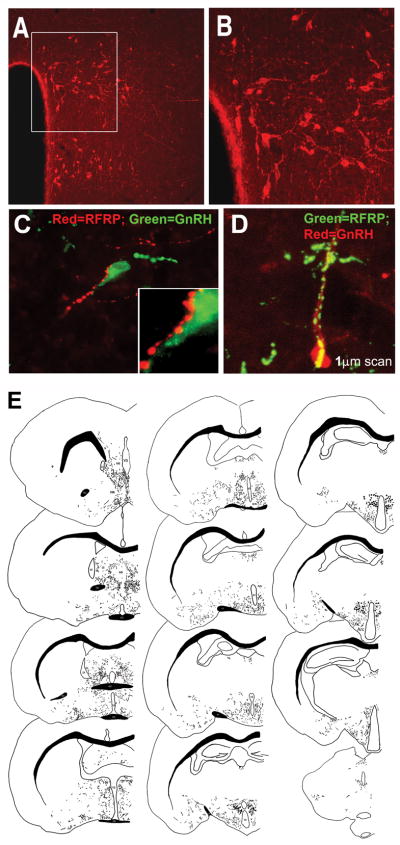
Figure 1. GnRH cells bodies are tightly clustered in the DMH and project throughout much of the brain of Syrian hamsters.
Medium-power photomicrograph depicting RFRP-3 cell bodies clustered in the dorsal and ventral regions of the DMH; bar=200 μm (A). The box in the left image outlines the cells bodies shown at high power; bar=100μm (B). A). GnRH cells were investigated using wide-field (C) and confocal microscopy (D). Both images (C, D) depict fibers for RFRP-3 ‘tracking’ the GnRH fiber and cell body, presumptive boutons are evident. (E) A tracing of the rostral-caudal extent of RFRP-3 fiber projections and cell bodies. Note that RFRP-3 cell bodies are clustered in the dorsomedial region of the hypothalamus with diffuse projections throughout most of the brain, with a concentration of terminals in midline brain regions. ac=anterior commisure; AH=anterior hypothalamus; AMG=amygdala; Arc=arcuate nuceleus; BNST=bed nucleus of the stria terminalis; DMH=dorsomedial hypothalamusl; LS=lateral septum; oc=optic chiasm; PAG=periaquaductal grey; POA=preoptic area; PVT=paraventricular nucleus of the thalamus. Modified from Kriegsfeld et al., 2006.
To confirm a role for mammalian RFRP-3 as a regulator of gonadotropin secretion in mammals, we injected the peptide intracerebroventricularly (ICV) or peripherally in hamsters to determine its effects on LH. Both routes of administration rapidly and markedly inhibit LH secretion (18). Given the pronounced inhibitory actions of RFRP-3 in mammalian brain, we examined the possibility that this peptide participates in mediating the negative feedback effects of sex steroids. We found that RFRP-3-ir cells express estrogen receptor-α (ERα) and respond to estrogen with increased FOS expression, suggesting activation by gonadal steroids (18). Combined with our results indicating direct projections upon GnRH cells, these findings suggest that mammalian RFRP-3 may mediate negative feedback effects of gonadal steroids at both the hypothalamic and pituitary levels. To our knowledge, no other studies to date have investigated the possibility that RFRP-3 cells express sex steroid receptors and participate in sex steroid negative feedback and this line of research represents an important area of further enquiry. Together, these recent findings pointed to a previously unidentified neurochemical pathway by which sex steroids act on the brain to regulate the reproductive axis. Additionally, the extensive fiber projections of RFRP-3 throughout the brain point to opportunity for investigating the role of this peptide in an array of sexual and other motivated behaviors.
To date, RFRP-3 has been identified in the brains of all mammalian species investigated, including cattle, sheep, rat, primate, and human (13, 15, 17, 19, 21–27). In mice, direct application of RFRP-3 to GnRH cells in cultured brain slices decreases firing rate in a subpopulation of cells, providing evidence for a direct action of RFRP-3 on GnRH neurons (28, 29). Likewise, administration of RFRP-3 suppressed GnRH neuronal activity, as measured by co-expression with the immediate early gene, c-Fos (30). Large animal models represent ideal systems to investigate the pattern of gonadotropin pulse frequency and amplitude following manipulations of the HPG axis. Clarke et al. (2008) capitalized on this system to examine the characteristics of LH secretion impacted by RFRP-3 administration in sheep (27). Peripheral administration of the RFRP-3 reduces ovine LH pulse amplitude and suppresses LH and FSH release in vitro, the latter finding providing converging evidence for a hypophysiotropic role of RFRP-3 in mammals. The inhibition of GnRH-stimulated LH and FSH release by RFRP-3 is associated with downregulation of LHβ- and FSHβ-subunit expression (26). In a large nonseasonal breeding animal model (bovine), repeated administration of RFRP-3 leads to reduced LH pulse frequency (31). In this species, the suppression of LH appears to be mediated, at least in part, at the level of the pituitary, as RFRP-3 inhibits GnRH-induced LH secretion from cultured bovine pituitary cells (31). Analogous results are seen in rats with RFRP-3 attenuating GnRH-induced LH release in cultured pituitary cells (32). Together, these findings further suggest that RFRP-3 is a functional mammalian ortholog, of avian GnIH, with a conserved mechanism of action across species. Curiously, in rats, peripheral injections of Fluoro-Gold, a technique used to retrogradely identify central hypophysiotropic cells, failed to label RFRP-3 neurons (33). In the same study, intravenous injections of RFRP-3 did not suppress LH secretion (33). Given these discrepant findings, whether or not RFRP-3 acts, endogenously, to impact gonadotropes directly requires further investigation.
To characterize fully the function of RFRP-3 and survey its role in reproductive axis control, it is necessary to observe the consequences of RFRP-3 receptor blockade. Until recently, such a strategy was not possible, as a selective receptor antagonist was not available. Recently, Pineda et al. investigated the role of RFRP-3 using a selective antagonist (RF9) of RFRP-3 and a related peptide in the same family, neuropeptide FF (NPFF). Alterations in NPFF and interactions with its receptor do not alter gonadotropin secretion (Pineda et al., unpublished observations), suggesting that effects of RF9 are likely mediated through blockade of RFRP-3’s actions (34). The role of RF9 was investigated in male and female mice and rats. As expected, i.c.v. injections of RF9 to cycling females led to a rapid and sustained, dose-dependent increase in LH both during estrus and diestrus. Analogous results were seen in males, with RF9 leading to a rapid and prolonged increase in LH and FSH. In rat pituitary cultures, RF9 did not alter LH secretion (34). Whether or not RF9 application synergizes with GnRH to potentiate LH release requires further investigation. Together, these findings provide additional, converging evidence for a prominent role of RFRP-3 in mammalian reproductive axis inhibition.
Role for RFRP-3 in Mammalian Reproductive Function and Behavior
Given the pronounced role of RFRP-3 in regulating gonadotropin secretion, we investigated the possibility that this peptide may be a key player in regulating ovulatory function. Throughout most of the Syrian hamster (and most other spontaneously ovulating rodents) ovulatory cycle, estrogen acts through negative feedback to maintain LH at low concentrations. However, at the time of the preovulatory LH surge, negative feedback fails and estrogen serves a permissive role, permitting the circadian system to stimulate the LH surge and initiate ovulation (35–37). We asked whether or not inhibitory drive on the reproductive axis throughout the ovulatory cycle is controlled by RFRP-3. First, we examined the pattern of RFRP-3 cellular activity and found that it was suppressed at the time of the LH surge, suggesting removal of negative feedback by RFRP-3 at this time (Figure 2) (20). Additionally, we found that the suprachiasmatic nucleus (SCN), the master circadian clock triggering ovulation, projects to a large proportion of RFRP-3 cells, providing a mechanism for timing removal of negative drive on the GnRH system (20). Finally, by exploiting a phenomenon in which activity of hamsters in constant light splits into two bouts, with each half of the SCN active in antiphase, we found that activation of the RFRP-3 system is also asymmetrical. Importantly, this asymmetry isopposite to that seen for the GnRH system, suggesting that the SCN concomitantly activates ipsilateral GnRH cells while removing the suppressive influence of RFRP-3 on the same side of the brain (20).
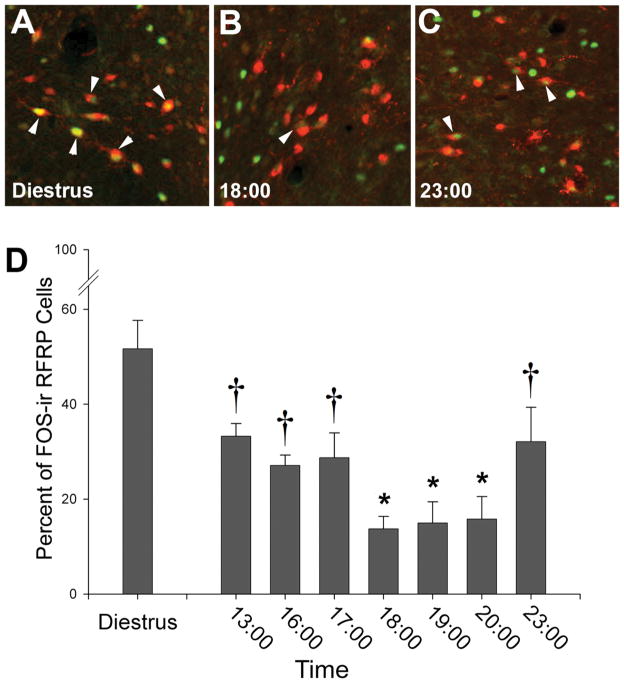
Figure 2. Activation of RFRP-3-ir cells is reduced during the LH surge and reinstated thereafter.
The percentage of RFRP-3-ir cells expressing FOS is reduced on the day of proestrus, around the time of the LH surge. A–C, Low-power photomicrographs of RFRP-3-ir cells expressing FOS on the day of diestrus (A) and during the trough (B) and peak (C) of expression on the day of proestrus. D, Mean (±SEM) percentage of RFRP-3-ir cells expressing FOS on the day of diestrus and throughout the day of proestrus. All bars not sharing a symbol differ significantly from each other at P < 0.05. Adapted from Gibson et al., 2008.
The neural circuits and neurochemical systems that monitor energetic status are intimately linked to those that control reproductive function, providing a means of optimizing reproductive success in fluctuating environments (38). As a result, it is likely that the RFRP-3 system plays a role in monitoring energy balance and relaying this information to the HPG axis to modulate reproductive behavior and physiology. Indeed, in rats, RFRP-3 facilitates food intake and suppresses male sexual behavior (19), consistent with the notion that high expression of this peptide is associated with a negative energy balance. In this study, male rats were gonadally intact, and it is unclear whether the impact of RFRP-3 on sexual behavior results from suppression of testosterone or a direct neuromodulatory effect of RFRP-3 on the neural loci mediating sexual motivation. More recently, Clarke and colleagues show that RFRP-3 neurons project to neuropeptide-Y, pro-opiomelanocortin, orexin and melanin concentrating cells in the ovine brain (39, 40), consistent with the notion that RFRP-3 cells communicate with key neurochemical systems regulating feeding, providing a neural locus for balancing reproductive effort and energy intake. In addition, both RFRP-3 and melanin-concentrating hormone block the excitatory effect of kisspeptin on vGluT2-GnRH neurons in mice, suggesting that RFRP-3 signaling may converge with energetic input at the level of this subpopulation of GnRH neurons (29, 41).
As with energetics, the stress axis reciprocally communicates with the reproductive system to drive long- and short-term changes in reproductive activity. In male rats, RFRP-3 cells express glucocorticoid receptors and RFRP-3 expression is upregulated by acute and chronic stress. The effects of stress on RFRP-3 activity are abolished in adrenalectomized animals (42), suggesting actions through glucocorticoids. Together with studies showing extensive projections of RFRP-3 in mammalian brain (e.g., (18, 23), these findings suggest that the RFRP-3 system is in a position to monitor internal and external status and integrate with/regulate the complex network of reproductive control.
In birds, GnIH and GPR147 are expressed in the gonads and accessory reproductive organs (43), suggesting a potential autocrine/paracrine role for this peptide in locally regulating gametogenesis or steroidogenesis. We recently found that RFRP-3 and its receptor are robustly expressed in Syrian hamster testis (Figure 3) (44), indicating that a role for GnIH/RFRP-3 in gonadal function may be common to all vertebrates. In hamsters, in situ hybridization revealed that expression is confined to seminiferous tubules, suggesting a role in spermatogenesis. In situ hybridization and immunohistochemical labeling indicate that spermatocytes and spermatids are the major cell types expressing RFRP-3 and GPR147. GPR147 is present in spermatids, with expression increased in late spermatocytes, implying the participation of RFRP-3 signaling during these times (44). Together, these findings suggest that RFRP-3 might be involved in the final maturation of spermatids, potentially necessary to counterbalance the influence of positive regulatory factors at this time.
Figure 3. Expression of RFRP peptide and its receptor protein (GPR147) in testicular seminiferous tubules.
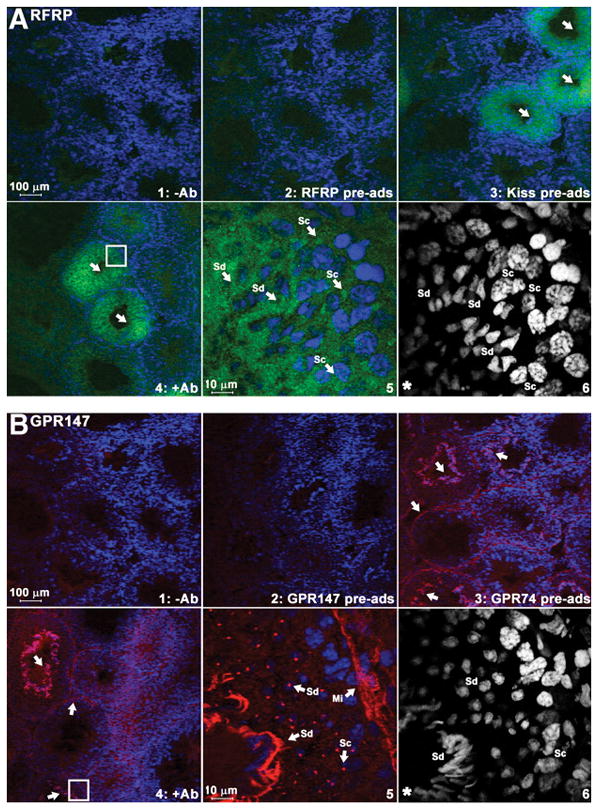
Immunohistochemistry using specific antibodies against RFRP-3 (A) and GPR147 (B). RFRP ligand (A, green) was found around the nucleus of spermatocytes (Sc) and spermatids (Sd). GPR147 receptor (B, red) was found in myoid cells (Mi) along the edge of tubules, spermatocytes, and both round and elongated spermatids. Nuclei were stained by DAPI (blue or white color). For each label, controls are shown as follows: no primary antibody (upper left), preadsorption with corresponding antigenic peptide (upper middle), and preadsorption with related peptides (upper right). Lower left, Primary antibody [RFRP (A), GPR147 (B)]. Lower middle, High-power image of area outlined in lower left. Arrows indicate positively stained tubules (upper right) or cells (lower middle). The highlighted DAPI channel for cell nuclear staining is shown in the lower right. Adapted from Zhao et al., 2010
RFRP-3 in Seasonal Regulation of Reproductive Function
Animals inhabiting temperate and boreal latitudes typically inhibit reproduction during winter to conserve scarce energetic resources. Given the interactions between the RFRP-3 system and the neurochemical systems mediating energy balance described previously, it is logical to speculate that the RFRP-3 system is a component of the neural circuitry coordinating seasonal changes in reproductive function. The first evidence for a role of RFamide peptides in seasonal breeding came from studies of photoperiodic song sparrows (7) and subsequent studies in quail in which the eyes and pineal gland were removed in order to eliminate endogenous melatonin (MEL), the principal hormone communicating day length information (7). These manipulations led to a marked reduction in GnIH mRNA and peptide expression, whereas MEL replacement reversed these effects. (45) This finding set the stage for investigating the role of GnIH and other RFamide peptides in seasonal reproduction across species. In Syrian hamsters, lesions of the DMH block short-day and melatonin-induced atrophy of the reproductive system (46–48), suggesting that the DMH is a key locus required for interpreting day length information communicated by the MEL signal in this species. The DMH also expresses androgen receptors (AR), and may be a site at which photoperiod/MEL modulate sensitivity to steroid negative feedback, a key mechanism contributing to winter reproductive decline. Additionally, in immortalized hypothalamic cell lines, melatonin administration leads to increased RFRP-3 expression, suggesting direct impact of melatonin on this cell phenotype (49). Given the location of RFRP-3 in the DMH across rodent species (18) and the critical role for the DMH in seasonal breeding in Syrian hamsters, the RFRP-3 system represented an attractive target cell phenotype for further exploration.
Two studies investigating the effects of photoperiod on RFRP-3 expression in Syrian hamsters have uncovered similar, but unexpected, findings. Contrary to expectation, extended exposure to inhibitory day lengths leads to reduced RFRP-3 immunoreactivity and mRNA (50, 51). Similar results are seen following 60 days of melatonin administration to long-day animals (50). Although the DMH has not been implicated in seasonal changes in reproduction in Siberian hamsters (52, 53), the same photoperiod-induced changes in RFRP-3 are observed following short day exposure in this species (50) (c.f., (54)). These findings are difficult to reconcile with a role for RFRP-3 in reproductive suppression, as it is lowest during maximal inhibition of this axis. One possibility is that Syrian hamsters require enhanced RFRP-3 expression to suppress GnRH during the initial period of regression, but that this level of inhibition is not necessary in hamsters with a fully regressed reproductive axis and low testosterone concentrations. Future studies examining the pattern of GnIH expression throughout the development of reproductive quiescence are necessary to examine this possibility. Alternatively, RFRP-3 may not serve a role in seasonal changes in reproductive function, and another peptide may serve this function in rodents. It is also possible that RFRP-3 is required to modulate reproductive function during the breeding season, as has been postulated for birds (Calisi et al., 2008).
In contrast to findings in rodents, in short-day-breeding sheep, RFRP-3 is decreased in the PVN and DMH during the breeding season relative to the non-breeding season (24). Similarly, RFRP-3 contacts upon GnRH neurons are decreased in the POA of breeding animals (24). Consistent with these findings, another study of sheep revealed that short, stimulatory day lengths lead to a marked decrease in RFRP-3 in ependymal cells surrounding the base of the third ventricle, relative to long-day animals (55). These observations in sheep more readily reconcilable with a role for RFRP-3 in seasonal breeding, although future studies using approaches to directly target RFRP-3 function/expression are necessary to begin to elucidate the role of this peptide in seasonal breeding.
In addition to acting at the level of the brain, as described previously, RFRP-3 and GPR147 are expressed in Syrian hamster testis, suggesting that local testicular expression of RFRP-3 may participate in seasonal changes in testicular function. Indeed, animals with regressed gonads exhibit low expression of RFRP-3 and GPR147 mRNA (44), suggesting a potential positive role for this peptide in spermatogenesis, possibly decreasing at a time when sperm production is not occurring. Alternatively, it is possible that RFRP-3 is responsible for balancing the actions of positive regulators of spermatogenesis. Consequently, at times when sperm are not being produced, negative regulation by RFRP-3 is not required and energetic resources can be conserved by downregulating the activity of this system.
Conclusions
Although research is still in its infancy, overwhelming converging evidence across mammalian species indicates that RFRP-3 is a key inhibitor of the HPG axis. In all species studied to date, direct or indirect evidence suggests a role for RFRP-3 at the level of the GnRH system and on pituitary gonadotropes. In rodents, application of RFRP-3 has inhibitory actions directly on GnRH neurons (28) and a number of studies suggest inhibitory modulation of GnRH-induced LH secretion at the level of the pituitary (27, 32). Likewise, the pattern of RFRP-3 fiber innervation at the level of the median eminence across studies suggests the potential for modulation of GnRH secretion into the hypophyseal portal system and for direct modulation of pituitary hormone release.
Whereas most studies to date have focused on confirming a role for RFRP-3 as an inhibitory regulator of the mammalian reproductive axis, several studies suggest a more pronounced role for RFRP-3 in behavioral regulation. These findings are consistent with the pronounced RFRP-3 projections seen throughout mammalians brain. In rats, RFRP-3 impacts sexual and feeding behavior (19). In ovine, RFRP-3 projects to key neurochemical cell phenotypes regulating energy balance and feeding (39), providing further evidence for an important role for RFRP-3 in non-sexual motivated behaviors. These initial findings, combined with the observation that RFRP-3 cells target hypothalamic and other limbic structures, suggests opportunity for exploring the role of this RFamide peptide in a host of behaviors.
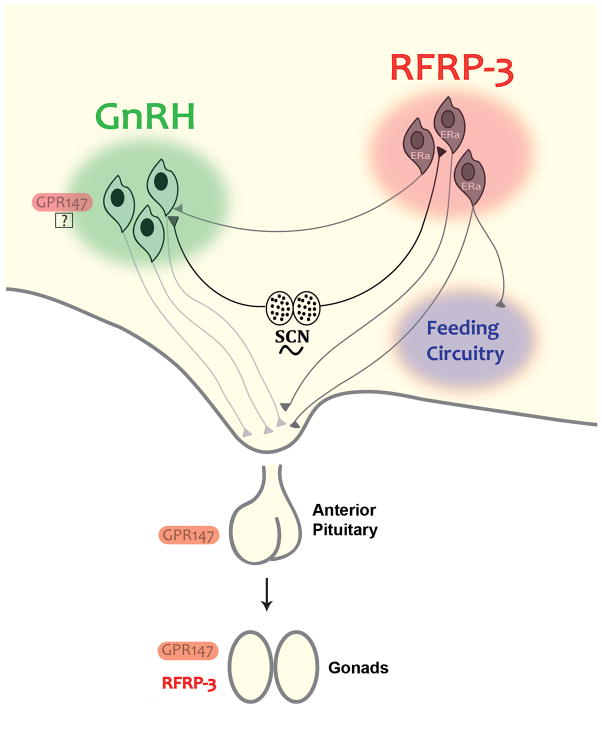
Figure 4. Schematic summarizing the mechanisms of RFRP-3 control of the mammalian reproductive axis and behavior.
According to the model, RFRP-3 cells project to GnRH cells and to the median eminence to regulate gonadotropin secretion. Recent evidence indicates that RFRP-3 impacts feeding and cells project to key neuropeptidergic cells in the arcuate nucleus regulating energy balance. Some actions of RFRP-3 occur directly on pituitary gonadotropes and GPR147 expression is seen in pituitary tissue. Finally, RFRP-3 and GPR147 expression is seen hamster testicular tissue, indicating a role local RFRP-3 secretion in paracrine/autocrine control of gonadal function.
Acknowledgments
The work presented herein was supported by the National Institutes of Health Grant HD-050470 (L.J.K.) and National Science Foundation Grant IOS-0641188 (G.E.B.)
References
- 1.Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun. 2000;275(2):661–7. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- 2.Price DA, Greenberg MJ. Structure of a molluscan cardioexcitatory neuropeptide. Science. 1977;197(4304):670–1. doi: 10.1126/science.877582. [DOI] [PubMed] [Google Scholar]
- 3.Dockray GJ, Reeve JR, Jr, Shively J, Gayton RJ, Barnard CS. A novel active pentapeptide from chicken brain identified by antibodies to FMRFamide. Nature. 1983;305(5932):328–30. doi: 10.1038/305328a0. [DOI] [PubMed] [Google Scholar]
- 4.Wong TM, Greenberg MJ, Tse SY. Cardiovascular effects of intraventricular injection of FMRFamide, Met-enkephalin and their common analogues in the rat. Comp Biochem Physiol C. 1985;81(1):175–9. doi: 10.1016/0742-8413(85)90111-2. [DOI] [PubMed] [Google Scholar]
- 5.Gayton RJ. Mammalian neuronal actions of FMRFamide and the structurally related opioid Met-enkephalin-Arg6-Phe7. Nature. 1982;298(5871):275–6. doi: 10.1038/298275a0. [DOI] [PubMed] [Google Scholar]
- 6.Ukena K, Ubuka T, Tsutsui K. Distribution of a novel avian gonadotropin-inhibitory hormone in the quail brain. Cell Tissue Res. 2003;312(1):73–9. doi: 10.1007/s00441-003-0700-x. [DOI] [PubMed] [Google Scholar]
- 7.Bentley GE, Perfito N, Ukena K, Tsutsui K, Wingfield JC. Gonadotropin-inhibitory peptide in song sparrows (Melospiza melodia) in different reproductive conditions, and in house sparrows (Passer domesticus) relative to chicken-gonadotropin-releasing hormone. J Neuroendocrinol. 2003;15(8):794–802. doi: 10.1046/j.1365-2826.2003.01062.x. [DOI] [PubMed] [Google Scholar]
- 8.Yin H, Ukena K, Ubuka T, Tsutsui K. A novel G protein-coupled receptor for gonadotropin-inhibitory hormone in the Japanese quail (Coturnix japonica): identification, expression and binding activity. J Endocrinol. 2005;184(1):257–66. doi: 10.1677/joe.1.05926. [DOI] [PubMed] [Google Scholar]
- 9.Osugi T, Ukena K, Bentley GE, O’Brien S, Moore IT, Wingfield JC, Tsutsui K. Gonadotropin-inhibitory hormone in Gambel’s white-crowned sparrow (Zonotrichia leucophrys gambelii): cDNA identification, transcript localization and functional effects in laboratory and field experiments. J Endocrinol. 2004;182(1):33–42. doi: 10.1677/joe.0.1820033. [DOI] [PubMed] [Google Scholar]
- 10.Bentley GE, Jensen JP, Kaur GJ, Wacker DW, Tsutsui K, Wingfield JC. Rapid inhibition of female sexual behavior by gonadotropin-inhibitory hormone (GnIH) Horm Behav. 2006;49(4):550–5. doi: 10.1016/j.yhbeh.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Ubuka T, Kim S, Huang YC, Reid J, Jiang J, Osugi T, Chowdhury VS, Tsutsui K, Bentley GE. Gonadotropin-inhibitory hormone neurons interact directly with gonadotropin-releasing hormone-I and -II neurons in European starling brain. Endocrinology. 2008;149(1):268–78. doi: 10.1210/en.2007-0983. [DOI] [PubMed] [Google Scholar]
- 12.Hinuma S, Shintani Y, Fukusumi S, Iijima N, Matsumoto Y, Hosoya M, Fujii R, Watanabe T, Kikuchi K, Terao Y, Yano T, Yamamoto T, Kawamata Y, Habata Y, Asada M, Kitada C, Kurokawa T, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat Cell Biol. 2000;2(10):703–8. doi: 10.1038/35036326. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida H, Habata Y, Hosoya M, Kawamata Y, Kitada C, Hinuma S. Molecular properties of endogenous RFamide-related peptide-3 and its interaction with receptors. Biochim Biophys Acta. 2003;1593(2–3):151–7. doi: 10.1016/s0167-4889(02)00389-0. [DOI] [PubMed] [Google Scholar]
- 14.Ukena K, Tsutsui K. A new member of the hypothalamic RF-amide peptide family, LPXRF-amide peptides: structure, localization, and function. Mass Spectrom Rev. 2005;24(4):469–86. doi: 10.1002/mas.20031. [DOI] [PubMed] [Google Scholar]
- 15.Ukena K, Iwakoshi E, Minakata H, Tsutsui K. A novel rat hypothalamic RFamide-related peptide identified by immunoaffinity chromatography and mass spectrometry. FEBS Lett. 2002;512(1–3):255–8. doi: 10.1016/s0014-5793(02)02275-5. [DOI] [PubMed] [Google Scholar]
- 16.Fukusumi S, Yoshida H, Fujii R, Maruyama M, Komatsu H, Habata Y, Shintani Y, Hinuma S, Fujino M. A new peptidic ligand and its receptor regulating adrenal function in rats. J Biol Chem. 2003;278(47):46387–95. doi: 10.1074/jbc.M305270200. [DOI] [PubMed] [Google Scholar]
- 17.Fukusumi S, Habata Y, Yoshida H, Iijima N, Kawamata Y, Hosoya M, Fujii R, Hinuma S, Kitada C, Shintani Y, Suenaga M, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. Characteristics and distribution of endogenous RFamide-related peptide-1. Biochim Biophys Acta. 2001;1540(3):221–32. doi: 10.1016/s0167-4889(01)00135-5. [DOI] [PubMed] [Google Scholar]
- 18.Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci U S A. 2006;103(7):2410–5. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson MA, Tsutsui K, Fraley GS. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav. 2007;51(1):171–80. doi: 10.1016/j.yhbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibson EM, Humber SA, Jain S, Williams WP, 3rd, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld LJ. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149(10):4958–69. doi: 10.1210/en.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ubuka T, Ukena K, Sharp PJ, Bentley GE, Tsutsui K. Gonadotropin-inhibitory hormone inhibits gonadal development and maintenance by decreasing gonadotropin synthesis and release in male quail. Endocrinology. 2006;147(3):1187–94. doi: 10.1210/en.2005-1178. [DOI] [PubMed] [Google Scholar]
- 22.Ubuka T, Morgan K, Pawson AJ, Osugi T, Chowdhury VS, Minakata H, Tsutsui K, Millar RP, Bentley GE. Identification of human GnIH homologs, RFRP-1 and RFRP-3, and the cognate receptor, GPR147 in the human hypothalamic pituitary axis. PLoS One. 2009;4(12):e8400. doi: 10.1371/journal.pone.0008400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ubuka T, Lai H, Kitani M, Suzuuchi A, Pham V, Cadigan PA, Wang A, Chowdhury VS, Tsutsui K, Bentley GE. Gonadotropin-inhibitory hormone identification, cDNA cloning, and distribution in rhesus macaque brain. J Comp Neurol. 2009;517(6):841–55. doi: 10.1002/cne.22191. [DOI] [PubMed] [Google Scholar]
- 24.Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149(11):5770–82. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith JT, Clarke IJ. Gonadotropin inhibitory hormone function in mammals. Trends Endocrinol Metab. 2010;21(4):255–60. doi: 10.1016/j.tem.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Sari IP, Rao A, Smith JT, Tilbrook AJ, Clarke IJ. Effect of RF-amide-related peptide-3 on luteinizing hormone and follicle-stimulating hormone synthesis and secretion in ovine pituitary gonadotropes. Endocrinology. 2009;150(12):5549–56. doi: 10.1210/en.2009-0775. [DOI] [PubMed] [Google Scholar]
- 27.Clarke IJ, Sari IP, Qi Y, Smith JT, Parkington HC, Ubuka T, Iqbal J, Li Q, Tilbrook A, Morgan K, Pawson AJ, Tsutsui K, Millar RP, Bentley GE. Potent action of RFamide-related peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology. 2008;149(11):5811–21. doi: 10.1210/en.2008-0575. [DOI] [PubMed] [Google Scholar]
- 28.Ducret E, Anderson GM, Herbison AE. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology. 2009;150(6):2799–804. doi: 10.1210/en.2008-1623. [DOI] [PubMed] [Google Scholar]
- 29.Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M. Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J Physiol. 2009;587(Pt 7):1401–11. doi: 10.1113/jphysiol.2008.166447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson GM, Relf HL, Rizwan MZ, Evans JJ. Central and peripheral effects of RFamide-related peptide-3 on luteinizing hormone and prolactin secretion in rats. Endocrinology. 2009;150(4):1834–40. doi: 10.1210/en.2008-1359. [DOI] [PubMed] [Google Scholar]
- 31.Kadokawa H, Shibata M, Tanaka Y, Kojima T, Matsumoto K, Oshima K, Yamamoto N. Bovine C-terminal octapeptide of RFamide-related peptide-3 suppresses luteinizing hormone (LH) secretion from the pituitary as well as pulsatile LH secretion in bovines. Domest Anim Endocrinol. 2009;36(4):219–24. doi: 10.1016/j.domaniend.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Murakami M, Matsuzaki T, Iwasa T, Yasui T, Irahara M, Osugi T, Tsutsui K. Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. J Endocrinol. 2008;199(1):105–12. doi: 10.1677/JOE-08-0197. [DOI] [PubMed] [Google Scholar]
- 33.Rizwan MZ, Porteous R, Herbison AE, Anderson GM. Cells expressing RFamide-related peptide-1/3, the mammalian gonadotropin-inhibitory hormone orthologs, are not hypophysiotropic neuroendocrine neurons in the rat. Endocrinology. 2009;150(3):1413–20. doi: 10.1210/en.2008-1287. [DOI] [PubMed] [Google Scholar]
- 34.Pineda R, Garcia-Galiano D, Sanchez-Garrido MA, Romero M, Ruiz-Pino F, Aguilar E, Dijcks FA, Blomenrohr M, Pinilla L, van Noort PI, Tena-Sempere M. Characterization of the Potent Gonadotropin-Releasing Activity of RF9, a Selective Antagonist of RF-Amide-Related Peptides and Neuropeptide FF Receptors: Physiological and Pharmacological Implications. Endocrinology. 2010 doi: 10.1210/en.2009-1259. [DOI] [PubMed] [Google Scholar]
- 35.Seegal RF, Goldman BD. Effects of photoperiod on cyclicity and serum gonadotropins in the Syrian hamster. Biol Reprod. 1975;12(2):223–31. doi: 10.1095/biolreprod12.2.223. [DOI] [PubMed] [Google Scholar]
- 36.Levine JE. New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biol Reprod. 1997;56(2):293–302. doi: 10.1095/biolreprod56.2.293. [DOI] [PubMed] [Google Scholar]
- 37.Karsch FJ, Bowen JM, Caraty A, Evans NP, Moenter SM. Gonadotropin-releasing hormone requirements for ovulation. Biol Reprod. 1997;56(2):303–9. doi: 10.1095/biolreprod56.2.303. [DOI] [PubMed] [Google Scholar]
- 38.Schneider JE. Energy balance and reproduction. Physiol Behav. 2004;81(2):289–317. doi: 10.1016/j.physbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Qi Y, Oldfield BJ, Clarke IJ. Projections of RFamide-related peptide-3 neurones in the ovine hypothalamus, with special reference to regions regulating energy balance and reproduction. J Neuroendocrinol. 2009;21(8):690–7. doi: 10.1111/j.1365-2826.2009.01886.x. [DOI] [PubMed] [Google Scholar]
- 40.Clarke IJ, Qi Y, Puspita Sari I, Smith JT. Evidence that RF-amide related peptides are inhibitors of reproduction in mammals. Front Neuroendocrinol. 2009;30(3):371–8. doi: 10.1016/j.yfrne.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Wu M, Dumalska I, Morozova E, van den Pol A, Alreja M. Melanin-concentrating hormone directly inhibits GnRH neurons and blocks kisspeptin activation, linking energy balance to reproduction. Proc Natl Acad Sci U S A. 2009;106(40):17217–22. doi: 10.1073/pnas.0908200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirby ED, Geraghty AC, Ubuka T, Bentley GE, Kaufer D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc Natl Acad Sci U S A. 2009;106(27):11324–9. doi: 10.1073/pnas.0901176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bentley GE, Ubuka T, McGuire NL, Chowdhury VS, Morita Y, Yano T, Hasunuma I, Binns M, Wingfield JC, Tsutsui K. Gonadotropin-inhibitory hormone and its receptor in the avian reproductive system. Gen Comp Endocrinol. 2008;156(1):34–43. doi: 10.1016/j.ygcen.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Zhao S, Zhu E, Yang C, Bentley GE, Tsutsui K, Kriegsfeld LJ. RFamide-related peptide and messenger ribonucleic acid expression in mammalian testis: association with the spermatogenic cycle. Endocrinology. 2010;151(2):617–27. doi: 10.1210/en.2009-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ubuka T, Bentley GE, Ukena K, Wingfield JC, Tsutsui K. Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. Proc Natl Acad Sci U S A. 2005;102(8):3052–7. doi: 10.1073/pnas.0403840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maywood ES, Hastings MH. Lesions of the iodomelatonin-binding sites of the mediobasal hypothalamus spare the lactotropic, but block the gonadotropic response of male Syrian hamsters to short photoperiod and to melatonin. Endocrinology. 1995;136(1):144–53. doi: 10.1210/endo.136.1.7828525. [DOI] [PubMed] [Google Scholar]
- 47.Maywood ES, Bittman EL, Hastings MH. Lesions of the melatonin- and androgen-responsive tissue of the dorsomedial nucleus of the hypothalamus block the gonadal response of male Syrian hamsters to programmed infusions of melatonin. Biol Reprod. 1996;54(2):470–7. doi: 10.1095/biolreprod54.2.470. [DOI] [PubMed] [Google Scholar]
- 48.Lewis D, Freeman DA, Dark J, Wynne-Edwards KE, Zucker I. Photoperiodic control of oestrous cycles in Syrian hamsters: mediation by the mediobasal hypothalamus. J Neuroendocrinol. 2002;14(4):294–9. doi: 10.1046/j.1365-2826.2002.00779.x. [DOI] [PubMed] [Google Scholar]
- 49.Gingerich S, Wang X, Lee PK, Dhillon SS, Chalmers JA, Koletar MM, Belsham DD. The generation of an array of clonal, immortalized cell models from the rat hypothalamus: analysis of melatonin effects on kisspeptin and gonadotropin-inhibitory hormone neurons. Neuroscience. 2009;162(4):1134–40. doi: 10.1016/j.neuroscience.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 50.Revel FG, Saboureau M, Pevet P, Simonneaux V, Mikkelsen JD. RFamide-related peptide gene is a melatonin-driven photoperiodic gene. Endocrinology. 2008;149(3):902–12. doi: 10.1210/en.2007-0848. [DOI] [PubMed] [Google Scholar]
- 51.Mason A, Duffy S, Zhao S, Ubuka T, Bentley G, Tsutsui K, Silver R, Kriegsfeld L. Photoperiod and Reproductive Condition are Associated with Changes in RFamide-Related Peptide (RFRP) Expression in Syrian Hamsters (Mesocricetus auratus) J Biol Rhythms. doi: 10.1177/0748730410368821. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bittman EL, Bartness TJ, Goldman BD, DeVries GJ. Suprachiasmatic and paraventricular control of photoperiodism in Siberian hamsters. Am J Physiol. 1991;260(1 Pt 2):R90–101. doi: 10.1152/ajpregu.1991.260.1.R90. [DOI] [PubMed] [Google Scholar]
- 53.Bartness TJ, Goldman BD, Bittman EL. SCN lesions block responses to systemic melatonin infusions in Siberian hamsters. Am J Physiol. 1991;260(1 Pt 2):R102–12. doi: 10.1152/ajpregu.1991.260.1.R102. [DOI] [PubMed] [Google Scholar]
- 54.Paul MJ, Pyter LM, Freeman DA, Galang J, Prendergast BJ. Photic and nonphotic seasonal cues differentially engage hypothalamic kisspeptin and RFamide-related peptide mRNA expression in Siberian hamsters. J Neuroendocrinol. 2009;21(12):1007–14. doi: 10.1111/j.1365-2826.2009.01924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dardente H, Birnie M, Lincoln GA, Hazlerigg DG. RFamide-related peptide and its cognate receptor in the sheep: cDNA cloning, mRNA distribution in the hypothalamus and the effect of photoperiod. J Neuroendocrinol. 2008;20(11):1252–9. doi: 10.1111/j.1365-2826.2008.01784.x. [DOI] [PubMed] [Google Scholar]