Abstract
Background
Stress incontinence in men is a rare, usually iatrogenic condition. Its prevalence can be expected to rise in the future because of the increasingly common performance of radical prostatectomy. Most men who have undergone prostatectomy experience a transient disturbance of urinary continence. Such disturbances are only rarely due to structural damage to the sphincter apparatus and therefore have a good prognosis for spontaneous recovery.
Method
Selective literature review.
Results
Pelvic floor training and/or pharmacotherapy can be used for more rapid restoration of subjectively satisfactory urinary continence. If the sphincter is intact, continence can also be regained in the early postoperative period through the submucosal injection of bulking agents. Incontinent patients whose urinary sphincter is dysfunctional because of denervation or direct injury to striated muscle can now be treated with a variety of surgical techniques. The implantation of an artificial sphincter is the gold standard of therapy. Properly selected and informed patients can also be treated with minimally invasive procedures, such as the creation of a male suburethral sling, although the experience with such procedures to date has not been extensive.
Conclusion
Post-prostatectomy incontinence has a good prognosis and should thus be treated conservatively at first. If it nonetheless persists, surgical treatment is indicated for patients who choose it after being fully informed about their options.
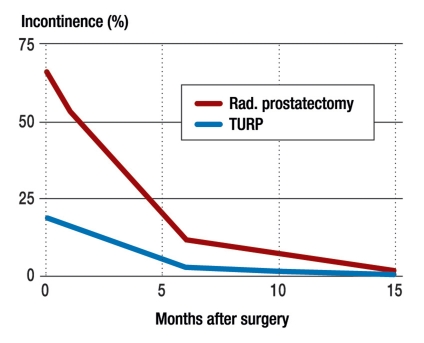
The most common cause of stress incontinence in men is iatrogenically or traumatically induced insufficiency of the external urethral sphincter (e1). The rate of incontinence after radical prostatectomy, as reported in the literature, ranges from just below 1% to 90% (1– 3). This very wide divergence of reported incontinence rates can be traced to differences in operative technique as well as to divergent definitions of incontinence. Prostate surgery has undergone major technical refinements in recent years. The nerves can be preserved more often today than in the past, because the relevant anatomy is better understood. Definitions of “continence” range from total dryness to the use of multiple incontinence pads per day. A further important aspect is the time point at which continence is assessed. Nearly all patients experience transient stress incontinence of the first or second degree. The problem is usually due, at first, to irritative symptoms and the urge incontinence associated with them; this is combined with weakness of the remaining external sphincter muscle, which faces a more difficult task than in the physiological situation, as structures adjacent to it that normally provide additional resistance have been surgically removed. In general, there is a good prognosis for the return of continence within one year after surgery (figure 1) (4, 5).
Figure 1.
The spontaneous course of urinary incontinence after prostatic surgery (from von Kampen et al. [5]).
TURP, transurethral resection of the prostate;
rad., radical
Stress incontinence markedly impairs the quality of life of the affected men, who regard the need to wear incontinence pads as even more bothersome than postoperative erectile dysfunction (6). As the population as a whole ages, pelvic surgery will become more common and we will be confronted with the problem of postoperative stress incontinence in ever greater numbers of men, despite improvements in surgical and radiotherapeutic technique. The current prevalence of stress incontinence in men is below 1% (7).
The desire for social continence is strong, and many different therapeutic innovations have been designed for this purpose. Social continence is defined as the ability of the patient to participate in his normal social activities without limitation. This usually corresponds to the use of no more than one incontinence pad per day.
We here present the treatment options for stress incontinence in men and discuss them critically. This article is based on a selective search for relevant literature in the database of the U. S. National Library of Medicine. We performed a general search for the terms “male stress incontinence” and “post-prostatectomy incontinence,” as well as specific searches for each of the individual therapeutic and surgical techniques under discussion. Prospective randomized studies are lacking for many of them, and thus the overall state of the evidence is weak.
Pathogenesis and diagnostic evaluation
Continence after prostatectomy depends on pre-, intra-, and postoperative factors. The preoperative factors include the patient’s age and continence status before surgery. Intraoperatively, nerve preservation, the surgeon’s experience, and preservation of the neck of the bladder all play an important role. Postoperatively, good patient compliance can be helpful; the pelvic floor can also undergo postoperative changes (sinking) (8).
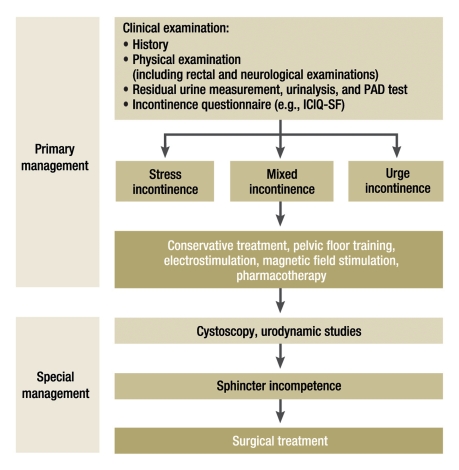
The most important step in the treatment of stress incontinence in men is active discussion of the subject—still sometimes wrongly regarded as taboo—between the physician and the patient. Men who have undergone pelvic surgery should be directly asked by their doctors about their continence situation. Thus, the initial diagnostic step is history-taking, including a medication history and any associated illnesses. Next, a physical examination is performed, including both a digital rectal examination and a directed neurological examination of the sacral region. Ultrasonography for the measurement of residual urine volume, urinalysis, a PAD test, a voiding diary, and an incontinence questionnaire all yield useful supplementary information (e.g., the Incontinence Questionnaire–Short Form, ICIQ-SF). After this basic diagnostic evaluation, some form of conservative therapy can be initiated, such as pelvic floor training, biofeedback, electrostimulation, or pharmacotherapy. If these measures are ineffective, further diagnostic assessment with cystoscopy, urodynamic testing, and (in some cases) radiological studies is performed, after which surgical treatment options may have to be considered. These range from minimally invasive techniques, such as the paraurethral injection of bulking agents, to sling plasties and to the implantation of artificial sphincter systems (figure 2) (e2).
Figure 2.
A diagnostic and therapeutic algorithm for the management of stress incontinence after radical prostatectomy (modified from the 2009 EAU guideline [e2]); ICIQ-SF, Incontinence Questionnaire–Short Form
Conservative treatment
Pelvic floor training, biofeedback, electrostimulation, magnetic field stimulation
The initial option in the treatment of post-surgical stress incontinence is pelvic floor training, which is sometimes combined with biofeedback. Two Cochrane meta-analyses from the years 2004 and 2007 involved a tabulation of results from 6 and 10 randomized studies, respectively, and yielded the finding that continence improved more rapidly in patients who underwent pelvic floor training than in control patients. This difference, however, was no longer significant 6 to 12 months after surgery (table 1) (9, 10). The main role of pelvic floor training is thus to shorten the period of incontinence that normally ends in any case when the condition takes its usual, favorable spontaneous course.
Table 1. Comparison of two Cochrane meta-analyses*1.
| Author | Number of studies | Treatment | Outcome |
| Hunter et al. 2004 (9) | 6 | Pelvic floor training Biofeedback Electrostimulation Control group | No significant difference at 6 or 12 months |
| Moore et al. 2007 (10) | 10 |
*1 These meta-analyses showed no significant advantage of conservative (non-surgical) treatment over the spontaneous course of the condition at 6 or 12 months
Electrical and magnetic field stimulation are two further options for physical therapy. In a three-armed study comparing pelvic floor training with electrical and magnetic field stimulation, the loss of urine was initially comparable in all three groups (more than 650 g per 24 hours in each). At 4 weeks, however, the magnetic and electrical stimulation groups were found to have a significantly smaller loss of urine than the pelvic floor training group (72 and 83 g/ 24 h, respectively, compared to 175 g/24 h). At 6 months, the three groups no longer differed from one another, with the daily loss of urine being less than 10 g in all three (11).
Pharmacotherapy
Many published case series concern the pharmacological treatment of stress incontinence in men. Table 2 provides an overview of the medications that have been tried. Even in the series with good results, the level of evidence is poor. The serotonin and noradrenaline reuptake inhibitor duloxetine is a new treatment option. It improves continence through relaxation of the detrusor muscle and simultaneous contraction of urethral smooth muscle, as well as increased tone in the striated muscle of the sphincter, which is mediated by Onuf’s nucleus in the sacral segments of the spinal cord. In a randomized study of 112 men who had undergone radical prostatectomy, the patients who were taking duloxetine had significantly better continence-related life quality parameters in the first 16 weeks than the patients in the control group (12). This drug, however, has not been approved for the treatment of stress incontinence in men, and is thus an off-label application at present. The patient must be informed of this fact, and the conversation should be documented in the patient’s chart, before any treatment with duloxetine is begun.
Table 2. Overview of clinical trials of pharmacotherapy for stress incontinence in men.
| Medication | n (Men) | Study type | Outcome | Author |
| Alpha agonists | ||||
| Ephedrine | 20 (+18 women) | Case series | 27 / 38 (71.1%) no incontinence or drops only | Diokno et al. (e3) |
| Midodrine | 5 | Case series | 5 / 5 (100%) improvement | Nito et al. (e4) |
| Beta agonists | ||||
| Glenbuterol | 14 | Cohort study | 9 / 14 (64.3%) reduced use of incontinence pads | Noguchi et al. (e5) |
| Glenbuterol | 72 | Case series | 55 / 72 (76.3%) fully continent | Zozikov et al. (e6) |
| Serotonin- and noradrenaline-reuptake inhibitors | ||||
| Imipramine | 5 | Case series | 3 / 5 (60%) improvement or cure | Reid et al. (e7) |
| Duloxetine | 20 | Case series | 7 / 18 (38.9%)* reduced use of incontinence pads | Schlenker et al. (e8) |
| Duloxetine | 18 | Case series | 46.7% less loss of urine (from 124 to 58 g by PAD test) | Zahariou et al. (e9) |
| Duloxetine | 112 | Randomized trial | 39 / 50 (78%) (treatment arm) vs. 27 / 52 (51.9%) (placebo arm)* Reduced use of incontinence pads at 16 weeks | Filocamo et al. (12) |
*1 significant differences
Surgical treatment
If primary conservative therapy fails and the findings of a further diagnostic evaluation warrant surgery, a variety of surgical procedures are available to treat postoperative stress incontinence.
Paraurethral injection therapy—sphincter injection therapy—bulking agents
For over 40 years, submucosal injections of many different substances have been tried as a means of adapting the urethral mucosa so that it can support the sphincter apparatus, with resulting urinary continence. A variety of biodegradable and non-biodegradable substances are in use. The most commonly used substances today are polydimethylsiloxane and dextranomer-hyaluronic acid copolymer. Many studies have shown that the injection of bulking agents improves continence by at least one degree in a large percentage of patients (up to 97%). It must be noted, however, that most of these studies were small, nonrandomized case series, and thus the overall level of evidence is low. Table 3 provides an overview of the relevant publications.
Table 3. Overview of paraurethral injection therapy for urethral bulking in the treatment of stress incontinence in men.
| Medication | N | Study type | Outcome | Author |
| Polydimethyl-siloxane | 50 | Case series | 34 / 50 (68%) improvement after the 1st injection, | Kylmala et al. (e10) |
| 42 / 50 (84%) after the 2nd injection | ||||
| Polydimethyl-siloxane | 23 | Randomized trial | 8 / 10 (80%) improvement of 1st- or 2nd-degree incontinence, | Imamoglu et al. (e11) |
| 3 / 13 (23%) improvement of 3rd-degree incontinence at 48 months | ||||
| Polydimethyl-siloxane | 44 | Case series | 14 / 15 (93%) improvement of 1st- or 2nd degree incontinence, | Schneider et al. (13) |
| 17 / 29 (59%) improvement of 3rd-degree incontinence at 26 months | ||||
| Dextranomer-hyaluronic acid copolymer | 72 | Case series | 70 / 72 (97%) improvement at 4–8 weeks | Alloussi et al. (e12) |
In our own case series of bulking with polydimethylsiloxane, improvement was obtained by 93% of men with first- or second-degree incontinence, but only 59% of men with third-degree incontinence. The timing of treatment also seems to be critical. Patients who are treated early with sphincter injection therapy (in order, one might say, to hasten the slow spontaneous improvement that would occur without it) apparently benefit the most from it, while those who have been incontinent for more than one year are less likely to respond to treatment (13). Loss of the therapeutic effect is another problem: A repeated intervention is often needed, yet reinjection of the substance can induce an inflammatory reaction leading to scarring of the submucosal vascular plexus and thus to an impairment of urethral elasticity and of the passive function of the closure apparatus. The final result may be a “frozen urethra,” which can make further treatment even more difficult (3). Injections of bulking agents cause no additional difficulties for the subsequent performance of alloplastic sphincter implantation, as long as the injections are performed for proper indications and with correct technique, i.e., under direct vision and exclusively in the area of the sphincter (14).
Paraurethral balloon compression
The system consists of a balloon connected by a tube 12 cm to 14 cm in length to an injection port through which the degree of balloon inflation can be adjusted. It is implanted through a perineal incision under x-ray guidance. One balloon is positioned on either side of the bladder neck, and 1 mL of contrast solution is instilled into each. The two ports are placed under the skin of the scrotum. Once the system has healed into place, the balloons are filled in small increments through their respective ports, until satisfactory continence is achieved. In two case series, continence improved in 59% and 90% of patients, but the revision rates were high: 27.4% and 30.6% (e13, e14). Serious complications of paraurethral balloon implantation, including rectal perforation, have also been described (e15).
Suburethral slings (“male slings”)
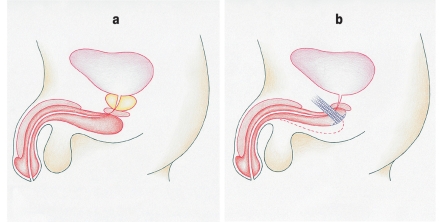
The first publication on sling plasty as a treatment of urinary incontinence in men appeared at the beginning of the 20th century. The method is based on the principle of passive, semi-circumferential urethral compression. This does not impair perfusion of the dorsal portion of the urethra and thus carries a lower risk of urethral atrophy than artificial sphincter implantation (figure 3) (15). Many modifications of the method have been described, involving the use of different materials for the spring as well as various different surgical approaches, positions for the sling, and methods for placing and anchoring it. Among the many sling systems available, we have selected a few for brief discussion in the following paragraphs.
Figure 3.
The underlying principle of sling plasty in the male patient: passive, semi-circumferential urethral compression
Autologous fascial strips
Thüroff et al. successfully used an autologous fascial sling, passed around the bulbous urethra and anchored to the rectus fascia, in 20 patients (e16). The average daily use of incontinence pads was reduced from 7.8 to 1.5. The drawback with this technically demanding operation is that it requires precise setting of the sling tension so that the patient can achieve adequate continence without overcorrection (4).
Sling systems anchored to bone
In systems of this type, a silicon-coated prolene mesh is anchored to the pubic bone with six bone screws in such a way that it passes around the bulbous urethra, raising the urethral resistance by 60 cm H2O. Compressing the urethra in this way improves continence.
Adjustable sling systems
In these systems, a prolene sling is connected to a so-called variotensor device that lies superficial to the rectus fascia. The sling tension can be adjusted afterward with a screw mechanism.
Trans-obtuator suburethral bands
Just as in the treatment of incontinence in women, a prolene sling can be pulled through the obturator foramen to correct post-surgical pelvic floor sinking in men. The system is passed around the bulbous urethra, and its two ends are pulled up. The pelvic floor is thereby elevated without tension and the urethra is restored to its normal position, with improved continence as the result. This mechanism is a unique feature of trans-obturator bands among all types of sling systems.
A number of case series of balloon compression and sling plasties are presented in Table 4. In summary, these minimally invasive surgical methods yield satisfactory rates of continence for mildly or moderately incontinent patients, with a low rate of complications. Their advantage over an artificial sphincter lies in the ease of implantation. Nonetheless, the currently available data do not permit any definitive judgment on these promising techniques.
Table 4. Overview of sling and balloon compression systems for the surgical treatment of stress incontinence in men.
| Type of compression system | n | Study type | Outcome | Author |
| Balloon | 117 | Case series | 105 / 117 (90%) improvement, | Hubner et al. (e13) |
| 32 / 117 (27,4%) revisions at 13 months | ||||
| 62 | Case series | 36 / 62 (59%) improvement, 19 / 62 (30,6%) revisions | Lebret et al. (e14) | |
| Autologous fascial sling | 20 | Case series | Use of incontinence pads reduced from 7.8 to 1.5 per day | Thüroff et al. (e16) |
| Sling attached to bone | 16 | Case series | 14 / 16 (88%) continent at 12.2 months, | Madjar et al. (e17) |
| no complications or non-responders | ||||
| 50 | Case series | 38 / 50 (76%) improvement, 12 / 50 (24%) non-responders | Fassi-Fehri et al. (e18) | |
| Adjustable sling | 51 | Case series | 43 / 51 (84,3%) improvement, | Sousa et al. (e19) |
| 8 / 51 (15,7%) non-responders at 32 months | ||||
| 48 | Case series | 35 / 48 (73%) cure rate, | Romano et al. (e20) | |
| no major complications at 7.5 months | ||||
| Trans-obturator sling | 20 | Case series | 17 / 20 (85%) improvement, no major complications | De Leval et al. (e21) |
| 20 | Case series | 14 / 20 (70%) improvement | Rheder et al. (e22) | |
| 67 | Case series | 60 / 67 (90%) improvement | Gozzi et al. (e23) |
Artificial urethral sphincter
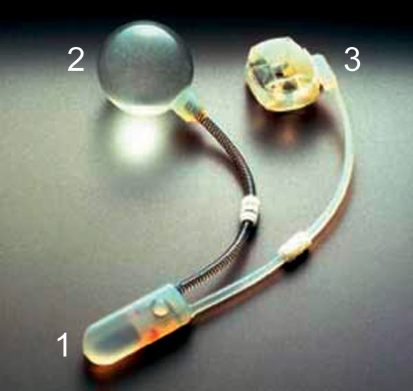
The artificial sphincter is the gold standard in the treatment of stress incontinence in men. No other method achieves comparable continence rates in patients with post-prostatectomy incontinence (8). The concept of treatment is to mimic the natural function of the sphincter muscle with circumferential compression of the bladder outlet. The first artificial sphincter system was implanted in 1972 by Scott, Bradley, and Timm (16). Their system has since undergone many modifications, leading up to the model that is currently used (figure 4). Other systems exist as well, such as that of Craggs (17), which includes a second pressure balloon that raises the pressure in the system when the intra-abdominal pressure rises. Unlike all other methods, which exert a steady, continuous pressure, the hydraulic sphincter exerts a variable degree of resistance to urinary outflow.
Figure 4.
The AMS 800 artificial sphincter consists of three components:
1. A pump to empty the cuff and permit micturition
2. A pressure-regulating balloon for the transmission of a defined pressure to the cuff
3. A cuff for urethral compression
The cuff is placed around the bulbous urethra to treat post-prostatectomy incontinence, or at the neck of the bladder to treat incontinence after transurethral resection of the prostate (TURP). The pressure-regulating balloon is best implanted intraperitoneally to enable optimal transmission of the abdominal pressure. Alternatively, it can be placed in the cavity of Retzius (retropubic space), as some surgeons prefer to do because of the shorter operating time. The pump mechanism and the control unit are placed in the scrotum.
The patient must be adequately informed before surgery in order for the implantation and use of a hydraulic sphincter to succeed. The patient will need to muster a certain degree of manual dexterity and mental compliance to be able to use the system independently after surgery. Contraindications include poor tissue quality at the site of the prospective implant, infection, subvesical obstruction with residual urine volume, detrusor hyperactivity, and a bladder capacity of less than 200 mL.
The observed functional outcome is an improvement of continence, with the use of no more than one incontinence pad per day, in 75% to 90% of patients undergoing the procedure (18– 20). There are, however, a number of potential complications. Artificial sphincters are fraught with a considerable risk of infection. One reason for this is the site of implantation (next to the urethra), another is the large volume of foreign material that has to be implanted. Reported infection rates in the literature range from 1.8% to 10% (21, 22). Furthermore, circumferential pressure on the urethra causes tissue atrophy in the long term, and this can actually worsen stress incontinence to an extent that necessitates reoperation. A need for revision owing to this intrinsic drawback of the system arises in as many as 9% of all patients so treated (23). Urethral erosion by the cuff, when it arises, is a serious complication that usually necessitates total removal of the system; its frequency, which partly depends on the surgeon’s experience with the system, has been reported at 1% to 8.1% (22, 24). The hydraulic sphincter, a mechanical system with three components, is subject to continual wear and tear. In a recent study, Kim et al. (22) documented a 23.4% rate of system defects, although rates in the older literature were approximately 8% (25). System defects are thus the reason for about half of all revision procedures for artificial sphincter systems.
Despite these rather high rates of complications and reinterventions, the subjective rate of satisfaction is 85% to 95% among patients with artificial sphincters for the treatment of incontinence after radical prostatectomy (19). The relevant studies are summarized in Table 5.
Table 5. Continence state and complications after the implantation of an artificial sphincter system.
| Author | n | Study type | Outcome |
| Perez et al. (19) | 75 | Case series | 56 / 75 (75%) max. 1 incontinence pad |
| Fleshner et al. (18) | 30 | Case series | 26 / 30 (87%) max. 1 incontinence pad |
| Trigo-Rocha et al. (20) | 40 | Prospective trial | 36 / 40 (90%) max. 1 incontinence pad, 8 / 40 (20%) revisions at 53 months |
| Montague et al. (21) | 166 | Case series | Up to 10% infection of the system by 41 months |
| Elliott et al. (24) | 400 | Case series | 1–3% urethral erosion at 68.8 months |
| Litwiller et al. (23) | 65 | Case series | 3–9% urethral atrophy at 23.4 months |
| Leo et al. (25) | 144 | Case series | 12 / 144 (8%) system defects by 28 months |
Overview
Pelvic floor training should always be used as primary treatment, with or without biofeedback. Even though this form of therapy does not yield a higher long-term success rate than the spontaneous course of the condition, it does shorten the period of time in which the patient is incontinent. Pharmacotherapy is currently a matter of great interest, but no general recommendation can be made yet on the basis of the available data. The same can be said of paraurethral injection therapy, which can sometimes shorten the postoperative period of incontinence but does not alter the long-term prognosis. Some of the current surgical treatments, including balloon compression and sling plasties, have shown promising results. The artificial sphincter remains the gold standard of treatment for intractable stress incontinence in men, despite its rather high complication rate and the availability of newer, less invasive techniques. Table 6 contains a summary of the evidence levels and recommendation grades of each of the treatments discussed in this article.
Table 6. Evidence levels and recommendation grades for different methods of treatment *1.
| Method of treatment | Evidence level | Recommendation grade |
| Pelvic floor training | 2 | B |
| Duloxetine | 3–4 | C |
| Paraurethral injection | 3 | B |
| Sling systems | 3 | B |
| Artificial sphincter systems | 1 | A |
*1 modified from Bauer et al. (8)
Case Illustration.
A 52-year-old man presented with recurrent stress incontinence twelve months after radical prostatectomy for localized prostatic carcinoma. He had suffered from stress incontinence after surgery and had undergone a transobturator sling plasty to treat it, with very good initial results. Later on in his postoperative course, a penile prosthesis was implanted to treat erectile dysfunction. From then onward, he noted increased involuntary loss of urine, particularly when the prosthesis was activated. To investigate this problem, A PAD test was performed and revealed 36 mL of urine loss per hour. Cystoscopy revealed a normal-appearing bladder, with a morphologically intact sphincter apparatus. A urodynamic study revealed a bladder capacity of 450 mL and no autonomous detrusor contractions. Provocation by coughing induced urinary dribbling, and the outlet resistance was diminished. On the basis of these findings, a neurogenic disturbance of the sphincter apparatus was diagnosed. Improved continence had been achieved, at first, by repositioning the bladder neck with the sling plasty; later, however, the bladder neck and the sling plasty were displaced caudally each time the cylinders of the implanted penile prosthesis were inflated, resulting in recurrent incontinence. This new problem was treated with the implantation of an artificial sphincter. Thereafter, the patient needed to use only one small incontinence pad per day. He said that he was losing only negligible amounts of urine, even when the penile prosthesis was activated. A PAD test revealed 1 to 2 mL of urine loss per hour.
Key Messages.
Many men suffer from stress incontinence after radical prostatectomy.
The subject of incontinence in men is still often wrongly regarded as taboo.
Stress incontinence after radical prostatectomy often takes a favorable spontaneous course.
Therefore, an adequate trial of conservative therapy should be given before any surgical procedure is considered.
Incontinence requires treatment.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
PD Dr. Sperling has received lecture honoraria from American Medical Systems. Dr. Kaufmann has received lecture honoraria from Medtronic.
Dr. Börgermann, Prof. Stöhrer, and Prof. Rübben state that they have no conflict of interest as defined by the International Committee of Medical Journal Editors.
References
- 1.Burkhard FC, Kessler TM, Fleischmann A, Thalmann GN, Schu-macher M, Studer UE. Nerve sparing open radical retropubic prostatectomy—does it have an impact on urinary continence? J Urol. 2006;176(1):189–195. doi: 10.1016/S0022-5347(06)00574-X. [DOI] [PubMed] [Google Scholar]
- 2.Rudy DC, Woodside JR, Crawford ED. Urodynamic evaluation of incontinence in patients undergoing modified Campbell radical retropubic prostatectomy: a prospective study. J Urol. 1984;132(4):708–712. doi: 10.1016/s0022-5347(17)49836-3. [DOI] [PubMed] [Google Scholar]
- 3.Wei JT, Dunn RL, Sandler HM, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20(2):557–566. doi: 10.1200/JCO.2002.20.2.557. [DOI] [PubMed] [Google Scholar]
- 4.Hampel C, Gillitzer R, Wiesner C, Thuroff JW. Established treatment options for male stress urinary incontinence. Urologe A. 2007;46(3):244–246. doi: 10.1007/s00120-007-1304-y. [DOI] [PubMed] [Google Scholar]
- 5.Van Kampen M, De Weerdt W, Van Poppel H, Baert L. Urinary incontinence following transurethral, transvesical and radical prostatectomy. Retrospective study of 489 patients. Acta Urol Belg. 1997;65(4):1–7. [PubMed] [Google Scholar]
- 6.Fowler FJ, Jr, Barry MJ, Lu-Yao G, Wasson J, Roman A, Wennberg J. Effect of radical prostatectomy for prostate cancer on patient quality of life: results from a Medicare survey. Urology. 1995;45(6):1007–1013. doi: 10.1016/s0090-4295(99)80122-8. [DOI] [PubMed] [Google Scholar]
- 7.Hampel C, Artibani W, Espuna PM, et al. Understanding the burden of stress urinary incontinence in Europe: a qualitative review of the literature. Eur Urol. 2004;46(1):15–27. doi: 10.1016/j.eururo.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Bauer RM, Bastian PJ, Gozzi C, Stief CG. Postprostatectomy incontinence: all about diagnosis and management. Eur Urol. 2009;55(2):322–333. doi: 10.1016/j.eururo.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Hunter KF, Moore KN, Cody DJ, Glazener CM. Conservative management for postprostatectomy urinary incontinence. Cochrane Database Syst Rev. 2004;(2) doi: 10.1002/14651858.CD001843.pub2. CD001843. [DOI] [PubMed] [Google Scholar]
- 10.Moore KN, Cody DJ, Glazener CM. Conservative management for post prostatectomy urinary incontinence. Cochrane Database Syst Rev. 2001;(2) doi: 10.1002/14651858.CD001843. CD001843. [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama T, Nishiguchi J, Watanabe T, et al. Comparative study of effects of extracorporeal magnetic innervation versus electrical stimulation for urinary incontinence after radical prostatectomy. Urology. 2004;63(2):264–267. doi: 10.1016/j.urology.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Filocamo MT, Li M, V, Del Popolo G, et al. Pharmacologic treatment in postprostatectomy stress urinary incontinence. Eur Urol. 2007;51(6):1559–1564. doi: 10.1016/j.eururo.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Schneider T, Sperling H, Rossi R, Schmidt S, Rubben H. Do early injections of bulking agents following radical prostatectomy improve early continence? World J Urol. 2005;23(5):338–342. doi: 10.1007/s00345-005-0010-x. [DOI] [PubMed] [Google Scholar]
- 14.Bross S, Kwon ST, Peter S, Honeck P. New techniques for surgical treatment of postoperative male stress incontinence. Urologe A. 2007;46(3):257–263. doi: 10.1007/s00120-007-1300-2. [DOI] [PubMed] [Google Scholar]
- 15.Hampel C, Hohenfellner M, Melchior S, Thuroff JW. Sling-plasty in therapy of female urinary incontinence. Urologe A. 2001;40(4):274–280. doi: 10.1007/s001200170036. [DOI] [PubMed] [Google Scholar]
- 16.Scott FB, Bradley WE, Timm GW. Treatment of urinary incontinence by implantable prosthetic sphincter. Urology. 1973;1(3):252–259. doi: 10.1016/0090-4295(73)90749-8. [DOI] [PubMed] [Google Scholar]
- 17.Craggs MD, Chaffey NJ, Mundy AR. A preliminary report on a new hydraulic sphincter for controlling urinary incontinence. J Med Eng Technol. 1991;15(2):58–62. doi: 10.3109/03091909109009969. [DOI] [PubMed] [Google Scholar]
- 18.Fleshner N, Herschorn S. The artificial urinary sphincter for post-radical prostatectomy incontinence: impact on urinary symptoms and quality of life. J Urol. 1996;155(4):1260–1264. [PubMed] [Google Scholar]
- 19.Perez LM, Webster GD. Successful outcome of artificial urinary sphincters in men with post-prostatectomy urinary incontinence despite adverse implantation features. J Urol. 1992;148(4):1166–1170. doi: 10.1016/s0022-5347(17)36850-7. [DOI] [PubMed] [Google Scholar]
- 20.Trigo-Rocha F, Gomes CM, Pompeo AC, Lucon AM, Arap S. Pro-spective study evaluating efficacy and safety of Adjustable Continence Therapy (ProACT) for post radical prostatectomy urinary incontinence. Urology. 2006;67(5):965–969. doi: 10.1016/j.urology.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Montague DK. The artificial urinary sphincter (AS 800): experience in 166 consecutive patients. J Urol. 1992;147(2):380–382. doi: 10.1016/s0022-5347(17)37242-7. [DOI] [PubMed] [Google Scholar]
- 22.Kim SP, Sarmast Z, Daignault S, Faerber GJ, McGuire EJ, Latini JM. Long-term durability and functional outcomes among patients with artificial urinary sphincters: a 10-year retrospective -review from the University of Michigan. J Urol. 2008;179(5):1912–1916. doi: 10.1016/j.juro.2008.01.048. [DOI] [PubMed] [Google Scholar]
- 23.Litwiller SE, Kim KB, Fone PD, White RW, Stone AR. Post-prostatectomy incontinence and the artificial urinary sphincter: a long-term study of patient satisfaction and criteria for success. J Urol. 1996;156(6):1975–1980. doi: 10.1016/s0022-5347(01)65408-9. [DOI] [PubMed] [Google Scholar]
- 24.Elliott DS, Barrett DM. Mayo Clinic long-term analysis of the functional durability of the AMS 800 artificial urinary sphincter: a review of 323 cases. J Urol. 1998;159(4):1206–1208. [PubMed] [Google Scholar]
- 25.Leo ME, Barrett DM. Success of the narrow-backed cuff design of the AMS800 artificial urinary sphincter: analysis of 144 patients. J Urol. 1993;150(5 Pt 11):412–414. doi: 10.1016/s0022-5347(17)35793-2. [DOI] [PubMed] [Google Scholar]
- e1.Nitti VW, Blaivas JG. Urinary Incontinence. In: Walsh P, Retik A, Vaughan ED, Wein A, editors. Campbell’s Urology. New York: Saunders; 2010. pp. 2047–2049. [Google Scholar]
- e2.Schröder A, Andersson K-E, Artibani W, et al. Guidlines on Urinary Incontinence. 2009. [Google Scholar]
- e3.Diokno AC, Taub M. Ephedrine in treatment of urinary incontinence. Urology. 1975;5(5):624–625. doi: 10.1016/0090-4295(75)90113-2. [DOI] [PubMed] [Google Scholar]
- e4.Nito H. Clinical effect of midodrine hydrochloride on the patients with urinary incontinence. Hinyokika Kiyo. 1994;40(1):91–94. [PubMed] [Google Scholar]
- e5.Noguchi M, Eguchi Y, Ichiki J, Yahara J, Noda S. Therapeutic efficacy of clenbuterol for urinary incontinence after radical prostatectomy. Int J Urol. 1997;4(5):480–483. doi: 10.1111/j.1442-2042.1997.tb00289.x. [DOI] [PubMed] [Google Scholar]
- e6.Zozikov B, Kunchev SI, Varlev C. Application of clenbuterol in the treatment of urinary incontinence. Int Urol Nephrol. 2001;33(3):413–416. doi: 10.1023/a:1019565229886. [DOI] [PubMed] [Google Scholar]
- e7.Reid GF, Fitzpatrick JM, Worth PH. The treatment of patients with urinary incontinence after prostatectomy. Br J Urol. 1980;52(6):532–534. doi: 10.1111/j.1464-410x.1980.tb03108.x. [DOI] [PubMed] [Google Scholar]
- e8.Schlenker B, Gratzke C, Reich O, Schorsch I, Seitz M, Stief CG. Preliminary results on the off-label use of duloxetine for the treatment of stress incontinence after radical prostatectomy or cystectomy. Eur Urol. 2006;49(6):1075–1078. doi: 10.1016/j.eururo.2006.01.038. [DOI] [PubMed] [Google Scholar]
- e9.Zahariou A, Papaioannou P, Kalogirou G. Is HCl duloxetine effective in the management of urinary stress incontinence after radical prostatectomy? Urol Int. 2006;77(1):9–12. doi: 10.1159/000092927. [DOI] [PubMed] [Google Scholar]
- e10.Kylmala T, Tainio H, Raitanen M, Tammela TL. Treatment of postoperative male urinary incontinence using transurethral macroplastique injections. J Endourol. 2003;17(2):113–115. doi: 10.1089/08927790360587450. [DOI] [PubMed] [Google Scholar]
- e11.Imamoglu MA, Tuygun C, Bakirtas H, Yigitbasi O, Kiper A. The comparison of artificial urinary sphincter implantation and endourethral macroplastique injection for the treatment of postprostatectomy incontinence. Eur Urol. 2005;47(2):209–213. doi: 10.1016/j.eururo.2004.08.019. [DOI] [PubMed] [Google Scholar]
- e12.Alloussi S. Preleminary results of non-animal stabilised hyaluronic acid/dextranomer (Nasha/.DX) gel for posprostatectomy incontinence. Eur Urol. 2005;4(Suppl) [Google Scholar]
- e13.Hubner WA, Schlarp OM. Treatment of incontinence after prostatectomy using a new minimally invasive device: adjustable continence therapy. BJU Int. 2005;96(4):587–594. doi: 10.1111/j.1464-410X.2005.05689.x. [DOI] [PubMed] [Google Scholar]
- e14.Lebret T, Cour F, Benchetrit J, Grise P, Bernstein J, Delaporte V, et al. Treatment of postprostatectomy stress urinary incontinence using a minimally invasive adjustable continence balloon device, ProACT: results of a preliminary, multicenter, pilot study. Urology. 2008;71(2):256–260. doi: 10.1016/j.urology.2007.08.062. [DOI] [PubMed] [Google Scholar]
- e15.Kempkensteffen C, Hinz S, Christoph F, Weikert S, Schrader M, Schostak M. Rectal perforation as a late complication of ProACT implantation. Urologe A. 2006;45(7):865–867. doi: 10.1007/s00120-006-1053-3. [DOI] [PubMed] [Google Scholar]
- e16.Thuroff JW, Hohenfellner M, Schultz-Lampel D. Operative Technik der Faszienzügelplastik beim Mann. Aktuel Urol. 1992 [Google Scholar]
- e17.Madjar S, Jacoby K, Giberti C, Wald M, Halachmi S, Issaq E, et al. Bone anchored sling for the treatment of post-prostatectomy incontinence. J Urol. 2001;165(1):72–76. doi: 10.1097/00005392-200101000-00018. [DOI] [PubMed] [Google Scholar]
- e18.Fassi-Fehri H, Badet L, Cherass A, Murat FJ, Colombel M, Martin X, et al. Efficacy of the InVance male sling in men with stress urinary incontinence. Eur Urol. 2007;51(2):498–503. doi: 10.1016/j.eururo.2006.08.042. [DOI] [PubMed] [Google Scholar]
- e19.Sousa-Escandon A, Cabrera J, Mantovani F, Moretti M, Ioanidis E, Kondelidis N, et al. Adjustable suburethral sling (male remeex system) in the treatment of male stress urinary incontinence: a multicentric European study. Eur Urol. 2007;52(5):1473–1479. doi: 10.1016/j.eururo.2007.05.017. [DOI] [PubMed] [Google Scholar]
- e20.Romano SV, Metrebian SE, Vaz F, Muller V, D’Ancona CA, Costa DE Souza EA, et al. An adjustable male sling for treating urinary incontinence after prostatectomy: a phase III multicentre trial. BJU Int. 2006;97(3):533–539. doi: 10.1111/j.1464-410X.2006.06002.x. [DOI] [PubMed] [Google Scholar]
- e21.de Leval J, Waltregny D. The Inside-Out Trans-Obturator Sling: A Novel Surgical Technique for the Treatment of Male Urinary Incontinence. Eur Urol. 2007 doi: 10.1016/j.eururo.2007.11.025. [DOI] [PubMed] [Google Scholar]
- e22.Rehder P, Gozzi C. Transobturator sling suspension for male urinary incontinence including post-radical prostatectomy. Eur Urol. 2007;52(3):860–866. doi: 10.1016/j.eururo.2007.01.110. [DOI] [PubMed] [Google Scholar]
- e23.Gozzi C, Becker AJ, Bauer R, Bastian PJ. Early results of transobturator sling suspension for male urinary incontinence following radical prostatectomy. Eur Urol. 2008;54(4):960–961. doi: 10.1016/j.eururo.2008.04.096. [DOI] [PubMed] [Google Scholar]