Abstract
EE is an emerging disease reported in children and adults of urbanized countries, where indoor insect allergens are major health risk factors. Review of our hospital patient database uncovered that a number of EE patients have hypersensitivity to indoor cat, dog, cockroach, and dust mite allergens. We tested the hypothesis whether inhaled indoor insect allergens are effective inducers of experimental EE. We delivered cat, dog, cockroach, and dust mite allergen extracts intranasally to wild-type and eotaxin-1/2-, CCR3-, and IL-5-deficient mice. Interestingly, wild-type mice exposed to cockroach or dust mite allergens develop a significant increase in the levels of esophageal eosinophils and mast cells compared with saline-challenged mice. The eosinophil numbers in the esophagus of cockroach- and dust mite-exposed mice were 18.3 ± 6.8/mm2 and 33.4 ± 11.1/mm2 compared with 2.3 ± 1.8/mm2 and 2.1 ± 1.2/mm2 in saline-challenged mice. Additionally, we observed an additive effect of these two allergens in inducing esophageal eosinophilia and mastocytosis. Histopathological analysis detected intraepithelial esophageal eosinophilia in mice exposed to both allergens. Furthermore, mice exposed to cockroach and/or dust mite had increased levels of total IgE and antigen-specific IgG1 in the blood and increased esophageal expression of eosinophil-active cytokines (IL-13) and chemokines (eotaxin-1). Notably, mice deficient in eotaxin-1/2, CCR3, and IL-5 showed ablated esophageal eosinophilia following cockroach or dust mite allergen exposure. These data indicate that indoor insect allergens are potent inducers of IL-5 and eotaxin-mediated esophageal eosinophilia. These experimental studies are in accordance with clinical data but may have some limitations inherent to animal models of human disease.
Keywords: cockroach, dust mite, eotaxin, eosinophils, mast cells
Introduction
It is well established that the indoor environment is a source of human health risk factors [1,2,3]. Indoor environments, especially homes, have been recognized as major sources of aeroallergen exposure in adults and children [4,5,6,7]. In particular, exposure to dust mite and cockroach allergens is an important indoor factor in the inner city, as these insects are ubiquitous and highly allergenic [1, 8]. In most industrialized countries, the modern home is a sealed and highly thermally insulated building that commonly has air quality compromised with indoor allergens. In animal models, unsensitized mice develop concomitant eosinophilic inflammation of esophagus following nine doses of intranasal aeroallergen Aspergillus fumigatus antigen, but not by OVA [9]. The Aspergillus-induced esophageal eosinophilia is accompanied by intraepithelial eosinophils, extracellular granule deposition, and epithelial cell hyperplasia, features that mimic the pathophysiological changes observed in individuals with various forms of EE [9,10,11,12,13,14,15]. It is important to note that the standard OVA/alum experimental asthma model does not induce EE, highlighting a special etiological role for aeroallergens in this disorder [9]. EE is an inflammatory, cell-mediated esophageal disease characterized by a high number of intraepithelial eosinophils and is triggered by allergens and overexpression of Th2 cytokines (IL-5 and IL-13) [9, 16, 17]. Food and aeroallergens have been implicated in the induction of human EE based on the presence of a high degree of food and aeroallergen hypersensitivity and the ability of food dietary restrictions to improve EE [13]. The prevalence of pediatric and adult EE continues to increase, especially in industrialized countries over the last decade [7, 12, 14, 18,19,20,21,22,23,24,25,26,27,28,29]. However, a causal relationship between insect allergens, such as dust mite or cockroach, and development of EE has not yet been demonstrated.
The patient dataset compiled by the CCED at CCHMC indicates that children with EE have a high prevalence of allergy to environment allergens, especially to indoor allergens, such as dust mite, cockroach, and mold. The inhaled dust mite or cockroach allergens stick to the mucous in the airway, and sensitized children and adults develop inflammatory reactions quickly [7, 30, 31]. In the present study, we performed a comprehensive analysis of factors that might be associated with EE, focusing on aeroallergens present in the inner city. We tested the hypothesis whether indoor allergens induce EE in mice. First, we analyzed EE patient clinical records maintained by our allergy clinic and the CCED. Our analysis included immediate hypersensitivity to cockroach, house dust mite, cat, and dog allergens, as measured by SPT. Second, we exposed mice to indoor allergens (cockroach, house dust mite, cat, and dog) and examined airway and esophageal eosinophilia to determine if EE had developed. Cockroach and dust mite allergens induced eosinophil-active chemokines and cytokines in the esophagus, and mice exposed to both of these allergens developed experimental EE in a time- and dose-dependent manner involving a mechanism dependent on IL-5, eotaxin, and CCR3. Based on these findings, the present study demonstrates that indoor insect allergens can induce experimental EE in mice and may play a role in development of human EE.
MATERIALS AND METHODS
Normal and EE patient characteristics
Normal individuals and EE patients (2–18 years old) were selected without regard to their atopic status or gender. Diagnosis was established based on the maximum eosinophil counts/hpf (×400) and the lack of response to acid suppression. [10, 26] “Normal” was defined as having 0 eosinophils/hpf and no basal layer expansion. The normal biopsies were obtained from patients who had symptoms typical of gastroesophageal reflux disease and EE but had completely normal findings for esophageal endoscopic and microscopic analyses. Patients with EE were defined as having more than 24 esophageal eosinophils/hpf and are consistent with the First International Gastrointestinal Eosinophil Research Symposium’s recommendation for research studies about EE [10]. The history of allergy in the EE patients was collected from medical records.
Evaluation of allergen sensitization
EE patients and atopic and nonatopic control groups received SPT for as many as 62 foods and 11 indoor and outdoor aeroallergen extracts. SPT was performed and interpreted as described previously [32, 33].
Mice
Specific pathogen-free BALB/c mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA). All of the experiments were performed on age- and gender-matched mice, 6–8 weeks of age. The eotaxin-1/2 double gene-deficient mice were generated in our laboratory as described previously [34, 35]. The CCR3-deficient mice (BALB/c background) were a kind gift of Drs. Alison A. Humbles and Craig J. Gerard, Harvard Medical School (Boston, MA, USA). The IL-5-deficient mice were obtained originally from Drs. Klaus I. Matthaei and Paul S. Foster of the John Curtin School of Medical Research, Australian National University (Canberra, Australia). All animals were maintained in a pathogen-free barrier facility and handled according to institutional guidelines.
Experimental EE induction
A mouse model of allergic EE was established using modified methods described previously [9]. In brief, mice were lightly anesthetized with isoflurane (Iso-Flo, Abbott Laboratories, North Chicago, IL, USA), and 100 μg (50 μl) dog, cat, cockroach (Bayer Pharmaceuticals, Spokane, WA, USA), or dust mite extract (Greer Laboratories, Lenoir, NC, USA) or 50 μl normal saline alone (or as mentioned in individual experiments) was applied to the nares using a micropipette at three treatments/week for 4 weeks. After allergen instillation, mice were held upright until alert. Mice were sacrificed between 18 and 20 h after the last allergen or saline challenge.
BALF collection and analysis
The mice were killed by CO2 inhalation. Immediately thereafter, a midline neck incision was made, and the trachea was cannulated. The lungs were lavaged two times with 1.0 ml PBS containing 1% FCS and 0.5 mM EDTA. The recovered BALF was centrifuged at 250 g for 5 min at 4°C and resuspended in 200 μl PBS containing 1% FCS and 0.5 mM EDTA. Lysis of RBCs was carried out using RBC lysis buffer (Sigma-Aldrich, St. Louis, MO, USA), according to the manufacturer’s recommendations. Total cell numbers were counted with a hemacytometer. Cytospin preparations of 5 × 104 cells were stained with Giemsa-Diff-Quick (Dade Diagnostics of P.R., Inc., Aguada, Puerto Rico), and differential cell counts were determined. The BALF eosinophil counts were expressed as an indication of lung eosinophilia.
Eosinophil analysis in the esophagus
Esophageal, 5 μm paraffin tissue sections were immunostained with antiserum against mouse eosinophil MBP (anti-MBP), as described previously [36, 37]. In brief, endogenous peroxide in the tissue was quenched with 0.3% hydrogen peroxide in methanol followed by nonspecific protein blocking with normal goat serum. Tissue sections were then incubated with rat anti-MBP (1:2000) overnight at 4°C, followed by a 1:200 dilution of biotinylated anti-rat IgG secondary antibody and avidin-peroxidase complex (Vector Laboratories, Burlingame, CA, USA) for 30 min each. These slides were developed further with nickel diaminobenzidine-cobalt chloride solution to form a black precipitate and counterstained with hematoxylin. Negative controls included replacing the primary antibody with normal rat serum.
Mast cell analysis
Esophageal, 5 μm paraffin tissue sections were de-paraffinized and stained with hexazonized new fuchsin (Sigma-Aldrich) with 4% sodium nitrate in naphthol-AS-D chloroacetate (Sigma-Aldrich) and PBS solution for 30 min and counterstained with hematoxylin. In addition, toludine blue staining was performed to identify degranulated mast cells in the tissue sections. The histological analysis was performed using light microscopy, and mast cells were counted and expressed as cells/hpf. Nonhexazonized new fuchsin was used as a negative control.
Quantification of eosinophils and mast cells
Eosinophils were quantified by counting the anti-MBP-positive cells in the epithelial mucosa and lamina propria. Combined eosinophil numbers in each esophageal tissue section were calculated with the assistance of digital morphometry using the Metamorph Imaging System (Universal Imaging Corp., West Chester, PA, USA) and expressed as eosinophils/mm2, as described previously [16, 17]. The CAE or toludine blue-positive mast cells in the epithelial mucosa and lamina propria were counted using light microscopy, and their combined numbers in each esophageal tissue section were expressed as mast cells/hpf.
Protease-free allergen
Protease activity of allergens was measured by the QuantiClevave™ protease assay kit (Pierce, Rockford, IL, USA), as described in the manufacturer’s protocol. This assay is based on the use of succinylated casein in conjugation with TNBSA. The protease will act on succinylated casein to cleave peptide bonds, exposing primary amines. TNBSA reacts with primary amines to produce an orange-yellow color that can be quantified at 450 nm by a spectrophotometer for optimum results. The cockroach and dust mite antigens were incubated with a dose-dependent antiprotease, such as trypsin, glutathione, and DTT. We found that 100 mM DTT inhibited maximum allergen protease activity. The allergen samples incubated with DTT were dialyzed overnight at 4°C using a 1000-m.w. cut-off size dialysis cassette (Pierce). Protein concentration was measured as described in the above section. The Balb/c mice were intranasally challenged with DTT-treated dust mite extract, original dust mite extract, and saline, as per the protocol described above. Additionally, the product data sheet of the cockroach and dust mite allergens indicates that both allergens have some LPS contamination. We measured LPS concentration in dust mite and cockroach extract using the Limulus amoebocyte lysate QCL-1000 (Cat. #50-647U, Lonza, Walkersville, MD, USA) product following the manufacturer’s provided protocol. LPS contamination range in both allergens was 1.1–1.5 ng/ml, indicating that we are introducing 0.05–0.07 ng LPS to the mice/challenge. This low LPS concentration will not affect our present hypothesis, as LPS induces Th1 responses mostly, not Th2 [38].
Real-time PCR analysis
The RNA samples (1 μg) were subjected to reverse transcription using iScript RT (Bio-Rad, Hercules, CA, USA), according to the manufacturer’s instructions. The cytokine and chemokine mRNA levels were quantified by real-time PCR using the LightCycler instrument and LightCycler FastStart DNA master SYBR Green I as a ready-to-use reaction mix (Roche, Indianapolis, IN, USA). Results were then normalized to β-actin, amplified from the same cDNA mix and expressed as fold induction compared with the controls. cDNA were amplified using the primers listed in Supplemental Table 1.
Serum IgE and antigen-specific IgG1
Total serum IgE levels were measured using the BD OptEIA ELISA set (BD Biosciences, San Diego, CA, USA) as per the manufacturer’s protocol. Similarly, antigen-specific IgG1 in mouse serum samples was measured as per the protocol described earlier [39]. Briefly, sample wells were coated with 100 μl cockroach or dust mite extract (50 μg/ml), blocked with 10% FBS in PBS, and washed with 0.05% Tween-20 in PBS. Serum samples were serially diluted with 10% FCS in PBS. After a 2-h incubation at room temperature, plates were washed with 0.05% Tween-20 in PBS, and 100 μl HRP-conjugated anti-mouse IgG1 (1:1500 dilution from 1 mg/ml, BD PharMingen, San Diego, CA, USA) was added. Using streptavidin-HRP detection (100 μl/well, TMB substrate regents, BD Biosciences), the reaction was stopped using 2 N H2SO4, and OD was read at 450 nm immediately.
Cytokine analysis
Levels of IL-4, IL-5, and IL-13 were determined by using the BD OptEIA ELISA set (BD Biosciences) as per the manufacturer’s protocol. Briefly, esophageal homogenate was applied to a 96-well ELISA plate precoated with cytokine-specific mAb and blocked against nonspecific protein binding with 10% FBS. The plate was incubated 2 h at room temperature and washed with 0.05% Tween-20-PBS. Biotinylated, cytokine-specific mAb was applied to each well, followed by avidin-HRP conjugate reagent. Finally, TMB substrate solution (BD PharMingen) was added to each well, the color was developed in the dark at room temperature, and the OD was read at 450 nm immediately. The cytokine concentration of each sample was calculated by using a standard curve and normalized to total tissue protein.
Statistical analysis
Data are expressed as mean ± sd. Statistical significance comparing different sets of mice was determined by unpaired InStat GraphPad t-test, and P < 0.05 is considered statistically significant.
RESULTS
EE patients have sensitivity to indoor insect allergens
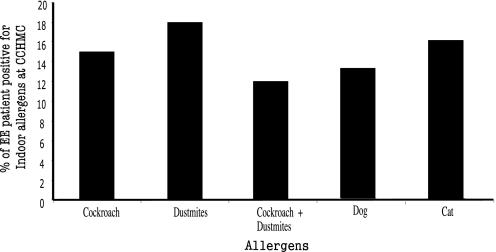
Active EE patients were identified as described in Materials and Methods. SPT was performed in 236 patients, and ∼85% of patients had SPT-positive reactions to food and inhaled aeroallergens (data not shown). The EE patient database indicates that ∼38% of the 236 patients were sensitive to indoor allergens and that a majority of these EE patients reacted to cockroach or dust mites. We found that ∼16% of EE patients were sensitive to cockroach allergen, and ∼19% were sensitive to dust mite allergen. Notably, ∼12% of EE patients were positive to cockroach and dust mites allergens. A comparable number of EE patients were found positive for dog or cat allergens (Fig. 1). Interestingly, ∼90% of cockroach and dust mite SPT-positive EE patients were white/Caucasian and had more than one allergic disease (Supplemental Table 2). The EE patients included in the analysis had no symptoms or sign of eosinophilic gastroenteritis, eosinophilic duodenitis, eosinophilic colitis, or hypereosinophilic syndrome.
Figure 1.
Clinical characteristics of EE patients. The 236 active human EE patients enrolled in the CCHMC allergy clinic were reviewed for indoor insect allergy. Percentages of cockroach and dust mite (SPT-positive) patients were analyzed and shown.
Intranasal indoor allergen induces airway and esophageal eosinophilia
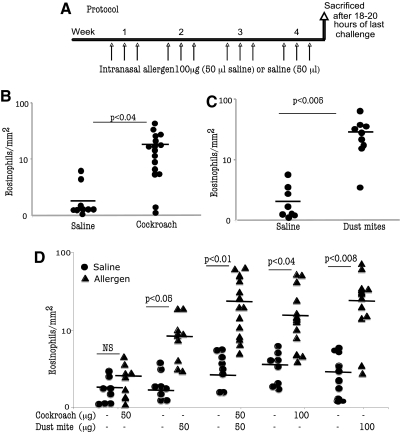
We next determined whether intranasal delivery of indoor allergen (cockroach, dust mite, cat, dog) to the lung was sufficient to induce EE. Allergens were delivered to the lung of BALB/c naive mice using doses and techniques reported previously [9] with slight modifications as shown in Figure 2A. The cat and dog allergen did not induce any significant lung or esophageal eosinophilia in the allergen-challenged mice compared with saline-challenged controls (data not shown). In contrast, mice that were challenged with 100 μg cockroach or dust mite allergen had an eight- to ten-fold increase in esophageal eosinophilia compared with saline-exposed mice (Fig. 2, B and C). The eosinophil numbers in the esophagus of mice challenged with cockroach or dust mite allergen were 18.3 ± 6.8/mm2 and 33.4 ± 11.1/mm2 compared with 2.3 ± 1.8/mm2 and 2.1 ± 1.2/mm2 in saline-challenged mice (mean±sd; n=14–16), respectively. Interestingly, mice exposed to a mixture of cockroach and dust mite allergens (50 μg each) showed an additive effect in inducing esophageal eosinophilia; esophageal eosinophilia in mice exposed to the mixture was comparable with that of mice exposed to 100 μg cockroach or dust mite allergen (Fig. 2D). As expected, mice evidencing esophageal eosinophilia after being challenged with cockroach and/or dust mite allergen(s) also have pulmonary and blood eosinophilia. The levels of eosinophils in the BALF of mice exposed to 100 μg cockroach, 100 μg dust mite, or 50 μg cockroach/50 μg dust mite mixture were 1.2 ± 0.6 × 105/lung, 6.1 ± 1.6 × 105/lung, and 9.3 ± 5.8 × 105/lung, respectively (mean±sd; n=9–10), compared with 0.07 ± 0.04 × 103/lung, 0.05 ± 0.04 × 103/lung, and 0.02 ± 0.02 × 103/lung (mean±sd; n=8–9) in the respective saline-challenged control mice. The blood eosinophilia in mice with saline, cockroach (100 μg), or dust mite (100 μg) exposure was 0.9 ± 1.2 × 104/ml, 2.6 ± 0.9 × 104/ml, and 4.4 ± 1.6 × 104/ml (mean±sd; n=8–9), respectively. These results establish that intranasal delivery of indoor allergens, specifically cockroach and dust mite allergens, promotes esophageal, pulmonary, and blood eosinophilia. Additionally, we tested whether allergen protease activity has any role in EE induction. We challenged mice with 100 μg of DTT-treated (100 mM) dust mite extract, 100 μg original dust mite extract, or saline and then examined for esophageal eosinophilia. Our data showed a comparable number of esophageal eosinophilia in mice exposed to DTT-treated dust mite extract versus original dust mite extract. The numbers of eosinophils in mice exposed to 100 μg DTT-treated dust mite extract or original dust mite extract were 21.2 ± 7.4/mm2 eosinophil/mm2 (mean±sd; n=6) and 27.7 ± 9.2/mm2 (mean±sd; n=6), respectively. Eosinophil counts of saline-challenged mice were 1.8 ± 2.6/mm2 (mean±sd; n=6).
Figure 2.
Eosinophil accumulation in the esophagus following indoor allergen exposure. Mice, intranasal, exposed to cockroach and/or dust mite, were analyzed for the number of eosinophils in the esophagus following the protocol shown (A). The numbers of eosinophils in the esophagus of mice challenged with cockroach (B), dust mite (C), or the mixture of cockroach and dust mite allergen(s) (D) are shown 18–20 h after the last allergen exposure. Saline-exposed mice are indicated by –. Data are expressed as mean ± sd; n = 10–16 mice/treatment group. NS, Not significant.
Intranasal cockroach and dust mite allergen induces a dose- and time-dependent increase in esophageal eosinophilia
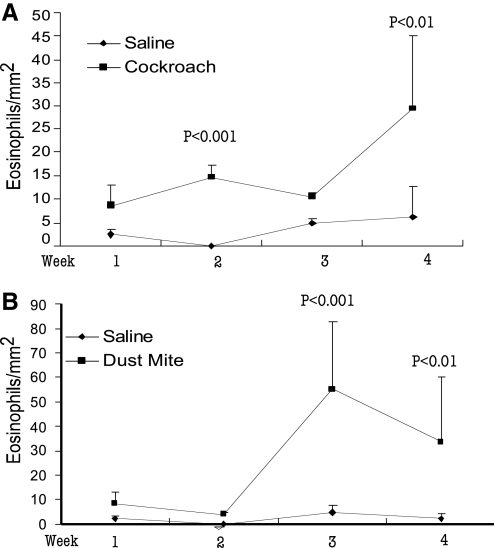
We were interested further in testing the hypothesis that esophageal eosinophilia increases in a dose- and time-dependent manner following exposure to cockroach or dust mite allergen. Mice received 100 μg cockroach or dust mite allergen or saline intranasally three times/week for 1, 2, 3, and 4 weeks. Weekly cohorts were examined for esophageal eosinophilia. A dose-dependent increase in esophageal eosinophilia was observed for cockroach (Fig. 3A) and dust mite (Fig. 3B) challenged mice. The numbers of eosinophils in the esophagus of mice at 1, 2, 3, and 4 weeks of cockroach allergen challenge were 8.52 ± 4.5/mm2, 14.7 ± 7.6/mm2, 10.5 ± 0.8/mm2 [5], and 29.3 ± 15.8/mm2 (mean±sd; n=6–10). The numbers of eosinophils in the esophagus of mice at 1, 2, 3, and 4 weeks of dust mite allergen challenge were 8.5 ± 4.5, 4.0 ± 0.7, 55.0 ± 28.1, and 33.4 ± 27.1 (mean±sd; n=6–10). As a control, mice treated with intranasal saline showed few baseline eosinophils in the esophagus. In contrast to the effect of intranasal cockroach and dust mite allergen on esophageal eosinophilia, the level of eosinophils in the stomach did not change. Saline- and allergen-challenged mice showed a comparable number of eosinophils in the stomach (data not shown).
Figure 3.
Kinetic time-dependent analysis of indoor insect allergen-induced esophageal eosinophilia. Naïve Balb/c mice were exposed to 100 μg cockroach allergen (A), dust mite allergen (B), or saline by an intranasal route three times/week for 4 weeks. The level of eosinophils in the esophagus was quantified weekly by performing anti-MBP immunohistochemistry and morphometric analysis. The data are expressed as mean ± sd; n = 6–10 mice/treatment group at each weekly time-points.
Esophageal mast cells are increased following cockroach and dust mite exposure
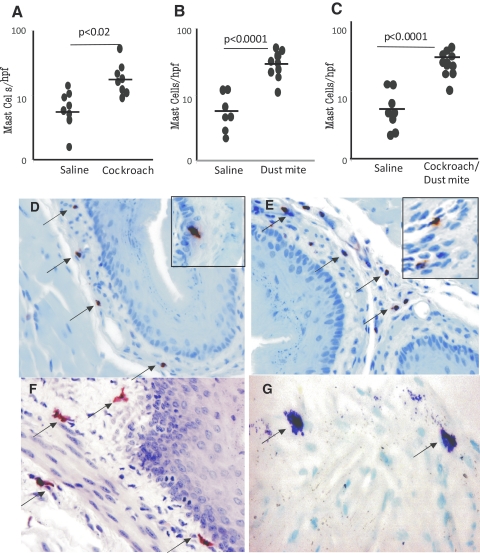
Recent clinical reports indicated that mast cell numbers increased in pediatric and adult EE [15, 40, 41]; we therefore measured mast cells in the esophagus by CAE and toludine blue staining. Cockroach (100 μg)- and dust mite (100 μg)-challenged mice showed an approximate four- and threefold increase in mast cells in the esophagus (Fig. 4, A and B). Mast cell numbers in the esophagus of saline- and cockroach-challenged mice were 6.8 ± 3.1/mm2 and 16.2 ± 6.2/mm2 (mean±sd; n=9–10) and of saline- and dust mite-challenged mice were 4.7 ± 1.4/mm2 and 32.4 ± 8.8/mm2 (mean±sd; n=9–10), respectively. Further, a greater than four-fold increase of mast cells was observed in mice challenged with the mixture of cockroach and dust mite allergens (50 μg each) compared with saline challenge (Fig. 4C). Additionally, the histopathological analysis of esophageal tissue sections identified a number of eosinophils and mast cells in the lamina propria of cockroach- and dust mite-challenged mice (Fig. 4, D–G). We detected a number of intraepithelial eosinophils in cockroach- and dust mite-challenged mice (Fig. 4, D and E) but did not observe any mast cell accumulation in the epithelial mucosa. However, an increased number of mast cells was detected in the esophageal lamina propria following CAE staining in dual allergen-challenged mice (Fig. 4F), and degranulated mast cells were identified by toludine blue staining in dual allergen-challenged mice (Fig. 4G). Surprisingly, we did not observe significantly induced basal cell hyperplasia in mice following 100 μg cockroach or dust mite allergen challenge.
Figure 4.
Mast cell accumulation in the esophagus following indoor allergen exposure. Mice exposed to intranasal cockroach and/or dust mite were analyzed for the number of mast cells in the esophagus. The numbers of mast cells in the esophagus of mice challenged with cockroach (A), dust mite (B), or the mixture of cockroach and dust mite (C) allergen(s) are shown 18–20 h after the last allergen exposure. Representative photomicrographs of anti-MBP-stained eosinophils in a cockroach-exposed esophageal tissue section (D) and dust mite-exposed esophageal tissue section (E), with arrows marking the eosinophils, are shown. Mast cells of both allergen-challenged mice were identified by performing CAE and toludine blue staining, and representative photomicrographs with esophageal mast cells marked by arrows are shown (F and G). The inserted area in D and E shows intraepithelial eosinophils in the esophagus. These photomicrographs are representative of three experiments. The original magnification was ×100. Data are expressed as mean ± sd; n = 9–10 mice/treatment group.
Antigen-specific antibodies are increased following cockroach and dust mite challenge
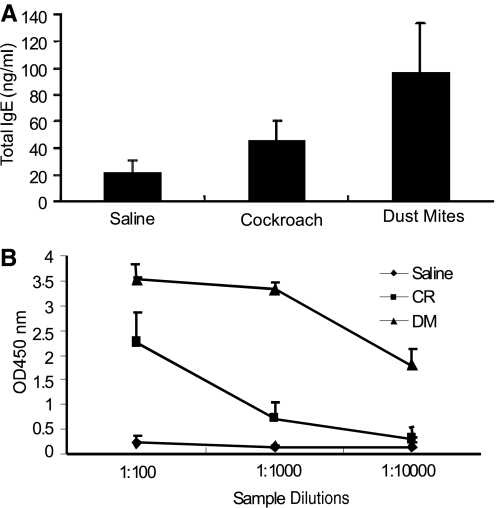
As allergen-induced total IgE and IgG have a critical role in mast cell-mediated allergic responses, we examined the levels of total IgE and antigen-specific IgG1 antibodies in cockroach- and dust mite-challenged mice. Our analysis indicated a significant increase in the levels of total IgE (Fig. 5A) and antigen-specific IgG1 (Fig. 5B) in mice exposed to cockroach and dust mite allergens.
Figure 5.
Induction of allergen-induced antibodies following cockroach and dust mite treatment. Naive Balb/c mice were exposed to 4 weeks of tri-weekly treatments of saline, cockroach (CR), and/or dust mite (DM) allergens. The levels of serum total IgE (A) and antigen-specific IgG1 (B) following saline or allergen exposure are shown. Data are obtained 18–20 h after the last treatment and are expressed as mean ± sd; n = 6–8 mice/treatment group.
Eosinophil-active chemokines and cytokines are induced following cockroach and dust mite challenge in the esophagus
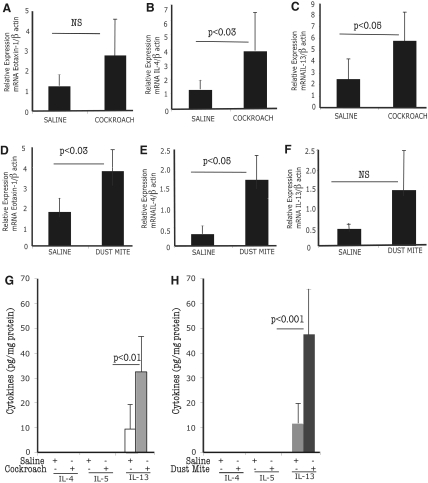
Next, we tested whether cockroach and dust mite exposure in mice induces the expression of eosinophil-active chemokines and cytokines in the esophagus. Accordingly, we performed quantitative real-time PCR analysis for eotaxin-1, IL-4, IL-5, and IL-13 and β-actin mRNA expression in the esophagus of saline-, cockroach-, and dust mite-challenged mice. The cockroach- and dust mite-challenged mice showed a two- to three-fold increase in the relative mRNA expression of esophageal eotaxin-1, IL-4, and IL-13 compared with the saline-challenged mice following β-actin normalization (Fig. 6, A–F). The levels of IL-5 and eotaxin-2 mRNA are induced in the esophagus; however, the statistical significance level was P < 0.06 between allergen- and saline-challenged mice (data not shown). Additionally, we examined the IL-4, IL-5, and IL-13 protein levels in the esophageal homogenates of saline-, cockroach-, or dust mite-challenged mice. The IL-4 and IL-5 levels were nondetectable in saline-, cockroach-, or dust mite allergen-challenged mice. However, only IL-13 levels were detected in the esophageal homogenates of saline-, cockroach-, or dust mite-challenged mice (Fig. 6, G and H). The IL-13 levels in cockroach- or dust mite-challenged mice were 32.4 ± 14.7 and 47.3 ± 18.5 pg/mg protein compared with levels of their respective saline-exposed mice, 9.3 ± 9.6 and 11.5 ± 8.6 pg/mg protein (mean±sd; n=6).
Figure 6.
Indoor insect allergen-induced mRNA expression of eotaxin-1, IL-5, and IL-13 in the esophagus. The quantitative real-time PCR analyses of eotaxin-1, IL-4, and IL-13 mRNA levels in mice following saline and cockroach exposure (A–C) and saline and dust mite exposure mice (D–F) are shown. The eosinophil-active cytokine protein levels of IL-4, IL-5, and IL-13 in esophageal homogenates are shown (G and H). Allergen- or saline-exposed mice were indicated by + and not exposed, by –. The data are expressed as mean ± sd; n = 7–8 mice in each group/experiment.
Cockroach and dust mite allergen-induced EE is dependent on eotaxins, its receptor CCR3, and IL-5
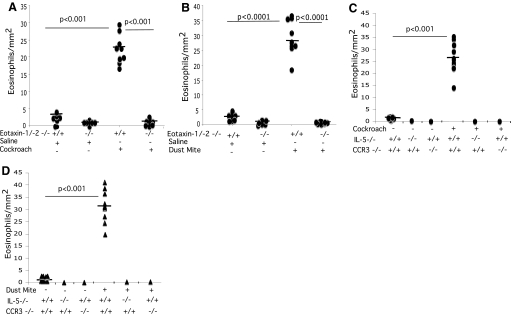
We next investigated whether eotaxin-1 and -2 had an essential role in promoting esophageal eosinophils following cockroach and dust mite challenge by inducing experimental EE in eotaxin-1/2-deficient mice via intranasal cockroach and dust mite treatment. The level of eosinophils in the esophagus was increased markedly in wild-type mice treated with either allergen compared with eotaxin-1/2-deficient mice. In the absence of eotaxin-1 and eotaxin-2, eosinophils were ablated in the esophagus of mice treated with cockroach or dust mite allergen compared with wild-type mice (Fig. 7, A and B). The numbers of eosinophils in the esophagus of cockroach and dust mites allergen-exposed, wild-type mice were 23.7 ± 12.8/mm2 and 29.2 ± 14.3/mm2, respectively, compared with those of the associated saline controls, 2.3 ± 2.1/mm2 and 1.9 ± 2.4/mm2 (mean±sd; n=8; P<0.001). The numbers of eosinophils in the esophagus of cockroach and dust mite allergen-exposed eotaxin-1/2-deficient mice were 0.4 ± 0.5/mm2 and 0.6 ± 0.4/mm2, respectively, compared with 0.5 ± 0.7/mm2 and 0.6 ± 0.6/mm2 in the associated saline-exposed, eotaxin-1/2-deficient mice. Additionally, we exposed wild-type, eosinophil growth, and survival factor IL-5-deficient and eotaxin receptor CCR3-deficient mice to saline, cockroach, or dust mite allergens. The numbers of eosinophils in the esophagus of cockroach- and dust mite allergen-exposed, wild-type mice were 26.8 ± 9.7/mm2 and 31.2 ± 11.8/mm2, respectively, compared with 1.7 ± 2.4/mm2 and 2.2 ± 1.8/mm2 (mean±sd; n=8 in saline-exposed, control mice, compared with undetectable in saline-, cockroach-, or dust mite allergen-exposed CCR3- and IL-5-deficient mice (Fig. 7, C and D).
Figure 7.
Indoor allergen-induced EE in eotaxin-1/2-, IL-5-, and CCR3-deficient mice. The levels of eosinophils in the esophagus of wild-type, eotaxin-1/2 double-, IL-5-, and CCR3-deficient mice were analyzed following intranasal saline, cockroach, or dust mite allergen exposure. The number of eosinophils in the esophagus was determined by anti-MBP immunoassayed and quantified in cockroach allergen-exposed wild-type mice (A), dust mite allergen-exposed wild-type mice (B), and cockroach- or dust mite-exposed, IL-5- or CCR3-deficient mice (C and D). Allergen- or saline-exposed mice were indicated by + and not exposed, by –. Wild-type mice, +/+; gene-deficient, −/−. The results are expressed as mean ± sd; n = 8 mice/group.
DISCUSSION
Eosinophil infiltration into the esophagus is a commonly observed medical problem in patients with diverse diseases, including gastroesophageal reflux, drug reactions, eosinophilic gastroenteritis, and EE [18, 19, 24, 42,43,44,45]. A number of reports show a link between the indoor environment and asthma [1, 2, 4, 6]; however, testing of the indoor environment’s role in induction of esophageal eosinophilia is lacking. In this study, we extend our understanding of the role of indoor allergens in EE pathogenesis. Our clinical database analysis shows that EE patients are sensitized to a number of indoor aeroallergens and food allergens. A large percentage of EE patients is SPT-positive to common indoor allergens, such as cat, dog, cockroach, and dust mite allergens. Based on the high reported concordance between indoor insect allergens and asthma in clinical studies, allergic lung responses induced by indoor allergens may trigger EE. Therefore, we tested whether indoor allergens induce experimental EE. We investigated this hypothesis by inducing allergen sensitization in mice via intranasal challenge with a number of indoor allergens (dog, cat, cockroach, and dust mite) and then examining the consequences on the esophageal inflammation.
We show that intranasal cockroach and dust mite delivery is sufficient for the elicitation of eosinophil trafficking to the esophagus, including accumulation of eosinophilis in the epithelial mucosa. In contrast, dog and cat allergens fail to induce airway or esophageal eosinophilia despite EE patients having hypersensitivity to these two allergens (data not shown). These data suggest that dog and cat allergens may not have the capacity to induce eosinophil-active cytokines or chemokines. We showed previously that Aspergillus-induced EE has similar characteristics to allergen-induced EE, including epithelial hyperplasia and remodeling [9, 46], a cardinal feature of primary EE that is consistent with the pathogenesis of human EE [10, 12, 43, 47]. The present study implicates indoor allergens in the EE pathogenesis, which has clinical significance, as our patient dataset demonstrates that 25–30% of EE patients are hypersensitive to indoor allergens.
In this study, we uncover several principles concerning the mechanism of indoor insect allergen-induced esophageal eosinophilia. First, we demonstrate a dose- and concentration-dependent ability of indoor insect allergens to induce esophageal eosinophilia; however, the magnitude of eosinophils in the esophagus following cockroach and/or dust mite allergens is lower than the previously reported Aspergillus allergen-induced esophageal eosinophilia [9]. Second, we show that both of these allergens are capable of inducing eosinophil-active cytokines and chemokines in the esophagus. Third, we show that cockroach and dust mite allergens induce a high number of mast cells in the esophageal mucosa and induce allergen-specific antibodies (IgE and IgG1) in the blood. Both of these characteristics have been reported recently in human EE [40, 41, 48]. Fourth, we further investigated the role of eosinophil responsiveness to endogenous chemokines expressed by the esophagus, such as the eotaxins and eotaxin receptors involved specifically in eosinophil trafficking to the esophagus [32].
Eosinophilic gastrointestinal diseases are primarily polygenic allergic disorders that involve mechanisms that fall between pure IgE-mediated and delayed lymphocyte responses [49,50,51]. Our data indicate that eosinophil-active cytokines and chemokines are induced by indoor insect allergens. Of note, eotaxins are constitutively expressed chemokines in the esophagus [37]. To evaluate the role of eotaxins in mediating indoor insect allergen-induced eosinophil trafficking to the esophagus, we examined mice that were genetically deficient in eotaxin-1/2, CCR3, or IL-5. Our studies revealed that eotaxins, CCR3, and IL-5 have a role in indoor insect allergen-mediated induction of esophageal eosinophilia. These data are consistent with our previous reports that indicate EE is dependent on eosinophil-active cytokines and chemokines [9, 16, 32]. Although IL-5 protein is not detected in the esophagus of saline- or allergen-challenged mice, that may be a result of lack of ELISA sensitivity for IL-5 detection. It is well established that IL-5 is a growth and survival factor for eosinophils [52], and our previous report implicated IL-5 in EE pathogenesis [9, 16, 32]. The importance of IL-5 is also evident from our present data that IL-5-deficient mice do not induce esophageal eosinophilia following insect allergen exposure. Furthermore, it is highly unlikely that the LPS in the cockroach and dust mite allergen extracts is mediating the effect observed in this study, as LPS is known to be a powerful stimulator of IL-12, which preferentially drives Th1 responses [38]. This conclusion is consistent with our previous report that endotoxin-contaminated allergen and endotoxin-free allergen have comparable levels of esophageal eosinophilia in mice [39].
Notably, our finding that the level of eosinophils in the stomach was unaffected by intranasal indoor allergens is consistent with aeroallergen-induced EE as reported previously [9]. The present experimental findings are in accordance with the human EE data, which show that most EE patients have no other eosinophilic gastrointestinal diseases, and support our earlier understanding that a local mechanism is operational in inducing esophageal eosinophilia. These investigations highlight the significance of further dissecting the role of indoor allergens in the pathogenesis of EE. These experimental studies are in accordance with the human clinical data collected from an existing database; however, it has some limitations, as no animal model mimics human disease completely. Interestingly, a number of human EE characteristics, such as intraepithelial eosinophils, deposition of extracelluar granules, and Th2 cytokine induction in the esophagus, are observed in cockroach and dust mite allergen-induced esophageal eosinophila in mice. In summary, our investigation implicates indoor insect allergens in the induction of EE and dissects the cellular and molecular mechanisms involved in indoor insect allergen-induced EE pathogenesis.
AUTHORSHIP
Madhavi Rayapudi performed experiments and data analysis related to cockroach allergens. Parm Mavi performed experiments and data analysis related to dust mite allergens. Xiang Zhu performed kinetic analysis experiments of cockroach and dust mite allergens. Akhilesh K. Pandey performed ELISA and protein analysis. J. Pablo Abonia summarized the EE patient dataset. Marc E. Rothenberg performed a critical review of the manuscript. Anil Mishra performed the study design, experiment and analysis supervision, and writing of the manuscript.
ACKNOWLEDGMENTS
This work was supported in part by the following grants and organizations: NIH RO1 DK067255 (A.M.), R01 AI080581 (A.M.), AI045898 (M.E.R.), the Digestive Health Center (DHC) grant DK078392, Campaign Urging Research for Eosinophilic Disease (CURED), Food Allergy Project, and the Buckeye Foundation. The authors thank Andrea Lippelman and Shawna Hottinger for editorial assistance and Drs. James and Nancy Lee (Mayo Clinic, Scottsdale, AZ, USA) for the generous supply of anti-MBP. The authors also thank the summer students Kalyan Rao, Sri Rajmouii, and Akanksha Mishra for their initial contributions to this project under NIH, ARRA grant DK067255-05S1 (A.M.).
Footnotes
Abbreviations: BALF=bronchoalveolar lavage fluid, CAE=chloroacetate esterase, CCED=Cincinnati Center of Eosinophilic Disorder, CCHMC= Cincinnati Children’s Hospital Medical Center, EE=eosinophilic esophagitis, hpf=high-power field, MBP=major basic protein, SPT=skin-prick testing, TMB=tetramethylbenzidine, TNBSA=trinitrobenzenesulfonic acid
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
References
- Gelber L E, Seltzer L H, Bouzoukis J K, Pollart S M, Chapman M D, Platts-Mills T A. Sensitization and exposure to indoor allergens as risk factors for asthma among patients presenting to hospital. Am Rev Respir Dis. 1993;147:573–578. doi: 10.1164/ajrccm/147.3.573. [DOI] [PubMed] [Google Scholar]
- Platts-Mills T A, Ward G W, Jr, Sporik R, Gelber L E, Chapman M D, Heymann P W. Epidemiology of the relationship between exposure to indoor allergens and asthma. Int Arch Allergy Appl Immunol. 1991;94:339–345. doi: 10.1159/000235398. [DOI] [PubMed] [Google Scholar]
- Munir A K. Environmental factors influencing the levels of indoor allergens. Pediatr Allergy Immunol. 1995;6:13–21. doi: 10.1111/j.1399-3038.1995.tb00379.x. [DOI] [PubMed] [Google Scholar]
- Richardson G, Eick S, Jones R. How is the indoor environment related to asthma?: literature review. J Adv Nurs. 2005;52:328–339. doi: 10.1111/j.1365-2648.2005.03591.x. [DOI] [PubMed] [Google Scholar]
- Rosen K G, Richardson G. Would removing indoor air particulates in children’s environments reduce rate of absenteeism—a hypothesis. Sci Total Environ. 1999;234:87–93. doi: 10.1016/s0048-9697(99)00266-1. [DOI] [PubMed] [Google Scholar]
- Munir A K, Kjellman N I, Bjorksten B. Exposure to indoor allergens in early infancy and sensitization. J Allergy Clin Immunol. 1997;100:177–181. doi: 10.1016/s0091-6749(97)70221-5. [DOI] [PubMed] [Google Scholar]
- Kapel R C, Miller J K, Torres C, Aksoy S, Lash R, Katzka D A. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology. 2008;134:1316–1321. doi: 10.1053/j.gastro.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Twarog F J, Picone F J, Strunk R S, So J, Colten H R. Immediate hypersensitivity to cockroach. Isolation and purification of the major antigens. J Allergy Clin Immunol. 1977;59:154–160. doi: 10.1016/0091-6749(77)90218-4. [DOI] [PubMed] [Google Scholar]
- Mishra A, Hogan S P, Brandt E B, Rothenberg M E. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest. 2001;107:83–90. doi: 10.1172/JCI10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta G T, Liacouras C A, Collins M H, Gupta S K, Justinich C, Putnam P E, Bonis P, Hassall E, Straumann A, Rothenberg M E, First International Gastrointestinal Eosinophil Research Symposium (FIGERS) Subcommittees Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–1363. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Noel R J, Putnam P E, Collins M H, Assa'ad A H, Guajardo J R, Jameson S C, Rothenberg M E. Clinical and immunopathologic effects of swallowed fluticasone for eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2004;2:568–575. doi: 10.1016/s1542-3565(04)00240-x. [DOI] [PubMed] [Google Scholar]
- Croese J, Fairley S K, Masson J W, Chong A K, Whitaker D A, Kanowski P A, Walker N I. Clinical and endoscopic features of eosinophilic esophagitis in adults. Gastrointest Endosc. 2003;58:516–522. doi: 10.1067/s0016-5107(03)01870-4. [DOI] [PubMed] [Google Scholar]
- Fox V L, Nurko S, Furuta G T. Eosinophilic esophagitis: it’s not just kid’s stuff. Gastrointest Endosc. 2002;56:260–270. doi: 10.1016/s0016-5107(02)70188-0. [DOI] [PubMed] [Google Scholar]
- Furuta G T. Clinicopathologic features of esophagitis in children. Gastrointest Endosc Clin N Am. 2001;11:683–715 (vii.). [PubMed] [Google Scholar]
- Straumann A, Bauer M, Fischer B, Blaser K, Simon H U. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol. 2001;108:954–961. doi: 10.1067/mai.2001.119917. [DOI] [PubMed] [Google Scholar]
- Mishra A, Hogan S P, Brandt E B, Rothenberg M E. IL-5 promotes eosinophil trafficking to the esophagus. J Immunol. 2002;168:2464–2469. doi: 10.4049/jimmunol.168.5.2464. [DOI] [PubMed] [Google Scholar]
- Mishra A, Rothenberg M E. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology. 2003;125:1419–1427. doi: 10.1016/j.gastro.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Cury E K, Schraibman V, Faintuch S. Eosinophilic infiltration of the esophagus: gastroesophageal reflux versus eosinophilic esophagitis in children—discussion on daily practice. J Pediatr Surg. 2004;39:e4–e7. doi: 10.1016/j.jpedsurg.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Attwood S E, Smyrk T C, Demeester T R, Jones J B. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci. 1993;38:109–116. doi: 10.1007/BF01296781. [DOI] [PubMed] [Google Scholar]
- Cantu P, Velio P, Prada A, Penagini R. Ringed oesophagus and idiopathic eosinophilic oesophagitis in adults: an association in two cases. Dig Liver Dis. 2005;37:129–134. doi: 10.1016/j.dld.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Morita A, Kobayashi H, Hamano K, Fujiwara Y, Hirai K, Yano M, Naka T, Saeki Y. Infiltrating eosinophils and eotaxin: their association with idiopathic eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2002;89:429–432. doi: 10.1016/S1081-1206(10)62047-9. [DOI] [PubMed] [Google Scholar]
- Munitiz V, Martinez de Haro L F, Ortiz A, Pons J A, Bermejo J, Serrano A, Molina J, Parrilla P. Primary eosinophilic esophagitis. Dis Esophagus. 2003;16:165–168. doi: 10.1046/j.1442-2050.2003.00319.x. [DOI] [PubMed] [Google Scholar]
- Lucendo A J, Carrion G, Navarro M, Pascual J M, Gonzalez P, Castillo P, Erdozain J C. Eosinophilic esophagitis in adults: an emerging disease. Dig Dis Sci. 2004;49:1884–1888. doi: 10.1007/s10620-004-9588-x. [DOI] [PubMed] [Google Scholar]
- Straumann A. [Eosinophilic esophagitis: a novel entity?] Praxis (Bern 1994) 2006;95:191–195. doi: 10.1024/0369-8394.95.6.191. [DOI] [PubMed] [Google Scholar]
- Orenstein S R, Shalaby T M, Di Lorenzo C, Putnam P E, Sigurdsson L, Kocoshis S A. The spectrum of pediatric eosinophilic esophagitis beyond infancy: a clinical series of 30 children. Am J Gastroenterol. 2000;95:1422–1430. doi: 10.1111/j.1572-0241.2000.02073.x. [DOI] [PubMed] [Google Scholar]
- Rothenberg M E, Mishra A, Collins M H, Putnam P E. Pathogenesis and clinical features of eosinophilic esophagitis. J Allergy Clin Immunol. 2001;108:891–894. doi: 10.1067/mai.2001.120095. [DOI] [PubMed] [Google Scholar]
- Noel R J, Putnam P E, Rothenberg M E. Eosinophilic esophagitis. N Engl J Med. 2004;351:940–941. doi: 10.1056/NEJM200408263510924. [DOI] [PubMed] [Google Scholar]
- Straumann A, Simon H U. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol. 2005;115:418–419. doi: 10.1016/j.jaci.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Prasad G A, Alexander J A, Schleck C D, Zinsmeister A R, Smyrk T C, Elias R M, Locke G R, III, Talley N J. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2009;7:1055–1061. doi: 10.1016/j.cgh.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell E M, Kunkel S L, Strieter R M, Lukacs N W. Temporal role of chemokines in a murine model of cockroach allergen-induced airway hyperreactivity and eosinophilia. J Immunol. 1998;161:7047–7053. [PubMed] [Google Scholar]
- Noah T L, Henderson F W, Henry M M, Peden D B, Devlin R B. Nasal lavage cytokines in normal, allergic, and asthmatic school-age children. Am J Respir Crit Care Med. 1995;152:1290–1296. doi: 10.1164/ajrccm.152.4.7551384. [DOI] [PubMed] [Google Scholar]
- Blanchard C, Wang N, Stringer K F, Mishra A, Fulkerson P C, Abonia J P, Jameson S C, Kirby C, Konikoff M R, Collins M H, Cohen M B, Akers R, Hogan S P, Assa'ad A H, Putnam P E, Aronow B J, Rothenberg M E. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116:536–547. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock J Z, Villanueva J M, Blanchard C, Filipovich A H, Putnam P E, Collins M H, Risma K A, Akers R M, Kirby C L, Buckmeier B K, Assa'ad A H, Hogan S P, Rothenberg M E. Interplay of adaptive th2 immunity with eotaxin-3/c-C chemokine receptor 3 in eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007;45:22–31. doi: 10.1097/MPG.0b013e318043c097. [DOI] [PubMed] [Google Scholar]
- Pope S M, Zimmermann N, Stringer K F, Karow M L, Rothenberg M E. The eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J Immunol. 2005;175:5341–5350. doi: 10.4049/jimmunol.175.8.5341. [DOI] [PubMed] [Google Scholar]
- Fulkerson P C, Fischetti C A, McBride M L, Hassman L M, Hogan S P, Rothenberg M E. A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. Proc Natl Acad Sci USA. 2006;103:16418–16423. doi: 10.1073/pnas.0607863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews A N, Friend D S, Zimmermann N, Sarafi M N, Luster A D, Pearlman E, Wert S E, Rothenberg M E. Eotaxin is required for the baseline level of tissue eosinophils. Proc Natl Acad Sci USA. 1998;95:6273–6278. doi: 10.1073/pnas.95.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Hogan S P, Lee J J, Foster P S, Rothenberg M E. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest. 1999;103:1719–1727. doi: 10.1172/JCI6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cates E C, Fattouh R, Wattie J, Inman M D, Goncharova S, Coyle A J, Gutierrez-Ramos J C, Jordana M. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J Immunol. 2004;173:6384–6392. doi: 10.4049/jimmunol.173.10.6384. [DOI] [PubMed] [Google Scholar]
- Mishra A, Schlotman J, Wang M, Rothenberg M E. Critical role for adaptive T cell immunity in experimental eosinophilic esophagitis in mice. J Leukoc Biol. 2007;81:916–924. doi: 10.1189/jlb.1106653. [DOI] [PubMed] [Google Scholar]
- Chehade M, Sampson H A, Morotti R A, Magid M S. Esophageal subepithelial fibrosis in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007;45:319–328. doi: 10.1097/MPG.0b013e31806ab384. [DOI] [PubMed] [Google Scholar]
- Vicario M, Blanchard C, Stringer K F, Collins M H, Mingler M K, Ahrens A, Putnam M E, Abonia J P, Santos J, Rothenberg M E. Local B cells and IgE production in the oesophageal mucosa in eosinophilic esophagitis. Gut. 2010;59:12–20. doi: 10.1136/gut.2009.178020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora A S, Yamazaki K. Eosinophilic esophagitis: asthma of the esophagus? Clin Gastroenterol Hepatol. 2004;2:523–530. doi: 10.1016/s1542-3565(04)00236-8. [DOI] [PubMed] [Google Scholar]
- Baxi S, Gupta S K, Swigonski N, Fitzgerald J F. Clinical presentation of patients with eosinophilic inflammation of the esophagus. Gastrointest Endosc. 2006;64:473–478. doi: 10.1016/j.gie.2006.03.931. [DOI] [PubMed] [Google Scholar]
- Furuta G T. Eosinophils in the esophagus: acid is not the only cause. J Pediatr Gastroenterol Nutr. 1998;26:468–471. doi: 10.1097/00005176-199804000-00021. [DOI] [PubMed] [Google Scholar]
- Spergel J M. Eosinophilic esophagitis in adults and children: evidence for a food allergy component in many patients. Curr Opin Allergy Clin Immunol. 2007;7:274–278. doi: 10.1097/ACI.0b013e32813aee4a. [DOI] [PubMed] [Google Scholar]
- Mishra A, Wang M, Pemmaraju V R, Collins M H, Fulkerson P C, Abonia J P, Blanchard C, Putnam P E, Rothenberg M E. Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology. 2008;134:204–214. doi: 10.1053/j.gastro.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straumann A, Bauer M, Fischer B, Blaser K, Simon H U. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol. 2001;108:954–961. doi: 10.1067/mai.2001.119917. [DOI] [PubMed] [Google Scholar]
- Kirsch R, Bokhary R, Marcon M A, Cutz E. Activated mucosal mast cells differentiate eosinophilic (allergic) esophagitis from gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2007;44:20–26. doi: 10.1097/MPG.0b013e31802c0d06. [DOI] [PubMed] [Google Scholar]
- Yunginger J W, Ahlstedt S, Eggleston P A, Homburger H A, Nelson H S, Ownby D R, Platts-Mills T A, Sampson H A, Sicherer S H, Weinstein A M, Williams P B, Wood R A, Zeiger R S. Quantitative IgE antibody assays in allergic diseases. J Allergy Clin Immunol. 2000;105:1077–1084. doi: 10.1067/mai.2000.107041. [DOI] [PubMed] [Google Scholar]
- Kulmburg P A, Huber N E, Scheer B J, Wrann M, Baumruker T. Immunoglobulin E plus antigen challenge induces a novel intercrine/chemokine in mouse mast cells. J Exp Med. 1992;176:1773–1778. doi: 10.1084/jem.176.6.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon A, Kleinman R E. Allergic gastroenteropathy in children. Ann Allergy Asthma Immunol. 1995;74:5–12. [PubMed] [Google Scholar]
- Rothenberg M E, Mishra A, Brandt E B, Hogan S P. Gastrointestinal eosinophils. Immunol Rev. 2001;179:139–155. doi: 10.1034/j.1600-065x.2001.790114.x. [DOI] [PubMed] [Google Scholar]