Introduction
Signaling through the TLR pathway has been implicated in the pathogenesis of autoimmune diseases including RA and SLE, as well as in atherosclerosis. Further, an increased incidence of atherosclerosis has been observed in RA and SLE, suggesting a pathogenic link. SLE is a disease that strongly affects young women, and it is in this group that atherosclerotic coronary heart disease is up to 50-fold increased [1]. In RA, the population at risk is older, however they are at increased risk for fatal myocardial infarction compared with age-matched controls [1]. This review will examine the potential role of signaling by endogenous TLR ligands in the pathogenesis of each of these diseases and will explore how they might be related.
OVERVIEW OF TLR SIGNALING
TLRs are a family of type 1 integral membrane glycoprotein PRRs, well known for their role in the recognition of microbial ligands. They are also critical for the development of adaptive immunity, activating DCs and macrophages as the initial step in generating T and B lymphocyte responses [2]. There are at least nine human TLRs expressed on the cell surface (TLR1, -2, -4, -5, and -6) or intracellularly on endosomal membranes (TRL3, -7, -8, and -9) [3]. The extracellular or lysosomal domains recognize specific PAMPs expressed by microbial pathogens, including bacteria, fungi, or viruses. Those TLRs on the cell surface recognize PAMPs that are on the surface of the microbe, and the endosomal TLRs recognize RNA or DNA from the organism. Other PRRs that recognize PAMPS include nucleotide-binding domain and leucine-rich repeat-containing receptor and retinoic acid-inducible gene 1-like receptors [2]. The primary sequences and the structure of the extracellular and lysosomal leucine-rich repeat motifs provide diversity and specificity in ligand recognition. TLR activation may also require heterodimerization to recognize PAMPs, such as TLR2 with TLR1 or TLR6 to recognize diacyl or triacyl lipoproteins or may need cofactors such as myeloid differentiation protein-2 and CD14 for TLR4 activation by LPS [3].
All TLRs share the intracellular domain with the IL-1R family, known as the TIR domain, which is responsible for recruitment of adaptor molecules that mediate signal transduction to nuclear transcription factors [4]. Four adaptor molecules interact with TIR domains to initiate TLR signaling: MyD88; Mal, also called TIRAP; TRIF; and TRAM [4]. TLR3 uses only TRIF, TLR2 uses MyD88 plus Mal, and TLR4 uses MyD88 plus Mal or TRIF plus TRAM, and TLR5, -7, -8, and -9 only use MyD88. Signaling through MyD88 results in NF-κB and MAPK activation, mediated by IRAK1 and -4 and TRAF6, which leads to the expression of proinflammatory chemokines and cytokines. In contrast, signaling through TRIF by TLR3 or TLR4 results in the activation of IFN regulatory factor-3, which results in the expression of type I IFNs, IFN-α and IFN-β.
Dynamic regulation of TLR signaling is necessary to maintain homeostasis to prevent chronic inflammation and tissue destruction. For example, following activation of TLR4 on a given cell by LPS, repeat stimulation results in tolerance, identified as hyporesponsiveness to second LPS challenge, which may affect MyD88-dependent and -independent pathways [5, 6]. Further, soluble TLR2 and TLR4 may antagonize signaling by inhibiting these receptors [7, 8]. Intracellularly, the expression of a variety of molecules may interfere with signaling by antagonizing the MyD88-IRAK-TRIF axis. MyD88s, a splice variant of MyD88, forms MyD88 s-MyD88 heterodimer, which prevents IRAK4 recruitment [9] and IRAK1 phosphorylation. IRAK-M, Toll-interacting protein and suppressor of cytokine signaling 1 may suppress IRAK activation [10]. A20, which is induced by NF-κB activation, deubiquitinates TRAF6, resulting in attenuated TLR signaling [11]. The membrane bound isoform of ST2 (ST2L) is a type I transmembrane receptor that regulates TLR4 and IL-1 signaling, and RP105 attenuates TLR4 signaling [12]. In summary, there are a number of intrinsic mechanisms that dampen the activation through TLRs. We are not aware that these mechanisms have been examined in patients with RA, SLE, or atherosclerosis.
ENDOGENOUS TLR SIGNALING IN RA
Overview
RA is a chronic, inflammatory, systemic disease that focuses on the joints. The chronic inflammation of the joint or synovial lining consists of macrophages, DCs, synovial fibroblasts, B cells, and T cells, each of which has been implicated in the observed joint destruction [13]. An increasing body of data supports the role of the innate immune system in the pathogenesis of RA [14]. In RA, TLRs are important for the generation of adaptive immunity, including the activation of T cells and B cells. However, the role of TLRs in the persistence of RA and the destruction of the joint by the chronic expression of proinflammatory cytokines and chemokines, such as TNF-α, IL-1β, IL-6, and CXCL8 (IL-8), are currently a matter of great interest. RA develops in genetically predisposed individuals who are exposed to an environmental insult. About 60% of patients with RA possess a common sequence in the third hypervariable region of the HLA-DRB1 allele, known as the shared epitope, which includes HLA-DRβ1*0401, *0404, and *0101 [15]. Some individuals who possess the shared epitope, when exposed to environmental stress, such as cigarette smoke, develop antibodies to CCPs (anti-CCP) [16]. Citrullination is a post-translational modification of proteins, such as vimentin and fibronectin, which occurs in response to environmental stress, regardless of the HLA type. The shared epitope is capable of avidly binding CCPs, initiating an immune response that results in the production of anti-CCP antibodies, which may deposit within the joint, initiating the pathogenesis of RA. However, instead of the normal dampening of the immune response [17] and the down-regulation of innate immunity in patients with RA, there is the persistent, pathogenic expression of the inflammatory cytokine, including TNF-α, IL-1, and IL-6, each of which has been targeted successfully in patients with RA. Synovial macrophages have been identified as the major source of these cytokines in the rheumatoid joint, and macrophage numbers in the joint tissue are a reliable barometer of disease activity. Therapies that are effective in RA consistently result in reduced numbers of macrophages in the synovial tissue [18]. An increasing body of data, which will be reviewed in this article, supports the role of TLRs in the persistent, progressive activation of macrophages that appears to drive this destructive inflammatory process.
THE ROLE OF TLR LIGANDS IN ANIMAL MODELS OF ARTHRITIS
A number of animal models of arthritis support the role of TLRs in chronic arthritis. Injection of SCWs into the joints of mice results in an acute, self-limited arthritis mediated by TLR2 [19]. However, repeated injections of SCW result in a chronic, destructive arthritis that becomes dependent on TLR4. As SCWs are a TLR2 ligand, this suggests that the repeated injections result in the induction of endogenous TLR4 ligands that contribute to the persistence and destruction. Another model of RA is collagen-induced arthritis, which develops following the immunization with type II collagen, a component of diarthrodial joints. Mice deficient in RP105, which is a TLR homologue that attenuates TLR4 signaling, had more severe collagen-induced arthritis, again supporting a role for TLR4 [20]. Arthritis may also be developed by the transfer of serum-containing antibodies to glucose-6-phosphate isomerase from K/BxN mice. In this model, antibodies gain access to the intra-articular space, resulting in an immune complex-mediated arthritis. The arthritis that develops following the transfer of serum is shortened in TLR4–/– mice [21], suggesting a potential role for endogenous TLR4 ligands in the persistence of this model. Finally, mice deficient in IL-1Ra develop a spontaneous arthritis that was ameliorated when IL-1Ra−/− mice were crossed with TLR4−/− mice or if the mice were treated with a TLR4 antagonist [22]. These studies also demonstrated that the source of the TLR4 agonist was the gut, as germ-free mice were protected. Together, these observations support a role for local endogenous TLR ligands or those from the gastrointestinal tract in the pathogenesis of these experimental models of arthritis.
LOCAL EXPRESSION OF TLRs IN PATIENTS WITH RA
TLRs are expressed by cells within the RA joint. TLR2, -3, -4, and -7 have been identified by immunohistochemistry [23,24,25], and TLR1–6 were identified by RT-PCR in RA synovial fibroblasts [26]. TLR2 was highly expressed at the sites of bone erosion, identified by in situ hybridization [27], suggesting the importance of TLR2 in bone destruction in RA. We examined macrophages isolated from the synovial fluid of patients with RA and demonstrated that TLR2 and TLR4 were increased compared with normal control monocytes differentiated in vitro into macrophages (control IVD macrophages) [28].
RESPONSE OF CELLS FROM THE RA JOINT TO MICROBIAL LIGANDS
RA synovial fibroblasts are an important source of cytokines, chemokines, and MMPs. RA synovial fibroblasts respond to microbial TLR2, TLR4, and TLR3 ligands but not to the TLR9 ligand CpG [29,30,31]. Following the up-regulation of TLR7, RA synovial fibroblasts also responded to a TLR7 agonist [32]. Therefore, synovial fibroblasts not only express TLRs but also respond to microbial TLR ligands.
The RA synovial macrophages demonstrated increased TNF-α and IL-8 in response to microbial TLR2 (peptidoglycan) and TLR4 (LPS) ligands compared with control IVD macrophages or those obtained from the joints of patients with other forms of inflammatory arthritis, such as psoriatic arthritis and ankylosing spondylitis [28]. The response of the control IVD macrophages to peptidoglycan correlated with the level of TLR2 expressed on the cell surface. In contrast, there was no correlation of cell-surface TLR2 expression on RA synovial macrophages with activation by the microbial TLR2 ligand, suggesting that other factors may be important. The increased response to microbial TLR ligands may be related to the increased expression of IFN-γ or the decreased expression of IL-10 by RA synovial macrophages, each of which may contribute to increased signaling through TLR ligands [33]. In this regard, we recently observed an increased IFN-γ expression by RA synovial fluid macrophages in response to peptidoglycan [34]. In summary, RA synovial fibroblasts respond to microbial TLR ligands, and RA synovial macrophages, the source of many of the cytokines that have been targeted successfully for therapy in RA, demonstrate increased responsiveness to microbial TLR ligands.
POTENTIAL ENDOGENOUS LIGANDS IN THE RA JOINT
Studies have documented a number of potential endogenous ligands in the joints of patients with RA. These molecules are expressed within the cell in response to inflammation, which are released under conditions of stress, even in the absence of apoptosis or necrosis, or they are ECM molecules that may be degraded as a result of inflammation. The potential endogenous TLR4 ligands include HSP22 [35], EDA fibronectin [36], low molecular weight fragments of hyaluronic acid [37], fibrinogen [38], and more recently, tenascin-C [39]. Ligands that activate TLR2 primarily include the gp96 [40] and serum amyloid A [41]. Ligands capable of activation of TLR2 and TLR4 include HSP60 [42, 43] and -70 [44, 45], HMGB1, and biglycan [46]. We suggest that for endogenous TLR ligands to activate cell-surface TLRs, such as TLR2 and TLR4, the ligands must be expressed locally in the inflamed joint and gain access to the extracellular milieu, which may be sampled by examining synovial fluid. In addition, they must bind the TLRs and be capable of activating cells within the joint that cause joint destruction such as synovial macrophages and synovial fibroblasts. Among the identified candidates, EDA fibronectin, tenacin-C, serum amyloid A, HMGB1, and gp96 have been identified within RA synovial fluid. Serum amyloid A, which is expressed in RA synovial tissue, activates NF-κB in synovial fibroblasts and up-regulates adhesion molecules [47]. We were not able to detect significant levels of HSP60, HSP70, or biglycan in the synovial fluid, although these molecules were readily detected in the lysates of cells taken from the rheumatoid joint (unpublished data). The molecular mechanisms by which these potential endogenous ligands, which may also be considered DAMPs, bind and activate TLRs remain to be determined.
A concern with some of these studies is the potential contamination of recombinant proteins with microbial ligands, such as endotoxin capable of activating TLR4, or lipoproteins or lipopeptides, which may activate through TLR2 [48, 49]. However, most recent studies have paid considerable attention to controls, including the use of control recombinant proteins generated and purified by the same techniques, the routine use of inhibitors of endotoxin, and protease digestion. Nonetheless, when recombinant proteins generated in bacteria are used, there may be the possibility of contamination by microbial TLR ligands. Nonetheless, studies that have used patient synovial fluids and tissues, which have not been exposed to microbial contamination, as mentioned in the next paragraph, support the relevance of endogenous TLR ligands in RA.
With all of these potential ligands, what data support that molecules expressed in the tissues or fluids are functionally active? RA synovial fluid has been shown to activate human embryonic kidney 293 cells expressing TLR4 [22]. Further, our unpublished data demonstrate that RA synovial fluid is capable of activating normal IVD macrophages to express TNF-α and IL-8 and that this activation can be suppressed by antibodies to TLR2 or TLR4. In similar experiments, culture supernatants from explants of RA synovial tissue also activated normal IVD macrophages in a MyD88- and Mal/TIRAP-dependent manner, suggesting that TLR ligands may have been involved [50]. The observations are consistent with the hypothesis that endogenous TLR ligands within the RA joint are capable of activating macrophages through TLR signaling. Exactly which ligand dominates in this process remains to be determined, however recent studies highlight the potential importance of gp96 and tenascin-C.
gp96 IN RA
Our recent observations support the potential role of gp96 as an important endogenous TLR ligand in RA. By immunohistochemistry, gp96 was increased in RA synovial tissue compared with osteoarthritis and arthritis-free control synovial tissues [40]. The expression of gp96 correlated strongly with inflammation and synovial lining thickness. gp96 was increased in synovial fluid from the joints of RA compared with patients with osteoarthritis, psoriatic arthritis, or ankylosing spondylitis. Recombinant gp96, as well as gp96 from macrophage lysates, bound to recombinant TLR2 and TLR4 molecules in pull-down experiments. Further, recombinant gp96 was a potent activator of macrophages, and the activation was mediated primarily through TLR2 signaling and to a lesser degree, through TLR4. The cellular response to gp96 was significantly stronger in RA synovial macrophages compared with peripheral blood monocytes from RA or healthy controls or control IVD macrophages. The transcription of TLR2, TNF-α, and IL-8, but not TLR4, was induced significantly by gp96, and the induction was significantly greater in purified RA synovial macrophages compared with control IVD macrophages. Providing in vivo relevance, the expression of TLR2, but not TLR4, on RA synovial fluid macrophages correlated with the level of gp96 in the synovial fluid in the same patient. These observations identify gp96 as a potential endogenous TLR ligand that might contribute to the persistent, destructive synovitis observed in RA.
TENACIN-C
Tenascin-C is an ECM glycoprotein that is not highly expressed in normal tissues but is increased at sites of inflammation, such as RA synovial tissue and in synovial fluid [39]. Recombinant tenascin-C, which was very low in endotoxin, was capable of activating normal IVD macrophages to produce TNF-α, IL-6, and IL-8 [51]. In addition, tenascin-C was capable of activating RA synovial fibroblasts to express IL-6, but not IL-8 or TNF-α. The effects of tenascin-C were mediated by the fibrinogen-like domain of tenascin-C and were mediated through TLR4 and MyD88. To document the potential role of tenascin-C in chronic inflammation, mice deficient in tenascin-C were used. Immune complex-mediated arthritis was induced in mice by injecting methylated BSA into the joints of immunized mice. Tenacin-C was induced in the joints of these mice. However, mice deficient in tenascin-C showed rapid resolution of acute joint inflammation and were protected from erosive arthritis. These observations suggest that during the initial acute inflammatory response induced by the deposition of immune complexes within the joint, tenascin-C was induced, and it was capable of activating cells locally within the joint, through TLR4, to promote chronic inflammation and joint destruction.
ENDOGENOUS TLR LIGANDS IN SLE
Overview
SLE is an autoimmune disease with a variable clinical presentation that may include rash, arthritis, pleuropericarditis, CNS involvement, cytopenias, and nephritis. Patients characteristically express circulating antinuclear antibodies, which may be composed of antibodies to DNA and RNA, including dsDNA, nucleosomes, and RNPs. Data support the role of these autoantibodies in the pathogenesis of SLE, as they are present at sights of pathology such as the kidney. The normal germ line contains Ig gene sequences, which following selection and maturation, develop high-affinity, pathogenic IgG autoantibodies. Decreased clearance of apoptotic cells may result in the increased release of DNA- and RNA-containing molecules in patients with lupus, potentially aided by enhanced antigen presentation, resulting in the generation of these pathogenic antibodies. In lupus, evidence suggests that the TLR system enhances the expression of pathogenic autoantibodies and possibly contributes to the IFN-α signature pattern that is characteristic of patients with active SLE, particularly by the activation of pDCs, which are programmed to express high levels of IFN-α following activation through endosomal TLRs [52].
TLRs CONTRIBUTE TO AUTOANTIBODY FORMATION AND LUPUS
A number of different murine models of lupus have been used to examine the role of TLRs in the generation of anti-DNA and -RNA complexes. MRL-lpr mice develop spontaneous lupus with splenomegaly and nephritis, as well as antibodies to dsDNA, chromatin, and Smith antigen. Deletion of TLR9 resulted in the reduction of antibodies to dsDNA and chromatin but not RNP, and importantly, there was no protection against disease [53]. In another study, deletion of TLR9 actually resulted in more severe disease in MRL-lpr mice [54]. Further, B6-lpr mice have less severe disease compared with MRL-lpr mice, and deletion of TLR9 in the B6-lpr mice resulted in reduced anti-DNA antibodies, but these mice also developed more severe nephritis [55]. TLR9–/– MRL-lpr mice demonstrated increased pDC maturation [56] and impaired T regulatory cells [54], identifying potential mechanisms for more severe disease, despite the reduction of anti-DNA antibodies.
The role of TLR7 in murine models of lupus has also been examined. MRL-lpr mice deficient in TLR7 demonstrated decreased antibodies to RNP, but there was no reduction of antibodies to dsDNA or nucleosomes [56]. The TLR7–/– mice demonstrated reduced pDC activation and less kidney disease. Recently, in MRL-lpr mice, deletion of TLR7 and TLR9 or MyD88 resulted in a marked reduction of antinucleosome and anti-RNA autoantibodies [57]. The TLR7/9–/– and MyD88–/– mice also demonstrated a marked reduction of kidney disease, although there was no reduction of spleen or lymph node size, dissociating the effects of autoantibody production from lymphocyte proliferation. In another model of lupus, the NZB autoimmunity two locus mice, there was an exacerbation of disease in TLR9–/– mice, and marked protection from lupus nephritis in the TLR7/9 double-deficient mice [58]. The TLR7–/– /9–/– mice also demonstrated a marked reduction of antibodies to dsDNA and RNA. These observations demonstrate that although TLR9 contributes to the regulation of antibodies to DNA and nucleosomes, its absence may actually worsen disease. In contrast, TLR7, which is critical for the expression of antibodies to RNA, is critical for the regulation of disease, even the disease exacerbation observed when TLR9 is absent.
Supporting the importance of TLR7 and -9, inhibition of TLR7 and TLR9, using immunoregulatory oligonucleotides, suppressed anti-dsDNA, antinucleosome, and anti-RNP antibodies and ameliorated nephritis in NZB × New Zealand White F1 mice [59]. Further, a deficiency of endosomal TLR signaling, which prevents binding to TLR3, -7, and -9, resulted in reduced antichromatin antibodies and reduced nephritis in B6-lpr and in BXSB (a unique recombinant inbreed strain derived from the mating of C57B/6L and SB/LB strains) mice [60]. Further, mice with a duplication of the TLR7 gene demonstrated enhanced antibodies to RNA and a more severe lupus-like disease [61]. These studies demonstrate the importance of TLR7 and TLR9 in the pathogenesis of murine models of SLE.
TLRs REGULATE AUTOANTIBODY PRODUCTION
The endosomal TLRs (3, 7, 8, and 9) recognize microbial dsRNA, single-stranded RNA, and DNA, in particular, hypomethylated CpG motifs. Therefore, studies have been performed to determine how TLR7 and TLR9 contribute to the production of antibodies to self-RNA and -DNA molecules in lupus. DNA or RNA, alone or bound to nuclear-binding proteins, may contribute to the generation of autoantibodies through effects on pDCs or B cells. Antibodies recognizing DNA or RNA form immune complexes in lupus. These complexes may bind to the FcRs on DCs, which results in the delivery of the complex to the endosome, where the DNA or RNA is recognized by TLR7 or TLR9. Alternatively, antigen containing DNA or RNA may bind to B cells through the BCR, which also results in the internalization of the antigen to the endosomal compartment.
Immune complexes containing IgG and DNA obtained from the circulation of patients with SLE activated pDC to produce inflammatory cytokines and chemokines [62]. This activation was mediated by the binding of the immune complexes to FcγRIIa (CD32), which internalized the complexes to the endosomal compartment containing TLR9. The DNA, which is recognized as an antigen in patients with SLE, may derive from apoptotic cells. Mammalian DNA has fewer CpG islands than expected by chance, and most are methylated. Nonetheless, hypomethylated CpG dsDNA was released from apoptotic cells [63]. The released, hypomethylated CpG dsDNA formed an immune complex with antibodies that recognized dsDNA. This complex was internalized by binding to the BCR and following localization to the endosome, resulted in B cell proliferation mediated by TLR9. Further, hypomethylated mammalian CpG DNA was also capable of activating pDC through TLR9 [64]. These observations support the potential role of mammalian DNA, which is released by apoptosis to activate pDC and B cells mediated through TLR9.
TLR7 also mediates B cell and pDC activation by autoantigens. Immune complexes containing antibodies to RNA were capable of activating B cells mediated through TLR7 [65]. Additionally, SLE serum containing anti-RNP antibodies, when added to apoptotic cells, activated PBMCs to produce IFN-α [66]. These observations support the role of RNA-containing immune complexes in activating pDCs and B cells mediated through TLR7.
TLR2 has also been implicated in the activation of macrophages and DCs by nucleosomes bound to HMGB1 [67], and HMGB1-containing nucleosomes activated macrophages and DCs and induced DC maturation, which was mediated through TLR2 and not TLR4, TLR9, or RAGE [67]. These observations suggest that TLR2 may also contribute to the generation of autoantibodies through the activation and maturation of APCs by apoptotic cells in patients with SLE.
In summary, TLR7 and TLR9 may contribute to the pathogenesis of lupus by promoting the expression of autoantibodies to RNA and DNA released from apoptotic cells, which serve as endogenous TLR ligands. Nucleic acid-containing immune complexes may activate pDC by activation of TLR7 and TLR9, resulting in more efficient antigen presentation to T cells, providing help to autoantibody-producing B cells. Additionally, the activation of pDC results in the expression of IFN-α, the signature cytokine in lupus. Finally, the binding of nucleic acid-containing autoantigens to the BCR may also result in B cell activation, mediated by TLR7 and TLR9, promoting the proliferation of autoantibody-producing B cells. Overall, the data suggest that DNA and RNA released from apoptotic cells serve as antigens that are critical in the pathogenesis of SLE. In addition, these ligands function as endogenous TLR7 and TLR9 ligands, which may accelerate the disease by enhancing pDC activation and the release of INF-α and by promoting B cell activation and proliferation.
ENDOGENOUS TLR LIGANDS IN ATHEROSCLEROSIS
Overview of pathogenesis
Atherosclerosis is an arterial disease that results in blood-vessel narrowing, a process that may culminate in thrombosis and occlusion, resulting in a myocardial infarction or stroke. Atherosclerosis is a chronic inflammatory disease of arteries resulting in the formation of a plaque. This process is characterized by activation of endothelial cells; proliferation of smooth muscle cells; infiltration with macrophages containing lipids (foam cells), DCs, and T cells; and the accumulation of lipids and necrotic and apoptotic material, together with the ECM, which organizes the lesion and forms a fibrous cap. The lesion begins with the formation of a fatty streak by the deposition of LDLs, which deposit in the subendothelial intima, where they are modified by oxLDLs. They are taken up by macrophages through PRRs, including SRA and CD36, to become foam cells, which may progress or resolve over time [68, 69]. The production of cytokines and chemokines by macrophages activates endothelial cells, increasing the expression of adhesion molecules such as VCAM-1, which promotes the adhesion of monocytes and lymphocytes. These cells are recruited into the lesion by chemokines, including MCP-1 and RANTES [68]. Monocytes differentiate into macrophages as a result of adhesion and M-CSF-1 [70]. Plaque growth and eventual rupture are associated with increased numbers of macrophages [71]. The T cells become activated, likely as a result of antigen presentation locally by macrophages and DCs. Macrophages are activated possibly by the expression of endogenous TLR ligands or DAMPs that are expressed locally (reviewed below). With the propagation of the plaque, the foam cells die releasing lipids, intracellular molecules, and necrotic debris, which can further activate the remaining macrophages. Macrophage activation results in the production of proteases, such as MMPs, which may disrupt the ECM of the lesion, resulting in plaque rupture, which contributes to thrombosis and ischemic damage distal to the lesion.
EXPRESSION OF TLRs IN HUMAN DISEASE
Supporting the potential role for TLRs in human atherosclerotic plaques, TLR1, TLR2, TLR6, TLR7, and TLR8 were increased significantly by RT-PCR compared with nondiseased segments, and TLR4 mRNA was also increased, although the difference was not significant [72]. By immunohistochemistry, TLR1, TLR2, and TLR4 were highly expressed, particularly on endothelial cells and macrophages, and weaker staining was observed for TLR3, TLR5, and TLR2. In contrast, in normal arteries, endothelial cells were generally negative for TLR staining, although when DCs were detected, they were generally positive, and the pattern of TLR expression varied within the vascular bed examined [73]. In these normal vessels, CD11b mRNA was not detected, suggesting that macrophages were not frequent [73]. Further, in the ApoE−/− model of atherosclerosis, the expression of TLR2 and TLR4 was negative initially but increased over time in diseased lesions [74]. These observations support a potential role of TLRs in the pathogenesis of atherosclerosis.
ANIMAL MODELS SUPPORT THE ROLE OF TLRs IN ATHEROSCLEROSIS
Supporting the role of TLR signaling in the development of atherosclerosis, the carotid arteries of rabbits transfected with cDNA encoding TLR2 or TLR4 developed atherosclerosis, and the induction of disease was synergistic if TLR2 and TLR4 were expressed [75]. More definitive studies have been performed with mice deficient in molecules critical for the prevention of atherosclerosis. Mice deficient in LDLR, which is necessary to clear LDL from the circulation, develop accelerated atherosclerosis when fed a high-fat diet, and mice deficient in ApoE, necessary for the clearance of LDL, also experience severe disease. All TLRs except TLR3, together with IL-1β and IL-18, signal through the adaptor molecule MyD88. When MyD88−/− mice were crossed with ApoE−/− mice, there was a marked diminution of atherosclerosis, including a reduction of lesional macrophages and chemokines [76, 77]. Additionally, the functional deficiency of IRAK4, which is downstream of MyD88, ameliorated the vascular lesions observed in ApoE−/− mice [78]. To determine the role of TLR4, TLR4−/− mice were crossed with those that were ApoE−/− [77]. These mice were also protected against the development of atherosclerosis, which was associated with a decrease of macrophages in the lesions, as well as a reduction of MCP-1, which is chemotactic for monocytes [77]. The protection observed in these studies was not a result of a reduction of circulating cholesterol. Endothelial cells from mice with a defect of TLR4 signaling (C3H/HeJ) demonstrated decreased production of MCP-1 and M-CSF in response to oxLDL [79], suggesting a pathogenic mechanism. These observations support the potential role of MyD88 and TLR4 in the pathogenesis of atherosclerosis.
To study the role of TLR2, TLR2−/− and ApoE−/− mice were crossed. These mice also exhibited decreased atherosclerotic plaques, decreased numbers of macrophages, and reduced MCP-1 [80]. However, the lack of TLR2 did not reduce the ability of macrophages to take up modified LDL. In another murine model of atherosclerosis, mice deficient in LDLr and TLR2 were also resistant to disease, when the experimental animals were fed a high-fat diet [81]. The use of bone marrow chimeras demonstrated that TLR2−/− on the non-bone marrow-derived cells, likely endothelial cells, protected against disease development. In the LDLr−/− mice, i.p. injection of a TLR2 agonist (Pam3CSK4) worsened disease, and the absence of TLR2 on bone marrow cells protected against disease exacerbation induced by Pam3CSK4. In the spontaneous disease in LDLr−/− mice, endothelial cell expression of TLR2 in regions of disturbed blood flow was one of the earliest findings [82]. It was in the area where endothelial TLR2 was expressed initially that endothelial cell damage occurred, and foam cells accumulated [82]. These observations suggest a role for TLR2 on endothelial cells and macrophages in the pathogenesis of atherosclerosis, however they suggest that endothelial cell TLR2 induced by turbulent bloodflow may be one of the earliest events in disease pathogenesis in this model.
ROLE OF TLR-MEDIATED NF-κB ACTIVATION IN ATHEROSCLEROSIS
In human atherosclerotic lesions, activated NF-κB is present in endothelial cells, smooth muscle cells, and macrophages when examined by immunohistochemistry, and NF-κB was not activated in arteries without disease [83], supporting the potential role of TLR signaling. Further, cells isolated from atherosclerotic plaques at the time of therapeutic endarterectomies, which included macrophages, smooth muscle cells, and T lymphocytes, demonstrated activation of the canonical NF-κB pathway, determined by the activation of p65, p50, and c-Rel, which resulted in the secretion of proinflammatory cytokines and MMPs [84]. This spontaneous activation was mediated through TLR2, but not TLR4 or IL-1R, suggesting a role for endogenous TLR2 ligands, when the disease is quite advanced [85].
Animal models also support the role of NF-κB signaling in atherosclerosis. The endothelial-specific deletion of the IKKγ/NF-κB essential modulator suppressed atherosclerosis in ApoE−/− mice [86]. Additionally, mice haplosufficient for A20, which suppresses TLR-mediated NF-κB activation, demonstrated reduced atherosclerosis in ApoE−/− mice [87]. In contrast, deletion of IKK2 in macrophages, which was not complete and only resulted in a 50% reduction of NF-κB activation, actually resulted in more atherosclerosis in LDLr−/− mice [88]. This may be a result of decreased IL-10 and/or increased necrosis within the plaques [88], each of which may result in increased TLR activation. We showed recently that when NF-κB activation is suppressed in macrophages, TLR4 ligation resulted in apoptotic cell death mediated by receptor-interacting protein 1 [89]. Together, these observations support the role of TLR-mediated NF-κB activation in endothelial cells and macrophages in the pathogenesis of atherosclerosis.
POTENTIAL ENDOGENOUS TLR LIGANDS IN ATHEROSCLEROSIS
A variety of potential endogenous TLR ligands has been identified in atherosclerotic lesions, including a variety of HSPs [68]. HSP60 is particularly interesting, as it has been shown to be increased in endothelial cells by fluid sheer stress [90], similar to TLR2 [82], and circulating HSP60 may be a biomarker for atherosclerosis [91]. Further, EDA fibronectin, a potential TLR4 ligand, and TLR4 each increased over time with the progression of the disease in ApoE−/− mice [74]. Serum amyloid A, which is highly expressed in RA, is capable of binding the high-density lipoproteins [92] and binding to and activating the high-density lipoprotein receptor scavenger receptor-class B type 1 [93], supporting a potential role in atherosclerosis. HMGB1 protein, which may activate cells through RAGE, TLR2, TLR4, or TLR9 [94], is expressed at low levels in endothelial cells and smooth muscle cells in normal and atherosclerotic arteries, and most macrophages in diseased arteries express HMGB1 highly [95]. Further, cytokines expressed locally, such as TNF-α, IFN-γ, and TGF-β, increase the secretion of HMGB1, setting up a local autocrine pathway to accelerate macrophage activation. Additionally, HMGB1 promotes the proliferation of smooth muscle cells [96]. Therefore, TLRs and endogenous TLR ligands may each be induced locally early in the disease pathogenesis, contributing to an autocrine loop.
Although there has been controversy over the role of lipids in the activation of TLR2 and TLR4, recent studies have clarified how oxLDL may contribute to activation of the TLR signaling pathway. PRRs, such as SRA and CD36, are well-characterized receptors, which bind oxLDL and transport lipids into the cell. CD36 binds diacyl- and triacylglycerides and serves as a coreceptor with TLR2, which may also heterodimerize with TLR1 or TLR6 [97,98,99]. Further, a recent study demonstrated that CD36 served as a coreceptor with TLR4 and TLR6, resulting in macrophage activation by oxLDL [100], which bound to CD36, resulting in the activation of Lyn, and Lyn activation induced the physical association of CD36 with TLR4 and TLR6, which then signaled through NF-κB in a MyD88- and TRIF-dependent manner, resulting in the expression of chemokines, including RANTES (CCL5), MIP-2 (CXCL2), and MIP-1γ (CCL9). In addition to these well-described pathways, it is possible that when necrotic cells release their contents, endosomal receptors such as TLR3 and TLR7 may be activated. TLR3 has been shown to be the sensor for necrotic cells in vitro [101].
In summary, TLRs are highly expressed in atherosclerotic lesions. Areas of turbulent bloodflow may induce TLR2 as well as endogenous TLR ligands by endothelial cells. The local induction of endogenous TLR ligands as well as the deposition of oxLDL may promote endothelial cell activation, which results in monocyte recruitment. Monocytes differentiate into macrophages, which become activated, resulting in further chemokine secretion and in the additional accumulation of inflammatory cells. This process results in further modification of LDL and in a self-perpetuating process, which may culminate in plaque rupture induced by the expression of MMPs, resulting in thrombosis.
THE CONNECTION BETWEEN AUTOIMMUNITY AND ATHEROSCLEROSIS
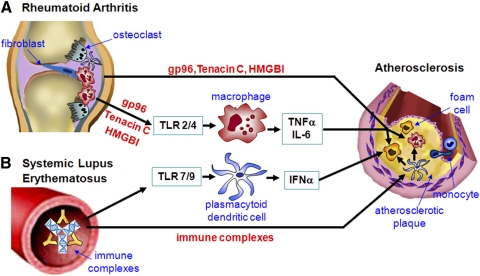
The association of RA or SLE with atherosclerosis may be, in part, a result of TLR signaling, which contributes to the pathogenesis of each condition (Fig. 1). RA and atherosclerosis exhibit a number of mechanistic similarities. Each is characterized by chronic inflammation, the accumulation of macrophages, DCs, and B and T lymphocytes. The accumulation of lesional macrophages is highly associated with disease progression or response to therapy in each disease. Each demonstrates the local expression of TLRs and potential endogenous TLR ligands, particularly those that signal through TLR2 and TLR4. They are distinct, as RA synovial tissue does not demonstrate infiltration with lipids, such as oxLDL or foam cells. Further, necrosis is not characteristic of RA synovial tissue, and apoptosis is limited [13].
Figure 1.
The connection between autoimmunity and atherosclerosis. The association of atherosclerosis with endogenous ligands mediated TLR signaling in RA or immune complex mediated TLR signaling in SLE is outlined in this figure. (A) Endogenous gp96, Tenacin C or HMGBI released from the inflamed synovial tissue of RA serve as TLR ligands to active macrophages and foam cells accumulated in the atherosclerotic plague. This activation is promoted by the inflammatory cytokines (such as TNFα and IL-6) from the rheumatoid joint mediated by macrophages, resulted in the increased occurrence and severity of atherosclerosis in patients with RA. (B) In SLE, the endogenous immune complexes, formed by anti RNA and DNA antibodies, active plasmacytoid dendritic cells mediated by TLR7 and TLR9 to release IFNα. The systemic release of IFNα further promotes the activation of macrophages and foam cells in the atherosclerotic plaques. In addition, the plasmacytoid dendritic in the plaque can be activated by direct contact with the RNA/DNA containing immune complexes, further inducing the local recruitment and activation of inflammatory cells, resulting in a self-perpetuating process of inflammation and plaque formation towards rupture.
It is possible that the increased occurrence and severity of atherosclerosis observed in patients with RA may be a result of the release of endogenous TLR ligands from the inflamed synovial tissue, which might then further activate macrophages in the atherosclerotic plaque. Additionally, the release of inflammatory cytokines, such as TNF-α or IL-6, from the rheumatoid joint, which may be promoted in part by local endogenous TLR ligands, may activate macrophages or other cell types in the atherosclerotic plaque.
The mechanisms by which SLE may result in increased atherosclerosis are many. Blood vessel inflammation or vasculitis is a characteristic feature of lupus, which may predispose vessels to atherosclerosis. Vasculitis may also be seen in a subset of patients with RA, who develop extraarticular manifestations or nodules [1]. Patients with active SLE demonstrate a type I IFN signature pattern, the expression of IFN-α-responsive genes in their lymphocytes, such as interferon-inducible double stranded dependent protein kinase (PRKP) and interferon-inducible 44 (IFI44) [102]. The expression of type I IFN was associated with reduced endothelial progenitor cells and decreased endothelial cell function in vitro in patients with SLE [103]. The depletion of endothelial progenitor cells may be mediated by IFN-α-induced endothelial progenitor cell apoptosis [104]. The release of IFN-α from pDC in patients with lupus may be mediated by activation of TLR7 and TLR9 by endogenous RNA and DNA, taken up as immune complexes by the pDCs. Further, the release of IFN-α systemically may also promote the activation of macrophages in the atherosclerotic plaques by endogenous TLR2 and TLR4 ligands that are expressed locally. Supporting this possibility, plaque tissue stimulated with CpG induced the lesional pDC to release IFN-α, which sensitized the plaque macrophages to become more responsive to activation by TLR4 ligation [105]. It is also possible that in patients with lupus and atherosclerosis, the plaque pDC may also become activated by coming into contact with RNA- or DNA-containing immune complexes. Therefore, IFN-α induced by endogenous TLR7 and TLR9 ligands may promote atherosclerosis by deleting endothelial progenitor cells and by sensitizing plaque macrophages to activation by endogenous TLR2 and TLR4 ligands.
In summary, the data presented support the potential role of endogenous TLR ligands or DAMPs, which may contribute to the chronic, persistent inflammation noted in RA and atherosclerosis. DAMPs arise at the site of the initial inflammatory lesions and may contribute to the activation of lesional cells, particularly macrophages mediated through TLR2 and TLR4. In contrast, the endogenous TLR ligands, indentified in lupus, may be generated from apoptotic cells. These ligands, which include DNA and RNA and activate pDC and B cells through endosomal TLR7 and TLR9, serve as antigens for the formation of immune complexes. Although the discussion above focused on the effects DAMPs arising as part of RA and SLE on atherosclerosis, it is also possible that necrotic or apoptotic material in atherosclerotic plaques may exacerbate lupus, and the release of endogenous TLR2/4 ligands might increase disease activity in RA. Further studies in experimental animals will be required to define further the mechanisms for the increase of atherosclerosis in patients with RA and SLE and whether atherosclerosis may affect the clinical course of RA or SLE.
AUTHORSHIP
Q.H. and R.M.P. contributed to this manuscript preparation.
ACKNOWLEDGMENTS
This work is supported by National Institutes of Health grant AR055240 and a Within Our Reach grant from the American College of Rheumatology. The authors thank Dr. Harris Perlman for his thoughtful review of the manuscript.
Footnotes
Abbreviations: –/–=deficient, ApoE=apolipoprotein E, CCP=cyclic-citrullinated peptide, DAMP=damage-associated molecular pattern, DC= dendritic cell, dsDNA=double-stranded DNA, ECM=extracellular matrix, EDA=extra domain A, gp96=96-kDa heat shock glycoprotein, HMGB1= high mobility group box chromosomal protein 1, HSP=heat shock protein, IKK=IκB kinase, IL-1Ra=IL-1R antagonist, IRAK=IL-1R-associated kinase, IVD macrophage=in vitro differentiated macrophage, LDL=low-density lipoprotein, LDLr=LDLR, Mal=MyD88 adaptor-like protein, MMP=matrix metalloproteinase, MyD88s=MyD88short, NZB=New Zealand Black, oxLDL=oxidized low-density lipoprotein, Pam3CSK4=palmitoyl-3-cysteine-serine-lysine-4, PAMP=pathogen-associated molecular pattern, pDC=plasmacytoid DC, PRR=pattern recognition receptor, RA=rheumatoid arthritis, RAGE=receptor for advanced glycation end-products, RNP=ribonucleoprotein, RP105=radioprotective 105, SCW=streptococcal cell wall, SLE=systemic lupus erythematosus, SRA=scavenger receptor A, TIR=Toll/IL-1R, TIRAP=Toll/IL-1R domain-containing adaptor protein, TRAF6=TNFR-associated factor 6, TRAM=TIR domain-containing adaptor-inducing IFN-α-related adaptor molecule, TRIF=Toll/IL-1R domain-containing adaptor-inducing IFN-α
References
- Haque S, Mirjafari H, Bruce I N. Atherosclerosis in rheumatoid arthritis and systemic lupus erythematosus. Curr Opin Lipidol. 2008;19:338–343. doi: 10.1097/MOL.0b013e328304b65f. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- de Vos A F, Pater J M, van den Pangaart P S, de Kruif M D, van 't Veer C, van der Poll T. In vivo lipopolysaccharide exposure of human blood leukocytes induces cross-tolerance to multiple TLR ligands. J Immunol. 2009;183:533–542. doi: 10.4049/jimmunol.0802189. [DOI] [PubMed] [Google Scholar]
- Medvedev A E, Sabroe I, Hasday J D, Vogel S N. Tolerance to microbial TLR ligands: molecular mechanisms and relevance to disease. J Endotoxin Res. 2006;12:133–150. doi: 10.1179/096805106X102255. [DOI] [PubMed] [Google Scholar]
- Iwami K I, Matsuguchi T, Masuda A, Kikuchi T, Musikacharoen T, Yoshikai Y. Cutting edge: naturally occurring soluble form of mouse Toll-like receptor 4 inhibits lipopolysaccharide signaling. J Immunol. 2000;165:6682–6686. doi: 10.4049/jimmunol.165.12.6682. [DOI] [PubMed] [Google Scholar]
- Raby A C, Le Bouder E, Colmont C, Davies J, Richards P, Coles B, George C H, Jones S A, Brennan P, Topley N, Labeta M O. Soluble TLR2 reduces inflammation without compromising bacterial clearance by disrupting TLR2 triggering. J Immunol. 2009;183:506–517. doi: 10.4049/jimmunol.0802909. [DOI] [PubMed] [Google Scholar]
- Burns K, Janssens S, Brissoni B, Olivos N, Beyaert R, Tschopp J. Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J Exp Med. 2003;197:263–268. doi: 10.1084/jem.20021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew F Y, Xu D, Brint E K, O'Neill L A. Negative regulation of Toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- Boone D L, Turer E E, Lee E G, Ahmad R C, Wheeler M T, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, McNally E, Pickart C, Ma A. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- Divanovic S, Trompette A, Petiniot L K, Allen J L, Flick L M, Belkaid Y, Madan R, Haky J J, Karp C L. Regulation of TLR4 signaling and the host interface with pathogens and danger: the role of RP105. J Leukoc Biol. 2007;82:265–271. doi: 10.1189/jlb.0107021. [DOI] [PubMed] [Google Scholar]
- Pope R M. Apoptosis as a therapeutic tool in rheumatoid arthritis. Nat Rev Immunol. 2002;2:527–535. doi: 10.1038/nri846. [DOI] [PubMed] [Google Scholar]
- Huang Q Q, Pope R M. The role of Toll-like receptors in rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:357–364. doi: 10.1007/s11926-009-0051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes J, Barton A. Recent advances in the genetics of RA susceptibility. Rheumatology (Oxford) 2008;47:399–402. doi: 10.1093/rheumatology/ken005. [DOI] [PubMed] [Google Scholar]
- Lundstrom E, Kallberg H, Alfredsson L, Klareskog L, Padyukov L. Gene-environment interaction between the DRB1 shared epitope and smoking in the risk of anti-citrullinated protein antibody-positive rheumatoid arthritis: all alleles are important. Arthritis Rheum. 2009;60:1597–1603. doi: 10.1002/art.24572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebe K, Janssen E, Beutler B. The interface between innate and adaptive immunity. Nat Immunol. 2004;5:971–974. doi: 10.1038/ni1004-971. [DOI] [PubMed] [Google Scholar]
- Wijbrandts C A, Vergunst C E, Haringman J J, Gerlag D M, Smeets T J, Tak P P. Absence of changes in the number of synovial sublining macrophages after ineffective treatment for rheumatoid arthritis: implications for use of synovial sublining macrophages as a biomarker. Arthritis Rheum. 2007;56:3869–3871. doi: 10.1002/art.22964. [DOI] [PubMed] [Google Scholar]
- Joosten L A, Abdollahi-Roodsaz S, Heuvelmans-Jacobs M, Helsen M M, van den Bersselaar L A, Oppers-Walgreen B, Koenders M I, van den Berg W B. T cell dependence of chronic destructive murine arthritis induced by repeated local activation of Toll-like receptor-driven pathways: crucial role of both interleukin-1β and interleukin-17. Arthritis Rheum. 2008;58:98–108. doi: 10.1002/art.23152. [DOI] [PubMed] [Google Scholar]
- Divanovic S, Trompette A, Atabani S F, Madan R, Golenbock D T, Visintin A, Finberg R W, Tarakhovsky A, Vogel S N, Belkaid Y, Kurt-Jones E A, Karp C L. Negative regulation of Toll-like receptor 4 signaling by the Toll-like receptor homolog RP105. Nat Immunol. 2005;6:571–578. doi: 10.1038/ni1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe J Y, Crain B, Wu S R, Corr M. Interleukin 1 receptor dependence of serum transferred arthritis can be circumvented by Toll-like receptor 4 signaling. J Exp Med. 2003;197:537–542. doi: 10.1084/jem.20021850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdollahi-Roodsaz S, Joosten L A, Koenders M I, Devesa I, Roelofs M F, Radstake T R, Heuvelmans-Jacobs M, Akira S, Nicklin M J, Ribeiro-Dias F, van den Berg W B. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J Clin Invest. 2008;118:205–216. doi: 10.1172/JCI32639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahashi M, Yamamura M, Aita T, Okamoto A, Ueno A, Ogawa N, Akashi S, Miyake K, Godowski P J, Makino H. Expression of Toll-like receptor 2 on CD16+ blood monocytes and synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum. 2004;50:1457–1467. doi: 10.1002/art.20219. [DOI] [PubMed] [Google Scholar]
- Radstake T R, Roelofs M F, Jenniskens Y M, Oppers-Walgreen B, van Riel P L, Barrera P, Joosten L A, van den Berg W B. Expression of Toll-like receptors 2 and 4 in rheumatoid synovial tissue and regulation by proinflammatory cytokines interleukin-12 and interleukin-18 via interferon-γ. Arthritis Rheum. 2004;50:3856–3865. doi: 10.1002/art.20678. [DOI] [PubMed] [Google Scholar]
- Sorensen L K, Havemose-Poulsen A, Sonder S U, Bendtzen K, Holmstrup P. Blood cell gene expression profiling in subjects with aggressive periodontitis and chronic arthritis. J Periodontol. 2008;79:477–485. doi: 10.1902/jop.2008.070309. [DOI] [PubMed] [Google Scholar]
- Ospelt C, Brentano F, Rengel Y, Stanczyk J, Kolling C, Tak P P, Gay R E, Gay S, Kyburz D. Overexpression of Toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: Toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum. 2008;58:3684–3692. doi: 10.1002/art.24140. [DOI] [PubMed] [Google Scholar]
- Seibl R, Birchler T, Loeliger S, Hossle J P, Gay R E, Saurenmann T, Michel B A, Seger R A, Gay S, Lauener R P. Expression and regulation of Toll-like receptor 2 in rheumatoid arthritis synovium. Am J Pathol. 2003;162:1221–1227. doi: 10.1016/S0002-9440(10)63918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Ma Y, Adebayo A, Pope R M. Increased macrophage activation mediated through Toll-like receptors in rheumatoid arthritis. Arthritis Rheum. 2007;56:2192–2201. doi: 10.1002/art.22707. [DOI] [PubMed] [Google Scholar]
- Cho M L, Ju J H, Kim H R, Oh H J, Kang C M, Jhun J Y, Lee S Y, Park M K, Min J K, Park S H, Lee S H, Kim H Y. Toll-like receptor 2 ligand mediates the upregulation of angiogenic factor, vascular endothelial growth factor and interleukin-8/CXCL8 in human rheumatoid synovial fibroblasts. Immunol Lett. 2007;108:121–128. doi: 10.1016/j.imlet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Jung Y O, Cho M L, Kang C M, Jhun J Y, Park J S, Oh H J, Min J K, Park S H, Kim H Y. Toll-like receptor 2 and 4 combination engagement upregulate IL-15 synergistically in human rheumatoid synovial fibroblasts. Immunol Lett. 2007;109:21–27. doi: 10.1016/j.imlet.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Kyburz D, Rethage J, Seibl R, Lauener R, Gay R E, Carson D A, Gay S. Bacterial peptidoglycans but not CpG oligodeoxynucleotides activate synovial fibroblasts by Toll-like receptor signaling. Arthritis Rheum. 2003;48:642–650. doi: 10.1002/art.10848. [DOI] [PubMed] [Google Scholar]
- Roelofs M F, Wenink M H, Brentano F, Abdollahi-Roodsaz S, Oppers-Walgreen B, Barrera P, van Riel P L, Joosten L A, Kyburz D, van den Berg W B, Radstake T R. Type I interferons might form the link between Toll-like receptor (TLR) 3/7 and TLR4 mediated synovial inflammation in rheumatoid arthritis (RA) Ann Rheum Dis. 2009;68:1486–1493. doi: 10.1136/ard.2007.086421. [DOI] [PubMed] [Google Scholar]
- Hu X, Chakravarty S D, Ivashkiv L B. Regulation of interferon and Toll-like receptor signaling during macrophage activation by opposing feedforward and feedback inhibition mechanisms. Immunol Rev. 2008;226:41–56. doi: 10.1111/j.1600-065X.2008.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrara S, Huang Q, Mandelin A M, II, Pope R M. TH-17 cells in rheumatoid arthritis. Arthritis Res Ther. 2008;10:R93. doi: 10.1186/ar2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs M F, Boelens W C, Joosten L A, Abdollahi-Roodsaz S, Geurts J, Wunderink L U, Schreurs B W, van den Berg W B, Radstake T R. Identification of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and potential involvement in the pathogenesis of rheumatoid arthritis. J Immunol. 2006;176:7021–7027. doi: 10.4049/jimmunol.176.11.7021. [DOI] [PubMed] [Google Scholar]
- Smiley S T, King J A, Hancock W W. Fibrinogen stimulates macrophage chemokine secretion through Toll-like receptor 4. J Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon J C. Oligosaccharides of hyaluronan activate dendritic cells via Toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura Y, Watari M, Jerud E S, Young D W, Ishizaka S T, Rose J, Chow J C, Strauss J F., III The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- Goh F G, Piccinini A M, Krausgruber T, Udalova I A, Midwood K S. Transcriptional regulation of the endogenous danger signal tenascin-C: a novel autocrine loop in inflammation. J Immunol. 2010;184:2655–2662. doi: 10.4049/jimmunol.0903359. [DOI] [PubMed] [Google Scholar]
- Huang Q Q, Sobkoviak R, Jockheck-Clark A R, Shi B, Mandelin A M, II, Tak P P, Haines G K, III, Nicchitta C V, Pope R M. Heat shock protein 96 is elevated in rheumatoid arthritis and activates macrophages primarily via TLR2 signaling. J Immunol. 2009;182:4965–4973. doi: 10.4049/jimmunol.0801563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N, He R, Tian J, Ye P P, Ye R D. Cutting edge: TLR2 is a functional receptor for acute-phase serum amyloid A. J Immunol. 2008;181:22–26. doi: 10.4049/jimmunol.181.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethke K, Staib F, Distler M, Schmitt U, Jonuleit H, Enk A H, Galle P R, Heike M. Different efficiency of heat shock proteins (HSP) to activate human monocytes and dendritic cells: superiority of HSP60. J Immunol. 2002;169:6141–6148. doi: 10.4049/jimmunol.169.11.6141. [DOI] [PubMed] [Google Scholar]
- Boog C J, de Graeff-Meeder E R, Lucassen M A, van der Zee R, Voorhorst-Ogink M M, van Kooten P J, Geuze H J, van Eden W. Two monoclonal antibodies generated against human hsp60 show reactivity with synovial membranes of patients with juvenile chronic arthritis. J Exp Med. 1992;175:1805–1810. doi: 10.1084/jem.175.6.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch J A, Bare O, Auron P E, Stevenson M A, Calderwood S K. Novel signal transduction pathway utilized by extracellular HSP70: role of Toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Schett G, Redlich K, Xu Q, Bizan P, Groger M, Tohidast-Akrad M, Kiener H, Smolen J, Steiner G. Enhanced expression of heat shock protein 70 (hsp70) and heat shock factor 1 (HSF1) activation in rheumatoid arthritis synovial tissue. Differential regulation of hsp70 expression and hsf1 activation in synovial fibroblasts by proinflammatory cytokines, shear stress, and antiinflammatory drugs. J Clin Invest. 1998;102:302–311. doi: 10.1172/JCI2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L, Babelova A, Kiss E, Hausser H J, Baliova M, Krzyzankova M, Marsche G, Young M F, Mihalik D, Gotte M, Malle E, Schaefer R M, Grone H J. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullan R H, Bresnihan B, Golden-Mason L, Markham T, O'Hara R, FitzGerald O, Veale D J, Fearon U. Acute-phase serum amyloid A stimulation of angiogenesis, leukocyte recruitment, and matrix degradation in rheumatoid arthritis through an NF-κB-dependent signal transduction pathway. Arthritis Rheum. 2006;54:105–114. doi: 10.1002/art.21518. [DOI] [PubMed] [Google Scholar]
- Wakelin S J, Sabroe I, Gregory C D, Poxton I R, Forsythe J L, Garden O J, Howie S E. “Dirty little secrets”—endotoxin contamination of recombinant proteins. Immunol Lett. 2006;106:1–7. doi: 10.1016/j.imlet.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Zahringer U, Lindner B, Inamura S, Heine H, Alexander C. TLR2—promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunobiology. 2008;213:205–224. doi: 10.1016/j.imbio.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Sacre S M, Andreakos E, Kiriakidis S, Amjadi P, Lundberg A, Giddins G, Feldmann M, Brennan F, Foxwell B M. The Toll-like receptor adaptor proteins MyD88 and Mal/TIRAP contribute to the inflammatory and destructive processes in a human model of rheumatoid arthritis. Am J Pathol. 2007;170:518–525. doi: 10.2353/ajpath.2007.060657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midwood K, Sacre S, Piccinini A M, Inglis J, Trebaul A, Chan E, Drexler S, Sofat N, Kashiwagi M, Orend G, Brennan F, Foxwell B. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15:774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- Baechler E C, Batliwalla F M, Karypis G, Gaffney P M, Ortmann W A, Espe K J, Shark K B, Grande W J, Hughes K M, Kapur V, Gregersen P K, Behrens T W. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen S R, Kashgarian M, Alexopoulou L, Flavell R A, Akira S, Shlomchik M J. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Peng S L. Toll-like receptor 9 signaling protects against murine lupus. Arthritis Rheum. 2006;54:336–342. doi: 10.1002/art.21553. [DOI] [PubMed] [Google Scholar]
- Lartigue A, Courville P, Auquit I, Francois A, Arnoult C, Tron F, Gilbert D, Musette P. Role of TLR9 in anti-nucleosome and anti-DNA antibody production in lpr mutation-induced murine lupus. J Immunol. 2006;177:1349–1354. doi: 10.4049/jimmunol.177.2.1349. [DOI] [PubMed] [Google Scholar]
- Christensen S R, Shupe J, Nickerson K, Kashgarian M, Flavell R A, Shlomchik M J. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Nickerson K M, Christensen S R, Shupe J, Kashgarian M, Kim D, Elkon K, Shlomchik M J. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. 2010;184:1840–1848. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Raber M L, Dunand-Sauthier I, Wu T, Li Q Z, Uematsu S, Akira S, Reith W, Mohan C, Kotzin B L, Izui S. Critical role of TLR7 in the acceleration of systemic lupus erythematosus in TLR9-deficient mice. J Autoimmun. 2010;34:339–348. doi: 10.1016/j.jaut.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Barrat F J, Meeker T, Chan J H, Guiducci C, Coffman R L. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur J Immunol. 2007;37:3582–3586. doi: 10.1002/eji.200737815. [DOI] [PubMed] [Google Scholar]
- Kono D H, Haraldsson M K, Lawson B R, Pollard K M, Koh Y T, Du X, Arnold C N, Baccala R, Silverman G J, Beutler B A, Theofilopoulos A N. Endosomal TLR signaling is required for anti-nucleic acid and rheumatoid factor autoantibodies in lupus. Proc Natl Acad Sci USA. 2009;106:12061–12066. doi: 10.1073/pnas.0905441106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisitkun P, Deane J A, Difilippantonio M J, Tarasenko T, Satterthwaite A B, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- Means T K, Latz E, Hayashi F, Murali M R, Golenbock D T, Luster A D. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest. 2005;115:407–417. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viglianti G A, Lau C M, Hanley T M, Miko B A, Shlomchik M J, Marshak-Rothstein A. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–847. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Richez C, Uccellini M B, Richards R J, Bonegio R G, Akira S, Monestier M, Corley R B, Viglianti G A, Marshak-Rothstein A, Rifkin I R. Requirement for DNA CpG content in TLR9-dependent dendritic cell activation induced by DNA-containing immune complexes. J Immunol. 2009;183:3109–3117. doi: 10.4049/jimmunol.0900399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C M, Broughton C, Tabor A S, Akira S, Flavell R A, Mamula M J, Christensen S R, Shlomchik M J, Viglianti G A, Rifkin I R, Marshak-Rothstein A. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bave U, Alm G V, Ronnblom L. The combination of apoptotic U937 cells and lupus IgG is a potent IFN-α inducer. J Immunol. 2000;165:3519–3526. doi: 10.4049/jimmunol.165.6.3519. [DOI] [PubMed] [Google Scholar]
- Urbonaviciute V, Furnrohr B G, Meister S, Munoz L, Heyder P, De Marchis F, Bianchi M E, Kirschning C, Wagner H, Manfredi A A, Kalden J R, Schett G, Rovere-Querini P, Herrmann M, Voll R E. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med. 2008;205:3007–3018. doi: 10.1084/jem.20081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A M, Hansson G K. Innate immune signals in atherosclerosis. Clin Immunol. 2010;134:5–24. doi: 10.1016/j.clim.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Hansson G K. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Liu H, Shi B, Huang C C, Eksarko P, Pope R M. Transcriptional diversity during monocyte to macrophage differentiation. Immunol Lett. 2008;117:70–80. doi: 10.1016/j.imlet.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollard K J, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edfeldt K, Swedenborg J, Hansson G K, Yan Z Q. Expression of Toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–1161. [PubMed] [Google Scholar]
- Pryshchep O, Ma-Krupa W, Younge B R, Goronzy J J, Weyand C M. Vessel-specific Toll-like receptor profiles in human medium and large arteries. Circulation. 2008;118:1276–1284. doi: 10.1161/CIRCULATIONAHA.108.789172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoneveld A H, Hoefer I, Sluijter J P, Laman J D, de Kleijn D P, Pasterkamp G. Atherosclerotic lesion development and Toll like receptor 2 and 4 responsiveness. Atherosclerosis. 2008;197:95–104. doi: 10.1016/j.atherosclerosis.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Hirata K, Yamashita T, Takaya T, Sasaki N, Shiraki R, Ueyama T, Emoto N, Inoue N, Yokoyama M, Kawashima S. Local overexpression of Toll-like receptors at the vessel wall induces atherosclerotic lesion formation: synergism of TLR2 and TLR4. Arterioscler Thromb Vasc Biol. 2007;27:2384–2391. doi: 10.1161/ATVBAHA.106.139253. [DOI] [PubMed] [Google Scholar]
- Bjorkbacka H, Kunjathoor V V, Moore K J, Koehn S, Ordija C M, Lee M A, Means T, Halmen K, Luster A D, Golenbock D T, Freeman M W. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- Michelsen K S, Wong M H, Shah P K, Zhang W, Yano J, Doherty T M, Akira S, Rajavashisth T B, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekhter M, Staschke K, Estridge T, Rutherford P, Jackson N, Gifford-Moore D, Foxworthy P, Reidy C, Huang X D, Kalbfleisch M, Hui K, Kuo M S, Gilmour R, Vlahos C J. Genetic ablation of IRAK4 kinase activity inhibits vascular lesion formation. Biochem Biophys Res Commun. 2008;367:642–648. doi: 10.1016/j.bbrc.2007.12.186. [DOI] [PubMed] [Google Scholar]
- Shi W, Haberland M E, Jien M L, Shih D M, Lusis A J. Endothelial responses to oxidized lipoproteins determine genetic susceptibility to atherosclerosis in mice. Circulation. 2000;102:75–81. doi: 10.1161/01.cir.102.1.75. [DOI] [PubMed] [Google Scholar]
- Liu X, Ukai T, Yumoto H, Davey M, Goswami S, Gibson F C, III, Genco C A. Toll-like receptor 2 plays a critical role in the progression of atherosclerosis that is independent of dietary lipids. Atherosclerosis. 2008;196:146–154. doi: 10.1016/j.atherosclerosis.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullick A E, Tobias P S, Curtiss L K. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullick A E, Soldau K, Kiosses W B, Bell T A, III, Tobias P S, Curtiss L K. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J Exp Med. 2008;205:373–383. doi: 10.1084/jem.20071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand K, Page S, Rogler G, Bartsch A, Brandl R, Knuechel R, Page M, Kaltschmidt C, Baeuerle P A, Neumeier D. Activated transcription factor nuclear factor-κ B is present in the atherosclerotic lesion. J Clin Invest. 1996;97:1715–1722. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco C, Andreakos E, Kiriakidis S, Mauri C, Bicknell C, Foxwell B, Cheshire N, Paleolog E, Feldmann M. Canonical pathway of nuclear factor κ B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc Natl Acad Sci USA. 2004;101:5634–5639. doi: 10.1073/pnas.0401060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco C, Gregan S M, Navin T J, Foxwell B M, Davies A H, Feldmann M. Toll-like receptor-2 mediates inflammation and matrix degradation in human atherosclerosis. Circulation. 2009;120:2462–2469. doi: 10.1161/CIRCULATIONAHA.109.851881. [DOI] [PubMed] [Google Scholar]
- Gareus R, Kotsaki E, Xanthoulea S, van der Made I, Gijbels M J, Kardakaris R, Polykratis A, Kollias G, de Winther M P, Pasparakis M. Endothelial cell-specific NF-κB inhibition protects mice from atherosclerosis. Cell Metab. 2008;8:372–383. doi: 10.1016/j.cmet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Wolfrum S, Teupser D, Tan M, Chen K Y, Breslow J L. The protective effect of A20 on atherosclerosis in apolipoprotein E-deficient mice is associated with reduced expression of NF-κB target genes. Proc Natl Acad Sci USA. 2007;104:18601–18606. doi: 10.1073/pnas.0709011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanters E, Pasparakis M, Gijbels M J, Vergouwe M N, Partouns-Hendriks I, Fijneman R J, Clausen B E, Forster I, Kockx M M, Rajewsky K, Kraal G, Hofker M H, de Winther M P. Inhibition of NF-κB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;112:1176–1185. doi: 10.1172/JCI18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Temkin V, Liu H, Pope R M. NF-κB protects macrophages from lipopolysaccharide-induced cell death: the role of caspase 8 and receptor-interacting protein. J Biol Chem. 2005;280:41827–41834. doi: 10.1074/jbc.M510849200. [DOI] [PubMed] [Google Scholar]
- Hochleitner B W, Hochleitner E O, Obrist P, Eberl T, Amberger A, Xu Q, Margreiter R, Wick G. Fluid shear stress induces heat shock protein 60 expression in endothelial cells in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2000;20:617–623. doi: 10.1161/01.atv.20.3.617. [DOI] [PubMed] [Google Scholar]
- Xu Q, Schett G, Perschinka H, Mayr M, Egger G, Oberhollenzer F, Willeit J, Kiechl S, Wick G. Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation. 2000;102:14–20. doi: 10.1161/01.cir.102.1.14. [DOI] [PubMed] [Google Scholar]
- Yamada T, Sato J, Okuda Y. Differential affinity of serum amyloid A1 isotypes for high-density lipoprotein. Amyloid. 2009;16:196–200. doi: 10.3109/13506120903421546. [DOI] [PubMed] [Google Scholar]
- Mullan R H, McCormick J, Connolly M, Bresnihan B, Veale D J, Fearon U. A role for the high-density lipoprotein receptor SR-B1 in synovial inflammation via serum amyloid-A. Am J Pathol. 2010;176:1999–2008. doi: 10.2353/ajpath.2010.090014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Avalos A M, Mao S Y, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, Hua J, An L L, Audoly L, La Rosa G, Bierhaus A, Naworth P, Marshak-Rothstein A, Crow M K, Fitzgerald K A, Latz E, Kiener P A, Coyle A J. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- Kalinina N, Agrotis A, Antropova Y, DiVitto G, Kanellakis P, Kostolias G, Ilyinskaya O, Tararak E, Bobik A. Increased expression of the DNA-binding cytokine HMGB1 in human atherosclerotic lesions: role of activated macrophages and cytokines. Arterioscler Thromb Vasc Biol. 2004;24:2320–2325. doi: 10.1161/01.ATV.0000145573.36113.8a. [DOI] [PubMed] [Google Scholar]
- Porto A, Palumbo R, Pieroni M, Aprigliano G, Chiesa R, Sanvito F, Maseri A, Bianchi M E. Smooth muscle cells in human atherosclerotic plaques secrete and proliferate in response to high mobility group box 1 protein. FASEB J. 2006;20:2565–2566. doi: 10.1096/fj.06-5867fje. [DOI] [PubMed] [Google Scholar]
- Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- Jimenez-Dalmaroni M J, Xiao N, Corper A L, Verdino P, Ainge G D, Larsen D S, Painter G F, Rudd P M, Dwek R A, Hoebe K, Beutler B, Wilson I A. Soluble CD36 ectodomain binds negatively charged diacylglycerol ligands and acts as a co-receptor for TLR2. PLoS One. 2009;4:e7411. doi: 10.1371/journal.pone.0007411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boullier A, Friedman P, Harkewicz R, Hartvigsen K, Green S R, Almazan F, Dennis E A, Steinberg D, Witztum J L, Quehenberger O. Phosphocholine as a pattern recognition ligand for CD36. J Lipid Res. 2005;46:969–976. doi: 10.1194/jlr.M400496-JLR200. [DOI] [PubMed] [Google Scholar]
- Stewart C R, Stuart L M, Wilkinson K, van Gils J M, Deng J, Halle A, Rayner K J, Boyer L, Zhong R, Frazier W A, Lacy-Hulbert A, Khoury J E, Golenbock D T, Moore K J. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavassani K A, Ishii M, Wen H, Schaller M A, Lincoln P M, Lukacs N W, Hogaboam C M, Kunkel S L. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205:2609–2621. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirou K A, Lee C, George S, Louca K, Papagiannis I G, Peterson M G, Ly N, Woodward R N, Fry K E, Lau A Y, Prentice J G, Wohlgemuth J G, Crow M K. Coordinate overexpression of interferon-α-induced genes in systemic lupus erythematosus. Arthritis Rheum. 2004;50:3958–3967. doi: 10.1002/art.20798. [DOI] [PubMed] [Google Scholar]
- Lee P Y, Li Y, Richards H B, Chan F S, Zhuang H, Narain S, Butfiloski E J, Sobel E S, Reeves W H, Segal M S. Type I interferon as a novel risk factor for endothelial progenitor cell depletion and endothelial dysfunction in systemic lupus erythematosus. Arthritis Rheum. 2007;56:3759–3769. doi: 10.1002/art.23035. [DOI] [PubMed] [Google Scholar]
- Denny M F, Thacker S, Mehta H, Somers E C, Dodick T, Barrat F J, McCune W J, Kaplan M J. Interferon-α promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood. 2007;110:2907–2915. doi: 10.1182/blood-2007-05-089086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessner A, Shin M S, Pryshchep O, Goronzy J J, Chaikof E L, Weyand C M. Synergistic proinflammatory effects of the antiviral cytokine interferon-α and Toll-like receptor 4 ligands in the atherosclerotic plaque. Circulation. 2007;116:2043–2052. doi: 10.1161/CIRCULATIONAHA.107.697789. [DOI] [PubMed] [Google Scholar]