We continue to investigate the nature of the host response to mycobacterial infection to be able to intervene with vaccination and immunotherapy and thereby, improve immunity. Animal models provide a tool to define pathways by which the vertebrate response to mycobacteria occurs, however making these observations relevant to human disease requires examination of these pathways in humans. In this edition of the Journal of Leukocyte Biology [1], the pathway by which Mtb induces an early IL-17 response from naive human peripheral blood cells is shown to involve the following: serine protease activity, the p38MAPK and Erk pathways, and IL-1R. In this model, peripheral blood cells from naive individuals with no known exposure to BCG or a history of positive, purified protein-derivative reaction were cultured with heat-killed Mtb for 7 days, and these cells produced IL-17A by Day 5. During exposure, the frequency of IFN-γ and IL-17 double-positive cells was found to increase tenfold, and although CD45RO-positive CD4 T cells made IL-17, and CD45RA-positive CD4 T cells did not; there was also a tenfold increase in the frequency of γδ T cells producing IL-17. By introducing inhibitors or gene deficiency into this model, the mannose receptor, NOD2, IL-6R, and Jnk pathway were excluded from having a role in this response. In contrast, strong inhibition of the IL-17 response was seen with an IL-1R antagonist as well as chemical inhibitors of the p38 MAPK and Erk pathways. In further support of a role for the activation of IL-1, inhibition of serine protease activity reduced the IL-17 response. Finally, dectin-1 and TLR4 were implicated in the IL-17A response by the ability of laminarin (which blocks dectin-1-dependent pathways [2]) and LPS from Bartonella quintana (which is a natural antagonist for TLR4 [3]) to reduce IL-17A production.
So why are we interested in the response of naive people to Mtb exposure? IL-17 has been seen to be a component of the human response to Mtb, but what does this mean? Is it useful? Is it detrimental? Is it always good/bad to have it (Fig. 1)? As we are still asking these questions about IFN-γ [4], a cytokine we have been studying for far longer, it is premature to expect definitive answers to these questions for IL-17. What the data reported in this issue [1] do tell us, however, is that the earliest interaction between Mtb and a naive human host results in an environment that allows for IL-17 production and that specific pathways are required.
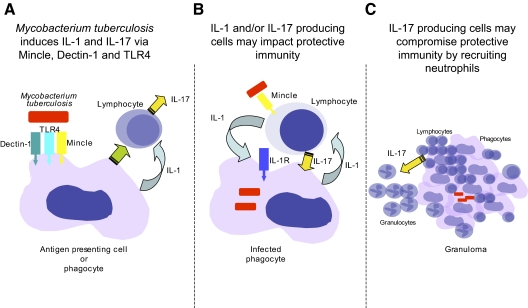
Figure 1.
The role of IL-17 in protective immunity to Mtb is still being determined. (A) We know that IL-17 can be produced by human peripheral blood cells with no known exposure to mycobacterial antigens in response to Mtb. This IL-17 response is dependent on signaling through lectins and IL-1R. (B) In the absence of IL-1 or IL-17, mice are more susceptible to Mtb infection; it is not clear which cells producing IL-1 or IL-17 are the most important nor what they are actually doing to mediate protection. Development of the granuloma may be one aspect of the protective response mediated by IL-17. (C) If antigen-specific, IL-17-producing T cells are induced, then, they may compromise immunity in the long-term, as they will promote neutrophil accumulation in the mononuclear granuloma. Even nonantigen-specific T cells may be stimulated to produce IL-17 in the presence of extracellular bacteria, as the newly described receptor for trehalose dimycolate, a major surface molecule of Mtb, is Mincle, and this is expressed by T cells (B).
How then does this paper help us in our goal of being able to intervene by vaccination or immunotherapy? A major area that is aided by this paper is not linked directly to Mtb but rather, to our ability to induce cellular responses effectively by vaccination. Although this ability lags behind our ability to induce antibody-mediated immunity, we are currently in a growth phase of adjuvant development and use. As we have come to understand the complexity of the immune response, we realize that effective and specific induction of cellular immunity requires defined adjuvants that are targeted exquisitely to induce specific subsets of cells. We know that complete Freund’s adjuvent (CFA) induces a cell-mediated inflammatory response but the pathways by which CFA induces this response are not fully defined. It has not been used in humans as a result of its broadly inflammatory nature, and surely, understanding how specific elements of CFA interact with human cells will improve adjuvant design. As the stimulant used in the paper under discussion here is heat-killed Mtb, and this is the major inflammatory ingredient in CFA, then the paper provides us with understanding of why and how CFA is inflammatory in humans. Combining the work of van de Veerdonks et al.[1] and some excellent recent work about defining the pathway by which one of the major components of Mtb—TDM—binds and stimulates cells, we are finally beginning to define more specifically the activity of CFA. In particular, although recent papers have demonstrated that TDM acts via Mincle [5, 6] and the Fcγ/Syk/CARD9 pathway [7] to induce inflammatory responses and Th1/Th17 acquired immunity, we now also know that IL-1R, dectin-1, and TLR4 are important in the induction of the IL-17 response in humans.
With regard to immunotherapy, it is important to know where IL-17 is coming from in the response to Mtb infection. Once we know this, we can induce or inhibit this response from specific cell types and determine the role of these cell types in the protective and the inflammatory response. van de Veerdonk et al. [1] identify IL-17-positive CD4 memory T cells and γδ T cells as potential sources of IL-17, but we know many cells can produce IL-17. However, what should we do with this information? Should we induce a memory response that augments IL-17 production upon infection? We might say this is a good thing, as we have shown that IL-17 is essential for the accelerated recall of memory IFN-γ-producing cells into the lung [8], however IL-17 responses may be detrimental in chronic infection as a result of increased neutrophil responses that can compromise immunity [9, 10]. A recent study using a prime-boost strategy to enhance cellular responses in BCG-vaccinated individuals found that multifunctional cells expressing IL-2, TNF, and IFN were expanded, but they did not see an increase in IL-17; they were not overly concerned with this, as they did not know whether induction of Th17 cells would result in more or less optimal immunity [11]. So what good would IL-17 do in the protective response to mycobacterial infection? To begin to answer this, we need to go to the animal models for definitive data. Although studies with BCG implicated IL-17 recruitment of neutrophils as important in the early inflammatory response and maintenance of Th1 responses [12], later work has implicated IL-17 in maintenance of the granuloma [13]. Although this work was interesting, one is always concerned when BCG is used as a surrogate for Mtb, as it lacks the early secretory antigenic target system 1/region-of-difference 1 virulence locus that has been implicated in the earliest interaction between vertebrate host phagocytes and the invading mycobacteria [14]. This being said, when IL-17-deficient mice are infected with Mtb in this model, they fail to develop significant inflammation and do not control bacterial growth [13]. The source of IL-17 in these models is largely γδ T cells with some CD4 T cell production, consistent with the data in the paper under discussion. Thus, we can hypothesize that early cellular responses to Mtb induce IL-17 and that this response aids in granuloma formation and ability to control bacterial growth. A second animal model using IL-1β-deficient mice has shown recently that the IL-1R pathway is essential for control of Mtb growth and survival [15], however in this model, in contrast to the work by van de Veerdonk et al. [1], caspase-1 activity was not required. Whether a deficiency of IL-17 occurs in the IL-1β-deficient animal model and is linked to this susceptibility was not addressed, but if this were the case, then the paper describing an essential role for IL-1R in the human IL-17 response to Mtb will provide important support for further investigation of this pathway in humans with TB.
TB can be a chronic disease, and although the early response may define later events, it is likely that the nature of the acquired cellular response to Mtb will have a great impact on the development and maintenance of immunity. In this regard, the cells studied in the accompanying paper were not assessed for antigen specificity, and it is possible that the memory CD4 T cells and the γδ T cells producing IL-17 were being stimulated nonspecifically (possibly by TDM stimulation of Mincle, as the authors suggest). Although we have made great strides in characterizing the acquired cellular response occurring in TB patients, we still do not know what constitutes a protective response at the site of infection. We know that patients with active TB have high levels of circulating γδ T cells producing IL-17 [16], however when a patient has active disease, there is more antigen and greater levels of inflammation that can drive more effector cell generation but equally, during active disease effector cells are more likely to be sequestered at the active site of disease. Interpreting the importance of specific cell types by their frequency in the peripheral blood is, therefore, a task fraught with complexity. Although IL-17 production may be required for early granuloma formation, it may also be detrimental in the long run, as it promotes neutrophil accumulation, and this may not be good for the generation of a stable mononuclear granuloma [17].
In conclusion, the accompanying paper provides an excellent mechanistic base for our understanding of how Mtb induces an early IL-17 response in naïve cells. This is helpful for our development of adjuvants as well as our understanding of the early response to Mtb in humans. What we still do not know, however, is whether an acquired specific and long-term IL-17 response is a good or a bad thing for human immunity to Mtb (Fig. 1).
Acknowledgments
Andrea Cooper is supported by the Trudeau Institute, Inc., and grants from the National Institutes of Health (AI46530, AI067721, and AI069121).
Footnotes
SEE CORRESPONDING ARTICLE ON PAGE 227
Abbreviations: BCG=bacille Calmette-Guerin, Mtb=Mycobacterium tuberculosis, TB= tuberculosis, TDM=trehalose dimycolate
References
- Van de Veerdonk F, Teirlinck A, Kleinnijenhui J, Jan Kullberg B, van Creval R, van der Meer J, Joosten L, Netea M. Mycobacterium tuberculosis induces IL-17A responses through TLR4 and dectin-1 and is critically dependent on endogenous IL-1. J Leukoc Biol. 2010;88:227–232. doi: 10.1189/jlb.0809550. [DOI] [PubMed] [Google Scholar]
- Gantner B N, Simmons R, Canavera S, Akira S, Underhill D. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa C, Abdollahi-Roodsaz S, Joosten L, Takahashi N, Sprong T, Matera G, Liberto M, Foca A, van Deuren M, Kullberg B, van den Berg W, van der Meer J, Netea M. Bartonella quintana lipopolysaccharide is a natural antagonist of Toll-like receptor 4. Infect Immun. 2007;75:4831–4837. doi: 10.1128/IAI.00237-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsack L, Kirman J. Half-truths and selective memory: interferon γ, CD4(+) T cells and protective memory against tuberculosis. Tuberculosis (Edinb) 2007;87:465–473. doi: 10.1016/j.tube.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Schoenen H, Bodendorfer B, Hitchens K, Manzanero S, Werninghaus K, Nimmerjahn F, Agger E, Stenger S, Andersen P, Ruland J, Brown G, Wells C, Lang R. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J Immunol. 2010;184:2756–2760. doi: 10.4049/jimmunol.0904013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa E, Ishikawa T, Morita Y, Toyonaga K, Yamada H, Takeuchi O, Kinoshita T, Akira S, Yoshikai Y, Yamasaki S. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med. 2009;206:2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werninghaus K, Babiak A, Gross O, Hölscher C, Dietrich H, Agger E, Mages J, Mocsai A, Schoenen H, Finger K, Nimmerjahn F, Brown G, Kirschning C, Heit A, Andersen P, Wagner H, Ruland J, Lang R. Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRγ-Syk-Card9-dependent innate immune activation. J Exp Med. 2009;206:89–97. doi: 10.1084/jem.20081445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader S A, Bell G, Pearl J, Fountain J, Rangel-Moreno J, Cilley G, Shen F, Eaton S, Gaffen S, Swain S, Locksley R, Haynes L, Randall T, Cooper A. IL-23 and IL-17 in establishment of protective pulmonary CD4+ T cell responses upon vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- Cooper A M. IL-17 and anti-bacterial immunity: protection versus tissue damage. Eur J Immunol. 2009;39:649–652. doi: 10.1002/eji.200839090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaraba R J. Experimental tuberculosis: the role of comparative pathology in the discovery of improved tuberculosis treatment strategies. Tuberculosis (Edinb) 2008;88:S35–S47. doi: 10.1016/S1472-9792(08)70035-0. [DOI] [PubMed] [Google Scholar]
- Abel B, Tameris M, Mansoor N, Gelderbloem S, Hughes J, Abrahams D, Makhethe L, Erasmus M, de Kock M, van der Merwe L, Hawkridge A, Veldsman A, Hatherill M, Schirru G, Pau M, Hendriks J, Weverling G, Goudsmit J, Sizemore D, McClain J, Goetz M, Gearhart J, Mahomed H, Hussey G, Sadoff J, Hanekom W. The novel TB vaccine, AERAS-402, induces robust and polyfunctional CD4 and CD8 T cells in adults. Am J Respir Crit Care Med. 2010 doi: 10.1164/rccm.200910-1484OC. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura M, Yahagi A, Hamada S, Begum M, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, Matsuzaki G. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- Okamoto Yoshida Y, Umemura M, Yahagi A, O'Brien R, Ikuta K, Kishihara K, Hara H, Nakae S, Iwakura Y, Matsuzaki G. Essential role of IL-17A in the formation of a Mycobacterial infection-induced granuloma in the lung. J Immunol. 2010;184:4414–4422. doi: 10.4049/jimmunol.0903332. [DOI] [PubMed] [Google Scholar]
- Davis J M, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Barber K, Barber D, Shenderov K, White S, Wilson M S, Cheever A, Kugler D, Hieny S, Caspar P, Núñez G, Schlueter D, Flavell R, Sutterwala F, Sher A. Cutting edge: caspase-1 independent IL-1{β} production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol. 2010;184:3326–3330. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M Y, Wang Z, Yao C, Jiang L, Jin Q, Wang J, Li B. Interleukin 17-producing γ δ T cells increased in patients with active pulmonary tuberculosis. Cell Mol Immunol. 2008;5:203–208. doi: 10.1038/cmi.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A M. Cell mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]