Abstract
Leukocytes and epithelial cells are fundamental to antimicrobial immunity. Their antimicrobial responses are an evolutionarily conserved component of the innate immune system and are influenced by the host’s response to external stimuli. The efficacy of host defense via antimicrobial responses derives from the ability of AMPs to rapidly identify and eradicate foreign microbes and activate proinflammatory pathways, and from the capacity of later innate and adaptive immune responses to amplify protection through distinct biochemical mechanisms. Recent advances in neuroimmunology have identified a direct link between the neuroendocrine and immune systems, where environmental stimuli are generally believed to promote a transient effect on the immune system in response to environmental challenges and are presumably brought back to baseline levels via neuroendocrine pathways. Stress is an environmental stimulus that flares from a variety of circumstances and has become engrained in human society. Small bouts of stress are believed to enhance the host’s immune response; however, prolonged periods of stress can be detrimental through excess production of neuroendocrine-derived mediators that dampen immune responses to invasive pathogens. Elucidation of the mechanisms behind stress-induced immune modulation of antimicrobial responses will ultimately lead to the development of more effective therapeutic interventions for pathologic conditions. It is the intent of this review to broaden the existing paradigm of how stress-related molecules dampen immune responses through suppression of antimicrobial mechanisms, and to emphasize that bacteria can use these factors to enhance microbial pathogenesis during stress.
Keywords: neuroimmunology, leukocytes, catecholamines, acetylcholine, glucocorticoids, antimicrobial peptide
Introduction
Mammals are under relentless bombardment by environmental stressors, including psychological anxiety, microorganisms, and physical insult. Although humans cannot distinguish between physical and psychological stressors, there exists a dramatic difference in the manner and degree to which stress is perceived and dealt with depending on the tolerance and coping mechanisms of the organism. As early as the 1970s, PS was implicated in the suppression of immune responses believed to contribute to a higher incidence of infection and cancer [1,2,3]. Stress has also been shown to exacerbate epithelial inflammatory diseases, such as AD and Crohn’s disease [4,5,6,7,8]. How the immune system responds specifically to stress remains elusive as a result of the complexity of the neuroimmune and stress response pathways. A major consequence of stress on immune function is its ability to rapidly promote dramatic shifts in leukocyte distribution among a variety of tissues, which is complicated further by the fact that stress can manifest as acute or chronic stress. Acute stress refers to stress that occurs for minutes or hours, whereas chronic stress persists for days, weeks, or months [9]. The endpoint of acute or chronic stress is related to diverse characteristics of the stress response: activation of the ANS and the HPA axis. Three major pathways of the stress response include catecholamines (i.e., adrenaline and noradrenaline) via adrenergic stimulation, GCs (i.e., cortisol) via activation of the HPA axis, and cholinergic stimulation via Ach [10].
Several theories have been proposed to explain the primary biological role for a physiologic stress response. Darwin and others [11, 12] have suggested that stress is an interference with evolved behavioral strategies or rather, a general warning in response to a shift in homeostatic balance, resulting in nonspecific neurophysiologic activation. The fundamental objective of a stress response is likely to promote survival of the individual via stress hormones and neurotransmitters that signal the immune system for potential challenges from injury or infection. An ideal stress response alerts and does not compromise the physiologic state of the organism; however, a prolonged or uncontrolled response can result in disease or illness through pathophysiological processes involving dysregulation of the immune system. Processive stressors are those that elicit the “fight-or-flight” reaction from facets within the host’s environment but do not cause harm directly, as they are perceived by the host as potential dangers. When a host senses danger, the pituitary gland responds involuntarily to the distress by releasing a surge of ACTH, which acts on the adrenal glands to release several stress hormones, including epinephrine (E) and cortisol [13]. In contrast, systemic stressors are those that pose a significant threat to the organism’s homeostasis, such as extreme pain, dehydration, or injury; these stressors require much less cognitive processing than processive stressors and often occur simultaneously with processive stressors.
This review expands the argument for the existence of a bidirectional interaction between the similarly complex immune and neuroendocrine systems and adds further to the existing evidence, suggesting that stress acts as a double-edged sword to augment and dampen host immune function. The objective of this review is to broaden the existing paradigm of how stress-related molecules modify host antimicrobial mechanisms and to highlight the notion that bacteria can use these factors to augment microbial pathogenesis during stress.
HOST RESPONSE TO STRESS
Neuroendocrine response to stress
Host responses to physical and psychological stressors are identical. Although the immune system was originally thought to act as an autonomous entity, the last few decades have revealed that the immune system and CNS communicate directly to mount sufficient responses to environmental stressors [14,15,16]. Stress represents a compilation of events to activate the fight-or-flight response via the SNS and the HPA axis, which was first described by Walter Cannon [17] in 1929 and comprises the peripheral branches of the stress response system that sustain basal homeostasis and normalize stress-induced dysregulation. Mobilization of major neuroendocrine mediators, including catecholamines, GCs, CRH, and ACTH during stress, induces an APR that occurs following the host’s response to pathogens and/or injury [18,19,20]. The APR is activated mainly in response to tissue damage and/or infection and activated primarily by cytokines [21]. However, catecholamines and GCs released during a normal stress response can augment the APR response to increase production of acute-phase proteins in the liver [22], signifying the convergence of several pathways within the neuroendocrine-immune systems. Furthermore, the type of stressor may induce different effects on GC or catecholamine secretion, such as acute versus chronic stress.
Autonomic response to stress
Two major pathways connect the CNS and the immune systems, which include the ANS and the HPA axis, as described above (Fig. 1). The ANS is a regulatory branch of the CNS that serves to control the function of any innervated tissue, with the exception of skeletal muscle, to provide the major motor (efferent) component of the peripheral nervous system. The ANS is subdivided into two distinct components: the parasympathetic (cholinergic) and sympathetic (adrenergic) system derived from the CNS. Adrenergic stimulation occurs via epinephrine (E) and norepinephrine (NE), and cholinergic stimulation occurs via Ach. Preganglionic sympathetic fibers secrete Ach, which activates further secretion of adrenaline (E) and to a lesser extent, noradrenaline (NE) from neuroendocrine tissues, such as the adrenal medulla. Ach binds to appropriate receptors to promote distinct effects through receptor-dependent mechanisms involving mAChRs and nAChRs, which are classified by the specificity of their respective agonists, muscarine and nicotine [23]. The major agonists of nAChRs include nicotine as an exogenous ligand or Ach as an endogenous ligand, whereas the major agonists of mAChRs include Ach and muscarine. NE and E target α- or β-adrenergic receptors that are categorized based on their subtype composition, similarly to nAChRs and mAChRs, which mediate a wide variety of immunological and homeostatic functions that have been described elsewhere [23,24,25].
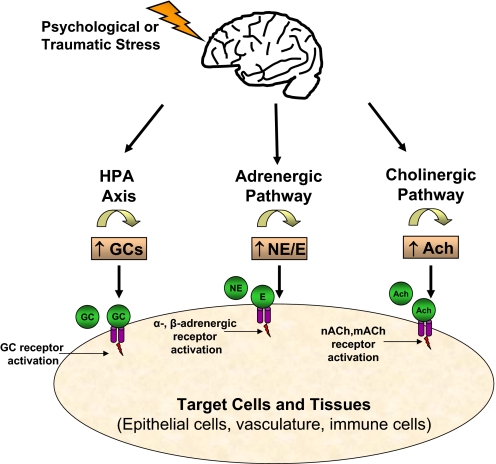
Figure 1.
Activation of stress pathways. Stress signals the brain to activate the three major neuroendocrine pathways: the HPA and the adrenergic and cholinergic pathways. Activation of the HPA promotes the release of GCs, and the activation of the adrenergic and cholinergic pathways promotes the release of NE/E and Ach, respectively. These molecules then act on distinct receptors expressed by a variety of tissues and cells that ultimately results in immunosuppression if prolonged.
PATHWAY INTERACTIONS BETWEEN THE NERVOUS AND IMMUNE SYSTEMS
The existence of a complete cholinergic system on immune cells, including the expression of receptors and the ability to synthesize and degrade Ach, is recognized as an extension of the neural system. Efferent activity in the vagus nerve was identified to inhibit local proinflammatory production of cytokines, as the vagus nerve innervates major organs involved in the response to endotoxin [26,27,28]; this response was later termed the “cholinergic anti-inflammatory pathway.” Acute stress promotes the release of Ach from the hippocampus and release of E and NE into plasma, although these responses do not parallel one another [29]. Ach release was observed as a biphasic response, and the NE and E levels remained elevated following immobilization stress, suggesting an adaptive mechanism to stress involving adrenergic pathways that may be acting against the cholinergic pathways. Furthermore, cholinergic and adrenergic activation yields distinct immune reactions through the cholinergic vagus nerve or adrenergic receptors, respectively. Receptors for adrenergic and cholinergic neurotransmitters are expressed on a variety of immune cells, and a significant amount of evidence demonstrates that the activity of a variety of immune cell subsets is associated directly with physiologic innervation and stress-related proteins (reviewed in refs. [30,31,32,33,34]).
BRIEF OVERVIEW OF CROSS-TALK BETWEEN STRESS PROTEINS AND INFLAMMATORY MEDIATORS
The nervous and immune systems mount an array of vital and sophisticated reactions when the host is threatened by injury or harm. As early as the 1970s, proteins that were functioning as hormonal mediators were identified as participating in crosstalk between the brain and immune systems, particularly the GC family [35,36,37]. Activation of immune responses has been shown to promote variable effects on neuronal mediators, including decreasing NE levels in spleen [35] and increasing plasma corticosterone levels [37]. There appears to be a direct correlation between cortisol secretion in diurnal adrenal responses and activation of proinflammatory responses during stress, as circulating levels of two major proinflammatory proteins, IL-6 and IL-8, were shown to be elevated after a period of acute stress without the activation of the anti-inflammatory cytokine IL-10 [38]. Furthermore, lymphocytes express functional α- and β-adrenergic receptors [30, 39] and cholinergic receptors [40, 41], in addition to neurotransmitters such as vasoactive intestinal peptide [42], substance P [43], and serotonin [44, 45]. Concurrently, primary and secondary lymphoid tissues were found to be innervated by parasympathetic and sympathetic nerve fibers [46,47,48], allowing each tissue to act as a sentinel for neurotransmitters to facilitate neural-immune interactions (reviewed in refs. [10, 49]). In response to stress, an inflammatory process may occur through the release of neuropeptides, particularly substance P, CRH, and ACTH, or additional inflammatory mediators. These peptides can be released following the activation of mast cells or other inflammatory cells, or directly, from sensory nerves. The neural signals sent back to the brain may initiate or block the inflammatory process, as many neuropeptides mediate an inflammatory and stress response. Cytokines and chemokines released by an inflammatory response or stress response may use parallel pathways activated by epithelial sensory signals to the brain.
Stress proteins and cutaneous epithelial barrier function and inflammation
The skin barrier provides a physical and chemical impediment to the external environment through multifunctional organelles, lamellar bodies (LB), which liberate endogenous lipids, enzymes, and proteins to the skin’s surface. The secreted lipids allow for the physical barrier, and secretion of AMPs allows for the chemical barrier against invading pathogens. There is limited data describing the effects of stress on AMP regulation in the skin, which will be discussed later in the review. However, appreciating how the epithelial barrier responds to stress is critical to our understanding of defects in antimicrobial activity of resident and infiltrating immune cells during stress, as the epithelial physical and antimicrobial barriers are codependent. Concurrently, stress precedes the development of many of the clinical manifestations of pathologic skin diseases, such as psoriasis, AD, and herpes virus infections (reviewed in ref. [50]).
Initial studies involving the impact of PS on cutaneous barrier function were conducted using immobilization or crowding stress prior to the analysis of barrier recovery, measured by the transepidermal water loss following skin barrier disruption via tape stripping [51, 52]. Barrier recovery was delayed in mice that were housed at high population densities compared with lower population densities, as well as in mice exposed to immobilization stress. Further probing revealed that plasma corticosterone levels (GCs) were increased markedly after stress. Pretreatment with a sedative or a GC antagonist blocked the delay in barrier recovery by systemic corticosterone treatment, change of cage, or immobilization, indicating that PS promotes elevated production of GCs to adversely influence cutaneous permeability homeostasis. As mentioned above, LB secreted by the resident epithelial cells of the skin, the keratinocytes, are important for the formation of the lipid physical barrier, as well as the storage and secretion of AMPs to the skin’s surface [53]. AMP and barrier lipid production are synchronized such that the expression of AMPs increases in parallel with the lipid metabolic response following barrier disruption [54]. Conversely, AMPs that reside within LB are also critical to normal permeability homeostasis, as mice deficient in the major AMP, cathelicidin, exhibit delayed barrier recovery and LB structure and secretion. The impact of PS on epidermal barrier function was determined to involve a multitude of alterations in epidermal homeostasis, including decreased epidermal cell proliferation, epidermal cell differentiation, smaller corneodesmosomes, and reduced production and secretion of LB [55]. These defects in permeability homeostasis via decreased LB production and secretion were reversed following GC blockade with a GC antagonist and following the addition of topical physiologic lipids [56]. This phenomenon was verified in humans in a study comprised of graduate students using two validated measures: the perceived stress scale and mood states [8]. Subjects demonstrated the most profound deterioration of cutaneous barrier function that paralleled the greatest increase in perceived PS, providing the first direct link that stress-induced defects in epithelial barrier function may trigger pathologic inflammation.
Langerhans’ cells are closely associated anatomically with nerve fibers in the epidermis and participate in neurogenic inflammation, which refers to the activation of inflammatory responses via neuropeptides. Langerhans’ cells express receptors for several neuropeptides, adrenergic mediators, and cholinergic mediators, which leads to inhibition of cytokine expression and migration. CGRP is a neuropeptide involved in vasodilation and antigen presentation and is associated with Langerhans’ cells during inflammation located within epidermal nerves and Merkel cells [57, 58]. Functional assays identified that CGRP-containing nerve fibers inhibited Langerhans’ cell antigen presentation [59], which was confirmed in a physiologic model of acute social stress showing increased epidermal CGRP levels and reduced Langerhans’ cell frequency [60]. Elimination of nerve fibers or the deletion of CGRP or substance P abolished the development of contact sensitivity in skin, which is thought to be mediated by Langerhans’ cells, despite observing a greater number of Langerhans’ cells at the site of contact [61]. The influence of nerve fibers on Langerhans’ cell antigen processing remains elusive and may yield more detailed evidence as to how innervation plays a role in immune surveillance and antigen presentation. These observations suggest a synchronized host defense network that uses neuropeptides as a mediator between the neural and immune systems.
Mast cells also play a physiological role in neurogenic inflammation, which includes vasodilation, protein extravasation, mast cell degranulation, and release of histamine. Nerve fibers can mediate the release of proinflammatory cytokines, in part, through the stimulation of mast cells [62]. Mast cell-nerve contacts are significantly higher in lesional skin samples from AD or psoriasis patients [63], which leads to the itch and plasma extravasation caused by histamine release and capillary permeability frequently associated with these inflammatory skin diseases. In human mast cell populations, mast cell degranulation by neuropeptides has only been seen in cutaneous mast cells, which emphasizes further the intricate relationship between the neural and cutaneous immune systems. The direct involvement of mast cells in cutaneous inflammation is supported by the evidence that antihistamines can block specific hallmarks of neurogenic inflammation, such as vasodilation and protein extravasation, and that neuropeptide-induced cutaneous inflammation is reduced dramatically in mast cell-deficient mice [64]. Mast cells express a variety of receptors that respond to mediators of the stress response, such as CRH, making mast cells a liaison to elicit proinflammatory effects via the release of chemokines, such as VEGF [65], in response to the production of stress proteins.
Stress and skin pathologies
AD is a common inflammatory disease of the skin that is believed to be initiated by environmental or psychological stressors. NC/Nga mice represent an animal model for AD, as these mice develop AD-like lesions and exhibit increased serum IgE and similar histopathology of the skin with aging [66]. NC/Nga mice exposed to PS in a pathogen-free environment developed AD-like skin lesions and elevated serum IgE, compared with their unstressed counterparts in a pathogen-free environment [67]. Interestingly, CRH pretreatment completely blocked the development of AD-like lesions following stress, indicating that cells expressing these CRH receptors are involved directly in the manifestation of AD lesions following stress. This increases AD-like parameters that may be mediated by mast cells, including eosinophil infiltration, epidermal thickness, and integrin expression on endothelial cells, in addition to an increase in the number of substance P-containing nerve fibers [68, 69]. Substance P has been shown to shift the TH-cytokine profile to favor that of a TH-2 response, typical of acute AD, or to TH-1, typical of chronic AD and psoriasis [70].
Psoriasis is also a chronic inflammatory disorder precipitated by stress, but is characterized by enhanced proliferation and differentiation of keratinocytes and manifests following T cell activation and aberrant local and systemic cytokine production [50]. Interestingly, human psoriatic skin lesions demonstrated a marked increase in CRH immunohistochemical expression within the epidermis, but no dramatic differences were observed in ACTH or α-MSH [71]. This indicates that CRH may be an important link between the development of many of these skin diseases and the stress response and represents a target for future studies designed to develop therapeutic treatments for these diseases.
Stress also appears to hasten the progression of a variety of tumors, particularly melanoma of the skin. NE directly increased the gene and protein expression of important proinflammatory mediators, VEGF, IL-8, and IL-6, in melanoma cell lines, a microenvironment that would stimulate the aggressive potential of melanoma tumor cells in vivo [72]. The cell proliferation and tyrosinase activity of melanoma cells were found to be dependent on the expression of adrenoreceptor subtypes [73], suggesting that the adrenoreceptor repertoire of melanoma tumors may influence its ability to progress and metastasize, proving to be detrimental in a stress environment. PS in mice and humans also showed a direct correlation between the extent of exposed or perceived stress, respectively, and the progression of melanoma tumor growth, indicating that the host’s resistance to tumor progression was attenuated by exposure to PS. Several other cancers are also influenced by stress and stress-related proteins, but they have been described elsewhere and are not within the scope of this review.
The altered interaction between the neural and immune communication seen in inflammatory-related pathologies and tumor progression likely shifts the balance to exacerbate various diseases. These data emphasize the neurogenic component involved in the development of cutaneous inflammatory diseases and may provide a therapeutic target for the suppression of inflammatory processes in individuals subjected to prolonged physiologic or psychologic stress.
Stress proteins and intestinal epithelial barrier function and inflammation
Stress proteins and/or neuropeptides can elicit inflammatory responses systemically or within specific tissues. PS, induced using restraint stress in mice, identified that intestinal eosinophils increase their expression of CRH, which was mediated by substance P and its receptors, neurokinin receptor-1 and -2, culminating in jejunum epithelial barrier dysfunction [74]. CRH increases ion secretion and mucosal permeability in intestinal epithelia, which contributes significantly to the mast cell hyperplasia/activation in intestinal mucosa following CRH administration [75]. CRH receptor activation subsequently stimulates the expression of proinflammatory chemokines, IL-8 and macrophage chemoattractant protein-1, in human colonic cells, whereas this inflammatory response was reduced dramatically in CRH receptor-2 null mice [76]. CRH receptor antagonists prevent intestinal mucosal dysfunction caused by acute stressors or peripheral CRH injection [77]. Similarly, animals subjected to stress or treated with the synthetic GC, dexamethasone, developed increased intestinal permeability that was absent in adrenalectomized animals or following pharmacological blockage with a GC antagonist [78]. The stress-induced barrier defects via neuroendocrine proteins, and their respective receptors may permit uptake of immunogenic material into the intestinal mucosa, which initiates or exacerbates intestinal inflammation frequently seen in recurring intestinal inflammatory diseases, such as Crohn’s disease. This suggests a direct role for adrenergic mediators in intestinal inflammation by increasing the secretion of proinflammatory molecules into the extracellular milieu, leading to epithelial dysfunction and subsequent pathologic inflammation and/or infection. Adrenergic innervation may also participate in the regulation of endothelial permeability and inflammatory response. Stimulation with adrenergic receptor antagonists specifically blocks the TNF-α-mediated expression of cell adhesion molecules in human microvascular endothelial cells [79].
Collectively, the convergent evidence suggests that stress-related proteins and their respective receptors are involved directly in stress-related alterations in tissue physiology. Neuropeptides promote a systemic stress response by activation of neuroendocrine pathways following the release of the stress hormones. The direct interaction among neuropeptides, nerve fibers, and immune cells, in combination with cytokines and chemokines induced by stress, promotes local and systemic inflammatory responses that are the cause of many pathological diseases that are worsened by stress, for instance, arthritis and psoriasis. Although the neural and immune systems are hard-wired to communicate signals for redistribution of immune cells and activation of proinflammatory responses, limited studies have been performed to identify the interaction between the stress response and antimicrobial activity. The remainder of this review will focus on studies that have addressed the consequence of stress on functions related to antimicrobial activity of leukocytes and epithelial cells. The various models of physiological and PS are summarized in Table 1.
TABLE 1.
Models of Psychological and Physiological Stress
| Model | Species | Acute vs. Chronic | Type | Duration | References |
|---|---|---|---|---|---|
| Insomnia | Mouse | Chronic | Psychological | 3–4 d | [76, 90, 95] |
| Insulin | Mouse | Acute | Physiological | 0.5-4 hrs | [61] |
| Restraint | Mouse, Rat | Acute | Psychological | 30 min–5 d | [84, 86, 88, 95] |
| Water avoidance | Rat | Chronic | Psychological | 10 d | [85] |
| Social stress | Mouse | Acute | Psychological | 6 cycles, 2hr each | [96] |
| Heat stress | Mouse | Acute | Physiological | 24 hrs | [90] |
| Anxiety | Human | Chronic | Psychological | 2–4 wks | [8] |
| Hyperoxic | Mouse | Acute | Physiological | 4 d | [105] |
| Endotoxin | Mouse | Acute | Physiologic | 5 d | [110] |
d, days; hrs, hours; min, minutes; wks, weeks.
INTRODUCTION TO AMPs
AMPs contribute significantly to innate host defense by inhibiting microbial growth promptly, a process that occurs prior to the adaptive response and nonadaptive components of acute inflammation [80,81,82]. AMPs have been established as fundamental components of the innate immune system. Several AMPs achieve protection by interfering with the lipids of the microbial outer core, leading to membrane destabilization and disruption [83, 84]. Furthermore, these peptides have expanded the repertoire of their antimicrobial activities by acting as direct signaling molecules to include microbicidal activity, inducing chemokine production, modulation of dendritic and/or T cell function, chemotactic activity, promotion of wound healing, and modulation of TLR pathways [85,86,87,88]. More thorough reviews about AMPs and their regulation have been published elsewhere [86, 88, 89]. For purposes of illustration only, a limited review of AMPs will be presented here.
AMPs are deployed rapidly upon injury and/or infection to provide immediate protection from impending pathogens and to initiate host defense mechanisms. Cathelicidins, defensins, and chromogranins represent three important families of AMPs that are expressed in a variety of immune and epithelial cells, including neutrophils, macrophages, mast cells, and keratinocytes. However, other AMPs that contribute significantly to epithelial defense of the skin, gut, or lung include psoriasin [90], RNase 7 [91, 92], and lysozyme [93, 94] and have been discussed elsewhere [89, 95, 96]. AMPs have demonstrated effective antimicrobial activity against a wide variety bacteria, fungi, and viruses at micromolar concentrations. AMPs provide protection against multiple bacterial pathogens, including GAS [97], Staphylococcus aureus [98, 99], Salmonella typhimurium [100], and Escherichia coli [90, 97,98,99], as well as viruses and fungi, including HSV [101], Haemophilus influenzae virus [102, 103], Candida albicans [104], and Aspergillus fumigatus [105].
Cathelicidin AMPs are small, cationic, amphipathic peptides that are highly conserved across invertebrate and vertebrate species. A single gene encodes an inactive precursor, termed human cathelicidin antimicrobial peptide 18, for human cathelicidins, which are named based on the highly conserved N-terminal domain identified as the cathelin domain. To liberate the inherent antimicrobial and immunomodulatory activity, cathelicidin proproteins are processed into smaller bioactive peptides by keratinocyte serine proteases and neutrophil proteases, such as kallikreins and proteinase-3, respectively [106]. Once processed from the inhibitory N-terminal domain, the mature form of human cathelicidin, LL-37, or mouse cathelicidin, CRAMP, can elicit potent, broad-spectrum, antimicrobial activity against invasive pathogens and stimulate proinflammatory pathways.
Defensins are also cationic peptides characterized by their conserved six cysteine residues that form intramolecular disulfide bonds and are classified into α-, β-, and θ-defensins based on their sequence homology and cysteine residues [107]. Like the cathelicidin family, defensins are proteolytically processed from an inactive proprotein into an active peptide. However, there is a lack of structural diversity generated post-translationally compared with cathelicidins.
The CHGA protein family is distributed in secretory granules of endocrine, neuroendocrine, and neuronal origin. Chromaffin cells present in the adrenal medulla contain secretory vesicles that are comprised of a complex mixture of nucleotides, histamine, catecholamines, and bioactive peptides, derived in part from CHGA, which are excreted into the extracellular milieu in response to stress [108,109,110]. Peptides generated from CHGA, as well as several other neuropeptides, including α-MSH, substance P, and neuropeptide Y, have been reported to have antimicrobial activity in vitro [111,112,113,114]. CHGA is proteolytically processed to produce several bioactive peptides, including Cst, which was characterized originally as a nAChR antagonist to block further secretion of catecholamine release during stress [115,116,117] but later shown to possess AMP activity against a wide variety of skin pathogens [99]. Cleavage of CHGA results in the generation of additional peptides with AMP activity, including chromacin I and II (bovine CHGA173–194 and bovine CHGA195–221), chromofungin (bovine CHGA47–66), and vasostatin I (bovine CHGA1–76).
The relevance of the antimicrobial activity of cationic peptides has been shown in mice, where an absence of cathelicidin increases the susceptibility to several forms of infection, including skin invasion by GAS [97], Citrobacter growth in the colon [118], and the development of E. coli urinary tract infections [119]. Induction of both classes of AMPs occurs following injury, microbial stimulation, chemokine/cytokine secretion, or hormone stimulation and is associated with resistance to invasive pathogens [120,121,122]. In contrast, suppression of AMP function is associated with an increase in susceptibility to infection and has been demonstrated only recently. PS and topical or systemic GCs were shown to suppress the expression of cutaneous AMPs in a murine model and resulted in susceptibility to invasive infection [123]. The ability of stress and its related metabolites to influence antimicrobial activity will be discussed below.
IMPACT OF ALTERNATE FORMS OF STRESS ON CELL AND TISSUE-SPECIFIC ANTIMICROBIAL MECHANISMS
Leukocytes and epithelial cells provide a rapid first line of defense against invading pathogens by producing and releasing AMPs and promoting various proinflammatory processes. PS is a major risk factor for the development of many of the clinical manifestations involved in inflammatory diseases, particularly in the gut and skin, and is associated with altered expression of AMPs. For instance, Crohn’s disease is characterized by a low gene copy number polymorphism of the hBD locus, resulting in an attenuated epithelial expression of β-defensin AMPs and the presence of mucosal-adherent bacteria [124, 125]. Immune cell recruitment to inflammatory sites is a major constituent of inflammation, is mast cell-dependent [126], and promotes the release of proinflammatory cytokines [127] and supports antimicrobial activity [128]. Overactivation of stress pathways during chronic stress results in a state of relative immunosuppression, and evidence points to HPA-derived stress proteins as primary mediators of this response. Until recently, most of the research related to stress and immunosuppression focused on chemokine and cytokine regulation. The remainder of this review will address the relevant remaining issues related to the impact of stress on direct antimicrobial mechanisms rather than inflammatory pathways.
Mast cell antimicrobial activity
Mast cells are important for allergic reactions, as well as innate and adaptive immune responses associated with inflammatory conditions that are exacerbated by stress. Mast cells are also potent producers of AMPs, particularly cathelicidin, which is released upon degranulation via the production of histamine and other neuropeptides during stress responses [129,130,131]. Mice, subjected to 10 days of PS, exhibited defects in barrier permeability within the ileum and colon and increased bacterial adhesion to intestinal epithelium, but presented with elevated neutrophil infiltration and myeloperoxidase activity [132]. Surprisingly, mast-cell deficient mice did not exhibit changes in intestinal inflammation or bacterial adherence, similar to sham-stressed mice. If the rate or extent of degranulation of mast cells is enhanced in the face of ongoing stress, this would ultimately limit the antimicrobial capacity of mast cells during subsequent infection by depleting mast cells of their antimicrobial contents early on, as AMPs comprise a significant portion of the excreted material of mast cells [133]. Inadvertently, the early release of mast cell granular contents in response to stress could exacerbate inflammatory responses directly, implicating a direct role for mast cells in stress-induced inflammation through the release of proinflammatory molecules, such as AMPs, from intracellular stores. This may be part of the fight-or-flight response that would allow for immediate defense against infection; however, depletion of AMP stores too quickly prior to infection may backfire and actually enhance susceptibility to infection. These observations suggest that although the distribution of leukocytes is augmented with PS, their antimicrobial capacity is diminished to allow for microbial colonization. Similar studies found that continuous exposure of rats to CRH, a stress-induced neuroendocrine protein, caused significant colonic barrier dysfunction, increased mast cell hyperplasia, and increased adherence of bacteria to colonic epithelium [75]. These effects were absent in mast cell-deficient mice and were blocked by addition of a CRH receptor antagonist, identifying the mast cell as a major contributor to mucosal barrier dysfunction during stress via defects in antimicrobial capacity. Acute stress was shown previously to stimulate intestinal mucous production by a mast cell-dependent mechanism [134], but the results from this study indicate that chronic PS may augment inflammation via a mast cell-dependent mechanism in intestinal epithelial. Activation of the HPA axis using restraint stress was also shown to enhance the susceptibility of isolated splenic macrophages to Mycobacterium avium, which was reversed by treatment with the GC receptor antagonist, RU-486 [135, 136]. Together, these findings suggest that PS can initiate inflammatory dysregulation and leukocyte distribution and simultaneously diminish the capacity of antimicrobial defenses that allow for increased microbial penetration into epithelium and lymphoid tissue.
Bladder epithelial antimicrobial activity
Bladder epithelial tissues are highly susceptible to invasive infection and have also been shown to display changes in antimicrobial activity subsequent to PS. Cathelicidin was found previously to be increased in urine and bladder epithelium following bacterial contact, and mice deficient in cathelicidin were more susceptible to E. coli infection [119]. Using a chronic stress model, female mice subjected to constant light and excess heat stress exhibited an increase in epithelial shedding of the bladder, which conferred protection against E. coli, presumably by activation of the HPA axis, as similar changes were also observed following i.p. injection of a NE/hydrocortisone combination [137]. In contrast, male mice subjected to similar stress conditions did not exhibit epithelial shedding and succumbed to infection more frequently than females. The protection observed in female mice was believed to be attributed to elevated mobilization of PMN cells into the bladder, along with the increased epithelial shedding that likely allows for bacteria to adhere and consequently, are washed out during urination. Sex differences may confer differential antimicrobial responses and require further scrutiny into the mechanism behind intrinsic hormonal balance in the regulation of antimicrobial responses during stress. In separate studies, acute restraint stress and administration of CRH induced mast cell-dependent VEGF release from bladder explants [138]. This likely contributes to unnecessary inflammation associated with stress-induced pathological diseases of the bladder, such as interstitial cystitis, and may be extrapolated to other diseases of epithelial tissues (i.e., Crohn’s disease and AD), where AMP expression and activity are impaired.
Cutaneous epithelial antimicrobial activity
PS negatively affects the epidermal permeability barrier homeostasis and healing capacity of epithelial tissues [8, 51], which is attributed to endogenous GC production and HPA-derived GCs. Mice subjected to PS for 3 days using crowding, constant light, and radio static showed a decrease in the murine cathelicidin CRAMP and the murine β-defensin 3 and resulted in more severe infections from a major skin pathogen, GAS [123]. Systemic administration of RU-486, a GC/progesterone receptor antagonist, was able to normalize AMP expression and resistance to bacterial challenge during PS. Furthermore, cutaneous permeability barrier function was also compromised during PS and following topical application of GCs, indicating GCs as a primary mechanism for PS-induced barrier defects. In a murine model of social disruption stress, an “intruder mouse” was added to the cage for 2 h periods for a total of six cycles, which increased the mortality of mice when challenged with bacterial LPS and resulted in extreme inflammatory damage to multiple organs [139]. Thus, the PS-induced reduction in AMPs could account for the correlation between PS and the susceptibility to cutaneous infections during stress. This phenomenon may be induced via increased GCs derived from the HPA and/or generated from endogenous skin cells to promote the activity of additional neuroendocrine mediators, including CRH and Ach. The impact of major stress-released neuroendocrine mediators on cutaneous antimicrobial function is summarized in Figure 2.
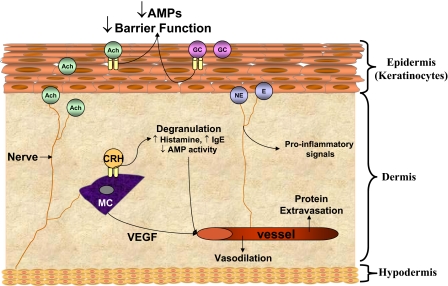
Figure 2.
Suppression of cutaneous antimicrobial activity following stress. Release of stress hormones and neurotransmitters, such as Ach, GCs, NE/E, and CRH, from nerves and epithelial cells results in suppression of AMP expression and impaired barrier function at the interface between the skin barrier and external environment. Activation of neuroendocrine receptors on mast cells (MC) promotes degranulation, consequently depleting AMP stores, while releasing proinflammatory molecules that are believed to contribute to stress-induced skin diseases. AMPs and other factors liberated from dermal cells exacerbate inflammation further through vasodilation and protein extravasation, as well as through the induction of detrimental proinflammatory mediators.
Further probing into the mechanism behind PS-induced AMP suppression identified a mechanism unknown previously though activation of the nAChR. Wild-type mice treated topically with nicotine or CHGA null (Chga−/−) mice lacking the endogenous nAChR antagonist, Cst exhibited less antimicrobial activity in skin extracts (unpublished results). Interestingly, GCs and/or corticosteroids augment Ach content in tissue [140] and enhance nAChR activity and sensitization [141], which may explain the restoration of AMPs in PS mice by RU-486 [123]. As CHGA-derived peptides are present within secretory chromaffin granules and are co-secreted together with catecholamines, their secretion during the stress response may function in a binary mechanism to augment the antimicrobial response and provide an immediate protective barrier against invasive pathogens, similar to other AMPs, while acting as a negative regulator of GC-induced suppression of AMPs during stress [142]. A recent study identified that two CHGA-derived peptides, chromofungin and Cst, stimulated PMN exocytosis of important inflammatory and innate immune factors by provoking a transient Ca+ entry into the cells via a calmodulin-dependent mechanism [143], suggesting a more direct role for CHGA and its proteolytic fragments in modulating leukocyte antimicrobial capacity.
Taken together, it appears that PS promotes the release of GC and Ach, which act upon their respective receptors to suppress antimicrobial mechanisms in epithelial tissues and leukocytes. GCs can further augment nAChR activation to exacerbate AMP suppression and lipid production required for optimal permeability barrier function. Blocking nAChR and GC receptor activation using specific antagonists may re-establish normal AMP and lipid synthesis during or following periods of prolonged stress to maintain an antimicrobial barrier and may prove to be an effective treatment for cutaneous infection and inflammatory conditions caused by abnormal AMP activity. Collectively, the stress studies conducted in skin act as a scaffold, upon which future experiments can be designed to investigate the impact of stress mediators on other epithelial known to use direct antimicrobial mechanisms to combat infection and maintain normal tissue homestasis.
Trauma-related stress and antimicrobial mechanisms
The neuroendocrine and inflammatory pathways are activated subsequent to traumatic injury, burn injury, or surgery, culminating in a host of metabolic and endocrine modifications following activation of the HPA and SNS. The magnitude of the response to physiologic stress is directly proportional to the severity of injury. In addition to increases in endocrine mediators such as cortisol, insulin, catecholamines, and Ach, APR becomes activated to minimize further tissue damage, provide antimicrobial activity, and promote wound repair [18, 22]. The APR is mediated, in part, by production of cytokines such as IL-1β, which produces fever and increases ACTH and GCs [144, 145]. α-MSH is produced from pituitary, monocytes, lymphocytes, and epithelial cells following activation of the HPA axis and is known to act as an antagonist of injury-induced APR [146]. Pretreatment of rats with α-MSH prior to tail shock stress was shown to block the APR without influencing HPA activation, suggesting that α-MSH may interact directly with IL-1βRs to block IL-1β-induced APR. Separate studies found α-MSH to suppress pulmonary antimicrobial immune responses following cerebral ischemia and thus, increase bacterial loads [147]. Administration of recombinant α-MSH was reported to increase bacterial loads in the lung, and systemic administration of an α-MSH receptor antagonist reduced pulmonary bacterial burden significantly. However, α-MSH receptor antagonist treatment did not influence pulmonary immune cell number or cytokine production, indicating that α-MSH may block the antimicrobial capacity of immune cells to protect against infection efficiently following acute injury and to impede the activation of the APR. Chronically stressed mice developed increased bacterial counts spontaneously in the lung and liver, which continued for up to 10 days after cessation of stress exposure [148]. It was determined that stress induced a deficient antimicrobial response to translocated commensal bacteria, as splenocytes from stressed animals exhibited a reduced ability to produce proinflammatory molecules when stimulated with LPS and increased release of the anti-inflammatory protein, IL-10. Traumatic stress appears to suppress the overall antimicrobial response to translocated commensals that contributes to the development of long-lasting or secondary infections frequently seen in animals and humans subjected to traumatic injury.
Hyperoxic stress is observed frequently in patients with acute lung injury and acute respiratory distress syndrome, which develops following burn or traumatic injury. Mice exposed to sublethal hyperoxia exhibited a significant impairment of phagocytic killing capacity and increased bacterial load in the lung [149]. This effect was found later to be reversed by pharmacologic treatment with GM-CSF, indicating that suppression of pulmonary innate antimicrobial activity during hyperoxic stress occurs through a reduction of GM-CSF by alveolar epithelial cells.
Septic shock manifests as a physiologic stress to stimulate a variety of immune mechanisms but is also associated with dysfunction of macrophages, which are important effector cells involved in innate immune function. MIF was identified originally as a pituitary-derived protein released in the presence of endotoxin or stress but was later identified in macrophages [150]. MIF induces robust proinflammatory processes and is highly prevalent in the circulation of severe sepsis patients. During septic shock, the host responds to LPS by releasing MIF to enhance the antimicrobial responses of macrophages. However, neutralization of MIF with anti-MIF antibodies or deletion of the MIF gene protected mice from lethal bacterial sepsis and toxic shock by LPS [151]. Thus, the stress response to bacterial infection seems to enhance macrophage antimicrobial capacity and function through MIF, but this leads to an overload of proinflammatory molecules that becomes detrimental to the host. LzMPC, derived from treating Lactobacillus with the AMP lysozyme, produce probiotic molecules that have been shown to enhance effector cell function of innate immunity. Oral delivery of LzMPC protected rats from lethal polymicrobial sepsis by increasing the expression of cathelicidin in macrophages and increased their bactericidal capacity [152]. Further probing into the mechanism using a neutralizing antibody showed that protection against sepsis by LzMPC was dependent on endogenous cathelicidin. This protective effect by LzMPC during sepsis may help to reverse the suppression of antimicrobial activity by HPA activation or ANS activation following the onset of physiologic stress.
Adrenergic and cholinergic activation and antimicrobial activity
Adrenergic and cholinergic pathways activated during stress contribute to antimicrobial dysfunction through the release of NE/E and/or Ach, respectively. β-Adrenergic agonists act by binding to specific receptors expressed on virtually all cell types, which leads to elevated levels of cAMP [153]. This activation of β-adrenergic receptors is associated with repression of neutrophil chemotaxis but not phagocytosis [154, 155]. Exogenous activation of nAChRs by nicotine induced suppression of bactericidal activity and cytokine responses of alveolar macrophages in response to Legionella pneumophila [156]. MH-S alveolar macrophages treated with nicotine following infection with L. pneumophila increased the replication of bacteria significantly within macrophages and selectively suppressed the production of proinflammatory cytokines induced by L. pneumophila. These effects were reversed in the presence of a nonselective antagonist, d-tubocurarine, suggesting that nAChRs are involved in the regulation of macrophage-immune function by nicotine and may contribute to the cigarette-induced risk factors for respiratory infections in smokers. These results parallel other work in keratinocytes, where stimulation with increasing concentrations of E or NE did not induce changes in the expression of the AMPs cathelicidin or hBD-2; however, stimulation of nAChR with Ach significantly reduced the gene and protein expression of cathelicidin and hBD-2 in cultured keratinocytes (unpublished observations). Activation of the cholinergic pathway by nicotine also suppressed TH-1 and TH-2 responses and neuroinflammation associated with experimental autoimmune encephalomyelitis [157] and attenuated the accumulation of neutrophils through in the spleen [158]. Thus, the outcome of cholinergic activation may be more pronounced with respect to changes in antimicrobial activity in a variety of cell types involved in immune function compared with adrenergic activation. Table 2 lists selected outcomes of stress on antimicrobial activity through HPA, adrenergic, or cholinergic activation.
TABLE 2.
Select Studies Reporting the Consequence of Stress Mediators on Antimicrobial Activity
| Factor | Species | Cell/Tissue type | Effect on AMP activity | References |
|---|---|---|---|---|
| PS | Mouse | Keratinocytes | ↓ AMP expression | [76] |
| GCs | ↓ Barrier function | |||
| PS | Rat | Intestinal epithelia | ↑ Neutrophil infiltration, MPO | [85, 86] |
| CRF | MC hyperplasia, | |||
| ↑ Bacterial adherence | ||||
| PS, NE/GC | Mouse | Bladder epithelia | ↑ PMN infiltration, | [ 90] |
| ↓ E. coli infection in females, | ||||
| ↑ E. coli infection in males | ||||
| PS | Humans | Neutrophil | ↓ Bactericidal activity, | [91,92,93] |
| ↓ Leukocyte opsonization | ||||
| Ach | Mouse | Keratinocytes | ↓ AMP expression, activity | Unpublished |
| α-MSH | Rats | Pulmonary epithelia | ↓ APR, AMP responses | [104] |
| NE/E | Human | Neutrophils | ↓ Bactericidal activity | [113, 114] |
| ↓Chemotaxis | ||||
| Nicotine | Mouse | Macrophages | ↓ Bactericidal activity | [115] |
Ach, acetylcholine; AMP, antimicrobial peptide; APR, acute phase response; CRF, corticotrophin releasing hormone; E, epinephrine; GC, glucocorticoid; MC, mast cell; MPO, myeloperoxidase; MSH, melanocyte stimulating hormone; NE, norepinephrine; PS, psychological stress.
INFLUENCE OF THE HOST STRESS RESPONSE ON BACTERIAL VIRULENCE
Bacterial pathogens encounter a wide variety of host microenvironments and must adjust their virulence to colonize successfully and provoke host pathological conditions. Host responses to stress stimulate endocrine and metabolic changes that influence the pathogenicity of various microorganisms. These host-pathogen interactions are complex and multifactorial. Host physiologic alterations allow pathogens to circumvent antimicrobial responses more easily by modification of membrane components or virulence factors to promote their survival. A short selection of these mechanisms will be highlighted in this review (for a more extensive review, see refs. [159,160,161,162]).
Influence of catecholamines on bacterial resistance to AMPs
Resistance of bacteria to AMPs is acquired by modifying bacterial cell wall components or expression of virulence factors, and the net result allows resistance to antimicrobial mechanisms. For example, trauma-related sepsis is associated with the ability of nonpathogenic commensal bacteria to disseminate and penetrate mucosal or epithelial barriers. Recognizing the ability of host neuroendocrine-derived hormones to directly influence the severity of sepsis and outcome of infection has opened a new field in microbiology that acknowledges the ability of microorganisms to exploit host responses to stressful events to benefit their survival and pathogenic ability.
Catecholamines include adrenaline and noradrenaline (i.e., E and NE) as part of the acute fight-or-flight response. Following traumatic stress, catecholamines released in response to traumatic injury can noticeably augment the severity of infection, as sepsis caused by proliferation and dissemination of bacteria exists as a major surgical complication and provides a mechanism for gut-derived sepsis. Partial hepatectomy in mice markedly increase levels of NE in the gut lumen, which supports the observation that adhesion of an opportunistic pathogen, Pseudomonas aeruginosa, is amplified following inoculation into hepatectomized mice [163].
Catecholamines have also been shown to promote bacterial growth by mediating communication with eukaryotic cells through quarum-sensing mechanisms [164], where E and NE synergistically augmented genes involved in virulence and motility [165]. E and NE increase the ability of E. coli to adhere to intestinal epithelium, which can be reversed by the presence of the β-adrenergic antagonist, propranolol [166, 167]. Treatment of mice with NE prior to intragastric challenge with Salmonella resulted in increased intestinal colonization and systemic spread of infection [168]. E and NE were also shown to play a role in the infectious cycle of Borrelia burgdorferi infection, as adrenergic stimulation in mice increases the expression of the virulence factor Osp A in B. burgdorferi, which was blocked following treatment with the β-adrenergic antagonist propranolol [169]. In this model, blocking of local catecholamine recognition in the skin by propranolol decreases the uptake of B. burgdorferi in infected mice by preventing the increase in the expression of Osp A that allows for transmission of B. burgdorferi from a mammalian host to uninfected ticks. Collectively, increased sympathetic stimulation, presumably during periods of stress, may increase the susceptibility to the host to pathogenic infection by influencing virulence capacity related to adhesion and antimicrobial defenses.
Effect of stress hormones on iron limitation as an antimicrobial defense
Iron limitation is a part of the host’s environmental signal for stress and can mediate resistance to host innate immune defenses by altering the expression of bacterial membrane proteins. The correlation among bacterial growth, iron limitation, and virulence has been recognized for over a decade, where low host iron reserves present as a major impediment to microbial growth. The catechol moiety in catecholamine hormones allows for the removal of iron from lactoferrin and Tf and consequent gain by bacteria, which accounts for the high rate of growth of commensal E. coli in the gut following traumatic injury [166]. NE can promote iron shuttling between Tf molecules that enable bacterial siderophores, which are high-affinity, iron-chelating compounds secreted by microorganisms, to obtain iron molecules more easily for proliferation [170]. Further studies determined that catecholamine-iron complexes reduce iron and liberate protein-complexed iron from Tf that is used by bacteria as a nutrient source [171]. However, it was determined that therapeutically applicable concentrations of catecholamines and iontropes directly influence the iron-binding capacity of serum Tf to actually suppress the growth of bacteria, which suggests that ionotropic therapy may be used in the future as an intervention for infection following traumatic injury to minimize secondary infection and sepsis.
IsdA is the predominant cell wall-bound bacterial surface protein under iron-starvation conditions. Under iron-deprivation conditions, IsdA renders S. aureus more hydrophilic [172]. The ability of S. aureus to become hydrophobic facilitates its resistance to the antimicrobial fatty acids and peptides present in skin. Interestingly, hepcidin is an anti-fungal peptide hormone produced by the liver, which is regulated by IL-6, and modulates iron metabolism and homeostasis [173]. Mice deficient in hepcidin develop iron overload, whereas mice overexpressing hepcidin develop severe iron-deficiency anemia [174, 175]. In parallel, rats subjected to foot shock PS showed changes in iron distribution and an increase in IL-6 hepcidin axis activation [176].
Together, PS acts to disrupt normal iron metabolism in the host via neuroendocrine hormones that result in metabolic disturbances, such as hypoferremia, which can increase the ability of invasive pathogens to proliferate and evade host antimicrobial mechanisms (Fig. 3). These data reflect the importance of understanding how host antimicrobial mechanisms and bacterial pathogenic mechanisms are influenced by stress hormones to design effectively more successful therapeutic treatments for localized infections and sepsis. The continued research in the field of microbial endocrinology will bring together clinicians and basic researchers who represent a wide range of interests in this scientific discipline to identify the receptors and response pathways involved in catecholamine-mediated bacterial pathogenesis. Of course, the bacterial response to stress not only involves catecholamines but also likely GCs, Ach, and other neuroendocrine effector molecules.
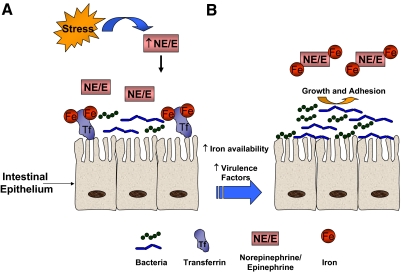
Figure 3.
Suppression of intestinal antimicrobial activity by modifying bacterial virulence and iron stores. (A) Normally high-affinity, iron-binding proteins, such as Tf, prevent free iron (Fe) being made available to microbes to limit their growth and dissemination into exogenous tissues. Concentrations of catecholamines (NE; E) increase in the intestinal lumen following traumatic stress. (B) The catechol moiety acts as a magnet to mediate sequestration of iron from Tf and shift the balance to an iron-rich environment. Quarum-sensing mechanisms signals the bacteria to increase the expression of bacterial virulence factors, siderophores, which facilitate the internalization of iron liberated from Tf by NE/E. Ultimately, iron use allows for greater microbial growth and an increased capacity of the bacteria to adhere and possibly disseminate to distal sites to cause infection.
CONCLUSIONS AND PERSPECTIVES
AMPs play a diverse role in the innate immune response in epithelia and leukocytes. Deposition of AMPs at epithelial and musocal surfaces maintains normal barrier function and mediates protection from infection by providing direct microbicidal activity and initiating proinflammatory responses. Further in vivo studies of AMP regulation in the face of acute and chronic stress are necessary to elucidate the impact of neuroendocrine mediators mobilized during the stress response on antimicrobial activity. The concentration and processing of AMPs determine their efficacy as bactericidal agents and mediators of the inflammatory response. These studies need to identify the specific means by which host defense is compromised during PS, whether to promote undesired inflammatory conditions or to inhibit essential antimicrobial responses. Catecholamines, Ach, GCs, and other neuroendocrine metabolites influence antimicrobial functions through direct and indirect mechanisms (Fig. 4). Of course, the presence of primary disease, such as diabetes or pulmonary insufficiency, confounds these variables, making it difficult to elucidate the specific effects of PS when the antimicrobial capacity of the individual is already compromised from such states of physiologic stress. Furthermore, microbial pathogens have evolved mechanisms to circumvent host antimicrobial responses in response to stress. Thus, a more comprehensive analysis of the effects of stress on antimicrobial function may channel the development of more vigorous therapeutic strategies for treating patients experiencing stress to prevent infection and minimize exacerbation of inflammatory diseases.
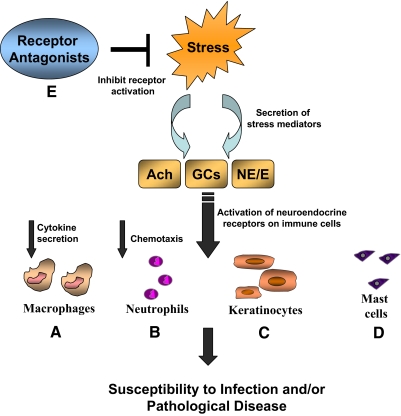
Figure 4.
Impact of stress on antimicrobial activity in immune cells. Physiological or PS promotes the secretion and release of neuroendocrine mediators, such as GCs, Ach, and catecholamines (NE; E). These molecules act on their respective receptors expressed on immune cells to suppress or enhance antimicrobial function and/or inflammation depending on the type of stress. Dysregulation of the antimicrobial activity may lead to a greater incidence of infection or inflammatory diseases. (A) Macrophage bactericidal capacity is diminished in addition to suppression of cytokine production when Ach receptors are activated. However, septic shock enhances macrophage antimicrobial activity, leading to an overload of proinflammatory mediators. (B) Neutrophil chemotaxis and bactericidal activity are reduced during periods of prolonged stress, although neutrophil infiltration is enhanced. (C) Keratinocyte expression of cathelicidin and defensins is reduced during chronic stress, in addition to a suppression of innate antimicrobial activity and lipid secretion. (D) Mast cell hyperplasia and greater tissue infiltration are evident during chronic stress, which implicate mast cells as an important contributor to stress-induced inflammation. (E) Receptor antagonists may be a useful treatment strategy, particularly for skin diseases, in patients with pathologic disease or infection caused by defects in antimicrobial protein regulation.
ACKNOWLEDGMENT
This work was supported by the National Institutes of Health grant NIH/NIAAA 1P30AA019373-01.
DISCLOSURE
The author states no conflict of interest.
Footnotes
Abbreviations: Ach=acetylcholine, ACTH=adrenocorticotropin hormone, AD=atopic dermatitis, AMP=antimicrobial peptide, ANS=autonomic nervous system, APR=acute-phase response, CGRP=calcitonin gene-related peptide, CHGA=chromogranin A, CRAMP=cathelin-related antimicrobial peptide, CRH=corticotrophin-releasing hormone, Cst=catestatin, GAS=Group A Streptococcus, GC=glucocorticoid, hBD=human β-defensin, HPA=hypothalamic-pituitary-adrenal axis, IsdA=iron-responsive surface determinant protein A, LzMPC=lysozyme-modified probiotic component(s), mAChR=muscarinic acetylcholine receptor, MIF=macrophage inhibitory factor, MSH=melanocyte-stimulating hormone, nAChR=nicotinic acetylcholine receptor, NE/E=norepinephrine/epinephrine, Osp A=outer surface protein A, PMN=peripheral mononuclear cells, PS=psychological stress, SNS=sympathetic nervous system, Tf=transferrin, VEGF=vascular endothelial growth factor
References
- Locke S E. Stress, adaptation, and immunity: studies in humans. Gen Hosp Psychiatry. 1982;4:49–58. doi: 10.1016/0163-8343(82)90027-5. [DOI] [PubMed] [Google Scholar]
- Fox B H. Premorbid psychological factors as related to cancer incidence. J Behav Med. 1978;1:45–133. doi: 10.1007/BF00846586. [DOI] [PubMed] [Google Scholar]
- Haggerty R J. Breaking the link between stress and illness in children. What role can physicians play? Postgrad Med. 1983;74:287–291. doi: 10.1080/00325481.1983.11698434. 294–295. [DOI] [PubMed] [Google Scholar]
- Koehler T, Weber D. Psychophysiological reactions of patients with atopic dermatitis. J Psychosom Res. 1992;36:391–394. doi: 10.1016/0022-3999(92)90075-d. [DOI] [PubMed] [Google Scholar]
- Garrett V D, Brantley P J, Jones G N, McKnight G T. The relation between daily stress and Crohn’s disease. J Behav Med. 1991;14:87–96. doi: 10.1007/BF00844770. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Gierens A, Hollig H, Hellhammer D H. Stress-induced immunomodulation is altered in patients with atopic dermatitis. J Neuroimmunol. 2002;129:161–167. doi: 10.1016/s0165-5728(02)00168-6. [DOI] [PubMed] [Google Scholar]
- Duffy L C, Zielezny M A, Marshall J R, Byers T E, Weiser M M, Phillips J F, Calkins B M, Ogra P L, Graham S. Relevance of major stress events as an indicator of disease activity prevalence in inflammatory bowel disease. Behav Med. 1991;17:101–110. doi: 10.1080/08964289.1991.9937553. [DOI] [PubMed] [Google Scholar]
- Garg A, Chren M M, Sands L P, Matsui M S, Marenus K D, Feingold K R, Elias P M. Psychological stress perturbs epidermal permeability barrier homeostasis: implications for the pathogenesis of stress-associated skin disorders. Arch Dermatol. 2001;137:53–59. doi: 10.1001/archderm.137.1.53. [DOI] [PubMed] [Google Scholar]
- Olff M. Stress, depression and immunity: the role of defense and coping styles. Psychiatry Res. 1999;85:7–15. doi: 10.1016/s0165-1781(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Brogden K A, Guthmiller J M, Salzet M, Zasloff M. The nervous system and innate immunity: the neuropeptide connection. Nat Immunol. 2005;6:558–564. doi: 10.1038/ni1209. [DOI] [PubMed] [Google Scholar]
- Troisi A. Gender differences in vulnerability to social stress: a Darwinian perspective. Physiol Behav. 2001;73:443–449. doi: 10.1016/s0031-9384(01)00459-0. [DOI] [PubMed] [Google Scholar]
- Ursin H, Eriksen H R. The cognitive activation theory of stress. Psychoneuroendocrinology. 2004;29:567–592. doi: 10.1016/S0306-4530(03)00091-X. [DOI] [PubMed] [Google Scholar]
- Herman J P, Cullinan W E. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Kinnman J, Fryden A, Eriksson S, Moller E, Link H. Tuberculous meningitis: immune reactions within the central nervous system. Scand J Immunol. 1981;13:289–296. doi: 10.1111/j.1365-3083.1981.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Cross R J, Jackson J C, Brooks W H, Sparks D L, Markesbery W R, Roszman T L. Neuroimmunomodulation: impairment of humoral immune responsiveness by 6-hydroxydopamine treatment. Immunology. 1986;57:145–152. [PMC free article] [PubMed] [Google Scholar]
- Cannon W. New York, NY, USA: Appleton; Bodily Changes in Pain, Hunger, Fear, and Rage. 1929 [Google Scholar]
- Milligan E D, Nguyen K T, Deak T, Hinde J L, Fleshner M, Watkins L R, Maier S F. The long term acute phase-like responses that follow acute stressor exposure are blocked by α-melanocyte stimulating hormone. Brain Res. 1998;810:48–58. doi: 10.1016/s0006-8993(98)00869-5. [DOI] [PubMed] [Google Scholar]
- Maier S F, Watkins L R. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Fulford A J, Harbuz M S. An introduction to the HPA. Steckler T, Kalin N H, Reul J M H M, editors. Amsterdam: Elsevier; Handbook of Stress and the Brain. 2005:43–66. [Google Scholar]
- Kushner I. Regulation of the acute phase response by cytokines. Perspect Biol Med. 1993;36:611–622. doi: 10.1353/pbm.1993.0004. [DOI] [PubMed] [Google Scholar]
- Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Grando S A, Pittelkow M R, Schallreuter K U. Adrenergic and cholinergic control in the biology of epidermis: physiological and clinical significance. J Invest Dermatol. 2006;126:1948–1965. doi: 10.1038/sj.jid.5700151. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Peng Y, Wang J. Immunoregulatory role of neurotransmitters. Adv Neuroimmunol. 1996;6:223–231. doi: 10.1016/s0960-5428(96)00018-6. [DOI] [PubMed] [Google Scholar]
- Venter J C, Fraser C M, Kerlavage A R, Buck M A. Molecular biology of adrenergic and muscarinic cholinergic receptors. A perspective. Biochem Pharmacol. 1989;38:1197–1208. doi: 10.1016/0006-2952(89)90325-0. [DOI] [PubMed] [Google Scholar]
- Saeed R W, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, Tracey K J, Al-Abed Y, Metz C N. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201:1113–1123. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov V A, Ochani M, Gallowitsch-Puerta M, Ochani K, Huston J M, Czura C J, Al-Abed Y, Tracey K J. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Natl Acad Sci USA. 2006;103:5219–5223. doi: 10.1073/pnas.0600506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernik T R, Friedman S G, Ochani M, DiRaimo R, Susarla S, Czura C J, Tracey K J. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg. 2002;36:1231–1236. doi: 10.1067/mva.2002.129643. [DOI] [PubMed] [Google Scholar]
- Tajima T, Endo H, Suzuki Y, Ikari H, Gotoh M, Iguchi A. Immobilization stress-induced increase of hippocampal acetylcholine and of plasma epinephrine, norepinephrine and glucose in rats. Brain Res. 1996;720:155–158. doi: 10.1016/0006-8993(96)00046-7. [DOI] [PubMed] [Google Scholar]
- Baerwald C, Graefe C, Muhl C, Von Wichert P, Krause A. β 2-Adrenergic receptors on peripheral blood mononuclear cells in patients with rheumatic diseases. Eur J Clin Invest. 1992;22:42–46. [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella C A, Tanovic M, Susarla S, Li J H, Yang H, Ulloa L, Al-Abed Y, Czura C J, Tracey K J. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Ado A D, Goldstein M M, Kravchenko S A, Fominova T I. M-cholinergic receptors in mice B-lymphocytes in the process of immune response. Allergol Immunopathol (Madr) 1986;14:237–239. [PubMed] [Google Scholar]
- Fedoseev G B, Zhikharev S S, Kotenko T V, Subbotina T F. Mechanisms of changes in adrenoreceptor activity of leukocytes during immune stimulation. Hum Physiol. 1984;10:102–104. [PubMed] [Google Scholar]
- Elenkov I J, Wilder R L, Chrousos G P, Vizi E S. The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Besedovsky H O, del Rey A, Sorkin E, Da Prada M, Keller H H. Immunoregulation mediated by the sympathetic nervous system. Cell Immunol. 1979;48:346–355. doi: 10.1016/0008-8749(79)90129-1. [DOI] [PubMed] [Google Scholar]
- Smith E M, Blalock J E. A molecular basis for interactions between the immune and neuroendocrine systems. Int J Neurosci. 1988;38:455–464. doi: 10.3109/00207458808990706. [DOI] [PubMed] [Google Scholar]
- Besedovsky H, Sorkin E, Keller M, Muller J. Changes in blood hormone levels during the immune response. Proc Soc Exp Biol Med. 1975;150:466–470. doi: 10.3181/00379727-150-39057. [DOI] [PubMed] [Google Scholar]
- Bjornsson G L, Thorsteinsson L, Gudmundsson K O, Jonsson H, Jr, Gudmundsson S, Gudbjornsson B. Inflammatory cytokines in relation to adrenal response following total hip replacement. Scand J Immunol. 2007;65:99–105. doi: 10.1111/j.1365-3083.2006.01872.x. [DOI] [PubMed] [Google Scholar]
- Hadden J W, Hadden E M, Middleton E., Jr Lymphocyte blast transformation. I. Demonstration of adrenergic receptors in human peripheral lymphocytes. Cell Immunol. 1970;1:583–595. doi: 10.1016/0008-8749(70)90024-9. [DOI] [PubMed] [Google Scholar]
- Tracey K J. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Borovikova L V, Ivanova S, Zhang M, Yang H, Botchkina G I, Watkins L R, Wang H, Abumrad N, Eaton J W, Tracey K J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Voice J K, Grinninger C, Kong Y, Bangale Y, Paul S, Goetzl E J. Roles of vasoactive intestinal peptide (VIP) in the expression of different immune phenotypes by wild-type mice and T cell-targeted type II VIP receptor transgenic mice. J Immunol. 2003;170:308–314. doi: 10.4049/jimmunol.170.1.308. [DOI] [PubMed] [Google Scholar]
- Scicchitano R, Stanisz A M, Payan D G, Kiyono H, McGhee J R, Bienenstock J. Expression of substance P and somatostatin receptors on a T helper cell line. Adv Exp Med Biol. 1987;216A:185–190. doi: 10.1007/978-1-4684-5344-7_21. [DOI] [PubMed] [Google Scholar]
- Aune T M, McGrath K M, Sarr T, Bombara M P, Kelley K A. Expression of 5HT1a receptors on activated human T cells. Regulation of cyclic AMP levels and T cell proliferation by 5-hydroxytryptamine. J Immunol. 1993;151:1175–1183. [PubMed] [Google Scholar]
- Hellstrand K, Hermodsson S. Serotonergic 5-HT1A receptors regulate a cell contact-mediated interaction between natural killer cells and monocytes. Scand J Immunol. 1993;37:7–18. doi: 10.1111/j.1365-3083.1993.tb01658.x. [DOI] [PubMed] [Google Scholar]
- Vizi E S, Elenkov I J. Nonsynaptic noradrenaline release in neuro-immune responses. Acta Biol Hung. 2002;53:229–244. doi: 10.1556/ABiol.53.2002.1-2.21. [DOI] [PubMed] [Google Scholar]
- Lorton D, Lubahn C, Felten S Y, Bellinger D. Norepinephrine content in primary and secondary lymphoid organs is altered in rats with adjuvant-induced arthritis. Mech Ageing Dev. 1997;94:145–163. doi: 10.1016/s0047-6374(96)01859-3. [DOI] [PubMed] [Google Scholar]
- Romano T A, Felten S Y, Olschowka J A, Felten D L. Noradrenergic and peptidergic innervation of lymphoid organs in the beluga, Delphinapterus leucas: an anatomical link between the nervous and immune systems. J Morphol. 1994;221:243–259. doi: 10.1002/jmor.1052210302. [DOI] [PubMed] [Google Scholar]
- Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol. 2004;5:575–581. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- Kimyai-Asadi A, Usman A. The role of psychological stress in skin disease. J Cutan Med Surg. 2001;5:140–145. doi: 10.1007/BF02737869. [DOI] [PubMed] [Google Scholar]
- Denda M, Tsuchiya T, Hosoi J, Koyama J. Immobilization-induced and crowded environment-induced stress delay barrier recovery in murine skin. Br J Dermatol. 1998;138:780–785. doi: 10.1046/j.1365-2133.1998.02213.x. [DOI] [PubMed] [Google Scholar]
- Denda M, Tsuchiya T, Elias P M, Feingold K R. Stress alters cutaneous permeability barrier homeostasis. Am J Physiol Regul Integr Comp Physiol. 2000;278:R367–R372. doi: 10.1152/ajpregu.2000.278.2.R367. [DOI] [PubMed] [Google Scholar]
- Braff M H, Di Nardo A, Gallo R L. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J Invest Dermatol. 2005;124:394–400. doi: 10.1111/j.0022-202X.2004.23443.x. [DOI] [PubMed] [Google Scholar]
- Aberg K M, Man M Q, Gallo R L, Ganz T, Crumrine D, Brown B E, Choi E H, Kim D K, Schroder J M, Feingold K R, Elias P M. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol. 2008;128:917–925. doi: 10.1038/sj.jid.5701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E H, Brown B E, Crumrine D, Chang S, Man M Q, Elias P M, Feingold K R. Mechanisms by which psychologic stress alters cutaneous permeability barrier homeostasis and stratum corneum integrity. J Invest Dermatol. 2005;124:587–595. doi: 10.1111/j.0022-202X.2005.23589.x. [DOI] [PubMed] [Google Scholar]
- Choi E H, Demerjian M, Crumrine D, Brown B E, Mauro T, Elias P M, Feingold K R. Glucocorticoid blockade reverses psychological stress-induced abnormalities in epidermal structure and function. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1657–R1662. doi: 10.1152/ajpregu.00010.2006. [DOI] [PubMed] [Google Scholar]
- Singaram C, Sengupta A, Stevens C, Spechler S J, Goyal R K. Localization of calcitonin gene-related peptide in human esophageal Langerhans cells. Gastroenterology. 1991;100:560–563. doi: 10.1016/0016-5085(91)90231-9. [DOI] [PubMed] [Google Scholar]
- Asahina A, Hosoi J, Grabbe S, Granstein R D. Modulation of Langerhans cell function by epidermal nerves. J Allergy Clin Immunol. 1995;96:1178–1182. doi: 10.1016/s0091-6749(95)70203-2. [DOI] [PubMed] [Google Scholar]
- Hosoi J, Murphy G F, Egan C L, Lerner E A, Grabbe S, Asahina A, Granstein R D. Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature. 1993;363:159–163. doi: 10.1038/363159a0. [DOI] [PubMed] [Google Scholar]
- Kleyn C E, Schneider L, Saraceno R, Mantovani C, Richards H L, Fortune D G, Cumberbatch M, Dearman R J, Terenghi G, Kimber I, Griffiths C E. The effects of acute social stress on epidermal Langerhans’ cell frequency and expression of cutaneous neuropeptides. J Invest Dermatol. 2008;128:1273–1279. doi: 10.1038/sj.jid.5701144. [DOI] [PubMed] [Google Scholar]
- Beresford L, Orange O, Bell E B, Miyan J A. Nerve fibers are required to evoke a contact sensitivity response in mice. Immunology. 2004;111:118–125. doi: 10.1111/j.1365-2567.2003.01786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saade N E, Massaad C A, Ochoa-Chaar C I, Jabbur S J, Safieh-Garabedian B, Atweh S F. Upregulation of proinflammatory cytokines and nerve growth factor by intraplantar injection of capsaicin in rats. J Physiol. 2002;545:241–253. doi: 10.1113/jphysiol.2002.028233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvikallio A, Harvima I T, Naukkarinen A. Mast cells, nerves and neuropeptides in atopic dermatitis and nummular eczema. Arch Dermatol Res. 2003;295:2–7. doi: 10.1007/s00403-002-0378-z. [DOI] [PubMed] [Google Scholar]
- Foreman J C. Substance P and calcitonin gene-related peptide: effects on mast cells and in human skin. Int Arch Allergy Appl Immunol. 1987;82:366–371. doi: 10.1159/000234229. [DOI] [PubMed] [Google Scholar]
- Cao J, Papadopoulou N, Kempuraj D, Boucher W S, Sugimoto K, Cetrulo C L, Theoharides T C. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174:7665–7675. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- Suto H, Matsuda H, Mitsuishi K, Hira K, Uchida T, Unno T, Ogawa H, Ra C. NC/Nga mice: a mouse model for atopic dermatitis. Int Arch Allergy Immunol. 1999;120:70–75. doi: 10.1159/000053599. [DOI] [PubMed] [Google Scholar]
- Amano H, Negishi I, Akiyama H, Ishikawa O. Psychological stress can trigger atopic dermatitis in NC/Nga mice: an inhibitory effect of corticotropin-releasing factor. Neuropsychopharmacology. 2008;33:566–573. doi: 10.1038/sj.npp.1301435. [DOI] [PubMed] [Google Scholar]
- Pavlovic S, Daniltchenko M, Tobin D J, Hagen E, Hunt S P, Klapp B F, Arck P C, Peters E M. Further exploring the brain-skin connection: stress worsens dermatitis via substance P-dependent neurogenic inflammation in mice. J Invest Dermatol. 2008;128:434–446. doi: 10.1038/sj.jid.5701079. [DOI] [PubMed] [Google Scholar]
- Peters E M, Kuhlmei A, Tobin D J, Muller-Rover S, Klapp B F, Arck P C. Stress exposure modulates peptidergic innervation and degranulates mast cells in murine skin. Brain Behav Immun. 2005;19:252–262. doi: 10.1016/j.bbi.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Herz U, Bunikowski R, Renz H. Role of T cells in atopic dermatitis. New aspects on the dynamics of cytokine production and the contribution of bacterial superantigens. Int Arch Allergy Immunol. 1998;115:179–190. doi: 10.1159/000023899. [DOI] [PubMed] [Google Scholar]
- Kim J E, Cho D H, Kim H S, Kim H J, Lee J Y, Cho B K, Park H J. Expression of the corticotropin-releasing hormone-proopiomelanocortin axis in the various clinical types of psoriasis. Exp Dermatol. 2007;16:104–109. doi: 10.1111/j.1600-0625.2006.00509.x. [DOI] [PubMed] [Google Scholar]
- Yang E V, Kim S J, Donovan E L, Chen M, Gross A C, Webster Marketon J I, Barsky S H, Glaser R. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progression. Brain Behav Immun. 2009;23:267–275. doi: 10.1016/j.bbi.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarparo A C, Sumida D H, Patrao M T, Avellar M C, Visconti M A, Maria de Lauro Castrucci A. Catecholamine effects on human melanoma cells evoked by α1-adrenoceptors. Arch Dermatol Res. 2004;296:112–119. doi: 10.1007/s00403-004-0488-x. [DOI] [PubMed] [Google Scholar]
- Zheng P Y, Feng B S, Oluwole C, Struiksma S, Chen X, Li P, Tang S G, Yang P C. Psychological stress induces eosinophils to produce corticotrophin releasing hormone in the intestine. Gut. 2009;58:1473–1479. doi: 10.1136/gut.2009.181701. [DOI] [PubMed] [Google Scholar]
- Teitelbaum A A, Gareau M G, Jury J, Yang P C, Perdue M H. Chronic peripheral administration of corticotropin-releasing factor causes colonic barrier dysfunction similar to psychological stress. Am J Physiol Gastrointest Liver Physiol. 2008;295:G452–G459. doi: 10.1152/ajpgi.90210.2008. [DOI] [PubMed] [Google Scholar]
- Kokkotou E, Torres D, Moss A C, O'Brien M, Grigoriadis D E, Karalis K, Pothoulakis C. Corticotropin-releasing hormone receptor 2-deficient mice have reduced intestinal inflammatory responses. J Immunol. 2006;177:3355–3361. doi: 10.4049/jimmunol.177.5.3355. [DOI] [PubMed] [Google Scholar]
- Tache Y, Perdue M H. Role of peripheral CRF signaling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Motil. 2004;16:137–142. doi: 10.1111/j.1743-3150.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- Meddings J B, Swain M G. Environmental stress-induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology. 2000;119:1019–1028. doi: 10.1053/gast.2000.18152. [DOI] [PubMed] [Google Scholar]
- Ohara Y, McCarron R M, Hoffman T T, Sugano H, Bembry J, Lenz F A, Spatz M. Adrenergic mediation of TNF α-stimulated ICAM-1 expression on human brain microvascular endothelial cells. Acta Neurochir Suppl. 2000;76:117–120. doi: 10.1007/978-3-7091-6346-7_24. [DOI] [PubMed] [Google Scholar]
- Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- Gallo R L, Nizet V. Endogenous production of antimicrobial peptides in innate immunity and human disease. Curr Allergy Asthma Rep. 2003;3:402–409. doi: 10.1007/s11882-003-0074-x. [DOI] [PubMed] [Google Scholar]
- Nizet V, Gallo R L. Cathelicidins and innate defense against invasive bacterial infection. Scand J Infect Dis. 2003;35:670–676. doi: 10.1080/00365540310015629. [DOI] [PubMed] [Google Scholar]
- Henzler-Wildman K A, Martinez G V, Brown M F, Ramamoorthy A. Perturbation of the hydrophobic core of lipid bilayers by the human antimicrobial peptide LL-37. Biochemistry. 2004;43:8459–8469. doi: 10.1021/bi036284s. [DOI] [PubMed] [Google Scholar]
- Brogden K A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- Braff M H, Gallo R L. Antimicrobial peptides: an essential component of the skin defensive barrier. Curr Top Microbiol Immunol. 2006;306:91–110. doi: 10.1007/3-540-29916-5_4. [DOI] [PubMed] [Google Scholar]
- Braff M H, Bardan A, Nizet V, Gallo R L. Cutaneous defense mechanisms by antimicrobial peptides. J Invest Dermatol. 2005;125:9–13. doi: 10.1111/j.0022-202X.2004.23587.x. [DOI] [PubMed] [Google Scholar]
- Brogden K A, Ackermann M, McCray P B, Jr, Tack B F. Antimicrobial peptides in animals and their role in host defences. Int J Antimicrob Agents. 2003;22:465–478. doi: 10.1016/s0924-8579(03)00180-8. [DOI] [PubMed] [Google Scholar]
- Lai Y, Gallo R L. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radek K, Gallo R. Antimicrobial peptides: natural effectors of the innate immune system. Semin Immunopathol. 2007;29:27–43. doi: 10.1007/s00281-007-0064-5. [DOI] [PubMed] [Google Scholar]
- Glaser R, Harder J, Lange H, Bartels J, Christophers E, Schroder J M. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat Immunol. 2005;6:57–64. doi: 10.1038/ni1142. [DOI] [PubMed] [Google Scholar]
- Kiehne K, Fincke A, Brunke G, Lange T, Folsch U R, Herzig K H. Antimicrobial peptides in chronic anal fistula epithelium. Scand J Gastroenterol. 2007;42:1063–1069. doi: 10.1080/00365520701320489. [DOI] [PubMed] [Google Scholar]
- Harder J, Schroder J M. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J Biol Chem. 2002;277:46779–46784. doi: 10.1074/jbc.M207587200. [DOI] [PubMed] [Google Scholar]
- Chen X, Niyonsaba F, Ushio H, Okuda D, Nagaoka I, Ikeda S, Okumura K, Ogawa H. Synergistic effect of antibacterial agents human β-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. J Dermatol Sci. 2005;40:123–132. doi: 10.1016/j.jdermsci.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Agerberth B, Grunewald J, Castanos-Velez E, Olsson B, Jornvall H, Wigzell H, Eklund A, Gudmundsson G H. Antibacterial components in bronchoalveolar lavage fluid from healthy individuals and sarcoidosis patients. Am J Respir Crit Care Med. 1999;160:283–290. doi: 10.1164/ajrccm.160.1.9807041. [DOI] [PubMed] [Google Scholar]
- Harder J, Schroder J M. Antimicrobial peptides in human skin. Chem Immunol Allergy. 2005;86:22–41. doi: 10.1159/000086650. [DOI] [PubMed] [Google Scholar]
- Ouellette A J, Selsted M E. Paneth cell defensins: endogenous peptide components of intestinal host defense. FASEB J. 1996;10:1280–1289. doi: 10.1096/fasebj.10.11.8836041. [DOI] [PubMed] [Google Scholar]
- Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner R A, Pestonjamasp V, Piraino J, Huttner K, Gallo R L. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- Braff M H, Zaiou M, Fierer J, Nizet V, Gallo R L. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect Immun. 2005;73:6771–6781. doi: 10.1128/IAI.73.10.6771-6781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radek K A, Lopez-Garcia B, Hupe M, Niesman I R, Elias P M, Taupenot L, Mahata S K, O'Connor D T, Gallo R L. The neuroendocrine peptide catestatin is a cutaneous antimicrobial and induced in the skin after injury. J Invest Dermatol. 2008;128:1525–1534. doi: 10.1038/sj.jid.5701225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman N H, Ghosh D, Huttner K M, Paterson Y, Bevins C L. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:522–526. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- Howell M D, Jones J F, Kisich K O, Streib J E, Gallo R L, Leung D Y. Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J Immunol. 2004;172:1763–1767. doi: 10.4049/jimmunol.172.3.1763. [DOI] [PubMed] [Google Scholar]
- Moser C, Weiner D J, Lysenko E, Bals R, Weiser J N, Wilson J M. β-Defensin 1 contributes to pulmonary innate immunity in mice. Infect Immun. 2002;70:3068–3072. doi: 10.1128/IAI.70.6.3068-3072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Wang Y, Kuang Y, Wang B, Li W, Gong T, Jiang Z, Yang D, Li M. Expression of mouse β-defensin-3 in MDCK cells and its anti-influenza-virus activity. Arch Virol. 2009;154:639–647. doi: 10.1007/s00705-009-0352-6. [DOI] [PubMed] [Google Scholar]
- Meyer J E, Harder J, Gorogh T, Weise J B, Schubert S, Janssen D, Maune S. Human β-defensin-2 in oral cancer with opportunistic Candida infection. Anticancer Res. 2004;24:1025–1030. [PubMed] [Google Scholar]
- Evans S E, Scott B L, Clement C G, Larson D T, Kontoyiannis D, Lewis R E, Lasala P R, Pawlik J, Peterson J W, Chopra A K, Klimpel G, Bowden G, Hook M, Xu Y, Tuvim M J, Dickey B F. Stimulated innate resistance of lung epithelium protects mice broadly against bacteria and fungi. Am J Respir Cell Mol Biol. 2010;42:40–50. doi: 10.1165/rcmb.2008-0260OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki K, Schauber J, Coda A, Lin H, Dorschner R A, Schechter N M, Bonnart C, Descargues P, Hovnanian A, Gallo R L. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006;20:2068–2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- Ganz T. Biosynthesis of defensins and other antimicrobial peptides. Ciba Found Symp. 1994;186:62–71. doi: 10.1002/9780470514658.ch4. discussion 71–76. [DOI] [PubMed] [Google Scholar]
- O'Connor D T. Chromogranin: widespread immunoreactivity in polypeptide hormone producing tissues and in serum. Regul Pept. 1983;6:263–280. doi: 10.1016/0167-0115(83)90145-3. [DOI] [PubMed] [Google Scholar]
- Mahata S K. Effect of insulin on serotonin, 5-hydroxyindole-acetic acid, norepinephrine, epinephrine and corticosterone contents in chick. Neurosci Lett. 1991;121:115–118. doi: 10.1016/0304-3940(91)90662-d. [DOI] [PubMed] [Google Scholar]
- Gayen J R, Saberi M, Schenk S, Biswas N, Vaingankar S M, Cheung W W, Najjar S M, O'Connor D T, Bandyopadhyay G, Mahata S K. A novel pathway of insulin sensitivity in chromogranin a null mice: a crucial role for pancreastatin in glucose homeostasis. J Biol Chem. 2009;284:28498–28509. doi: 10.1074/jbc.M109.020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz-Boutigue M H, Kieffer A E, Goumon Y, Aunis D. Innate immunity: involvement of new neuropeptides. Trends Microbiol. 2003;11:585–592. doi: 10.1016/j.tim.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Cutuli M, Cristiani S, Lipton J M, Catania A. Antimicrobial effects of α-MSH peptides. J Leukoc Biol. 2000;67:233–239. doi: 10.1002/jlb.67.2.233. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Shigeri Y, Tatsu Y, Yoshikawa S, Yumoto N. Enhancement of antimicrobial activity of neuropeptide Y by N-terminal truncation. Antimicrob Agents Chemother. 1998;42:2745–2746. doi: 10.1128/aac.42.10.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox F E, Kubin M, Cassin M, Niu Z, Hosoi J, Torii H, Granstein R D, Trinchieri G, Rook A H. Calcitonin gene-related peptide inhibits proliferation and antigen presentation by human peripheral blood mononuclear cells: effects on B7, interleukin 10, and interleukin 12. J Invest Dermatol. 1997;108:43–48. doi: 10.1111/1523-1747.ep12285627. [DOI] [PubMed] [Google Scholar]
- Mahata S K, O'Connor D T, Mahata M, Yoo S H, Taupenot L, Wu H, Gill B M, Parmer R J. Novel autocrine feedback control of catecholamine release. A discrete chromogranin A fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Invest. 1997;100:1623–1633. doi: 10.1172/JCI119686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahata S K, Mahata M, Yoo S H, Taupenot L, Wu H, Aroda V R, Livsey C V, Taulane J P, Goodman M, Parmer R J, O'Connor D T. A novel, catecholamine release-inhibitory peptide from chromogranin A: autocrine control of nicotinic cholinergic-stimulated exocytosis. Adv Pharmacol. 1998;42:260–264. doi: 10.1016/s1054-3589(08)60743-7. [DOI] [PubMed] [Google Scholar]
- Taupenot L, Harper K L, O'Connor D T. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- Iimura M, Gallo R L, Hase K, Miyamoto Y, Eckmann L, Kagnoff M F. Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. J Immunol. 2005;174:4901–4907. doi: 10.4049/jimmunol.174.8.4901. [DOI] [PubMed] [Google Scholar]
- Chromek M, Slamova Z, Bergman P, Kovacs L, Podracka L, Ehren I, Hokfelt T, Gudmundsson G H, Gallo R L, Agerberth B, Brauner A. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12:636–641. doi: 10.1681/01.asn.0000926856.92699.53. [DOI] [PubMed] [Google Scholar]
- Schauber J, Dorschner R A, Coda A B, Buchau A S, Liu P T, Kiken D, Helfrich Y R, Kang S, Elalieh H Z, Steinmeyer A, Zugel U, Bikle D D, Modlin R L, Gallo R L. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorschner R A, Pestonjamasp V K, Tamakuwala S, Ohtake T, Rudisill J, Nizet V, Agerberth B, Gudmundsson G H, Gallo R L. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol. 2001;117:91–97. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- Schauber J, Oda Y, Buchau A S, Yun Q C, Steinmeyer A, Zugel U, Bikle D D, Gallo R L. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J Invest Dermatol. 2008;128:816–824. doi: 10.1038/sj.jid.5701102. [DOI] [PubMed] [Google Scholar]
- Aberg K M, Radek K A, Choi E H, Kim D K, Demerjian M, Hupe M, Kerbleski J, Gallo R L, Ganz T, Mauro T, Feingold K R, Elias P M. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest. 2007;117:3339–3349. doi: 10.1172/JCI31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehkamp J, Salzman N H, Porter E, Nuding S, Weichenthal M, Petras R E, Shen B, Schaeffeler E, Schwab M, Linzmeier R, Feathers R W, Chu H, Lima H, Jr, Fellermann K, Ganz T, Stange E F, Bevins C L. Reduced Paneth cell α-defensins in ileal Crohn’s disease. Proc Natl Acad Sci USA. 2005;102:18129–18134. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellermann K, Stange D E, Schaeffeler E, Schmalzl H, Wehkamp J, Bevins C L, Reinisch W, Teml A, Schwab M, Lichter P, Radlwimmer B, Stange E F. A chromosome 8 gene-cluster polymorphism with low human β-defensin 2 gene copy number predisposes to Crohn disease of the colon. Am J Hum Genet. 2006;79:439–448. doi: 10.1086/505915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H, Wershil B K, Arizono N, Galli S J. Substance P-induced augmentation of cutaneous vascular permeability and granulocyte infiltration in mice is mast cell dependent. J Clin Invest. 1989;84:1276–1286. doi: 10.1172/JCI114295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ramos B F, Jakschik B A. Neutrophil recruitment by tumor necrosis factor from mast cells in immune complex peritonitis. Science. 1992;258:1957–1959. doi: 10.1126/science.1470922. [DOI] [PubMed] [Google Scholar]
- Echtenacher B, Mannel D N, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- Raithel M, Schneider H T, Hahn E G. Effect of substance P on histamine secretion from gut mucosa in inflammatory bowel disease. Scand J Gastroenterol. 1999;34:496–503. doi: 10.1080/003655299750026236. [DOI] [PubMed] [Google Scholar]
- Church M K, el-Lati S, Caulfield J P. Neuropeptide-induced secretion from human skin mast cells. Int Arch Allergy Appl Immunol. 1991;94:310–318. doi: 10.1159/000235393. [DOI] [PubMed] [Google Scholar]
- Eutamene H, Theodorou V, Fioramonti J, Bueno L. Acute stress modulates the histamine content of mast cells in the gastrointestinal tract through interleukin-1 and corticotropin-releasing factor release in rats. J Physiol. 2003;553:959–966. doi: 10.1113/jphysiol.2003.052274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderholm J D, Yang P C, Ceponis P, Vohra A, Riddell R, Sherman P M, Perdue M H. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology. 2002;123:1099–1108. doi: 10.1053/gast.2002.36019. [DOI] [PubMed] [Google Scholar]
- Di Nardo A, Vitiello A, Gallo R L. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003;170:2274–2278. doi: 10.4049/jimmunol.170.5.2274. [DOI] [PubMed] [Google Scholar]
- Castagliuolo I, Wershil B K, Karalis K, Pasha A, Nikulasson S T, Pothoulakis C. Colonic mucin release in response to immobilization stress is mast cell dependent. Am J Physiol. 1998;274:G1094–G1100. doi: 10.1152/ajpgi.1998.274.6.G1094. [DOI] [PubMed] [Google Scholar]
- Brown D H, Zwilling B S. Activation of the hypothalamic-pituitary-adrenal axis differentially affects the anti-mycobacterial activity of macrophages from BCG-resistant and susceptible mice. J Neuroimmunol. 1994;53:181–187. doi: 10.1016/0165-5728(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Zahwa H, Yorty J L, Bonneau R H. Elevated maternal corticosterone during lactation hinders the neonatal adaptive immune response to herpes simplex virus (HSV) infection. Brain Behav Immun. 2008;22:339–353. doi: 10.1016/j.bbi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Dalal E, Medalia O, Harari O, Aronson M. Moderate stress protects female mice against bacterial infection of the bladder by eliciting uroepithelial shedding. Infect Immun. 1994;62:5505–5510. doi: 10.1128/iai.62.12.5505-5510.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Boucher W, Kempuraj D, Donelan J M, Theoharides T C. Acute stress and intravesical corticotropin-releasing hormone induces mast cell dependent vascular endothelial growth factor release from mouse bladder explants. J Urol. 2006;176:1208–1213. doi: 10.1016/j.juro.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Quan N, Avitsur R, Stark J L, He L, Shah M, Caligiuri M, Padgett D A, Marucha P T, Sheridan J F. Social stress increases the susceptibility to endotoxic shock. J Neuroimmunol. 2001;115:36–45. doi: 10.1016/s0165-5728(01)00273-9. [DOI] [PubMed] [Google Scholar]
- Reinheimer T, Munch M, Bittinger F, Racke K, Kirkpatrick C J, Wessler I. Glucocorticoids mediate reduction of epithelial acetylcholine content in the airways of rats and humans. Eur J Pharmacol. 1998;349:277–284. doi: 10.1016/s0014-2999(98)00185-x. [DOI] [PubMed] [Google Scholar]
- Valera S, Ballivet M, Bertrand D. Progesterone modulates a neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci USA. 1992;89:9949–9953. doi: 10.1073/pnas.89.20.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov I J, Webster E L, Torpy D J, Chrousos G P. Stress, corticotropin-releasing hormone, glucocorticoids, and the immune/inflammatory response: acute and chronic effects. Ann N Y Acad Sci. 1999;876:1–11. doi: 10.1111/j.1749-6632.1999.tb07618.x. discussion 11–13. [DOI] [PubMed] [Google Scholar]
- Zhang D, Shooshtarizadeh P, Laventie B J, Colin D A, Chich J F, Vidic J, de Barry J, Chasserot-Golaz S, Delalande F, Van Dorsselaer A, Schneider F, Helle K, Aunis D, Prevost G, Metz-Boutigue M H. Two chromogranin A-derived peptides induce calcium entry in human neutrophils by calmodulin-regulated calcium independent phospholipase A2. PLoS One. 2009;4:e4501. doi: 10.1371/journal.pone.0004501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger M J. Fever: role of pyrogens and cryogens. Physiol Rev. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar S K, Becker K J, Cierpial M A, Carpenter M D, Rankin L A, Fleener S L, Ritchie J C, Simson P E, Weiss J M. Intracerebroventricular infusion of interleukin 1 rapidly decreases peripheral cellular immune responses. Proc Natl Acad Sci USA. 1989;86:6398–6402. doi: 10.1073/pnas.86.16.6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J M, Sundar S K, Cierpial M A, Ritchie J C. Effects of interleukin-1 infused into brain are antagonized by α-MSH in a dose-dependent manner. Eur J Pharmacol. 1991;192:177–179. doi: 10.1016/0014-2999(91)90087-7. [DOI] [PubMed] [Google Scholar]
- Schulte-Herbruggen O, Quarcoo D, Brzoska T, Klehmet J, Meisel A, Meisel C. α-MSH promotes spontaneous post-ischemic pneumonia in mice via melanocortin-receptor-1. Exp Neurol. 2008;210:731–739. doi: 10.1016/j.expneurol.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Kiank C, Daeschlein G, Schuett C. Pneumonia as a long-term consequence of chronic psychological stress in BALB/c mice. Brain Behav Immun. 2008;22:1173–1177. doi: 10.1016/j.bbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Baleeiro C E, Wilcoxen S E, Morris S B, Standiford T J, Paine R., III Sublethal hyperoxia impairs pulmonary innate immunity. J Immunol. 2003;171:955–963. doi: 10.4049/jimmunol.171.2.955. [DOI] [PubMed] [Google Scholar]
- Bernhagen J, Calandra T, Mitchell R A, Martin S B, Tracey K J, Voelter W, Manogue K R, Cerami A, Bucala R. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- Calandra T, Froidevaux C, Martin C, Roger T. Macrophage migration inhibitory factor and host innate immune defenses against bacterial sepsis. J Infect Dis. 2003;187:S385–S390. doi: 10.1086/374752. [DOI] [PubMed] [Google Scholar]
- Bu H F, Wang X, Zhu Y Q, Williams R Y, Hsueh W, Zheng X, Rozenfeld R A, Zuo X L, Tan X D. Lysozyme-modified probiotic components protect rats against polymicrobial sepsis: role of macrophages and cathelicidin-related innate immunity. J Immunol. 2006;177:8767–8776. doi: 10.4049/jimmunol.177.12.8767. [DOI] [PubMed] [Google Scholar]
- Rivkin I, Rosenblatt J, Becker E L. The role of cyclic AMP in the chemotactic responsiveness and spontaneous motility of rabbit peritoneal neutrophils. The inhibition of neutrophil movement and the elevation of cyclic AMP levels by catecholamines, prostaglandins, theophylline and cholera toxin. J Immunol. 1975;115:1126–1134. [PubMed] [Google Scholar]
- Llewellyn-Jones C G, Stockley R A. The effects of β 2-agonists and methylxanthines on neutrophil function in vitro. Eur Respir J. 1994;7:1460–1466. doi: 10.1183/09031936.94.07081460. [DOI] [PubMed] [Google Scholar]
- Silvestri M, Oddera S, Lantero S, Rossi G A. β 2-Agonist-induced inhibition of neutrophil chemotaxis is not associated with modification of LFA-1 and Mac-1 expression or with impairment of polymorphonuclear leukocyte antibacterial activity. Respir Med. 1999;93:416–423. doi: 10.1053/rmed.1999.0584. [DOI] [PubMed] [Google Scholar]
- Matsunaga K, Klein T W, Friedman H, Yamamoto Y. Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages to Legionella pneumophila infection by nicotine. J Immunol. 2001;167:6518–6524. doi: 10.4049/jimmunol.167.11.6518. [DOI] [PubMed] [Google Scholar]
- Nizri E, Irony-Tur-Sinai M, Lory O, Orr-Urtreger A, Lavi E, Brenner T. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J Immunol. 2009;183:6681–6688. doi: 10.4049/jimmunol.0902212. [DOI] [PubMed] [Google Scholar]
- Huston J M, Rosas-Ballina M, Xue X, Dowling O, Ochani K, Ochani M, Yeboah M M, Chatterjee P K, Tracey K J, Metz C N. Cholinergic neural signals to the spleen down-regulate leukocyte trafficking via CD11b. J Immunol. 2009;183:552–559. doi: 10.4049/jimmunol.0802684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichhart T, Haidinger M, Horl W H, Saemann M D. Current concepts of molecular defense mechanisms operative during urinary tract infection. Eur J Clin Invest. 2008;38:29–38. doi: 10.1111/j.1365-2362.2008.02006.x. [DOI] [PubMed] [Google Scholar]
- Prost L R, Sanowar S, Miller S I. Salmonella sensing of anti-microbial mechanisms to promote survival within macrophages. Immunol Rev. 2007;219:55–65. doi: 10.1111/j.1600-065X.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- Bottomley M J, Muraglia E, Bazzo R, Carfi A. Molecular insights into quorum sensing in the human pathogen Pseudomonas aeruginosa from the structure of the virulence regulator LasR bound to its autoinducer. J Biol Chem. 2007;282:13592–13600. doi: 10.1074/jbc.M700556200. [DOI] [PubMed] [Google Scholar]
- Freestone P P, Sandrini S M, Haigh R D, Lyte M. Microbial endocrinology: how stress influences susceptibility to infection. Trends Microbiol. 2008;16:55–64. doi: 10.1016/j.tim.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Alverdy J, Holbrook C, Rocha F, Seiden L, Wu R L, Musch M, Chang E, Ohman D, Suh S. Gut-derived sepsis occurs when the right pathogen with the right virulence genes meets the right host: evidence for in vivo virulence expression in Pseudomonas aeruginosa. Ann Surg. 2000;232:480–489. doi: 10.1097/00000658-200010000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzer K, Williams P. Quorum sensing and the regulation of virulence gene expression in pathogenic bacteria. Int J Med Microbiol. 2001;291:131–143. doi: 10.1078/1438-4221-00110. [DOI] [PubMed] [Google Scholar]
- Bansal T, Englert D, Lee J, Hegde M, Wood T K, Jayaraman A. Differential effects of epinephrine, norepinephrine, and indole on Escherichia coli O157:H7 chemotaxis, colonization, and gene expression. Infect Immun. 2007;75:4597–4607. doi: 10.1128/IAI.00630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freestone P P, Williams P H, Haigh R D, Maggs A F, Neal C P, Lyte M. Growth stimulation of intestinal commensal Escherichia coli by catecholamines: a possible contributory factor in trauma-induced sepsis. Shock. 2002;18:465–470. doi: 10.1097/00024382-200211000-00014. [DOI] [PubMed] [Google Scholar]
- Chen C, Brown D R, Xie Y, Green B T, Lyte M. Catecholamines modulate Escherichia coli O157:H7 adherence to murine cecal mucosa. Shock. 2003;20:183–188. doi: 10.1097/01.shk.0000073867.66587.e0. [DOI] [PubMed] [Google Scholar]
- Methner U, Rabsch W, Reissbrodt R, Williams P H. Effect of norepinephrine on colonization and systemic spread of Salmonella enterica in infected animals: role of catecholate siderophore precursors and degradation products. Int J Med Microbiol. 2008;298:429–439. doi: 10.1016/j.ijmm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Scheckelhoff M R, Telford S R, Wesley M, Hu L T. Borrelia burgdorferi intercepts host hormonal signals to regulate expression of outer surface protein A. Proc Natl Acad Sci USA. 2007;104:7247–7252. doi: 10.1073/pnas.0607263104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freestone P P, Haigh R D, Williams P H, Lyte M. Involvement of enterobactin in norepinephrine-mediated iron supply from transferrin to enterohemorrhagic Escherichia coli. FEMS Microbiol Lett. 2003;222:39–43. doi: 10.1016/S0378-1097(03)00243-X. [DOI] [PubMed] [Google Scholar]
- Sandrini S M, Shergill R, Woodward J, Muralikuttan R, Haigh R D, Lyte M, Freestone P P. Elucidation of the mechanism by which catecholamine stress hormones liberate iron from the innate immune defense proteins transferrin and lactoferrin. J Bacteriol. 2010;192:587–594. doi: 10.1128/JB.01028-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S R, Mohamed R, Bian L, Routh A F, Kokai-Kun J F, Mond J J, Tarkowski A, Foster S J. The Staphylococcus aureus surface protein IsdA mediates resistance to innate defenses of human skin. Cell Host Microbe. 2007;1:199–212. doi: 10.1016/j.chom.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Ganz T. The role of hepcidin in iron sequestration during infections and in the pathogenesis of anemia of chronic disease. Isr Med Assoc J. 2002;4:1043–1045. [PubMed] [Google Scholar]
- Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, Vaulont S. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci USA. 2001;98:8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas G, Bennoun M, Porteu A, Mativet S, Beaumont C, Grandchamp B, Sirito M, Sawadogo M, Kahn A, Vaulont S. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci USA. 2002;99:4596–4601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Chen J, Wang W, Wang L, Ma L, Shen H, Li M. Psychological stress induces hypoferremia through the IL-6-hepcidin axis in rats. Biochem Biophys Res Commun. 2008;373:90–93. doi: 10.1016/j.bbrc.2008.05.166. [DOI] [PubMed] [Google Scholar]