Abstract
This review attempts to provide some sense of our current knowledge of water including overall patterns of intake and some factors linked with intake, the complex mechanisms behind water homeostasis, the effects of variation in water intake on health and energy intake, weight, and human performance and functioning. Water represents a critical nutrient whose absence will be lethal within days. Water’s importance for prevention of nutrition-related noncommunicable diseases has emerged more recently because of the shift toward large proportions of fluids coming from caloric beverages. Nevertheless, there are major gaps in knowledge related to measurement of total fluid intake, hydration status at the population level, and few longer-term systematic interventions and no published random-controlled longer-term trials. We suggest some ways to examine water requirements as a means to encouraging more dialogue on this important topic.
Keywords: water, hydration, water intake, water measurement, recommended daily intake, water adequacy
I. INTRODUCTION
Water is essential for life. From the time that primeval species ventured from the oceans to live on land, a major key to survival has been prevention of dehydration. The critical adaptations cross an array of species, including man. Without water, humans can survive only for days. Water comprises from 75% body weight in infants to 55% in elderly and is essential for cellular homeostasis and life.1 Nevertheless there are many unanswered questions about this most essential component of our body and our diet. This review attempts to provide some sense of our current knowledge of water including overall patterns of intake and some factors linked with intake, the complex mechanisms behind water homeostasis, the effects of variation in water intake on health and energy intake, weight, and human performance and functioning.
Recent statements on water requirements have been based on retrospective recall of water intake from food and beverages among healthy non-institutionalized individuals. We provide examples of water intake assessment in populations to clarify the need for experimental studies. Beyond these circumstances of dehydration, we do not truly understand how hydration affects health and well-being, even the impact of water intakes on chronic diseases. Recently, Jéquier and Constant addressed this question based on the human physiology.2 We need to know more about the extent that water intake might be important for disease prevention and health promotion.
As we note later, few countries have developed water requirements and those that do base them on weak population-level measures of water intake and urine osmolality.3, 4 The European Food Safety Authority (EFSA) has been recently asked to revise existing recommended intakes of essential substances with a physiological effect including water since this nutrient is essential for life and health.5
The US Dietary Recommendations for water are based on median water intakes with no use of measurements of dehydration status of the population to assist. One-time collection of blood samples for the analysis of serum osmolality has been used by NHANES. At the population level we have no accepted method of assessing hydration status and one measure some scholars use, hypertonicity, is not even linked with hydration in the same direction for all age groups.6 Urine indices are used often but reflect recent volume of fluid consumed rather than a state of hydration.7 Many scholars use urine osmolality to measure recent hydration status.8–12 Deuterium dilution techniques (isotopic dilution with D2O or deuterium oxide) allows measurement of total body water but not water balance status.13 Currently we feel there are no adequate biomarkers to measure hydration status at the population level.
When we speak of water we are essentially focusing first and foremost on all types of water, be they soft or hard, spring or well, carbonated or distilled water. Furthermore we get water not only directly as a beverage but from food and to a very small extent also from oxidation of macronutrients (metabolic water). The proportion of water that comes from beverages and food varies with the proportion of fruits and vegetables in the diet. We present the ranges of water in various foods (Table 1). In the United States it is estimated that about 22% of water comes from our food intake while it would be much higher in European countries, particularly a country like Greece with its higher intake of fruits and vegetables or South Korea.3, 14, 15 The only in-depth study of water use and water intrinsic to food in the US found a 20.7% contribution from food water;16, 17 however as we show later, this research was dependent on poor overall assessment of water intake.
Table 1.
The Water Content Range for Selected Foods
| Percentage | Food Item |
|---|---|
| 100% | Water |
| 90–99% | Fat-free milk, cantaloupe, strawberries, watermelon, lettuce, cabbage, celery, spinach, pickles, squash (cooked) |
| 80–89% | Fruit juice, yogurt, apples, grapes, oranges, carrots, broccoli (cooked), pears, pineapple |
| 70–79% | Bananas, avocados, cottage cheese, ricotta cheese, potato (baked), corn (cooked), shrimp |
| 60–69% | Pasta, legumes, salmon, ice cream, chicken breast |
| 50–59% | Ground beef, hot dogs, feta cheese, tenderloin steak (cooked) |
| 40–49% | Pizza |
| 30–39% | Cheddar cheese, bagels, bread |
| 20–29% | Pepperoni sausage, cake, biscuits |
| 10–19% | Butter, margarine, raisins |
| 1–9% | Walnuts, peanuts (dry roasted), chocolate chip cookies, crackers, cereals, pretzels, taco shells, peanut butter |
| 0% | Oils, sugars |
Source: The USDA National Nutrient Database for Standard Reference, Release 21 provided in Altman.127
This review considers water requirements in the context of recent efforts to assess water intake in US populations. Relationship of water and calorie intake is explored both for insights into the possible displacement of calories from sweetened beverages by water and also to examine the possibility that water requirements would be better expressed in relation to calorie/energy requirements with the dependence of the latter on age, size, gender, and physical activity level. We review current understanding of the exquisitely complex and sensitive system which protects land animals against dehydration and comment on the complications of acute and chronic dehydration in man against which a better expression of water requirements might complement the physiological control of thirst. Indeed, the fine intrinsic regulation of hydration and water intake in individuals mitigates against prevalent underhydration in populations and effects on function and disease.
Regulation of fluid intake
To prevent dehydration reptiles, birds, vertebrates, and all land animals have evolved an exquisitely sensitive network of physiological controls to maintain body water and fluid intake by thirst. Humans may drink for various reasons, particularly for hedonic ones but most of drinking is due to water deficiency which triggers the so called regulatory or physiological thirst. The mechanism of thirst is quite well understood today and the reason non-regulatory drinking is often encountered is related to the large capacity of kidneys to rapidly eliminate excesses of water or reduce urine secretion to temporarily economize on water.1 But this excretory process can only postpone the necessity for drinking or for stopping drinking an excess of water. Non regulatory drinking is often confusing, particularly in wealthy societies facing highly palatable drinks or fluids that contain other substance that the drinker seeks. The most common of them are sweeteners or alcohol to which water is served as a vehicle. Drinking these beverages isn’t due to excessive thirst or hyperdipsia as it can be shown by offering pure water instead and finding out that the same drinker is in fact hypodipsic (Characterized by abnormally diminished thirst).1
Fluid balance of the two compartments
Maintaining a constant water and mineral balance requires the coordination of sensitive detectors at different sites in the body linked by neural pathways with integrative centers in the brain that process this information. These centers are also sensitive to humoral factors (neurohormones) produced for the adjustment of diuresis, natriuresis and blood pressure (angiotensin mineralocorticoids, vasopressin, atrial natriuretic factor). Instructions from the integrative centers to the “executive organs” (kidney, sweat glands and salivary glands) and to the part of the brain responsible for corrective actions such as drinking are conveyed by certain nerves in addition to the above mentioned substances.1
Most of the components of fluid balance are controlled by homeostatic mechanisms responding to the state of body water. These mechanisms are sensitive and precise, and are activated with deficits or excesses of water amounting to only a few hundred milliliters. A water deficit produces an increase in the ionic concentration of the extracellular compartment, which takes water from the intracellular compartment causing cells to shrink. This shrinkage is detected by two types of brain sensors, one controlling drinking and the other controlling the excretion of urine by sending a message to the kidneys mainly via the antidiuretic hormone vasopressin to produce a smaller volume of more concentrated urine.18 When the body contains an excess of water, the reverse processes occur: the lower ionic concentration of body fluids allows more water to reach the intracellular compartment. The cells imbibe, drinking is inhibited and the kidneys excrete more water.
The kidneys thus play a key role in regulating fluid balance. As discussed later, the kidneys function more efficiently in the presence of an abundant water supply. If the kidneys economize on water, producing a more concentrated urine, these is a greater cost in energy and more wear on their tissues. This is especially likely to occur when the kidneys are under stress, for example when the diet contains excessive amounts of salt or toxic substances that need to be eliminated. Consequently, drinking enough water helps protect this vital organ.
Regulatory drinking
Most drinking obeys signals of water deficit. Apart from urinary excretion, the other main fluid regulatory process is drinking, mediated through the sensation of thirst. There are two distinct mechanisms of physiological thirst: the intracellular and the extracellular mechanisms. When water alone is lost, ionic concentration increases. As a result, the intracellular space yields some of its water to the extracellular compartment. One again, the resulting shrinkage of cells is detected by brain receptors that send hormonal messages to induce drinking. This association with receptors that govern extracellular volume is therefore accompanied by an enhancement of salt appetite. Thus, people who have been sweating copiously prefer drinks that are relatively rich in Na+ salts rather than pure water. As previously mentioned, it is always important to supplement drinks with additional salt when excessive sweating is experienced.
The brain’s decision to start or stop drinking and to choose the appropriate drink is made before the ingested fluid can reach the intra- and extracellular compartments. The taste buds in the mouth send messages to the brain about the nature, and especially the salt of the ingested fluid, and neuronal responses are triggered as if the incoming water had already reached the bloodstream. These are the so-called anticipatory reflexes: they cannot be entirely “cephalic reflexes” because they arise from the gut as well as the mouth.1
The anterior hypothalamus and pre-optic area are equipped with osmo-receptors related to drinking. Neurons in these regions show enhanced firing when the inner milieu gets hyperosmotic. Their firing decreases when water is loaded in the carotid artery that irrigates the neurons. It is remarkable that the same decrease in firing in the same neurons takes place when the water load is applied on the tongue instead of being injected in the carotid artery. This anticipatory drop in firing is due to a mediation neural pathways departing from the mouth and by converging on to the neurons which simultaneously sense of the inner milieu (blood).
Non-regulatory drinking
Although everyone experiences thirst from time to time, it plays little day-to-day role in the control of water intake in healthy people living in temperate climates. We generally consume fluids not to quench our thirst, but as components of everyday foods (e.g. soup, milk), as beverages used as mild stimulants (tea, coffee) and for pure pleasure. As common example is alcohol consumption which can increase individual pleasure and stimulate social interaction. Drinks are also consumed for their energy content, as in soft drinks and milk, and are used in warm weather for cooling and in cold weather for warming. Such drinking seems also to be mediated through the taste buds, which communicate with the brain in a kind of “reward system” the mechanisms of which are just beginning to be understood. This bias in the way human beings rehydrate themselves may be advantageous because it allows water losses to be replaced before thirst-producing dehydration takes place. Unfortunately, this bias also carries some disadvantages. Drinking fluids other than water can contribute to an intake of caloric nutrients in excess of requirements, or in alcohol consumption that in some people may insidiously bring about dependence. For example, total fluid intake increased from 79 fluid ounces in 1989 to 100 fluid ounces in 2002 among US adults, all from caloric beverages.19
Effects of aging on fluid intake regulation
The thirst and fluid ingestion responses of older persons to a number of stimuli have been compared to those seen in younger persons.20 Following water deprivation older persons are less thirsty and drink less fluid compared to younger persons.21, 22 The decrease in fluid consumption is predominantly due to a decrease in thirst as the relationship between thirst and fluid intake is the same in young and old persons. Older persons drink insufficient water following fluid deprivation to replenish their body water deficit.23 When dehydrated older persons are offered a highly palatable selection of drinks, this also failed to result in an increased fluid intake.23 The effects of increased thirst in response to an osmotic load have yielded variable responses with one group reporting reduced osmotic thirst in older individuals24 and one failing to find a difference. In a third study, young individuals ingested almost twice as much fluid as old persons, despite the older subjects having a much higher serum osmolality.25
Overall these studies support small changes in the regulation of thirst and fluid intake with aging. Defects in both osmoreceptors and baroreceptors appear to exist as well as changes in the central regulatory mechanisms mediated by opioid receptors.26 Because of their low water reserves, it may be prudent for the elderly to learn to drink regularly when not thirsty and to moderately increase their salt intake when they sweat. Better education on these principles may help prevent sudden hypotension and stroke or abnormal fatigue can lead to a vicious circle and eventually hospitalization.
Thermoregulation
Hydration status is critical to the body’s process of temperature control. Body water loss through sweat is an important cooling mechanism in hot climates and in physical activity. Sweat production is dependent upon environmental temperature and humidity, activity levels, and type of clothing worn. Water losses via skin (both insensible perspiration and sweating) can range from 0.3 L/h in sedentary conditions to 2.0 L/h in high activity in the heat and intake requirements range from 2.5 to just over 3 L/d in adults under normal conditions, and can reach 6 L/d with high extremes of heat and activity.27, 28 Evaporation of sweat from the body results in cooling of the skin. However, if sweat loss is not compensated for with fluid intake, especially during vigorous physical activity, a hypohydrated state can occur with concomitant increases in core body temperature. Hypohydration from sweating results in a loss in electrolytes, as well as a reduction in plasma volume, and can lead to increased plasma osmolality. During this state of reduced plasma volume and increased plasma osmolality, sweat output becomes insufficient to offset increases in core temperature. When fluids are given to maintain euhydration, sweating remains an effective compensation for increased core temperatures. With repeated exposure to hot environments, the body adapts to heat stress, and cardiac output and stroke volume return to normal, sodium loss is conserved, and the risk for heat-stress related illness is reduced.29 Increasing water intake during this process of heat acclimatization will not shorten the time needed to adapt to the heat, but mild dehydration during this time may be of concern and is associated with elevations in cortisol, increased sweating, and electrolyte imbalances.29
Children and the elderly have differing responses to ambient temperature and different thermoregulatory concerns than healthy adults. Children in warm climates may be more susceptible to heat illness than adults due to greater surface area to body mass ratio, lower rate of sweating, and slower rate of acclimatization to the heat.30, 31 Children may respond to hypohydration during activity with a higher relative increase in core temperature than adults do,32 and sweat less, thus losing some of the benefits of evaporative cooling. However, it has been argued that children can dissipate a greater proportion of body heat via dry heat loss, and the concomitant lack of sweating provides a beneficial means of conserving water under heat stress.30 Elders, in response to cold stress, show impairments in thermoregulatory vasoconstriction and body water is shunted from plasma into the interstitial and intracellular compartments.33, 34 With respect to heat stress, water lost through sweating decreases water content of plasma, and the elderly are less able to compensate for increased blood viscosity.33 Not only do they have a physiological hypodipsia, but this can be exaggerated by central nervous system disease 35 and by dementia 36. In addition, illness and limitations in activities of daily living can further limit fluid intake. Coupled with reduced fluid intake, with advancing age there is a decrease in total body water. Older individuals have impaired renal fluid conservation mechanisms and, as noted above, have impaired responses to heat and cold stress 33, 34. All of these factors contribute to an increased risk of hypohydration and dehydration in the elderly.
II. PHYSIOLOGICAL EFFECTS OF DEHYDRATION
In this section, the role of water in health is generally characterized in terms of deviations from an ideal hydrated state, generally in comparison to dehydration. The concept of dehydration encompasses both the process of losing body water and also the state of dehydration. Much of the research on water and physical or mental functioning compares a euhydrated state, usually achieved by provision of water sufficient to overcome water loss, to a dehydrated state, which is achieved via withholding of fluids over time and during periods of heat stress or high activity. In general, provision of water is beneficial in those with a water deficit, but little research supports the notion that additional water in adequately hydrated individuals confers any benefit.
Physical performance
The role of water and hydration in physical activity, particularly in athletes and in the military, has been of considerable interest and is well-described in the scientific literature.37–39 During challenging athletic events, it is not uncommon for athletes to lose 6–10% of body weight in sweat loss, thus leading to dehydration if fluids have not been replenished. However, decrements in physical performance in athletes have been observed under much lower levels of dehydration, as little as 2%.38 Under relatively mild levels of dehydration, individuals engaging in rigorous physical activity will experience decrements in performance related to reduced endurance, increased fatigue, altered thermoregulatory capability, reduced motivation, and increased perceived effort.40, 41 Rehydration can reverse these deficits, and also reduce oxidative stress induced by exercise and dehydration.42 Hypohydration appears to have a more significant impact on high-intensity and endurance activity such as tennis43 and long-distance running44 than on anaerobic activities45 such as weight lifting or on shorter-duration activities, such as rowing.46
During exercise, individuals may not hydrate adequately when allowed to drink according to thirst.32 After periods of physical exertion, voluntary fluid intake may be inadequate to offset fluid deficits.1 Thus, mild to moderate dehydration can therefore persist for some hours after the conclusion of physical activity. Research in athletes suggests that, principally at the beginning of the season, they are at particular risk for dehydration due to lack of acclimatization to weather conditions or suddenly increased activity levels.47, 48 A number of studies show that performance in temperate and hot climates is affected to a greater degree than performance in cold temperatures.41, 49, 50 Exercise in hot conditions with inadequate fluid replacement is associated with hyperthermia, reduced stroke volume and cardiac output, decreases in blood pressure, and reduced blood flow to muscle.51
During exercise, children may be at greater risk for voluntary dehydration. Children may not recognize the need to replace lost fluids, and both children as well as coaches need specific guidelines for fluid intake.52 Additionally, children may require longer acclimation to increases in environmental temperature than do adults.30, 31 Recommendations are for child athletes or children in hot climates to begin athletic activities in a well-hydrated state and to drink fluids over and above the thirst threshold.
Cognitive performance
Water, or its lack (dehydration), can influence cognition. Mild levels of dehydration can produce disruptions in mood and cognitive functioning. This may be of special concern in the very young, very old, those in hot climates, and those engaging in vigorous exercise. Mild dehydration produces alterations in a number of important aspects of cognitive function such as concentration, alertness and short-term memory in children (10–12 y),32 young adults (18–25y)53–56 and in the oldest adults, 50–82y.57 As with physical functioning, mild to moderate levels of dehydration can impair performance on tasks such as short-term memory, perceptual discrimination, arithmetic ability, visuomotor tracking, and psychomotor skills.53–56 However, mild dehydration does not appear to alter cognitive functioning in a consistent manner.53, 54, 56, 58 In some cases, cognitive performance was not significantly affected in ranges from 2–2.6% dehydration.56, 58 Comparing across studies, performance on similar cognitive tests was divergent under dehydration conditions.54, 56 In studies conducted by Cian and colleagues,53, 54 participants were dehydrated to approximately 2.8% either through heat exposure or treadmill exercise. In both studies, performance was impaired on tasks examining visual perception, short-term memory, and psychomotor ability. In a series of studies using exercise in conjunction with water restriction as a means of producing dehydration, D’Anci and colleagues56 observed only mild decrements in cognitive performance in healthy young men and women athletes. In these experiments, the only consistent effect of mild dehydration was significant elevations of subjective mood score, including fatigue, confusion, anger, and vigor. Finally, in a study using water deprivation alone over a 24-h period, no significant decreases in cognitive performance were seen with 2.6% dehydration 58. It is possible therefore, that heat-stress may play a critical role in the effects of dehydration on cognitive performance.
Reintroduction of fluids under conditions of mild dehydration can reasonably be expected to reverse dehydration-induced cognitive deficits. Few studies have examined how fluid reintroduction may alleviate dehydration’s negative effects on cognitive performance and mood. One study59 examined how water ingestion affected arousal and cognitive performance in young people following a period of 12-h water restriction. While cognitive performance was not affected by either water restriction or water consumption, water ingestion affected self-reported arousal. Participants reported increased alertness as a function of water intake. Rogers and coworkers60 observed a similar increase in alertness following water ingestion in both high- and low-thirst participants. Water ingestion, however, had opposite effects on cognitive performance as a function of thirst. High-thirst participants’ performance on a cognitively demanding task improved following water ingestion, but low-thirst participants’ performance declined. In summary, hydration status consistently affected self-reported alertness, but effects on cognition were less consistent.
Several recent studies have examined the utility of providing water to school children on attentiveness and cognitive functioning in children.61–63 In these experiments, children were not fluid restricted prior to cognitive testing, but were allowed to drink as usual. Children were then provided with a drink or no drink 20–45 minutes before the cognitive test sessions. In the absence of fluid restriction and without physiological measures of hydration status, the children in these studies should not be classified as dehydrated. Subjective measures of thirst were reduced in children given water,62 and voluntary water intake in children varied from 57 ml to 250 ml. In these studies, as in the studies in adults, the findings were divergent and relatively modest. In the research led by Edmonds and colleagues,61, 62 children in the groups given water showed improvements in visual attention. However, effects on visual memory were less consistent, with one study showing no effects of drinking water on a spot-the-difference task in 6–7 year old children 61 and the other showing a significant improvement in a similar task in 7–9 year old children62 In the research described by Benton and Burgess,63 memory performance was improved by provision of water but sustained attention was not altered with provision of water in the same children.
Taken together these studies indicate that low to moderate dehydration may alter cognitive performance. Rather than indicating that the effects of hydration or water ingestion on cognition are contradictory, many of the studies differ significantly in methodology and in measurement of cognitive behaviors. These variances in methodology underscore the importance of consistency when examining relatively subtle chances in overall cognitive performance. However, in those studies in which dehydration were induced, most combined heat and exercise, thus it is difficult to disentangle the effects of dehydration on cognitive performance in temperate conditions, from the effects of heat and exercise. Additionally, relatively little is known about the mechanism of mild dehydration’s effects on mental performance. It has been proposed that mild dehydration acts as a physiological stressor which competes with and draws attention from cognitive processes64. However, research on this hypothesis is limited and merits further exploration.
Dehydration and delirium
Dehydration is a risk factor for delirium and delirium presenting as dementia in the elderly and in the very ill.65–67 Recent work shows that dehydration is one of several predisposing factors in observed confusion in long-term care residents,67 although in this study daily water intake was used as a proxy measure for dehydration rather than other, more direct clinical assessments such as urine or plasma osmolality. Older people have been reported as having reduced thirst and hypodypsia relative to younger people. In addition, fluid intake and maintenance of water balance can be complicated by factors such as disease, dementia, incontinence, renal insufficiency, restricted mobility, and drug side effects. In response to primary dehydration, older people have less thirst sensation and reduced fluid intakes in comparison to younger people. However, in response to heat stress, while older people still display a reduced thirst threshold, they do ingest comparable amounts of fluid as younger people.20
Gastrointestinal function
Fluids in the diet are generally absorbed in the proximal small intestine, and absorption rate is determined by the rate of gastric emptying to the small intestine. Therefore, the total volume of fluid consumed will eventually be reflected in water balance, but the rate at which rehydration occurs is dependent upon factors which affect the rate of delivery of fluids to the intestinal mucosa. Gastric emptying rate is generally accelerated by the total volume consumed and slowed by higher energy density and osmolality.68 In addition to water consumed in food (1 L/d) and beverages (~2–3 L/d), digestive secretions account for a far greater portion of water that passes through and is absorbed by the gastrointestinal tract (~8 L/d).69 The majority of this water is absorbed by the small intestine, with a capacity of up to 15 L/d with the colon absorbing some 5 L/d.69
Constipation, characterized by slow gastrointestinal transit, small, hard stools, and difficulty in passing stool, has a number of causes including medication use, inadequate fiber intake, poor diet, and illness.70 Inadequate fluid consumption is touted as a common culprit in constipation, and increasing fluid intake is a frequently recommended treatment. Evidence suggests, however, that increasing fluids is only of usefulness in individuals in a hypohydrated state, and is of little utility in euhydrated individuals.70 In young children with chronic constipation, increasing daily water intake by 50% did not affect constipation scores.71 For Japanese women with low fiber intake, concomitant low water intake in the diet is associated with increased prevalence of constipation.72 In older individuals, low fluid intake is a predictor for increased levels of acute constipation73, 74 with those consuming the least amount of fluid having over twice the frequency of constipation episodes than those consuming the most fluid. In one trial, researchers compared the utility of carbonated mineral water in reducing functional dyspepsia and constipation scores to tap water in individuals with functional dyspepsia.75 When comparing carbonated mineral water to tap water, participants reported improvements in subjective gastric symptoms, but there were no significant improvements in gastric or intestinal function. The authors indicate that it is not possible to determine to what degree the mineral content of the two waters contributed to perceived symptom relief, as the mineral water contained greater levels of magnesium and calcium than the tap water. The available evidence suggests that increased fluid intake should only be indicated in individuals in a hypohydrated state.71, 69
Significant water loss can occur through the gastrointestinal tract, and this can be of great concern in the very young. In developing countries, diarrheal diseases are a leading cause of death in children resulting in approximately 1.5–2.5 million deaths per year.76 Diarrheal illness results not only in a reduction in body water, but also in potentially lethal electrolyte imbalances. Mortality in such cases can many times be prevented with appropriate oral rehydration therapy, by which simple dilute solutions of salt and sugar in water can replace fluid lost by diarrhea. Many consider application of oral rehydration therapy to be one of the signal public health developments of the last century.77
Kidney function
As noted above, the kidney is crucial in regulating water balance and blood pressure as well as removing waste from the body. Water metabolism by the kidney can be classified into regulated and obligate. Water regulation is hormonally mediated, with the goal of maintaining a tight range of plasma osmolality between 275 to 290 mOsm/kg. Increases in plasma osmolality, and activation of osmoreceptors (intracellular) and baroreceptors (extracellular) stimulate hypothalamic release of arginine vasopressin (AVP). AVP acts at the kidney to decrease urine volume and promote retention of water, and the urine becomes hypertonic. With decreased plasma osmolality, vasopressin release is inhibited, and the kidney increases hypotonic urinary output.
In addition to regulating fluid balance, the kidneys require water for the filtration of waste from the blood stream and excretion via urine. Water excretion via the kidney removes solutes from the blood, and a minimum obligate urine volume is required to remove the solute load with a maximum output volume of 1 L/h.78 This obligate volume is not fixed, but is dependent upon the amount of metabolic solutes to be excreted and levels of AVP. Depending on the need for water conservation, basal urine osmolality ranges from 40 mOsm/kg up to a maximum of 1400 mOsm/kg.78 The ability to both concentrate and dilute urine decreases with age, with a lower value of 92 mOsm/kg and an upper range falling between 500–700 mOsm/kg for individuals over 70.79–81 Under typical conditions, in an average adult, urine volume of 1.5 to 2.0 L/d would be sufficient to clear a solute load of 900 to 1200 mOsm/d. During water conservation and the presence of AVP, this obligate volume can decrease to 0.75–1.0 L/d and during maximal diuresis can require up to 20 L/d to remove the same solute load.78, 80, 81 In cases of water loading, if the volume of water ingested cannot be compensated for with urine output, having overloaded the kidney’s maximal output rate, an individual can enter a hyponatremic state as described above.
Heart function and hemodynamic response
Blood volume, blood pressure, and heart rate are closely linked. Blood volume is normally tightly regulated by matching water intake and water output, as described in the section on kidney function. In healthy individuals, slight changes in heart rate and vasoconstriction act to balance the effect of normal fluctuations in blood volume on blood pressure.82 Decreases in blood volume can occur, through blood loss (or blood donation), or loss of body water through sweat, as seen with exercise. Blood volume is distributed differently relative to the position of the heart whether supine or upright, and moving from one position to the other can lead to increased heart rate, a fall in blood pressure and, in some cases, lead to syncope. This postural hypotension (or orthostatic hypotension) can be mediated by drinking 300–500 ml of water.83, 84 Water intake acutely reduces heart rate and increases blood pressure in both normotensive and hypertensive individuals.85 These effects of water intake on the pressor effect and heart rate occur within 15–20 minutes of drinking water and can last for up to 60 minutes. Water ingestion is also beneficial in preventing vasovagal reaction with syncope in blood donors at high risk for post-donation syncope.86 The effect of water drinking in these situations is thought to be due to effects on the sympathetic nervous system rather than to changes in blood volume.83, 84 Interestingly, in rare cases, individuals may experience bradycardia and syncope after swallowing cold liquids.87–89 While swallow syncope can be seen with other substances than water, swallow syncope further supports the notion that the result of water ingestion in the pressor effect has both a neural component as well as a cardiac component.
Headache
Water deprivation and dehydration can lead to the development of headache.90 Although this observation is largely unexplored in the medical literature, some observational studies indicate that water deprivation, in addition to impairing concentration and increasing irritability, can serve as a trigger for migraine and also prolong migraine.91, 92 In those with water deprivation-induced headache, ingestion of water provided relief from headache in most individuals within 30 min to 3 h.92 It is proposed that water deprivation-induced headache is the result of intracranial dehydration and total plasma volume. Although provision of water may be useful in relieving dehydration related headache, the utility of increasing water intake for the prevention of headache is less well documented.
The folk wisdom that drinking water can stave off headaches has been relatively unchallenged, and has more traction in the popular press than in the medical literature. Recently, one study examined increased water intake and headache symptoms in headache patients.93 In this randomized trial, patients with a history of different types of headache, including migraine and tension headache, were either assigned to a placebo condition (a non-drug tablet) or the increased water condition. In the water condition, participants were instructed to consume an additional volume of 1.5 L water/day on top of what they already consumed in foods and fluids. Water intake did not affect number of headache episodes, but was modestly associated with reduction in headache intensity and reduced duration of headache. The data from this study suggest that water is limited as prophylaxis in headache sufferers, and the ability of water to reduce or prevent headache in a broader population remains unknown.
Skin
One of the more pervasive myths regarding water intake is the improvement of the skin or complexion. By improvement, it is generally understood that individuals are seeking to have a more “moisturized” look to the surface skin, or to minimize acne or other skin conditions. Numerous lay sources such as beauty and health magazines as well as the Internet suggest that drinking 8–10 glasses of water a day will “flush toxins from the skin” and “give a glowing complexion” despite a general lack of evidence94, 95 to support these proposals. The skin, however, is important in maintaining body water levels and preventing water loss into the environment.
The skin contains approximately 30% water, which contributes to plumpness, elasticity, and resiliency. The overlapping cellular structure of the stratum corneum and lipid content of the skin serves as “waterproofing” for the body.96 Loss of water through sweat is not indiscriminate across the total surface of the skin, but is carried out by eccrine sweat glands, which are evenly distributed over most of the body surface.97 Skin dryness is usually associated with exposure to dry air, prolonged contact with hot water and scrubbing with soap (both strip oils from the skin), medical conditions and medications. While more serious levels of dehydration can be reflected in reduced skin turgor,98, 99 with tenting of the skin as a flag for dehydration, overt skin turgor in individuals with adequate hydration is not altered. Water intake, particularly in individuals with low initial water intake, can improve skin thickness and density as measured by sonogram,100 and offsets transepidermal water loss, and can improve skin hydration.101 Adequate skin hydration, however, is not sufficient to prevent wrinkles or other signs of aging, which are related to genetics, and sun and environmental damage. Of more utility to individuals already consuming adequate fluids, the use of topical emollients will improve skin barrier function and improve the look and feel of dry skin.102, 103
Hydration and chronic diseases
Many chronic diseases have multifactorial origins. In particular, differences in lifestyle and the impact of environment are known to be involved and constitute risk factors that are still being evaluated. Water is quantitatively the most important nutrient. In the past, scientific interest with regard to water metabolism was mainly directed toward the extremes of severe dehydration and water intoxication. There is evidence, however, that mild dehydration may also account for some morbidities.4, 104 There is currently no consensus on a “gold standard” for hydration markers, particularly for mild dehydration. As a consequence, the effects of mild dehydration on the development of several disorders and diseases have not been well documented.
There is strong evidence showing that good hydration reduces the risk of urolithiasis (See Table 2 for evidence categories). Less strong evidence links good hydration with reduced incidence of constipation, exercise asthma, hypertonic dehydration in the infant, and hyperglycemia in diabetic ketoacidosis. Good hydration is associated with a reduction in urinary tract infections, hypertension, fatal coronary heart disease, venous thromboembolism, and cerebral infarct but all these effects need to be confirmed by clinical trials. For other conditions such as bladder or colon cancer, evidence of a preventive effect of maintaining good hydration is not consistent (see Table 3).
Table 2.
Categories of evidence in evaluating the quality of reports
| Category | Classification | Description |
|---|---|---|
| Ia | Strong | Evidence from a meta-analysis of randomized, controlled trials |
| Ib | Strong | Evidence from at least one randomized, controlled trial |
| IIa | Less Strong | Evidence from at least one controlled study without randomization |
| IIb | Less Strong | Evidence from at least one other type of quasi-experimental study |
| III | Weaker | Evidence from descriptive studies, such as comparative studies, correlation studies, and case control studies |
| IV | Weaker | Evidence from expert committee reports, opinions or clinical experience of respected authorities, or both |
| Inconsistent | Evidence from small studies that are inconsistent in outcome | |
| Speculative | Posited relationships are based essentially on extrapolation from mechanism |
Adapted from Manz 104
Table 3.
Hydration Status and Chronic Diseases
| Chronic Disease | Evidence Level* | Findings |
|---|---|---|
| Urolithiasis | Strong | Increased urine volume from increased fluid intake reduces stone recurrence. Favorable associations between increased hydration status and lower stone recurrence rate. |
| Bronchopulmonary Disorders | Strong | Exercise related asthma is linked with low fluid intake.128 |
| Hypertonic Dehydration in Infants | Less strong | In infants with gastroenteritis, a high urine osmolality due to a high protein and sodium content of formula and weaning foods increases the risk of hypertonic dehydration.129 |
| Diabetic Hyper glycemia and Ketoacidosis | Less strong | In diabetics, experimentally induced dehydration promotes development of hyperglycemia.130 Higher serum osmolality at time of hospital admission was the most important predictor of death in children with diabetic ketoacidosis.131 |
| Morphological and Functional Changes in the Kidney | Weaker | In patients with polycystic kidney disease and chronic renal failure, sustained high urine volumes with urine osmolalities below plasma osmolality accelerate the decline of glomerular filtration rate.132 |
| Hypertension | Weaker | In diabetic patients, lower urine flow and sodium excretion rates are associated with higher blood pressure during the day and a reduced fall in blood pressure at night.133 In a study of 1688 healthy men, a low urine production day-to-night ratio was not associated with hypertension.134 In one study, eight male hypertensive volunteers and eight controls were exercised in a hot environment with or without water ingestion. In hypertensive men, water ingestion increased exercise-related differences in their systolic and diastolic blood pressure. |
| Fatal Coronary Heart Disease | Weaker | High intake of water is associated with lower risk of fatal heart disease.135 |
| Venous Thromboembolism | Weaker | High serum osmolality after stroke is associated with increased rate of thromboembolism.136 |
| Cerebral Infarct (Stroke) | Weaker | Increased serum osmolality or hematocrit is associated with increased risk of stroke morbidity/mortality.137, 138 Stroke patients with initial mid-range hematocrit have better discharge outcomes.139 |
| Dental Diseases | Weaker | Salivary output decreases with dehydration. Hypohydration may be linked with dental disease. |
| Urinary Tract Infection (UTI) | Weaker | Occurrence of UTI is associated with low fluid intake or low urine output.140, 141 No definitive evidence links susceptibility to UTI to fluid intake. |
| Bladder and Colon Cancer | Inconsistent | Generally show no association between fluid intake and cancer risk or tumor recurrence.142–144 |
| Gallstone | Speculative | Water intake induces gallbladder emptying suggestive that a high daily water intake may prevent gallstone formation.145 |
| Mitral Valve Prolapse | Speculative | Mitral valve prolapse developed after dehydration in one in 10 healthy men.146 |
| Glaucoma | Speculative | Dehydration reduces intraocular pressure and elevated colloid osmotic pressure.147 Intraocular pressure increases minutes after water ingestion and remains elevated above baseline for up to 45 minutes post-ingestion.148 |
Categories of evidence: Described in Table 2
III. Water consumption and requirements and relationships to total energy intake
Water consumption, water requirements and energy intake are linked in fairly complex ways. This is partially because physical activity and energy expenditures affect the need for water but also because a large shift in beverage consumption over the past century or more has led to consumption of a significant proportion of our energy intake from caloric beverages. Nonregulatory beverage intake, as noted earlier, has assumed a much greater role for individuals.19 In this section we first review current patterns of water intake, then refer to a full meta-analysis of the effects of added water on energy intake. This includes adding water to the diet and water replacement for a range of caloric and diet beverages, including sugar-sweetened beverages, juice, milk, and diet beverages. The third component is a discussion of water requirements and suggestions for considering the use of ml water/kcal energy intake as a metric.
A. Patterns and trends of water consumption
Measurement of total fluid water consumption in free-living individuals is fairly new in focus. As a result, the state of the science is poorly developed, data are most likely fairly incomplete, and adequate validation of the measurement techniques used are not available. We first present varying patterns and trends of water intake for the United States over the past three decades and review briefly the work on water intake in Europe.
We have really no information to allow us to assume that consumption of water alone or beverage containing water affects hydration differentially.3, 105 Some epidemiological data suggests water might have differential metabolic effects when consumed as water alone rather than water contained in caffeinated or flavored or sweetened beverages but these data are at best suggestive of an issue deserving of exploration.106, 107 We do show below from the research of Ershow that beverages not consisting of solely water do contain less than 100% water.
One study in the United States has attempted to examine all the sources of water in our diet.16, 17 These data are cited in Table 4 as the Ershow study and were based on National Food Consumption Survey food and fluid intake data from 1977–78. These data are presented in Table 4 for children 2–18 (Panel A) and for adults 19 and older (Panel B). Ershow and colleagues spent a great deal of time working out ways to convert USDA dietary data into water intake, including water absorbed during the cooking process, water in food, and all sources of drinking water.16, 17
Table 4.
Beverage Pattern Trends in the United States for Children aged 2–18 and Adults Aged 19 and older, Nationally Representative
| Panel A. The Amount of Beverages Consumed in Milliliters per Capita for Children | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1965 | Ershow | Original | 1988–1994 | 1989–1991 | 1994–1998 | 1999–2002 | 2005–2006 | |
| 1977–1978 | 1977–1978 | |||||||
| Water intrinsic to food | 393 | 393 | ||||||
| Water added during cooking | 95 | 95 | ||||||
| Water as a beverage | 624 | 624 | 835 | 520 | 531 | 715 | 552 | |
| Water added to other beverages | 186 | |||||||
| Water intrinsic to other beverages | 594 | |||||||
| Water total from all sources | 1892 | |||||||
| Unsweetened coffee & tea | 72 | 62 | 27 | 39 | 22 | 18 | 19 | |
| Low fat milk | 20 | 21 | 42 | 52 | 66 | 66 | 70 | |
| Diet | 5 | 11 | 30 | 31 | 34 | 25 | 41 | |
| Nutrients | 566 | 506 | 427 | 447 | 418 | 425 | 406 | |
| Caloric | 212 | 250 | 436 | 311 | 455 | 525 | 442 | |
| Total milliliters of beverages | 875 | 1961 | 1798 | 1400 | 1527 | 1774 | 1530 | |
|
Panel B. The Amount of Beverages Consumed in Milliliters per Capita for Adults | ||||||||
| 1965 | Ershow | Original | 1988–1994 | 1989–1991 | 1994–1998 | 1999–2002 | 2005–2006 | |
| 1977–1978 | 1977–1978 | |||||||
| Water intrinsic to food | 488 | 488 | ||||||
| Water added during cooking | 95 | 95 | ||||||
| Water as a beverage | 736 | 736 | 1248 | 792 | 856 | 1352 | 1127 | |
| Water added to other beverages | 630 | |||||||
| Water intrinsic to other beverages | 429 | |||||||
| Water total from all sources | 2377 | |||||||
| Unsweet coffee & tea | 640 | 544 | 551 | 484 | 464 | 428 | 452 | |
| Low fat milk | 14 | 18 | 54 | 53 | 57 | 68 | 62 | |
| Diet | 13 | 27 | 117 | 95 | 119 | 159 | 220 | |
| Nutrients | 306 | 328 | 419 | 317 | 340 | 431 | 458 | |
| Caloric | 136 | 201 | 418 | 264 | 402 | 549 | 474 | |
| Total milliliters of beverages | 1109 | 2437 | 2808 | 2005 | 2238 | 2987 | 2793 | |
Note: The data are age and sex adjusted to 1965; for 1977–78 the 488, 95, and 736 come from the Ershow calculations.
These created a number of categories and used a range of factors measured in other studies to estimate the water categories. The water that is found in food, based on food composition table data, was 393ml for children. The water that was added as a result of cooking (e.g., rice) was 95 ml. That consumed as a beverage directly as water was 624 ml. The water found in other fluids as noted comprised the remainder of the ml with the most water coming from whole fat milk and juices (506 ml). There is a small discrepancy between the Ershow total fluid intake measures for these children and that of the normal USDA figure. That is because USDA does not remove milk fats and solids, fiber and other food constituents found in beverages, particularly, juice and milk.
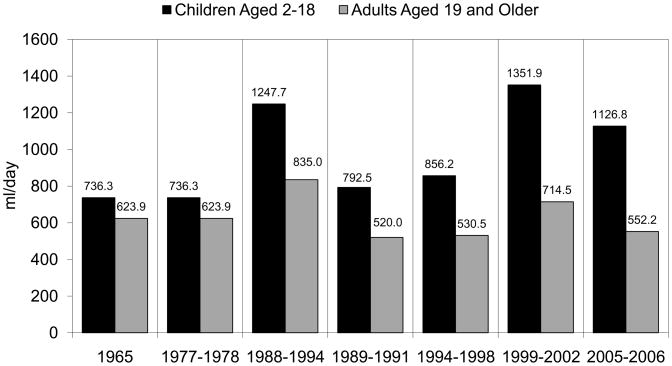
A key point to be seen in these nationally representative US data is enormous variability in the amount of water consumed between survey waves (see Figure 1 which highlights that large variation in water intake measured in these surveys). Although adult and child water intake moved up and down at the same time, for reasons we cannot explain, the variation is greater among children than adults. This is partly that the questions asked have varied and there has not been detailed probing for water intake as the focus has been on obtaining measures of macro-and micronutrients. Dietary survey methods in the past have focused on obtaining foods and beverages containing nutrient and nonnutritive sweeteners but not on water. Related are the huge differences between the NHANES 1988–1994 and 1999 and later surveys and the USDA surveys. In addition even the NHANES1999–2002 and the 2003–6 surveys differ greatly. This represents a shift in mode of questioning to inclusion of water intake as part of a standard 24-hour recall rather than as standalone questions. Water was not even measured in 1965, and the way the questions have been asked and the limitation on probes for water intake are very clear from a review of the questionnaires plus these data. Essentially in the past people were asked how much water they consumed in a day and now they are asked these questions as part of a 24-hour recall survey. However, unlike other caloric and diet beverages, there are limited probes for water. These must be viewed as crude approximations of total water intake without any strong research to show if they are over- or underestimated. We know from our own research with several studies of water and two on-going random controlled trials that probes that include consideration of all beverages including water as a separate item provide more complete data.
Figure 1.
Water Consumption Trends from USDA and NHANES Surveys (ml/day/capita), weighted to be nationally representative
Note: this includes water from fluids only, excluding water in foods. Sources for 1965, 77–78, 89- are USDA. Others are NHANES and 2005–6 is joint USDA and NHANES
Water consumption data for Europe are collected far more selectively than even the crude water intake questions from NHANES. The recent EFSA report provides measures of water consumption from a range of studies in Europe.4, 105, 108, 109 Essentially what these studies show is that total water intake is lower across Europe than the United States. As with the United States data, none are based on long-term carefully measured or even repeated 24-hour recall measures of water intake from food and beverages. In unpublished work Popkin and Jebb110 are examining water intake in UK adults in 1986–87 and 2001–2. Their intake increased by 226 ml/d over this time period but still is only 1787 ml/d in the latter period, far below the US figure for 2005–6 of 2793 or earlier figures for comparably aged adults.
There are a few studies in the US and Europe that utilize 24-hour urine and serum osmolality measures to determine total water turnover and this measure of hydration status. These studies suggest that US adults consume over 2100 ml of water per day while those from Europe consume less than a half liter or more.4, 111 Data on total urine collection would appear to be another useful measure for examining total water intake. Of course few studies aside from the Donald adolescent cohort in Germany have collected such data on population levels for large samples.109
B. The effects of water consumption on overall energy intake
There is an extensive literature that focuses on the impact of sugar-sweetened beverages on weight and risk of obesity, diabetes and heart disease; however the perspective of providing more water and its impact on health has not been examined. The water literature does not address portion sizes but rather focuses mainly on water ad libitum or in selected portions compared with other caloric beverages. Elsewhere we have prepared a detailed meta-analysis of the effects of water intake alone (adding additional water), replacing sugar-sweetened beverages, juice, milk and diet beverages.112
In general, the results of this review suggest that water, when replacing sugar-sweetened beverages, juice and milk is linked with reduced energy intake. This comes mainly from the clinical feeding studies but also from one very good random controlled school intervention and several other epidemiological and intervention studies. Aside from portion sizes, there are issues of timing of the beverage intake and meals (delay time from the beverage to the meal) and types of caloric sweeteners that remain to be considered. However when beverages are consumed in a normal free-living situations where 5–8 eating occasions are the norm, the delay time from the beverage to the meal may matter less.113–115
The literature on children is extremely limited as it relates to water intake. However, the excellent German school intervention with water would suggest the effects of water on overall energy intake of children might be comparable to that of adults.116 In this German study children were educated on the value of water and provided in school with special filtered drinking fountains and water bottles. The intervention school children increased by 1.1 glasses/day (P<0.001) and reduced their risk of overweight by 31% (OR=0.69, P=0.40).
C. Water requirements: Evaluation of the adequacy of water intake
Classically, water data are examined in terms of milliliters (or some other measure of water volume consumed per capita per day by age group). This measure does not link fluid and caloric intake. Disassociation of fluid and calorie intake difficult for clinicians dealing with an older person who has reduced caloric intake. This ml water measure assumes some mean body size (or surface area) and a mean level of physical activity – both determinants of not only energy expenditure, but also of water balance. Children are dependent on adults for access to water and studies suggest that a larger surface area to volume ratio makes them susceptible to changes in skin temperatures, linked with ambient temperature shifts.117 One option utilized by some scholars is to explore in ml/kcal of food and beverage intake as was done in the 1989 US RDAs.4, 118 This is an option interpretable for clinicians and does incorporate in some sense body size or surface area and activity. It has one disadvantage, namely that water as consumed with caloric beverages, affects both the numerator and denominator; however we do not know a measure that could be independent of this direct effect on body weight and/or total caloric intake.
Despite its critical importance in health and nutrition, as noted earlier, the array of available research that serves as a basis for determining requirements for water or fluid intake, or even rational recommendations for populations, is limited compared to most other nutrients. While this deficit may be partly explained by the highly sensitive set of neurophysiological adaptations and adjustments that occur over a large range of fluid intake to protect body hydration and osmolarity, this deficit remains a challenge for the nutrition and public health community. The latest official effort at recommending water intake for different subpopulations was a part of the Dietary Reference Intake process of the Institute of Medicine of the National Academy of Science’s Report on Dietary Reference Intakes on Water and Electrolytes as reported in 2005.3 As a graphic acknowledgement of the limited database upon which to express Estimated Average Requirements for water for different population groups, the Committee and the Institute of Medicine were forced to state “While it might appear useful to estimate an average requirement (an EAR) for water, an EAR based on data is not possible”. Given the extreme variability in water needs that are not solely based on differences in metabolism, but also on environmental conditions and activities, there is not a single level of water intake that would assure adequate hydration and optimum health for half of all apparently healthy persons in all environmental conditions. Thus, an Adequate Intake (AI) is established in place of an EAR for water.
The AI for different population groups was set as the median water intake in populations from the National Health and Nutrition Examination Survey, whose intake levels varied greatly based on the survey years (e.g., NHANES 1988–94 vs NHANES 1999–2002) and also were much higher than the USDA surveys (e.g., 1989–91, 1994–98, or 2005–6). If the AI for adults as expressed in Table 5 is taken as a recommended intake, we question the wisdom of the conversion of an AI into recommended water or fluid intake. The first problem is the almost certain inaccuracy of the fluid intake information from the national surveys, even though that problem may also exist for other nutrients. More importantly, from the standpoint of translating an AI into recommended fluid intake for individuals or populations, is the decision in setting the AI to add an additional roughly 20% of water intake, which is derived from some foods in addition to water and beverages. While this may be a legitimate effort to express total water intake as a basis for setting the AI, the recommendations that derive from this IOM report would better be directed at recommendations for water and other fluid intake on the assumption that the water content of foods would be a “passive” addition to total water intake. In this case, the observations of the DRI committee that water intake needs to meet needs imposed by not only metabolism and environmental conditions, but also body size, gender and physical activity. Those are the well studied factors which allow a rather precise measurement and determination of energy intake requirements. It is only logical that those same factors might underlie recommendations to meet water intake needs in the same populations and individuals, and therefore that consideration be given and data gathering be done by experimental and population research, to the possibility that water intake needs would best be expressed relative to the calorie requirements, as is done regularly in the clinical setting.
Table 5.
Water requirements expressed in relation to energy recommendations
| Sex | Age | Kcals/d Estimated Energy Requirement | Al for fluid intake (ml/d) | Ratio AI ml/d: EER Kcal/d |
|---|---|---|---|---|
| Child | 2–3 | 1000–1400 | 1300 | 0.93 |
| Female | 4–8 | 1400–1600 | 1700 | 1.06 |
| 9–13 | 1600–2000 | 2100 | 1.05 | |
| 14–18 | 2000 | 2300 | 1.15 | |
| 19–30 | 2000–2200 | 2700 | 1.23 | |
| 31–50 | 2000 | 2700 | 1.35 | |
| 50+ | 1800 | 2700 | 1.5 | |
| Male | 4–8 | 1400–1600 | 1700 | 1.06 |
| 9–13 | 1800–2000 | 2400 | 1.20 | |
| 14–18 | 2400–2800 | 3300 | 1.18 | |
| 19–30 | 2600–2800 | 3700 | 1.32 | |
| 31–50 | 2400–2600 | 3700 | 1.42 | |
| 50+ | 2200–2400 | 3700 | 1.54 |
AI for total fluids derived from dietary reference intakes for water, potassium, sodium, chloride, and sulfate
Ratios for water intake based on the AI for water in liters/day calculated using EER for each range of physical activity. EER adapted from the Institute of Medicine Dietary Reference Intakes Macronutrients Report, 2002.
It is important to note that only a few countries even include water on the list of nutrients.119 The European Food Safety Authority is developing a European wide standard.105 At present only the United States and Germany provide Adequate intake (AI) values but no other country does that.3, 120
Another way of considering an approach to the estimation of water requirements beyond the limited usefulness of the AI or estimated mean intake is to express water intake requirements in relation to energy requirements in ml/kcal. An argument for this approach includes the observation that energy requirements are strongly evidence-based in each age and gender group on extensive research which takes into account body size, and activity level which are crucial determinants of energy expenditure which must be met by dietary energy intake. Such measures of expenditure have used highly accurate methods such as doubly-labeled water and thus EERs (Estimated Energy Requirements) have been set based on solid data rather than the compromise inherent in the AIs for water. Those same determinants of energy expenditure and recommended intake are also applicable to water utilization and balance and this provides an argument for pegging water/fluid intake recommendations to the better studied energy recommendations. The extent to which water intake and requirements are determined by energy intake and expenditure is understudied but in the clinical setting it has long been practice to supply 1 ml per kcal administered by tube to patients unable to take in food or fluids. Factors such as fever or other drivers of increased metabolism affect both energy expenditure and fluid loss and are thus linked in clinical practice. This concept may well deserve consideration in the setting of population intake goals.
Finally, for decades there has been discussion of expressing nutrient requirements per 1000 kcal so that a single number would apply reasonable across the spectrum of age groups. This idea, which has never been adopted by the Institute of Medicine (IOM) and National Academy of Science, may lend itself to an improved expression of water/fluid intake requirements which must replace the AIs eventually. Table 5 presents the IOM water requirements and then develops a ratio of ml/kcal based on them. The European Food Safety Agency refers positively to the possibility of expressing water intake recommendations in ml/kcal as a function of energy requirements.105 Outliers in the adult male categories which reach ratios as high as 1.5 may well be based on American AI data which are above those in the more moderate and likely more accurate European recommendations.
Exploring the topic of utilizing ml/kcal as the way to examine water intake and water gaps, we take the full set of water intake AIs for each age-gender grouping and examine total intake in Table 6. They suggest a high level of fluid deficiency. Since a large proportion of fluids in the US are based on caloric beverages and this proportion has changed markedly over the past 30 years, fluid intake increases both the numerator and denominator of this ml/kcal relationship. Nevertheless even using 1 ml/kcal as the AI would leave a gap for all children and adolescents. We then utilize the NHANES physical activity data translated into METS/day to categorize all individuals by physical activity level and thus varying caloric requirements. Using these measures show a fairly large fluid gap, particularly for adult males as well as children.
Table 6.
Water intake and water intake gaps based on US Water Adequate Intake Recommendations (based on utilization of water and physical activity data from NHANES 2005–6.
| Sex | Age Group | Total ml | Ml/kcal | Gap Assuming 1 ml/kcal AI (liters) | Gaps based on actual METS activity (liters) | |||
|---|---|---|---|---|---|---|---|---|
| Sedentary | Moderate | Active | Total USa | |||||
| Child | 2–3 | 1076 | 0.76 | −0.38 | −0.82 | NA | NA | −0.82 |
| Female | 4–8 | 1085 | 0.67 | −0.63 | −1.35 | NA | NA | −1.35 |
| 9–13 | 1384 | 0.80 | −0.54 | −1.20 | −0.98 | −0.40 | −1.12 | |
| 14–18 | 1701 | 1.03 | −0.22 | −0.83 | −0.36 | 0.00 | −0.63 | |
| 19–30 | 2336 | 1.37 | 0.32 | −0.51 | 0.27 | 0.54 | −0.37 | |
| 31–50 | 2513 | 1.77 | 0.64 | −0.34 | 0.47 | 0.32 | −0.24 | |
| 51+ | 2219 | 1.49 | 0.58 | −0.60 | 0.29 | 0.11 | −0.51 | |
| Male | 4–8 | 1214 | 0.67 | −0.67 | −1.07 | NA | NA | −1.07 |
| 9–13 | 1523 | 0.74 | −0.67 | −1.45 | −0.75 | −0.96 | −1.35 | |
| 14–18 | 2450 | 0.94 | −0.44 | −2.04 | −1.2 | −0.86 | −1.52 | |
| 19–30 | 3189 | 1.21 | 0.29 | −1.30 | −0.65 | −0.60 | −1.09 | |
| 31–50 | 3361 | 1.28 | 0.48 | −1.54 | −0.65 | −0.09 | −1.37 | |
| 51+ | 2595 | 1.20 | 0.28 | −1.65 | −1.45 | −1.16 | −1.61 | |
| METS based proportion at each activity level | 85% | 7% | 8% | |||||
A weighted average for the proportion of individuals in each METS-based activity level
Note: Recommended water intake for actual activity level is the upper end of the range for moderate and active.
IV. DISCUSSION
This review has pointed out a number of issues related to water, hydration and health. As undoubtedly the most important nutrient and the only one whose absence will be lethal within days, understanding of water measurement and requirements are very important. The effects of water on daily performance and short and long-term health are quite clear. There are few negative effects of water intake and the evidence of positive effects is quite clear from the literature.
Little work has been done to measure total fluid intake systematically and there is no understanding of measurement error and best methods of understanding fluid intake. The most definitive US and European documents on total water requirements as based on these extant intake data.3, 105 We feel that absence of validation of methods for water consumption intake levels and patterns represents a major gap. Little exploration of even varying methods of probing to collect better water recall data have been conducted.
Of course, the other half of the issue is the need for understanding total hydration status. We have no acceptable biomarkers of hydration status at the population level. Controversy exists about current knowledge of hydration status among older Americans.6, 121 This represents a topic understudied at the population level though certainly scholars are focused on attempting to create biomarkers for measurement of hydration status.
As we have noted, the importance of understanding the role of fluid intake on health has emerged as a more important topic partially because of the shift toward large proportions of fluids coming from caloric beverages. We summarized briefly a related systematic review of the clinical, epidemiological and intervention literature on the effects of added water on health. As a replacement for SSB’s, juice, or whole milk there are clear effects in that energy intake is reduced by about 10–13% of total energy intake. However on these topics, there are only a few longer-term systematic interventions and no published random-controlled longer-term trials. There is very minimal evidence on the effects of just adding water to the diet and of replacing water with diet beverages.
Limitations to this review are many. One certainly is the omission of discussions of the issue of potential differences in the metabolic functioning of different types of beverages122. There is little basis at this point for delving into this sparse literature. Another is omission of the potential effects of fructose (from all caloric sweeteners) when consumed in caloric beverages on abdominal fat and subsequently all the metabolic conditions directly linked with this (e.g., diabetes).123–126 We do not review in any detail all the array of biomarkers being considered to measure hydration status as there is just no sense in the field today that there is a measurement that covers more than a very short time period except for 24-hour total urine collection.
We suggest some ways to examine water requirements as a means to encouraging more dialogue on this important topic. Given the importance of water to our health and of caloric beverages to our total energy intake and potential risks of nutrition-related non communicable diseases, understanding both the requirements for water related to energy requirements, and the differential effects of water vs. other caloric beverages remain important outstanding issues.
In the end, this review has attempted to provide some sense of the importance of water to our health, its role in relationship to the rapid increases of obesity and other related diseases, and our gaps in understanding measurement and requirements. Water is essential to our survival and to our civilizations’ and we hope this critical role will sharpen our focus on water in human health.
Acknowledgments
Funding was provided by the Nestlé Waters, Issy-les-Moulineaux, France, 5ROI AGI0436 from the National Institute on Aging Physical Frailty Program, and NIH R01- CA109831 and R01-CA121152. We also wish to thank Ms. Frances L. Dancy for administrative assistance, Mr. Tom Swasey for graphics support, Dr. Melissa Daniels for assistance, and Florence Constant (Nestle’s Water Research) for advice and references.
Contributor Information
Barry M. Popkin, Department of Nutrition, University of North Carolina, Chapel Hill, NC
Kristen E. D’Anci, Department of Psychology, Tufts University, Medford, MA
Irwin H. Rosenberg, Nutrition and Neurocognition Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, MA
References
- 1.Nicolaidis S. Physiology of thirst. In: Arnaud MJ, editor. Hydration Throughout Life. Montrouge: John Libbey Eurotext; 1998. p. 247. [Google Scholar]
- 2.Jequier E, Constant F. Water as an essential nutrient: the physiological basis of hydration. Eur J Clin Nutr. 2010;64:115–123. doi: 10.1038/ejcn.2009.111. [DOI] [PubMed] [Google Scholar]
- 3.Panel on Dietary Reference Intakes for Electrolytes and Water. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington DC: National Academy Press; 2005. [Google Scholar]
- 4.Manz F, Wentz A. Hydration status in the United States and Germany. Nutr Rev. 2005;63:S55–62. doi: 10.1111/j.1753-4887.2005.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 5.Scientific Opinion of the Panel on Dietetic Products Nutrition and Allergies. Draft Dietary reference values for water. The EFSA Journal. 2008:2–49. [Google Scholar]
- 6.Stookey JD. High prevalence of plasma hypertonicity among community-dwelling older adults: results from NHANES III. J Am Diet Assoc. 2005;105:1231–1239. doi: 10.1016/j.jada.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong LE. Hydration assessment techniques. Nutr Rev. 2005;63:S40–54. doi: 10.1111/j.1753-4887.2005.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 8.Bar-David Y, Urkin J, Kozminsky E. The effect of voluntary dehydration on cognitive functions of elementary school children. Acta Paediatr. 2005;94:1667–1673. doi: 10.1080/08035250500254670. [DOI] [PubMed] [Google Scholar]
- 9.Shirreffs S. Markers of hydration status. J Sports Med Phys Fitness. 2000;40:80–84. [PubMed] [Google Scholar]
- 10.Popowski L, Oppliger R, Lambert G, Johnson R, Johnson A, Gisolfi C. Blood and urinary measures of hydration status during progressive acute dehydration. Med Sci Sports Exerc. 2001;33:747–753. doi: 10.1097/00005768-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Bar-David Y, Landau D, Bar-David Z, Pilpel D, Philip M. Urine osmolality among elementary schoolchildren living in a hot climate: implications for dehydration. Ambulatory Child Health. 1998;4:393–397. [Google Scholar]
- 12.Fadda R, Rapinett G, Grathwohl D, Parisi M, Fanari R, Schmitt J. International Society for Developmental Psychobiology; 2008. Washington, DC: 2008. The benefits of drinking supplementary water at school on cognitive performance in children. [Google Scholar]
- 13.Eckhardt CL, Adair LS, Caballero B, et al. Estimating body fat from anthropometry and isotopic dilution: a four-country comparison. Obes Res. 2003;11:1553–1562. doi: 10.1038/oby.2003.207. [DOI] [PubMed] [Google Scholar]
- 14.Moreno LA, Sarria A, Popkin BM. The nutrition transition in Spain: a European Mediterranean country. Eur J Clin Nutr. 2002;56:992–1003. doi: 10.1038/sj.ejcn.1601414. [DOI] [PubMed] [Google Scholar]
- 15.Lee MJ, Popkin BM, Kim S. The unique aspects of the nutrition transition in South Korea: the retention of healthful elements in their traditional diet. Public Health Nutr. 2002;5:197–203. doi: 10.1079/PHN2001294. [DOI] [PubMed] [Google Scholar]
- 16.Ershow AG, Brown LM, Cantor KP. Intake of tapwater and total water by pregnant and lactating women. Am J Public Health. 1991;81:328–334. doi: 10.2105/ajph.81.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ershow AG, Cantor KP. Total Water and Tapwater Intake in the United States: Population-Based Estimates of Quantities and Sources. Bethesda, Md: FASEB/LSRO; 1989. [Google Scholar]
- 18.Ramsay DJ. Homeostatic control of water balance. In: Arnaud MJ, editor. Hydration Throughout Life. Montrouge: John Libbey Eurotext; 1998. pp. 9–18. [Google Scholar]
- 19.Duffey K, Popkin BM. Shifts in patterns and consumption of beverages between 1965 and 2002. Obesity. 2007;15:2739–2747. doi: 10.1038/oby.2007.326. [DOI] [PubMed] [Google Scholar]
- 20.Morley JE, Miller DK, Zdodowski C, Guitierrez B, Perry HM., III . Fluid intake, hydration and aging. In: Arnaud M, editor. Hydration throughout life: International conference Vittel (France) Montrouge: John Libbey Eurotext; 1998. p. 247. [Google Scholar]
- 21.Phillips PA, Rolls BJ, Ledingham JG, et al. Reduced thirst after water deprivation in healthy elderly men. N Engl J Med. 1984;311:753–759. doi: 10.1056/NEJM198409203111202. [DOI] [PubMed] [Google Scholar]
- 22.Mack GW, Weseman CA, Langhans GW, Scherzer H, Gillen CM, Nadel ER. Body fluid balance in dehydrated healthy older men: thirst and renal osmoregulation. J Appl Physiol. 1994;76:1615–1623. doi: 10.1152/jappl.1994.76.4.1615. [DOI] [PubMed] [Google Scholar]
- 23.Phillips PA, Johnston CI, Gray L. Disturbed fluid and electrolyte homoeostasis following dehydration in elderly people. Age Ageing. 1993;22:S26–33. doi: 10.1093/ageing/22.suppl_1.s26. [DOI] [PubMed] [Google Scholar]
- 24.Phillips PA, Bretherton M, Johnston CI, Gray L. Reduced osmotic thirst in healthy elderly men. Am J Physiol. 1991;261:R166–171. doi: 10.1152/ajpregu.1991.261.1.R166. [DOI] [PubMed] [Google Scholar]
- 25.Davies I, O’Neill PA, McLean KA, Catania J, Bennett D. Age-associated alterations in thirst and arginine vasopressin in response to a water or sodium load. Age Ageing. 1995;24:151–159. doi: 10.1093/ageing/24.2.151. [DOI] [PubMed] [Google Scholar]
- 26.Silver AJ, Morley JE. Role of the opioid system in the hypodipsia associated with aging. J Am Geriatr Soc. 1992;40:556–560. doi: 10.1111/j.1532-5415.1992.tb02102.x. [DOI] [PubMed] [Google Scholar]
- 27.Sawka MN, Latzka WA, Matott RP, Montain SJ. Hydration effects on temperature regulation. Int J Sports Med. 1998;19 (Suppl 2):S108–110. doi: 10.1055/s-2007-971971. [DOI] [PubMed] [Google Scholar]
- 28.Sawka MN, Cheuvront SN, Carter R., 3rd Human water needs. Nutr Rev. 2005;63:S30–39. doi: 10.1111/j.1753-4887.2005.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong LE, editor. Heat acclimatization: Internet Society for Sport Science. 1998. [Google Scholar]
- 30.Falk B, Dotan R. Children’s thermoregulation during exercise in the heat: a revisit. Appl Physiol Nutr Metab. 2008;33:420–427. doi: 10.1139/H07-185. [DOI] [PubMed] [Google Scholar]
- 31.Bytomski JR, Squire DL. Heat illness in children. Curr Sports Med Rep. 2003;2:320–324. doi: 10.1249/00149619-200312000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Bar-Or O, Dotan R, Inbar O, Rotshtein A, Zonder H. Voluntary hypohydration in 10- to 12-year-old boys. J Appl Physiol. 1980;48:104–108. doi: 10.1152/jappl.1980.48.1.104. [DOI] [PubMed] [Google Scholar]
- 33.Vogelaere P, Pereira C. Thermoregulation and aging. Rev Port Cardiol. 2005;24:747–761. [PubMed] [Google Scholar]
- 34.Thompson-Torgerson CS, Holowatz LA, Kenney WL. Altered mechanisms of thermoregulatory vasoconstriction in aged human skin. Exerc Sport Sci Rev. 2008;36:122–127. doi: 10.1097/JES.0b013e31817bfd47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller PD, Krebs RA, Neal BJ, McIntyre DO. Hypodipsia in geriatric patients. Am J Med. 1982;73:354–356. doi: 10.1016/0002-9343(82)90726-4. [DOI] [PubMed] [Google Scholar]
- 36.Albert SG, Nakra BR, Grossberg GT, Caminal ER. Drinking behavior and vasopressin responses to hyperosmolality in Alzheimer’s disease. Int Psychogeriatr. 1994;6:79–86. doi: 10.1017/s104161029400164x. [DOI] [PubMed] [Google Scholar]
- 37.Maughan RJ, Shirreffs SM, Watson P. Exercise, heat, hydration and the brain. J Am Coll Nutr. 2007;26:604S–612S. doi: 10.1080/07315724.2007.10719666. [DOI] [PubMed] [Google Scholar]
- 38.Murray B. Hydration and physical performance. J Am Coll Nutr. 2007;26:542S–548S. doi: 10.1080/07315724.2007.10719656. [DOI] [PubMed] [Google Scholar]
- 39.Sawka MN, Noakes TD. Does dehydration impair exercise performance? Med Sci Sports Exerc. 2007;39:1209–1217. doi: 10.1249/mss.0b013e318124a664. [DOI] [PubMed] [Google Scholar]
- 40.Montain SJ, Coyle EF. Influence of graded dehydration on hyperthermia and cardiovascular drift during exercise. J Appl Physiol. 1992;73:1340–1350. doi: 10.1152/jappl.1992.73.4.1340. [DOI] [PubMed] [Google Scholar]
- 41.Cheuvront SN, Carter R, 3rd, Sawka MN. Fluid balance and endurance exercise performance. Curr Sports Med Rep. 2003;2:202–208. doi: 10.1249/00149619-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Paik IY, Jeong MH, Jin HE, et al. Fluid replacement following dehydration reduces oxidative stress during recovery. Biochem Biophys Res Commun. 2009;383:103–107. doi: 10.1016/j.bbrc.2009.03.135. [DOI] [PubMed] [Google Scholar]
- 43.Kovacs MS. A review of fluid and hydration in competitive tennis. Int J Sports Physiol Perform. 2008;3:413–423. doi: 10.1123/ijspp.3.4.413. [DOI] [PubMed] [Google Scholar]
- 44.Cheuvront SN, Montain SJ, Sawka MN. Fluid replacement and performance during the marathon. Sports Med. 2007;37:353–357. doi: 10.2165/00007256-200737040-00020. [DOI] [PubMed] [Google Scholar]
- 45.Cheuvront SN, Carter R, 3rd, Haymes EM, Sawka MN. No effect of moderate hypohydration or hyperthermia on anaerobic exercise performance. Med Sci Sports Exerc. 2006;38:1093–1097. doi: 10.1249/01.mss.0000222838.74015.15. [DOI] [PubMed] [Google Scholar]
- 46.Penkman MA, Field CJ, Sellar CM, Harber VJ, Bell GJ. Effect of hydration status on high-intensity rowing performance and immune function. Int J Sports Physiol Perform. 2008;3:531–546. doi: 10.1123/ijspp.3.4.531. [DOI] [PubMed] [Google Scholar]
- 47.Bergeron MF, McKeag DB, Casa DJ, et al. Youth football: heat stress and injury risk. Med Sci Sports Exerc. 2005;37:1421–1430. doi: 10.1249/01.mss.0000174891.46893.82. [DOI] [PubMed] [Google Scholar]
- 48.Godek SF, Godek JJ, Bartolozzi AR. Hydration status in college football players during consecutive days of twice-a-day preseason practices. Am J Sports Med. 2005;33:843–851. doi: 10.1177/0363546504270999. [DOI] [PubMed] [Google Scholar]
- 49.Cheuvront SN, Carter R, 3rd, Castellani JW, Sawka MN. Hypohydration impairs endurance exercise performance in temperate but not cold air. J Appl Physiol. 2005;99:1972–1976. doi: 10.1152/japplphysiol.00329.2005. [DOI] [PubMed] [Google Scholar]
- 50.Kenefick RW, Mahood NV, Hazzard MP, Quinn TJ, Castellani JW. Hypohydration effects on thermoregulation during moderate exercise in the cold. Eur J Appl Physiol. 2004;92:565–570. doi: 10.1007/s00421-004-1079-4. [DOI] [PubMed] [Google Scholar]
- 51.Maughan RJ, Watson P, Shirreffs SM. Heat and cold: what does the environment do to the marathon runner? Sports Med. 2007;37:396–399. doi: 10.2165/00007256-200737040-00032. [DOI] [PubMed] [Google Scholar]
- 52.American Academy of Pediatrics. Climatic heat stress and the exercising child and adolescent. American Academy of Pediatrics. Committee on Sports Medicine and Fitness. Pediatrics. 2000;106:158–159. [PubMed] [Google Scholar]
- 53.Cian C, Barraud PA, Melin B, Raphel C. Effects of fluid ingestion on cognitive function after heat stress or exercise-induced dehydration. Int J Psychophysiol. 2001;42:243–251. doi: 10.1016/s0167-8760(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 54.Cian C, Koulmann PA, Barraud PA, Raphel C, Jimenez C, Melin B. Influence of variations of body hydration on cognitive performance. J Psychophysiol. 2000;14:29–36. [Google Scholar]
- 55.Gopinathan PM, Pichan G, Sharma VM. Role of dehydration in heat stress-induced variations in mental performance. Arch Environ Health. 1988;43:15–17. doi: 10.1080/00039896.1988.9934367. [DOI] [PubMed] [Google Scholar]
- 56.D’Anci KE, Vibhakar A, Kanter JH, Mahoney CR, Taylor HA. Voluntary dehydration and cognitive performance in trained college athletes. Percept Mot Skills. 2009;109:251–269. doi: 10.2466/PMS.109.1.251-269. [DOI] [PubMed] [Google Scholar]
- 57.Suhr JA, Hall J, Patterson SM, Niinisto RT. The relation of hydration status to cognitive performance in healthy older adults. Int J Psychophysiol. 2004;53:121–125. doi: 10.1016/j.ijpsycho.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 58.Szinnai G, Schachinger H, Arnaud MJ, Linder L, Keller U. Effect of water deprivation on cognitive-motor performance in healthy men and women. Am J Physiol Regul Integr Comp Physiol. 2005;289:R275–280. doi: 10.1152/ajpregu.00501.2004. [DOI] [PubMed] [Google Scholar]
- 59.Neave N, Scholey AB, Emmett JR, Moss M, Kennedy DO, Wesnes KA. Water ingestion improves subjective alertness, but has no effect on cognitive performance in dehydrated healthy young volunteers. Appetite. 2001;37:255–256. doi: 10.1006/appe.2001.0429. [DOI] [PubMed] [Google Scholar]
- 60.Rogers PJ, Kainth A, Smit HJ. A drink of water can improve or impair mental performance depending on small differences in thirst. Appetite. 2001;36:57–58. doi: 10.1006/appe.2000.0374. [DOI] [PubMed] [Google Scholar]
- 61.Edmonds CJ, Jeffes B. Does having a drink help you think? 6–7-Year-old children show improvements in cognitive performance from baseline to test after having a drink of water. Appetite. 2009;53:469–472. doi: 10.1016/j.appet.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Edmonds CJ, Burford D. Should children drink more water?: the effects of drinking water on cognition in children. Appetite. 2009;52:776–779. doi: 10.1016/j.appet.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 63.Benton D, Burgess N. The effect of the consumption of water on the memory and attention of children. Appetite. 2009;53:143–146. doi: 10.1016/j.appet.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 64.Cohen S. After effects of stress on human performance during a heat acclimatization regimen. Aviat Space Environ Med. 1983;54:709–713. [PubMed] [Google Scholar]
- 65.Culp KR, Wakefield B, Dyck MJ, Cacchione PZ, DeCrane S, Decker S. Bioelectrical impedance analysis and other hydration parameters as risk factors for delirium in rural nursing home residents. J Gerontol A Biol Sci Med Sci. 2004;59:813–817. doi: 10.1093/gerona/59.8.m813. [DOI] [PubMed] [Google Scholar]
- 66.Lawlor PG. Delirium and dehydration: some fluid for thought? Support Care Cancer. 2002;10:445–454. doi: 10.1007/s00520-001-0334-z. [DOI] [PubMed] [Google Scholar]
- 67.Voyer P, Richard S, Doucet L, Carmichael PH. Predisposing factors associated with delirium among demented long-term care residents. Clin Nurs Res. 2009;18:153–171. doi: 10.1177/1054773809333434. [DOI] [PubMed] [Google Scholar]
- 68.Leiper JB. Intestinal water absorption--implications for the formulation of rehydration solutions. Int J Sports Med. 1998;19 (Suppl 2):S129–132. doi: 10.1055/s-2007-971977. [DOI] [PubMed] [Google Scholar]
- 69.Ritz P, Berrut G. The importance of good hydration for day-to-day health. Nutr Rev. 2005;63:S6–13. doi: 10.1111/j.1753-4887.2005.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 70.Arnaud MJ. Mild dehydration: a risk factor of constipation? Eur J Clin Nutr. 2003;57:S88–S95. doi: 10.1038/sj.ejcn.1601907. [DOI] [PubMed] [Google Scholar]
- 71.Young RJ, Beerman LE, Vanderhoof JA. Increasing oral fluids in chronic constipation in children. Gastroenterol Nurs. 1998;21:156–161. doi: 10.1097/00001610-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 72.Murakami K, Sasaki S, Okubo H, et al. Association between dietary fiber, water and magnesium intake and functional constipation among young Japanese women. Eur J Clin Nutr. 2007;61:616–622. doi: 10.1038/sj.ejcn.1602573. [DOI] [PubMed] [Google Scholar]
- 73.Lindeman RD, Romero LJ, Liang HC, Baumgartner RN, Koehler KM, Garry PJ. Do elderly persons need to be encouraged to drink more fluids? J Gerontol A Biol Sci Med Sci. 2000;55:M361–365. doi: 10.1093/gerona/55.7.m361. [DOI] [PubMed] [Google Scholar]
- 74.Robson KM, Kiely DK, Lembo T. Development of constipation in nursing home residents. Dis Colon Rectum. 2000;43:940–943. doi: 10.1007/BF02237354. [DOI] [PubMed] [Google Scholar]
- 75.Cuomo R, Grasso R, Sarnelli G, et al. Effects of carbonated water on functional dyspepsia and constipation. Eur J Gastroenterol Hepatol. 2002;14:991–999. doi: 10.1097/00042737-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 76.Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81:197–204. [PMC free article] [PubMed] [Google Scholar]
- 77.Atia AN, Buchman AL. Oral rehydration solutions in non-cholera diarrhea: a review. Am J Gastroenterol. 2009;104:2596–2604. doi: 10.1038/ajg.2009.329. quiz 2605. [DOI] [PubMed] [Google Scholar]
- 78.Schoen EJ. Minimum urine total solute concentration in response to water loading in normal men. J Appl Physiol. 1957;10:267–270. doi: 10.1152/jappl.1957.10.2.267. [DOI] [PubMed] [Google Scholar]
- 79.Sporn IN, Lancestremere RG, Papper S. Differential diagnosis of oliguria in aged patients. N Engl J Med. 1962;267:130–132. doi: 10.1056/NEJM196207192670304. [DOI] [PubMed] [Google Scholar]
- 80.Brenner BM. In: Brenner and Rector’s The Kidney. 8. Brenner BM, editor. Philadelphia, PA: Saunders Elsevier; 2007. [Google Scholar]
- 81.Lindeman RD, Van Buren HC, Raisz LG. Osmolar renal concentrating ability in healthy young men and hospitalized patients without renal disease. N Engl J Med. 1960;262:1306–1309. doi: 10.1056/NEJM196006302622603. [DOI] [PubMed] [Google Scholar]
- 82.Shirreffs SM, Maughan RJ. Control of blood volume: Long term and short term regulation. In: Arnaud MJ, editor. Hydration Throughout Life. Montrouge: John Libbey Eurotext; 1998. pp. 31–39. [Google Scholar]
- 83.Schroeder C, Bush VE, Norcliffe LJ, et al. Water drinking acutely improves orthostatic tolerance in healthy subjects. Circulation. 2002;106:2806–2811. doi: 10.1161/01.cir.0000038921.64575.d0. [DOI] [PubMed] [Google Scholar]
- 84.Lu CC, Diedrich A, Tung CS, et al. Water ingestion as prophylaxis against syncope. Circulation. 2003;108:2660–2665. doi: 10.1161/01.CIR.0000101966.24899.CB. [DOI] [PubMed] [Google Scholar]
- 85.Callegaro CC, Moraes RS, Negrao CE, et al. Acute water ingestion increases arterial blood pressure in hypertensive and normotensive subjects. J Hum Hypertens. 2007;21:564–570. doi: 10.1038/sj.jhh.1002188. [DOI] [PubMed] [Google Scholar]
- 86.Ando SI, Kawamura N, Matsumoto M, et al. Simple standing test predicts and water ingestion prevents vasovagal reaction in the high-risk blood donors. Transfusion. 2009 doi: 10.1111/j.1537-2995.2009.02189.x. [DOI] [PubMed] [Google Scholar]
- 87.Brick JE, Lowther CM, Deglin SM. Cold water syncope. South Med J. 1978;71:1579–1580. doi: 10.1097/00007611-197812000-00040. [DOI] [PubMed] [Google Scholar]
- 88.Farb A, Valenti SA. Swallow syncope. Md Med J. 1999;48:151–154. [PubMed] [Google Scholar]
- 89.Casella F, Diana A, Bulgheroni M, et al. When water hurts. Pacing Clin Electrophysiol. 2009;32:e25–27. doi: 10.1111/j.1540-8159.2009.02521.x. [DOI] [PubMed] [Google Scholar]
- 90.Shirreffs SM, Merson SJ, Fraser SM, Archer DT. The effects of fluid restriction on hydration status and subjective feelings in man. Br J Nutr. 2004;91:951–958. doi: 10.1079/BJN20041149. [DOI] [PubMed] [Google Scholar]
- 91.Blau J. Water deprivation: a new migraine precipitant. Headache. 2005;45:757–759. doi: 10.1111/j.1526-4610.2005.05143_3.x. [DOI] [PubMed] [Google Scholar]
- 92.Blau JN, Kell CA, Sperling JM. Water-deprivation headache: a new headache with two variants. Headache. 2004;44:79–83. doi: 10.1111/j.1526-4610.2004.04014.x. [DOI] [PubMed] [Google Scholar]
- 93.Spigt MG, Kuijper EC, Schayck CP, et al. Increasing the daily water intake for the prophylactic treatment of headache: a pilot trial. Eur J Neurol. 2005;12:715–718. doi: 10.1111/j.1468-1331.2005.01081.x. [DOI] [PubMed] [Google Scholar]
- 94.Valtin H. “Drink at least eight glasses of water a day.” Really? Is there scientific evidence for “8 × 8”? Am J Physiol Regul Integr Comp Physiol. 2002;283:R993–1004. doi: 10.1152/ajpregu.00365.2002. [DOI] [PubMed] [Google Scholar]
- 95.Negoianu D, Goldfarb S. Just add water. J Am Soc Nephrol. 2008;19:1041–1043. doi: 10.1681/ASN.2008030274. [DOI] [PubMed] [Google Scholar]
- 96.Madison KC. Barrier function of the skin: “la raison d’etre” of the epidermis. J Invest Dermatol. 2003;121:231–241. doi: 10.1046/j.1523-1747.2003.12359.x. [DOI] [PubMed] [Google Scholar]
- 97.Champion RH, Burton JL, Ebling FJG, editors. Textbook of Dermatology (Rook) Oxford: Blackwell; 1992. [Google Scholar]
- 98.Vivanti A, Harvey K, Ash S, Battistutta D. Clinical assessment of dehydration in older people admitted to hospital: what are the strongest indicators? Arch Gerontol Geriatr. 2008;47:340–355. doi: 10.1016/j.archger.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 99.Colletti JE, Brown KM, Sharieff GQ, Barata IA, Ishimine P, Committee APEM. The Management of Children with Gastroenteritis and Dehydration in the Emergency Department. J Emerg Med. 2009 doi: 10.1016/j.jemermed.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 100.Williams S, Krueger N, Davids M, Kraus D, Kerscher M. Effect of fluid intake on skin physiology: distinct differences between drinking mineral water and tap water. Int J Cosmet Sci. 2007;29:131–138. doi: 10.1111/j.1467-2494.2007.00366.x. [DOI] [PubMed] [Google Scholar]
- 101.Mac-Mary S, Creidi P, Marsaut D, et al. Assessment of effects of an additional dietary natural mineral water uptake on skin hydration in healthy subjects by dynamic barrier function measurements and clinic scoring. Skin Res Technol. 2006;12:199–205. doi: 10.1111/j.0909-752X.2006.00160.x. [DOI] [PubMed] [Google Scholar]
- 102.Warner RR, Stone KJ, Boissy YL. Hydration disrupts human stratum corneum ultrastructure. J Invest Dermatol. 2003;120:275–284. doi: 10.1046/j.1523-1747.2003.12046.x. [DOI] [PubMed] [Google Scholar]
- 103.Loden M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am J Clin Dermatol. 2003;4:771–788. doi: 10.2165/00128071-200304110-00005. [DOI] [PubMed] [Google Scholar]
- 104.Manz F, Wentz A. The importance of good hydration for the prevention of chronic diseases. Nutr Rev. 2005;63:S2–5. doi: 10.1111/j.1753-4887.2005.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 105.Panel on Dietetic Products Nutrition and Allergies. Dietary reference values for water Scientific Opinion of the Panel on Dietetic Products, Nutrition and Allergies (Question No EFSA-Q-2005-015a) The EFSA Journal. 2009 [Google Scholar]
- 106.Stookey JD, Constant F, Gardner C, Popkin B. Replacing sweetened caloric beverages with drinking water is associated with lower energy intake. Obesity. 2007;15:3013–3022. doi: 10.1038/oby.2007.359. [DOI] [PubMed] [Google Scholar]
- 107.Stookey JD, Constant F, Gardner C, Popkin BM. Drinking water is associated with weight loss. Obesity. 2008;16:2481–2488. doi: 10.1038/oby.2008.409. [DOI] [PubMed] [Google Scholar]
- 108.Turrini A, Saba A, Perrone D, Cialfa E, D’Amicis D. Food consumption patterns in Italy: the INN-CA Study 1994–1996. Eur J Clin Nutr. 2001;55:571–588. doi: 10.1038/sj.ejcn.1601185. [DOI] [PubMed] [Google Scholar]
- 109.Sichert-Hellert W, Kersting M, Manz F. Fifteen year trends in water intake in German children and adolescents: results of the DONALD Study. Dortmund Nutritional and Anthropometric Longitudinally Designed Study. Acta Paediatr. 2001;90:732–737. [PubMed] [Google Scholar]
- 110.Popkin BM, Jebb S. Beverage patterns and trends in the United Kingdom. University of North Carolina; Chapel Hill: 2010. [Google Scholar]
- 111.Raman A, Schoeller DA, Subar AF, et al. Water turnover in 458 American adults 40–79 yr of age. Am J Physiol Renal Physiol. 2004;286:F394–401. doi: 10.1152/ajprenal.00295.2003. [DOI] [PubMed] [Google Scholar]
- 112.Daniels MC, Popkin BM. The impact of water intake on energy intake and weight status: a review. Nutr Rev. 2009 doi: 10.1111/j.1753-4887.2010.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Piernas C, Popkin B. Snacking trends in U.S. adults between 1977 and 2006. doi: 10.3945/jn.109.112763. (Under Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Piernas C, Popkin BM. Trends in snacking among U.S. children. Health Affairs. 2010;29:398–404. doi: 10.1377/hlthaff.2009.0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Popkin B, Duffey K. Does the physiological basis for eating exist any more? Continuous caloric intake is becoming the norm. Under Review. [Google Scholar]
- 116.Muckelbauer R, Libuda L, Clausen K, Toschke AM, Reinehr T, Kersting M. Promotion and provision of drinking water in schools for overweight prevention: randomized, controlled cluster trial. Pediatrics. 2009;123:e661–667. doi: 10.1542/peds.2008-2186. [DOI] [PubMed] [Google Scholar]
- 117.Bar-or O. Temperature regulation during exercise in children and aolescents. In: Gisolfi C, Lamb DR, editors. Youth, exercise, and sport: Symposium: Papers and discussions; 1989; Indianapolis: Benchmark; 1989. pp. 335–367. [Google Scholar]
- 118.National Research Council. Recommended dietary allowances. Washington D.C: National Academy Press; 1989. [Google Scholar]
- 119.Prentice A, Branca F, Decsi T, et al. Energy and nutrient dietary reference values for children in Europe: methodological approaches and current nutritional recommendations. Br J Nutr. 2004;92 (Suppl 2):S83–146. doi: 10.1079/bjn20041159. [DOI] [PubMed] [Google Scholar]
- 120.German Nutrition Society Austrian Nutrition Society Swiss Society for nutrition research swiss nutrition association. Reference Values for Nutrient Intake (in English) 1. Frankfurt: Umschau Brau; 2002. [Google Scholar]
- 121.Stookey JD, Pieper CF, Cohen HJ. Is the prevalence of dehydration among community-dwelling older adults really low? Informing current debate over the fluid recommendation for adults aged 70+years. Public Health Nutr. 2005;8:1275–1285. doi: 10.1079/phn2005829. [DOI] [PubMed] [Google Scholar]
- 122.Malik V, Popkin B, Bray G, Depres J-P, Willett W, Hu F, editors. Dept of Nutrition. Harvard SPH; Boston: 2009. Sugar Sweetened Beverages and Cardiometabolic Risk: A Meta-analysis. [Google Scholar]
- 123.Teff KL, Grudziak J, Townsend RR, et al. Endocrine and Metabolic Effects of Consuming Fructose- and Glucose-Sweetened Beverages with Meals in Obese Men and Women: Influence of Insulin Resistance on Plasma Triglyceride Responses. J Clin Endocrinol Metab. 2009;94:1562–1569. doi: 10.1210/jc.2008-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stanhope KL. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Investigat. 2009;119:1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stanhope KL, Havel PJ. Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose, or high-fructose corn syrup. Am J Clin Nutr. 2008;88:1733S–1737. doi: 10.3945/ajcn.2008.25825D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stanhope KL, Griffen SC, Bair BR, Swarbrick MM, Keim NL, Havel PJ. Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. Am J Clin Nutr. 2008;87:1194–1203. doi: 10.1093/ajcn/87.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Altman P. Blood and Other Body Fluids. Washington DC: Federation of American Societies for Experimental Biology; 1961. [Google Scholar]
- 128.Kalhoff H. Mild dehydration: a risk factor of broncho-pulmonary disorders? Eur J Clin Nutr. 2003;57 (Suppl 2):S81–87. doi: 10.1038/sj.ejcn.1601906. [DOI] [PubMed] [Google Scholar]
- 129.Taitz LS, Byers HD. High calorie-osmolar feeding and hypertonic dehydration. Arch Dis Child. 1972;47:257–260. doi: 10.1136/adc.47.252.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Burge MR, Garcia N, Qualls CR, Schade DS. Differential effects of fasting and dehydration in the pathogenesis of diabetic ketoacidosis. Metabolism. 2001;50:171–177. doi: 10.1053/meta.2001.20194. [DOI] [PubMed] [Google Scholar]
- 131.Jayashree M, Singhi S. Diabetic ketoacidosis: predictors of outcome in a pediatric intensive care unit of a developing country. Pediatr Crit Care Med. 2004;5:427–433. doi: 10.1097/01.pcc.0000137987.74235.5e. [DOI] [PubMed] [Google Scholar]
- 132.Hebert LA, Greene T, Levey A, Falkenhain ME, Klahr S. High urine volume and low urine osmolality are risk factors for faster progression of renal disease. Am J Kidney Dis. 2003;41:962–971. doi: 10.1016/s0272-6386(03)00193-8. [DOI] [PubMed] [Google Scholar]
- 133.Bankir L, Bardoux P, Mayaudon H, Dupuy O, Bauduceau B. Impaired urinary flow rate during the day: a new factor possibly involved in hypertension and in the lack of nocturnal dipping. Arch Mal Coeur Vaiss. 2002;95:751–754. [PubMed] [Google Scholar]
- 134.Blanker MH, Bernsen RM, Ruud Bosch JL, et al. Normal values and determinants of circadian urine production in older men: a population based study. J Urol. 2002;168:1453–1457. doi: 10.1016/S0022-5347(05)64472-2. [DOI] [PubMed] [Google Scholar]
- 135.Chan J, Knutsen SF, Blix GG, Lee JW, Fraser GE. Water, other fluids, and fatal coronary heart disease: the Adventist Health Study. Am J Epidemiol. 2002;155:827–833. doi: 10.1093/aje/155.9.827. [DOI] [PubMed] [Google Scholar]
- 136.Kelly J, Hunt BJ, Lewis RR, et al. Dehydration and venous thromboembolism after acute stroke. QJM. 2004;97:293–296. doi: 10.1093/qjmed/hch050. [DOI] [PubMed] [Google Scholar]
- 137.Bhalla A, Sankaralingam S, Dundas R, Swaminathan R, Wolfe CD, Rudd AG. Influence of raised plasma osmolality on clinical outcome after acute stroke. Stroke. 2000;31:2043–2048. doi: 10.1161/01.str.31.9.2043. [DOI] [PubMed] [Google Scholar]
- 138.Longo-Mbenza B, Phanzu-Mbete LB, M’Buyamba-Kabangu JR, et al. Hematocrit and stroke in black Africans under tropical climate and meteorological influence. Ann Med Interne (Paris) 1999;150:171–177. [PubMed] [Google Scholar]
- 139.Diamond PT, Gale SD, Evans BA. Relationship of initial hematocrit level to discharge destination and resource utilization after ischemic stroke: a pilot study. Arch Phys Med Rehabil. 2003;84:964–967. doi: 10.1016/s0003-9993(03)00009-1. [DOI] [PubMed] [Google Scholar]
- 140.Mazzola BL, von Vigier RO, Marchand S, Tonz M, Bianchetti MG. Behavioral and functional abnormalities linked with recurrent urinary tract infections in girls. J Nephrol. 2003;16:133–138. [PubMed] [Google Scholar]
- 141.Wilde MH, Carrigan MJ. A chart audit of factors related to urine flow and urinary tract infection. J Adv Nurs. 2003;43:254–262. doi: 10.1046/j.1365-2648.2003.02708.x. [DOI] [PubMed] [Google Scholar]
- 142.Altieri A, La Vecchia C, Negri E. Fluid intake and risk of bladder and other cancers. Eur J Clin Nutr. 2003;57 (Suppl 2):S59–68. doi: 10.1038/sj.ejcn.1601903. [DOI] [PubMed] [Google Scholar]
- 143.Donat SM, Bayuga S, Herr HW, Berwick M. Fluid intake and the risk of tumor recurrence in patients with superficial bladder cancer. J Urol. 2003;170:1777–1780. doi: 10.1097/01.ju.0000091803.35049.da. [DOI] [PubMed] [Google Scholar]
- 144.Radosavljevic V, Jankovic S, Marinkovic J, Djokic M. Fluid intake and bladder cancer. A case control study. Neoplasma. 2003;50:234–238. [PubMed] [Google Scholar]
- 145.Math MV, Rampal PM, Faure XR, Delmont JP. Gallbladder emptying after drinking water and its possible role in prevention of gallstone formation. Singapore Med J. 1986;27:531–532. [PubMed] [Google Scholar]
- 146.Aufderheide S, Lax D, Goldberg SJ. Gender differences in dehydration-induced mitral valve prolapse. Am Heart J. 1995;129:83–86. doi: 10.1016/0002-8703(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 147.Martin B, Harris A, Hammel T, Malinovsky V. Mechanism of exercise-induced ocular hypotension. Invest Ophthalmol Vis Sci. 1999;40:1011–1015. [PubMed] [Google Scholar]
- 148.Brucculeri M, Hammel T, Harris A, Malinovsky V, Martin B. Regulation of intraocular pressure after water drinking. J Glaucoma. 1999;8:111–116. [PubMed] [Google Scholar]