Abstract
Hospitalized infants are exposed to numerous devices containing the plasticizer di-(2-ethylhexyl) phthalate. Urinary levels of the phthalate metabolite, mono-(2-ethylhexyl) phthalate (MEHP), are markedly elevated in premature infants. Phthalates inactivate PPAR-γ, a nuclear transcription factor that mediates the resolution of inflammation, a process impaired in neonates. We speculate that this increases their susceptibility to MEHP, and this was analyzed. MEHP inhibited neutrophil apoptosis; neonatal cells were more sensitive than adult cells. In neonatal, but not in adult neutrophils, MEHP also inhibited chemotaxis, stimulated oxidative metabolism, and upregulated expression of NADPH oxidase-1. In both adult and neonatal neutrophils, MEHP stimulated IL-1β and VEGF production, while IL-8 production was stimulated only in adult cells. In contrast, MEHP inhibited production of MIP-1β by adult cells, and RANTES by neonatal neutrophils. The effects of MEHP on apoptosis and oxidative metabolism in neonatal cells were reversed by the PPAR-γ agonist, troglitazone. Whereas troglitazone had no effect on MEHP-induced alterations in inflammatory protein or chemokine production, constitutive IL-8 and MIP-1β production was reduced in adult neutrophils, and RANTES and MIP-1β in neonatal cells. These findings suggest that neonatal neutrophils are more sensitive to phthalate-mediated inhibition of PPAR-γ, which may be related to decreased anti-inflammatory signaling.
INTRODUCTION
Di-(2-ethylhexyl) phthalate (DEHP) is the only plasticizer approved by the U.S. Food and Drug Administration for medical use. Consequently, most polyvinyl chloride (PVC)-rich medical devices contain DEHP. The release of DEHP from PVC occurs at a rate that depends on temperature, storage time, flow rates of solution through tubing, the percentage of DEHP present, and the lipophilic nature of the solution in contact with the PVC plastic (1, 2). Because hospitalized infants are administered fluids and medications through DEHP-containing tubing and catheters, their level of exposure to phthalates is significantly greater than any other population. In this regard, urinary metabolites of DEHP, which are indicators of internal exposure to phthalates, are several-fold higher in hospitalized infants than in the general pediatric population (3). Neonates also have reduced renal clearance, which may result in even greater body burden of DEHP. DEHP is rapidly metabolized in blood and tissues to several biologically active metabolites, including mono-(2-ethylhexyl) phthalate (MEHP) (4, 5).
In addition to well-documented adverse effects on development and reproduction (6–9), DEHP and its metabolites have been shown to exhibit pro-inflammatory activity. Thus, DEHP upregulates CD11b expression on neutrophils (10) and stimulates the release of lysosomal enzymes and interleukin-1 by mononuclear cells (11, 12). Elevated phthalate levels during pregnancy have been reported to cause placental inflammation, increasing the risk of preterm delivery (13). These findings raise particular concerns in hospitalized premature neonates, who are developmentally susceptible to chronic inflammatory diseases such as bronchopulmonary dysplasia and necrotizing enterocolitis (14). The Food and Drug Administration has suggested a potential link between exposure to phthalates and the development of lung disease in premature infants (15, 16), and the American Academy of Pediatrics has expressed concern about DEHP exposure in children, calling for further study to quantify exposure and examine its health effects (17). Towards this goal, the present studies compared the effects of MEHP on inflammatory activity, apoptosis, and antioxidant expression in adult and neonatal neutrophils, and the potential role of PPAR-γ on these responses.
MATERIALS AND METHODS
Reagents
DMEM, dextran, N-formyl-methionyl-leucyl-phenylalanine (fMLP), TgT, BSA and Hanks balanced salt solution (HBSS) were purchased from Sigma Chemical Co. (St. Louis, MO). Ficoll-paque was from GE Healthcare (Piscataway, NJ). Annexin V-APC, 7-actinomycin D (7-AAD) and cytometric bead array flex sets were from BD Biosciences (San Jose, CA). MEHP was from TCI America (Portland, OR) and Amplex Red and horseradish peroxidase from Molecular Probes (Carlsbad, CA).
Subjects and neutrophil isolation
All studies were approved by the Institutional Review Board of UMDNJ-RWJ Medical School and informed consent obtained. Umbilical cord blood was obtained from healthy term infants (≥ 37 weeks gestation) delivered by elective cesarean section prior to labor between January 2007 and April 2009. Subjects were excluded with clinical evidence of chorioamnionitis or other perinatal infections (maternal fever, uterine tenderness, or foul-smelling amniotic fluid). Subjects experiencing labor were excluded because labor is associated with an inflammatory phenotype in neonatal neutrophils (18). Neutrophils were isolated by dextran sedimentation, followed by Ficoll gradient centrifugation and hypotonic lysis of erythrocytes (19). Peripheral venous blood neutrophils were collected from antecubital veins of healthy adult volunteers.
Chemotaxis
A 96-well microchemotaxis chamber was used to assess cellular migration (20). HBSS (30 µl) containing 0.5% BSA and 2.4 mg/ml HEPES (HBSS/BSA), with or without fMLP (5 × 10−8 M) or MEHP (500 µM), were placed in each well of the lower chamber. A 5 µm pore-size polycarbonate filter was then placed over the wells and the upper chamber set into place. Fifty µl of neutrophils (1 × 105 cells) were added to the upper wells. After incubation for 45 min at 37°C, the filter containing adhered, migrated neutrophils was removed and stained with Wright-Giemsa. Chemotaxis was quantified as the number of cells that migrated through the filter in 10 oil immersion fields. Neutrophils were treated with MEHP (500 µM), TgT (2 µM), MEHP + TgT, or medium control for 1 hr prior to the chemotaxis assay.
Apoptosis
Neutrophils were resuspended in DMEM containing 10% fetal bovine serum (1 × 106 cells/ml), and incubated with MEHP (100–500 µM), TgT (2 µM) or PBS control for 24 hr at 37°C. Cells were then suspended in Annexin binding buffer and incubated with Annexin V (1:20) and 7-AAD (1:10) (15 min, room temperature). Cells were analyzed by flow cytometry on a FACS Array Bioanalyzer (BD Biosciences, San Jose, CA). Viable, apoptotic and necrotic neutrophil populations were gated electronically and data analyzed using quadrant statistics based on relative Annexin V and 7-AAD fluorescence (21).
Hydrogen Peroxide (H2O2) Production
Neutrophils were plated in 96-well dishes (5 × 104 cells/well). A reaction mixture (50 µl) containing Amplex Red (25 µM) and horseradish peroxidase (1.07 U/ml) was added to each well, followed by MEHP (500 µM) or PBS control, with or without TgT (2 µM). Fluorescent product was measured spectrophotometrically at 1 min intervals for 30 min at 540 nm excitation and 590 nm emission on a Perkin Elmer HTS 7000 Bio Assay Reader (22).
RNA expression
Neutrophils were incubated with control, MEHP (500 µM), and/or TgT (2 µM) for 4 hr. RNA was isolated using an RNeasy Mini kit (Qiagen). cDNA was synthesized using a High Capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Real time PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems) and amplified on an ABI Prism 7900 sequence detection system, using GAPDH as standard. Full-length coding sequences were obtained from GenBank™. Primers were designed using Primer Express software (Applied Biosystems). Forward and reverse primers used were: GAPDH, tgggctacactgagcaccag and gggtgtcgctgttgaagtca; superoxide dismutase (SOD), gtcgtagtctcctgcagcgtc and ctggttccgaggactgcaa; catalase, cggagattcaacactgccaa and gaatgcccgcacctgagtaa; and NOX1, ccttgcaccggtcattcttt and cggtaaaaccggaggatcct.
Inflammatory proteins
Culture supernatants were incubated with premixed flex set beads coated with antibodies to IL-1β, IL-6, RANTES, VEGF, MIP-1β and IL-8 for 1 hr in 96-well filtration plates. Premixed phycoerythrin-labeled detection reagent was then added to the wells. The plates were incubated at room temperature in the dark for 2 hr. Bead/protein complexes were then washed and analyzed for fluorescence intensity using a BD FACS Array Bioanalyzer. Data were analyzed using BD FCAP software (v 2.0) with 5 parameter curve fitting.
Statistical analysis
Experiments were repeated 6–15 times. Data were analyzed using Statistica 5.5 (StatSoft, Inc., Tulsa, OK). The effects of treatments were compared pairwise by t-test.
RESULTS
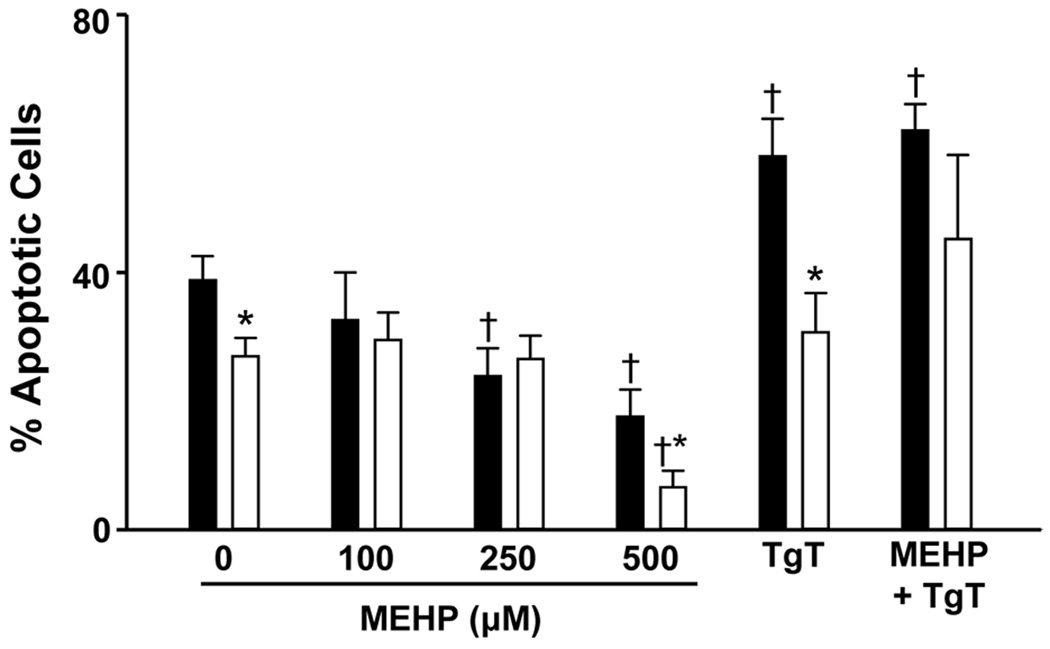
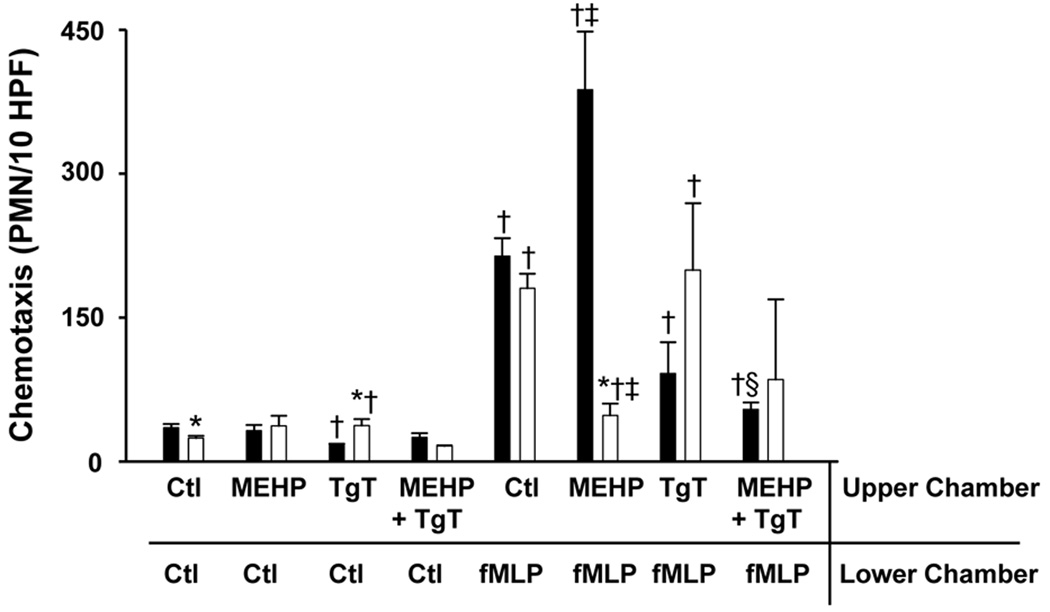
Initially, we compared the effects of MEHP on adult and neonatal neutrophil apoptosis. Consistent with previous studies (14), we found that spontaneous apoptosis was reduced in neonatal neutrophils relative to adult cells (Fig. 1). Treatment of both adult and neonatal neutrophils with MEHP inhibited apoptosis, but only at the higher doses of MEHP. The effects of MEHP (500 µM) on apoptosis were significantly greater in neonatal, relative to adult cells. Phthalates have been reported to bind PPAR-γ, inhibiting the activity of this anti-inflammatory signaling molecule (23). To analyze the role of PPAR-γ in MEHP-induced suppression of apoptosis, we used the PPAR-γ agonist, TgT (24). TgT was found to reverse the suppressive effects of MEHP on apoptosis in both adult and neonatal neutrophils. Interestingly, TgT by itself stimulated apoptosis, but only in adult cells. We next compared the effects of MEHP on chemotaxis in adult and neonatal neutrophils. The bacterially-derived peptide fMLP readily induced chemotaxis in both cell types; in accord with previous studies (25), cells from adults were more responsive than cells from neonates (Fig. 2). Pretreatment of adult neutrophils with MEHP resulted in a significant increase in fMLP-induced chemotaxis. In contrast, MEHP inhibited this activity in neonatal cells. MEHP by itself had no effect on random migration in either cell type. The PPAR-γ agonist, TgT, reduced fMLP-induced chemotaxis in both control and MEHP-treated adult neutrophils, but had no significant effects on neonatal cells.
Figure 1. Effects of MEHP on neutrophil apoptosis.
Adult (■) and neonatal (□) neutrophils were treated with MEHP (0–500 µM), TgT (10 µM), or MEHP (500 µM) + TgT for 24 h and then analyzed for apoptosis by flow cytometry using BD FACSArray quadrant and two-dimensional histogram statistics based on relative fluorescence. Each bar represents the mean ± SE (n=3–8). *Significantly different (p < 0.05) from adult; †Significantly different (p < 0.05) from control.
Figure 2. Effects of MEHP on neutrophil chemotaxis.
Chemotaxis of adult (■) and neonatal (□) neutrophils was assayed using microwell chambers. Cells were treated with MEHP (500 µM), TgT (2 µM), MEHP + TgT, or control (Ctl) for 1 h prior to measurement of chemotaxis toward fMLP or medium control. Each bar represents the mean ± SE (n = 3). *Significantly different (p<0.05) from adult; †Significantly different (p<0.05) from Ctl/Ctl; ‡Significantly different (p<0.05) from Ctl/fMLP; §Significantly different (p<0.05) from MEHP/fMLP.
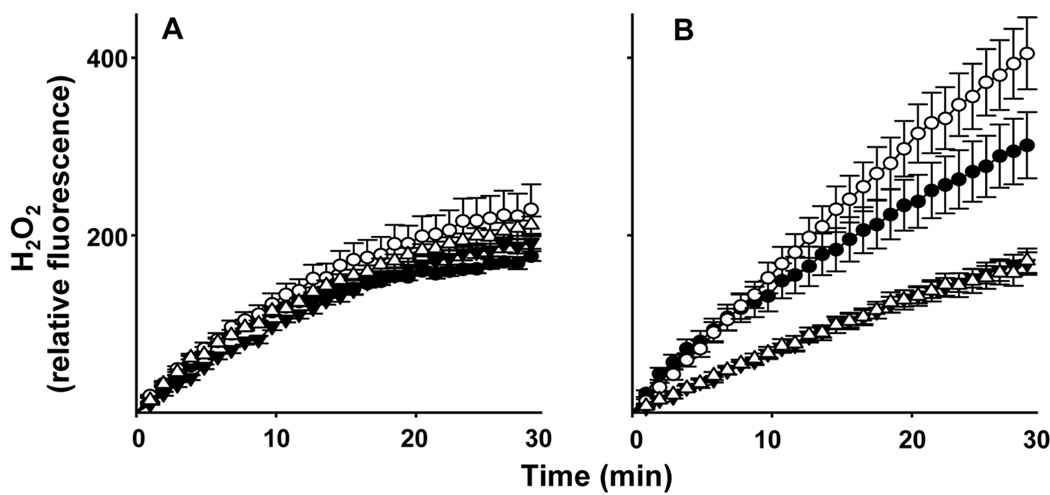
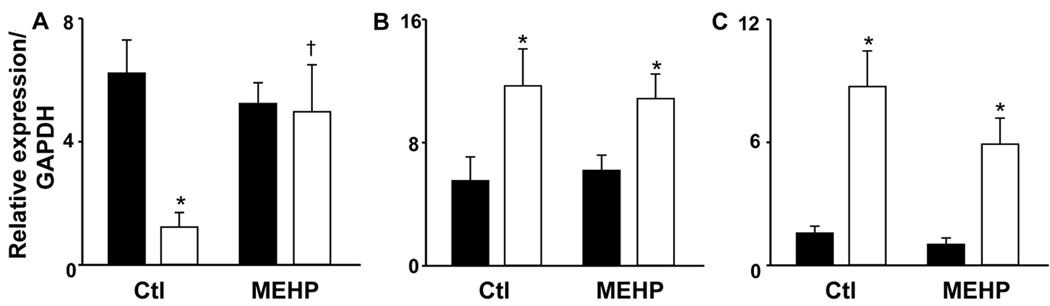
In further studies we compared the effects of MEHP on production of H2O2 by adult and neonatal neutrophils. In the absence of stimulation, the rate of H2O2 production was 2 times greater in neonatal, relative to adult neutrophils (Fig. 3). Moreover, after 30 min, neonatal cells produced 1.7 times more H2O2 than adult cells. MEHP significantly increased both the rate (1.8 fold) and total amount (1.3 fold) of H2O2 produced by neonatal, but not adult neutrophils. TgT suppressed both basal and MEHP-induced H2O2 production by neonatal cells, with no effect on adult neutrophils. Consistent with these results, MEHP was found to upregulate mRNA expression of NOX1, which catalyzes the production of superoxide anion, in neutrophils from neonates, but not adults (Fig. 4). However, basal levels of NOX1 were greater in adult cells.
Figure 3. MEHP stimulates hydrogen peroxide production by neonatal neutrophils.
Adult (panel A) and neonatal (panel B) neutrophils (5 × 104 cells/well) were incubated with Amplex Red (25 µM) and horseradish peroxidise, in the absence or presence of MEHP (500 µM), and TgT or control. H2O2 production was quantified at 1 min intervals for 30 min. Each point represents the mean ± SE (n = 6). MEHP significantly increased H2O2 production in neonatal cells at times >13 min with no effect on adult cells; TgT significantly attenuated this response in control and MEHP treated neonatal cells at all time points.
Control,●; MEHP,○; TgT,▼; TgT + MEHP, Δ
Figure 4. Effects of MEHP on expression of NOX1, SOD, and catalase in neutrophils.
Adult (■) and neonatal (□) neutrophils were incubated with MEHP (500 µM), or medium control (Ctl) for 4 h. mRNA expression of the (A) NOX1, (B) SOD, and (C) catalase genes were quantified by real-time PCR. Results were normalized to GAPDH expression. Each bar represents the mean ± SE (n=15). *Significantly different (P<0.05) from adult; †Significantly different (P<0.05) from Ctl.
In our next series of studies, we compared the effects of MEHP on mRNA expression of SOD and catalase which are known to play a role in protecting against cytotoxicity and tissue damage (26). Constitutive mRNA expression of both antioxidants was greater in neonatal, when compared to adult neutrophils (Fig. 4). Treatment with MEHP had no effect on expression of these antioxidants in either cell type.
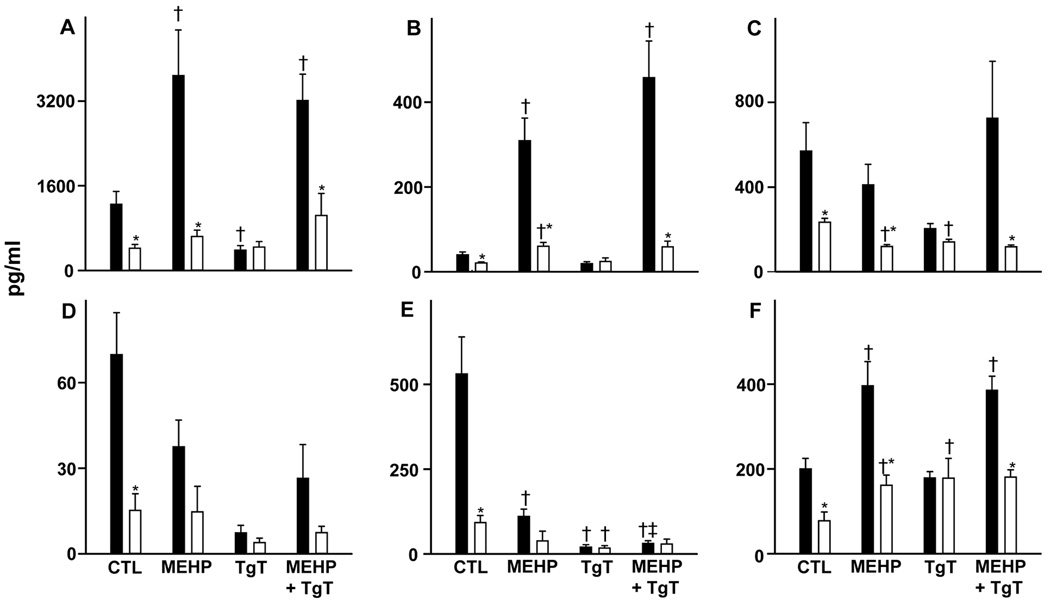
We also analyzed the effects of MEHP on neutrophil production of inflammatory proteins and chemotactic cytokines. Constitutive production of IL-1β, VEGF, IL-6, IL-8, MIP-1β, and RANTES was significantly reduced in neonatal, when compared to adult neutrophils (Fig. 5). MEHP stimulates IL-1β and VEGF production by both cell types. MEHP also stimulated IL-8 production, but only in adult neutrophils. In contrast, MEHP inhibited MIP-1β production by adult neutrophils, and RANTES production by neonatal cells. No significant effects of MEHP were noted on IL-6 production in either cell type. Although TgT had no effect on MEHP-induced alterations in inflammatory protein or chemokine production in either cell type, changes were noted in constitutive production. Thus, TgT by itself reduced IL-8 and MIP-1β production by adult cells, and RANTES and MIP-1β by neonatal neutrophils. In contrast, TgT stimulated VEGF production, but only in neonatal cells.
Figure 5. Effects of MEHP on inflammatory mediator production.
Adult (■) and neonatal (□) neutrophils were incubated in the presence or absence of MEHP (500 µM) and/or TgT (10 µM) for 24 h. The inflammatory mediators (A) IL-8, (B) IL-1β, (C) RANTES, (D) IL-6, (E) MIP-1β, and (F) VEGF were measured in culture supernatants using cytometric bead array analysis. Each bar represents the mean ± SE (n=10). *Significantly different (P<0.05) from adult; †Significantly different (P<0.05) from control; ‡Significantly different from MEHP.
DISCUSSION
Exposure of hospitalized newborns to phthalates is a major health concern because large quantities are infused intravenously with procedures such as blood transfusions (27–29). DEHP exposure may also occur via mechanical ventilator tubes and lipid infusions, resulting in exposures more than three orders of magnitude higher in neonates relative to non-hospitalized infants and children (3, 30–32). We hypothesized that MEHP exerts pro-inflammatory activity in neutrophils, and that this may increase susceptibility to chronic diseases. To test this, we compared the effects of MEHP on inflammatory activity of adult and neonatal neutrophils. Because serum phthalate levels in hospitalized neonates have not been reported, MEHP concentrations used in this study were calculated based on measurements in adults after prolonged exposure. Platelet apheresis can provide up to approximately 2 mg of DEHP (33). Extrapolating to a continuous intravenous infusion in a 1 kg infant with a blood volume of 100 ml, and assuming rapid metabolism to MEHP, this would yield a serum concentration of approximately 1 mM. While the rate of metabolism of MEHP in neonates is not known, it has a half-life of 6.3 hr in mature animals (34). Therefore, doses of 100 to 500 µM are expected to be within the magnitude of levels to which neutrophils may be exposed in the blood of NICU neonates.
Increased susceptibility of newborns to bronchopulmonary dysplasia and other inflammatory diseases is thought to be due to impaired resolution of inflammation and clearance of neutrophils by apoptosis (35, 36). Consistent with this, we have previously reported that apoptosis is reduced in neonatal neutrophils relative to adult cells; moreover, this is related to decreased responsiveness to Fas ligand and anti-inflammatory eicosanoids (14). Findings in the present studies that MEHP suppresses apoptosis, and that this effect is greater in neonatal, when compared to adult neutrophils, suggest that exposure of neonates to phthalates may further impair the clearance of neutrophils, exacerbating inflammatory conditions in these patients.
Neutrophils accumulate in tissues in response to chemokines generated at sites of infection or injury. IL-8 is a potent neutrophil chemoattractant; it also up-regulates expression of cell adhesion molecules (37). The present studies demonstrate that MEHP augmented fMLP-induced chemotaxis in adult, but not neonatal cells. This correlated with increased production of IL-8 by adult neutrophils. MEHP has also been shown to induce calcium flux and up-regulate expression of the integrin, CD11b, which are important in cell motility (10, 38). It has previously been reported that calcium mobilization and expression of CD11b are developmentally impaired in neonatal neutrophils (25, 39). These defects, together with the inability of MEHP to stimulate IL-8 production in neonatal cells, may contribute to the inhibitory effects of MEHP on chemotactic responses in these cells.
Inflammatory cytokines and bacterial-derived products trigger the generation of reactive oxygen species (40). The present studies show that both adult and neonatal neutrophils constitutively generate significant quantities of H2O2. However, the rate of production and total amount of H2O2 generated were greater in neonatal cells, which is consistent with our previous observations (18). MEHP was found to stimulate H2O2 production by neonatal, but not adult neutrophils. These findings are in accord with reports that phthalates induce oxidative metabolism in neonatal neutrophils (41). We also found that MEHP up-regulated NOX1 expression, but only in neonatal cells. NOX1 is the major enzymatic mediator of superoxide anion generation in neutrophils (42). Increased expression of NOX1 by neonatal neutrophils is consistent with previous reports that in response to inflammatory stimuli, these cells produce greater amounts of superoxide anion relative to adult cells (43). Of note is our observation that constitutive expression of NOX1 was lower in neonatal relative to adult cells. These findings are in accord with reports that constitutive production of superoxide anion is low or undetectable in these cells (44). Interestingly, constitutive expression of SOD and catalase was elevated in neonatal neutrophils, when compared to adult cells, which supports the idea that these antioxidants are key to protecting neonates from reactive oxygen species generated in fetal and maternal circulation (45). In contrast to its stimulatory effects on NOX1 and oxidative metabolism in neonatal neutrophils, MEHP had no effect on expression of SOD or catalase. MEHP-induced increases in NOX1 and production of reactive oxygen species occur in the absence of increases in antioxidant production. Consistent with the idea that oxidative activity is increased in neonates, the proportion of phthalates excreted as mono (2-ethyl-5-carboxypentyl) phthalate, an oxidative metabolite of DEHP, is markedly increased in neonates when compared to adults (31). Increased reactive oxygen species may also contribute to increased susceptibility of neonates to oxidant-induced tissue injury, characterized by sustained inflammation leading to cytotoxicity, apoptosis, and fibrosis.
Additional comparative studies revealed that constitutive production of IL-1β, IL-6, IL-8, MIP-1β, RANTES, and VEGF was significantly reduced in neonatal neutrophils when compared to adult cells. These findings are in accord with previous reports that the generation of cyokines and chemokines is developmentally impaired in neonatal cells (46). Whereas in adult neutrophils, MEHP suppressed the production of MIP-1β, in neonatal cells, it suppressed RANTES production. MIP-1β and RANTES are CC chemokines that act primarily on monocytes and macrophages (47). Down regulation of these mediators by MEHP suggests that the pro-inflammatory effects of MEHP are mainly directed towards neutrophils. This is supported by our findings that MEHP stimulated IL-8 production in adult cells. The fact that this was not evident in neonatal cells is in accord with our findings that MEHP blocks chemotaxis in these cells. MEHP was also found to up-regulate IL-1β and VEGF production in both cell types. IL-1β is a marker of neutrophil activation during chronic inflammatory disease (48), and VEGF has been shown to mediate neutrophil adhesion and migration (49). Increased production of these mediators may represent an important mechanism contributing to MEHP-induced neutrophilic inflammation in both adults and neonates.
PPAR-γ is a nuclear transcription factor important in down-regulating the production of pro-inflammatory cytokines and reactive nitrogen species during the resolution phase of inflammation (50). PPAR-γ agonists have been shown to reduce neutrophil-mediated lung and liver injury during endotoxemia (51). Previous studies have shown that phthalates bind to PPAR-γ, suppressing its activity (23). The PPAR-γ agonist TgT attenuated MEHP-mediated suppression of apoptosis and stimulation of oxidative metabolism by neonatal neutrophils. These data suggest that MEHP modulates these activities in neonates by inhibiting anti-inflammatory signaling via PPAR-γ. In contrast, TgT had no effect on MEHP-induced alterations in production of inflammatory proteins or chemokines by adult or neonatal neutrophils, indicating that these effects are mediated by PPAR-γ-independent pathways. Interestingly, TgT suppressed the effects of MEHP on chemotaxis in adult neutrophils, suggesting that that the role of PPAR-γ signaling in chemotaxis is developmentally regulated. Developmental alterations in PPAR-γ signaling are also supported by our observation that adult and neonatal neutrophils display differential sensitivity to the effects of TgT alone. For example, TgT stimulated apoptosis and inhibited IL-8 production in adult, but not neonatal cells. It may be that PPAR-γ plays a role in regulating neutrophil longevity and migration in adult neutrophils, and that these pathways are impaired in neonatal cells. It has been reported that prostaglandin J2 and other eicosanoids may be endogenous ligands for PPAR-γ, but the role of these mediators in neonatal disease is not known (52, 53).
Clinical case reports indicate that phthalates may contribute to neonatal inflammatory disease. For example, high levels of DEHP have been reported in the gastrointestinal tissue of infants who succumbed to necrotizing enterocolitis (54), and phthalate exposure has been implicated in chronic lung disease in premature infants (55). The present studies demonstrate that MEHP induces oxidative metabolism and up regulates expression of NOX1 in neonatal neutrophils. This is associated with reduced apoptosis and chemotaxis. Taken together, these data suggest that neonatal neutrophils are more sensitive to phthalate-mediated inhibition of PPAR-γ signaling, which may be related to decreased basal anti-inflammatory signaling via this pathway. Understanding the inflammatory effects of phthalates in neonates may support efforts to limit or discontinue the use of phthalate-containing medical devices in neonates.
Acknowledgments
Supported by National Institutes of Health grants HD042036, HD058019, GM034310, ES004738, ES005022, CA100994, CA132624 and AR055073.
ABBREVIATIONS
- DEHP
di-(2-ethylhexyl) phthalate
- fMLP
N-formyl-methionyl-leucyl-phenylalanine
- MEHP
mono-(2-ethylhexyl) phthalate
- NOX1
NADPH oxidase-1
- PPAR-γ
peroxisome proliferator-activated receptor-γ
- PVC
polyvinyl chloride
- TgT
troglitazone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kambia K, Dine T, Gressier B, Bah S, Germe AF, Luyckx M, Brunet C, Michaud L, Gottrand F. Evaluation of childhood exposure to di(2-ethylhexyl) phthalate from perfusion kits during long-term parenteral nutrition. Int J Pharm. 2003;262:83–91. doi: 10.1016/s0378-5173(03)00335-1. [DOI] [PubMed] [Google Scholar]
- 2.Loff S, Subotic U, Reinicke F, Wischmann H, Brade J. Extraction of di-ethylhexyl-phthalate from perfusion lines of various material, length and brand by lipid emulsions. J Pediatr Gastroenterol Nutr. 2004;39:341–345. doi: 10.1097/00005176-200410000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Calafat AM, Needham LL, Silva MJ, Lambert G. Exposure to di-(2-ethylhexyl) phthalate among premature neonates in a neonatal intensive care unit. Pediatrics. 2004;113:e429–e434. doi: 10.1542/peds.113.5.e429. [DOI] [PubMed] [Google Scholar]
- 4.Stroheker T, Cabaton N, Nourdin G, Regnier JF, Lhuguenot JC, Chagnon MC. Evaluation of anti-androgenic activity of di-(2-ethylhexyl)phthalate. Toxicology. 2005;208:115–121. doi: 10.1016/j.tox.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Kavlock R, Barr D, Boekelheide K, Breslin W, Breysse P, Chapin R, Gaido K, Hodgson E, Marcus M, Shea K, Williams P. NTP-CERHR Expert Panel Update on the Reproductive and Developmental Toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol. 2006;22:291–399. doi: 10.1016/j.reprotox.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, Gray LE., Jr The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci. 2000;58:339–349. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- 7.Moore RW, Rudy TA, Lin TM, Ko K, Peterson RE. Abnormalities of sexual development in male rats with in utero and lactational exposure to the antiandrogenic plasticizer Di(2-ethylhexyl) phthalate. Environ Health Perspect. 2001;109:229–237. doi: 10.1289/ehp.01109229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray LE, Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58:350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal DK, Eustis S, Lamb JC, Reel JR, Kluwe WM. Effects of di(2-ethylhexyl) phthalate on the gonadal pathophysiology, sperm morphology, and reproductive performance of male rats. Environ Health Perspect. 1986;65:343–350. doi: 10.1289/ehp.8665343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gourlay T, Samartzis I, Stefanou D, Taylor K. Inflammatory response of rat and human neutrophils exposed to di-(2-ethyl-hexyl)-phthalate-plasticized polyvinyl chloride. Artif Organs. 2003;27:256–260. doi: 10.1046/j.1525-1594.2003.07107.x. [DOI] [PubMed] [Google Scholar]
- 11.Calo L, Fracasso A, Cantaro S, Cozzi E, De Silvestro G, Plebani M, Bazzato G, Borsatti A. Plasticizers induced mononuclear cells interleukin 1 production: implications with peritoneal sclerosis. Clin Nephrol. 1993;40:57. [PubMed] [Google Scholar]
- 12.Bally MB, Opheim DJ, Shertzer HG. Di-(2-ethylhexyl) phthalate enhances the release of lysosomal enzymes from alveolar macrophages during phagocytosis. Toxicology. 1980;18:49–60. doi: 10.1016/0300-483x(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 13.Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, Mazzeo P. In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ Health Perspect. 2003;111:1783–1785. doi: 10.1289/ehp.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanna N, Vasquez P, Pham P, Heck DE, Laskin JD, Laskin DL, Weinberger B. Mechanisms underlying reduced apoptosis in neonatal neutrophils. Pediatr Res. 2005;57:56–62. doi: 10.1203/01.PDR.0000147568.14392.F0. [DOI] [PubMed] [Google Scholar]
- 15.Calafat AM, McKee RH. Integrating biomonitoring exposure data into the risk assessment process: phthalates [diethyl phthalate and di(2-ethylhexyl) phthalate] as a case study. Environ Health Perspect. 2006;114:1783–1789. doi: 10.1289/ehp.9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.United States Food and Drug Administration Safety Assessment of Di(2-ethylhexyl) Phthalate (DEHP) Released from PVC Medical Devices. [Accessed March 16, 2010]; Available at: http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM080457.pdf.
- 17.Shea KM. Pediatric exposure and potential toxicity of phthalate plasticizers. Pediatrics. 2003;111:1467–1474. doi: 10.1542/peds.111.6.1467. [DOI] [PubMed] [Google Scholar]
- 18.Weinberger B, Vetrano AM, Syed K, Murthy S, Hanna N, Laskin JD, Laskin DL. Influence of labor on neonatal neutrophil apoptosis, and inflammatory activity. Pediatr Res. 2007;61:572–577. doi: 10.1203/pdr.0b013e318045be38. [DOI] [PubMed] [Google Scholar]
- 19.Ferrante A, Thong YH. Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leucocytes from human blood by the Hypaque-Ficoll method. J Immunol Methods. 1980;36:109–117. doi: 10.1016/0022-1759(80)90036-8. [DOI] [PubMed] [Google Scholar]
- 20.Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sgonc R, Gruber J. Apoptosis detection: an overview. Exp Gerontol. 1998;33:525–533. doi: 10.1016/s0531-5565(98)00031-x. [DOI] [PubMed] [Google Scholar]
- 22.Mohanty JG, Jaffe JS, Schulman ES, Raible DG. A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J Immunol Methods. 1997;202:133–141. doi: 10.1016/s0022-1759(96)00244-x. [DOI] [PubMed] [Google Scholar]
- 23.Hurst CH, Waxman DJ. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol Sci. 2003;74:297–308. doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- 24.Naito Y, Yoshikawa T. Thiazolidinediones: a new class of drugs for the therapy of ischemia-reperfusion injury. Drugs Today (Barc) 2004;40:423–430. doi: 10.1358/dot.2004.40.5.850490. [DOI] [PubMed] [Google Scholar]
- 25.Weinberger B, Laskin DL, Mariano TM, Sunil VR, DeCoste CJ, Heck DE, Gardner CR, Laskin JD. Mechanisms underlying reduced responsiveness of neonatal neutrophils to distinct chemoattractants. J Leukoc Biol. 2001;70:969–976. [PMC free article] [PubMed] [Google Scholar]
- 26.Asikainen TM, White CW. Pulmonary antioxidant defenses in the preterm newborn with respiratory distress and bronchopulmonary dysplasia in evolution: implications for antioxidant therapy. Antioxid Redox Signal. 2004;6:155–167. doi: 10.1089/152308604771978462. [DOI] [PubMed] [Google Scholar]
- 27.Kamrin MA. Phthalate risks, phthalate regulation, and public health: a review. J Toxicol Environ Health B Crit Rev. 2009;12:157–174. doi: 10.1080/10937400902729226. [DOI] [PubMed] [Google Scholar]
- 28.Weisbach V, Koch HM, Angerer J, Eckstein R. Di(2-ethylhexyl)phthalate exposure of apheresis donors is procedure-related. Transfusion. 2006;46:1457–1458. doi: 10.1111/j.1537-2995.2006.00920.x. author reply 1459. [DOI] [PubMed] [Google Scholar]
- 29.Inoue K, Kawaguchi M, Yamanaka R, Higuchi T, Ito R, Saito K, Nakazawa H. Evaluation and analysis of exposure levels of di(2-ethylhexyl) phthalate from blood bags. Clin Chim Acta. 2005;358:159–166. doi: 10.1016/j.cccn.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 30.Latini G, Avery GB. Materials degradation in endotracheal tubes: a potential contributor to bronchopulmonary dysplasia. Acta Paediatr. 1999;88:1174–1175. doi: 10.1080/08035259950168333. [DOI] [PubMed] [Google Scholar]
- 31.Silva MJ, Reidy JA, Preau JL, Samandar E, Needham LL, Calafat AM. Measurement of eight urinary metabolites of di(2-ethylhexyl) phthalate as biomarkers for human exposure assessment. Biomarkers. 2006;11:1–13. doi: 10.1080/13547500500382868. [DOI] [PubMed] [Google Scholar]
- 32.Plonait SL, Nau H, Maier RF, Wittfoht W, Obladen M. Exposure of newborn infants to di-(2-ethylhexyl)-phthalate and 2-ethylhexanoic acid following exchange transfusion with polyvinylchloride catheters. Transfusion. 1993;33:598–605. doi: 10.1046/j.1537-2995.1993.33793325058.x. [DOI] [PubMed] [Google Scholar]
- 33.Koch HM, Angerer J, Drexler H, Eckstein R, Weisbach V. Di(2-ethylhexyl)phthalate (DEHP) exposure of voluntary plasma and platelet donors. Int J Hyg Environ Health. 2005;208:489–498. doi: 10.1016/j.ijheh.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Ljungvall K, Tienpont B, David F, Magnusson U, Torneke K. Kinetics of orally administered di(2-ethylhexyl) phthalate and its metabolite, mono(2-ethylhexyl) phthalate, in male pigs. Arch Toxicol. 2004;78:384–389. doi: 10.1007/s00204-004-0558-z. [DOI] [PubMed] [Google Scholar]
- 35.Speer CP. Pulmonary inflammation and bronchopulmonary dysplasia. J Perinatol. 2006;26:S57–S62. doi: 10.1038/sj.jp.7211476. discussion S63-54. [DOI] [PubMed] [Google Scholar]
- 36.Stefanutti G, Lister P, Smith VV, Peters MJ, Peters MJ, Klein NJ, Pierro A, Eaton S. P-selectin expression, neutrophil infiltration, and histologic injury in neonates with necrotizing enterocolitis. J Pediatr Surg. 2005;40:942–947. doi: 10.1016/j.jpedsurg.2005.03.027. discussion 947–948. [DOI] [PubMed] [Google Scholar]
- 37.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 38.Niggli V. Signaling to migration in neutrophils: importance of localized pathways. Int J Biochem Cell Biol. 2003;35:1619–1638. doi: 10.1016/s1357-2725(03)00144-4. [DOI] [PubMed] [Google Scholar]
- 39.Abughali N, Berger M, Tosi MF. Deficient total cell content of CR3 (CD11b) in neonatal neutrophils. Blood. 1994;83:1086–1092. [PubMed] [Google Scholar]
- 40.Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- 41.Latini G. Potential hazards of exposure to di-(2-ethylhexyl)-phthalate in babies. a review. Biol Neonate. 2000;78:269–276. doi: 10.1159/000014278. [DOI] [PubMed] [Google Scholar]
- 42.Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsukimori K, Komatsu H, Yoshimura T, Hikino S, Hara T, Wake N, Nakano H. Increased inflammatory markers are associated with early periventricular leukomalacia. Dev Med Child Neurol. 2007;49:587–590. doi: 10.1111/j.1469-8749.2007.00587.x. [DOI] [PubMed] [Google Scholar]
- 44.Newburger PE. Superoxide generation by human fetal granulocytes. Pediatr Res. 1982;16:373–376. doi: 10.1203/00006450-198205000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Rosenfeld W, Concepcion L. Endogenous antioxidant defenses in neonates. J Free Radic Biol Med. 1986;2:295–298. doi: 10.1016/s0748-5514(86)80013-7. [DOI] [PubMed] [Google Scholar]
- 46.Carr R. Neutrophil production and function in newborn infants. Br J Haematol. 2000;110:18–28. doi: 10.1046/j.1365-2141.2000.01992.x. [DOI] [PubMed] [Google Scholar]
- 47.Baggiolini M, Loetscher P, Moser B. Interleukin-8 and the chemokine family. Int J Immunopharmacol. 1995;17:103–108. doi: 10.1016/0192-0561(94)00088-6. [DOI] [PubMed] [Google Scholar]
- 48.Kotecha S. Cytokines in chronic lung disease of prematurity. Eur J Pediatr. 1996;155:S14–S17. doi: 10.1007/BF01958074. [DOI] [PubMed] [Google Scholar]
- 49.Scaldaferri F, Vetrano S, Sans M, Arena V, Straface G, Stigliano E, Repici A, Sturm A, Malesci A, Panes J, Yla-Herttuala S, Fiocchi C, Danese S. VEGF-A Links Angiogenesis and Inflammation in Inflammatory Bowel Disease Pathogenesis. Gastroenterology. 2009;136:585–595. doi: 10.1053/j.gastro.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 50.Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, Fruchart JC, Chapman J, Najib J, Staels B. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998;273:25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- 51.Collin M, Patel NS, Dugo L, Thiemermann C. Role of peroxisome proliferator-activated receptor-gamma in the protection afforded by 15-deoxydelta12,14 prostaglandin J2 against the multiple organ failure caused by endotoxin. Crit Care Med. 2004;32:826–831. doi: 10.1097/01.ccm.0000114821.25573.e7. [DOI] [PubMed] [Google Scholar]
- 52.Kuhn H, O'Donnell VB. Inflammation and immune regulation by 12/15-lipoxygenases. Prog Lipid Res. 2006;45:334–356. doi: 10.1016/j.plipres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 53.Scher JU, Pillinger MH. 5d-PGJ2: the anti-inflammatory prostaglandin? Clin Immunol. 2005;114:100–109. doi: 10.1016/j.clim.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 54.Hillman LS, Goodwin SL, Sherman WR. Identification and measurement of plasticizer in neonatal tissues after umbilical catheters and blood products. N Engl J Med. 1975;292:381–386. doi: 10.1056/NEJM197502202920801. [DOI] [PubMed] [Google Scholar]
- 55.Roth B, Herkenrath P, Lehmann HJ, Ohles HD, Homig HJ, Benz-Bohm G, Kreuder J, Younossi-Hartenstein A. Di-(2-ethylhexyl)-phthalate as plasticizer in PVC respiratory tubing systems: indications of hazardous effects on pulmonary function in mechanically ventilated, preterm infants. Eur J Pediatr. 1988;147:41–46. doi: 10.1007/BF00442609. [DOI] [PubMed] [Google Scholar]