Abstract
Mucopolysaccharidosis type IIIA (MPS IIIA), which is a lysosomal storage disorder (LSD) caused by inherited deficiency of sulfamidase, is characterized by severe, progressive central nervous system (CNS) dysfunction. Enzyme replacement therapy (ERT) to treat CNS storage is challenging, because the access of enzymes to the brain is restricted by the blood–brain barrier (BBB). In a prior study, we found that phosphorylated β-glucuronidase (P-GUS) could be transcytosed across the BBB in newborn mice by the mannose 6-phosphate (M6P) receptor. In order to determine whether sulfamidase can utilize this pathway, we examined brain influx and the specificity of uptake of sulfamidase after intravenous (IV) injection in 2-day-old and 8-week-old mice. [131I]Sulfamidase was transported across the BBB in neonates at rates higher than that of simultaneously injected [125I]albumin. In contrast, the transport of [131I]sulfamidase was negligible in 8-week-old mice, thereby showing that the BBB transport mechanism is developmentally downregulated. Capillary depletion revealed that 83.7% of the [131I]sulfamidase taken up by the brain was in the parenchyma, demonstrating transfer across the capillary wall. The uptake of [131I]sulfamidase into the brain was significantly reduced by co-injections of M6P and P-GUS. That is, the transport of sulfamidase into the brain parenchyma in early postnatal life is mediated by the M6P receptor, which is shared with P-GUS and is likely accessible to other M6P-containing lysosomal enzymes.
INTRODUCTION
Sulfamidase (N-sulfoglucosamine sulfohydrolase; EC 3.10.1.1) cleaves N-linked sulfates from nonreducing terminals of heparan sulfates. The native enzyme forms a 115 kd dimer, composed of two 62 kd monomers,1,2 each with five N-glycosylation sites.3 An inherited deficiency of sulfamidase causes mucopolysaccharidosis type IIIA (MPS IIIA, Sanfilippo syndrome type A), an autosomal recessive lysosomal storage disorder (LSD)4 characterized by severe progressive central nervous system (CNS) dysfunction in animals and humans.5,6 Patients with MPS IIIA have coarse facial features, mental retardation, hyperactivity, aggressive behavior, insomnia, diarrhea, and pulmonary infections, leading to premature death.4,7
Sulfamidase is taken up by receptor-mediated endocytosis through the mannose 6-phosphate/insulin-like growth factor 2 (M6P/IGF2) receptor, and prevents lysosomal storage in vitro.8 However, the entry from blood into the brain is restricted at the blood–brain barrier (BBB) which is made up of capillary endothelial cells, pericytes, perivascular astrocytic endfoot, and the basal membrane. The delivery of a lysosomal enzyme into the brain will likely require the elucidation of some transport mechanism for the therapeutic protein.
Intracerebral injection of sulfamidase into the brains of adult rodents reduces lysosomal storage in the CNS.9 Repeated intravenous (IV) administration of sulfamidase initiated at birth also reduces abnormal lysosomal storage in the CNS, and prevents the impairment of memory in MPS IIIA mice.10 However, enzyme replacement therapy (ERT) is effective only if begun at birth. No histological improvement is observed in the brain if ERT is initiated as late as 6 weeks after birth. One possible explanation for this phenomenon appeared to be that the enzyme leaks across an immature BBB. However, we as well as others have shown that the BBB is not leaky to other proteins at birth.11-13 Further, we demonstrated that M6P-containing β-glucuronidase, but not nonphosphorylated β-glucuronidase, crosses the neonatal BBB through a developmentally regulated receptor-mediated transport mechanism. Here we report that recombinant human sulfamidase crosses the neonatal BBB through the M6P/IGF2 receptor–mediated transcytosis pathway, a transporter it shares with phosphorylated β-glucuronidase (P-GUS).
RESULTS
Recombinant sulfamidase production and cellular uptake
Recombinant human sulfamidase was prepared from over-expressing Chinese Hamster Ovary (CHO) cells secretion medium as described in the Materials and Methods section (Supplementary Table S1). The purified enzyme had a specific activity of 15,000 U/mg. Analysis using reducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis revealed 62 kd (major) and 56 kd (minor) components (Supplementary Figure S1). The results presented in Supplementary Table S2 indicate that the enzyme is susceptible to endocytosis by human fibroblasts through the M6P receptor. Figure 1 shows the experiment to test the ability of purified sulfamidase to inhibit the M6P-dependent endocytosis of P-GUS. We concluded that the recombinant human sulfamidase is mannose 6-phosphorylated as was reported by Bielicki et al. (Supplementary Table S2).1
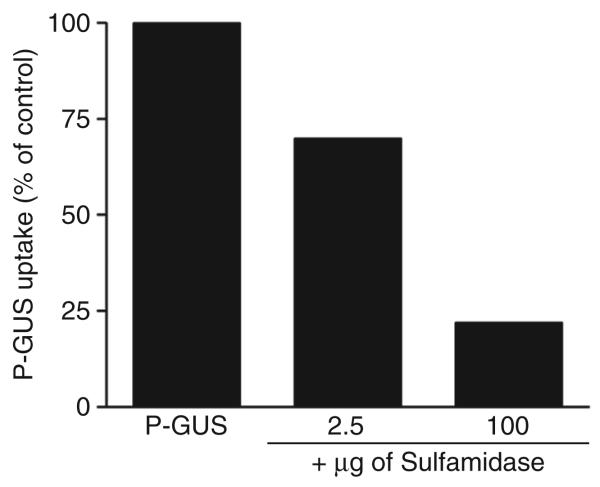
Figure 1. Inhibition of phosphorylated β-glucuronidase (P-Gus) uptake by sulfamidase.
Human mucopolysaccharidosis type VII fibroblasts were incubated with P-GUS ± 2.5 or 100 μg of sulfamidase. The addition of the sulfamidase inhibited the mannose 6-phosphate receptor–mediated endocytosis of P-GUS in a dose-dependent manner.
Brain influx of [131I]sulfamidase from serum after IV injection
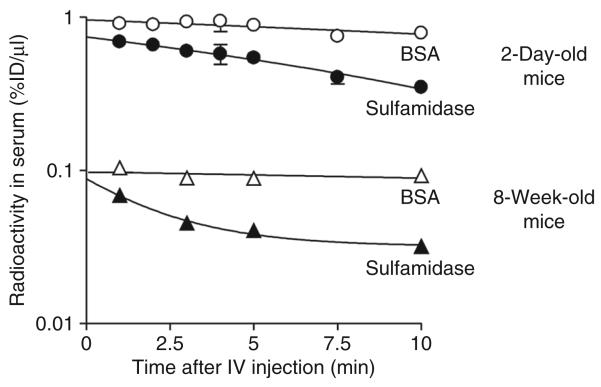
The concentration–time profile of [131I]sulfamidase and [125I]albumin in the serum after the IV injection is shown in Figure 2. The serum concentration of [131I]sulfamidase declined linearly with time after IV injection into 2-day-old and 8-week-old mice. In contrast, the levels of [125I]albumin in serum were sustained throughout the experiment, regardless of age.
Figure 2. Time-course of radioactivity in serum after intravenous (IV) co-injection of [131I]sulfamidase and [125I]albumin in 2-day-old and 8-week-old mice.
Filled circles and triangles indicate the levels of [131I]sulfamidase in 2-day-old and 8-week-old mice, respectively. Open circles and triangles indicate the levels of [125I]albumin in 2-day-old and 8-week-old mice, respectively. Each point represents the mean value ± SE from four mice. BSA, bovine serum albumin.
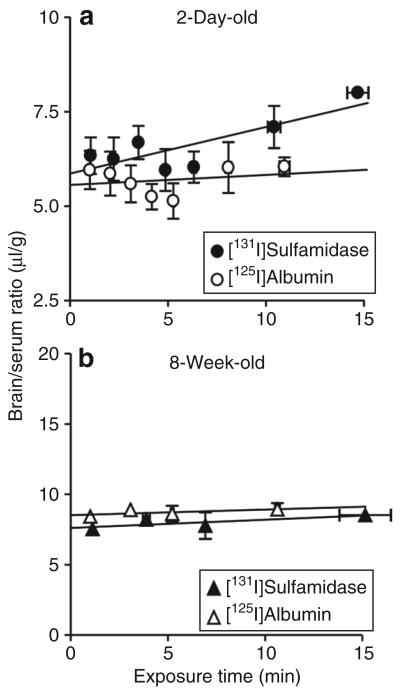
The brain/serum ratios of [131I]sulfamidase and [125I]albumin in the 2-day-old and 8-week-old mice are presented in Figure 3. The brain/serum ratios for [131I]sulfamidase increased linearly with exposure times in 2-day-old mice, and the slope of the regression line deviated significantly from zero (P < 0.05). Table 1 summarizes the Kin and Vi values. The Kin value for [131I]sulfamidase was 0.123 ± 0.038 μl/g-min. In contrast, [125I]albumin showed a flat regression line (not statistically deviating from zero), giving the Kin value of 0.027 ± 0.046 μl/g-min. The Vi value, estimated from the y-intercept, in 2-day-old mice was 5.86 ± 0.29 for [131I] sulfamidase and 5.56 ± 0.27 μl/g for [125I] albumin. In 8-week-old mice, there was no significant brain uptake of either ligand, thereby suggesting that the BBB transport mechanism for sulfamidase is subject to developmental downregulation. The larger Vi values for 8-week-old versus 2-day-old mice are consistent with growth and increased vascularization of the brain.11
Figure 3. Multiple-time regression analyses of [131I]sulfamidase and [125I]albumin after IV injection in (a) 2-day-old and (b) 8-week-old mice.
Each point represents the mean value ± SE from four mice. The brain/serum ratios were plotted against the respective exposure times calculated by Equation 1 in Materials and Methods.
Table 1.
Kin and Vi values of [131I]sulfamidase and [125I]albumin for brain after IV co-injection in mice at the age of 2 days and 8 weeks
| [131I]Sulfamidase | [125I]Albumin | |
|---|---|---|
| 2-Day-old | ||
| Kin (μl/g-min) | 0.123 ± 0.038* | 0.027 ± 0.046NS |
| Vi (μl/g) | 5.86 ± 0.29 | 5.56 ± 0.27 |
| 8-Week-old | ||
| Kin (μl/g-min) | 0.058 ± 0.032NS | 0.040 ± 0.030NS |
| Vi (μl/g) | 7.63 ± 0.28 | 8.53 ± 0.19 |
Abbreviations: IV, intravenous; NS, not signifcantly deviated from zero.
Kin and Vi values were calculated from the slope and y-intercept in Figure 3.
Values are the mean ± SE of four to six determinations.
Asterisk indicates a signifcant deviation in Kin value from fat line (no brain infux) in multiple-time regression analysis, P < 0.05.
In vivo stability of [131I]sulfamidase
In order to determine whether [131I]sulfamidase remains intact after IV injection, its stability in the brain and serum was examined using high-performance liquid chromatography (HPLC). Supplementary Figure S2 shows typical HPLC chromatograms of radioactivity for serum and brain extracts 10 minutes after the IV injection of [131I]sulfamidase in 2-day-old mice. Intact [131I]sulfamidase and free [131I]Na eluted at 7 and 13 minutes as single peaks, respectively. Intact [131I]sulfamidase in the serum and brain extract accounted for 97.1 ± 0.1 and 81.9 ± 2.45% of the total radioactivity, respectively. In the brains from other mice, we determined that degradation of [131I]sulfamidase during extraction was 5.46 ± 0.32% of the total radioactivity. From these data, we estimated that 87.4% of [131I]sulfamidase in the brain was intact at 10 minutes after IV injection in 2-day-old mice.
Specificity of [131I]sulfamidase uptake by brain and peripheral tissues
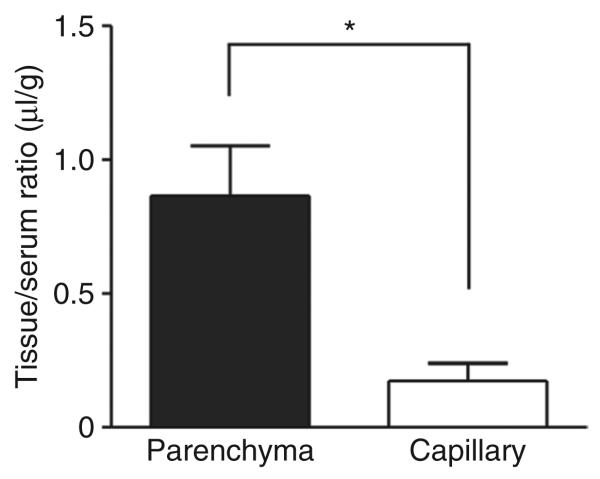
Capillary depletion was conducted in 2-day-old mice to determine how much of the [131I]sulfamidase in the brain actually was in the brain parenchymal fraction (Figure 4). The tissue/serum ratio of sulfamidase in brain parenchyma was 0.87 ± 0.19 μl/g and in the capillary fraction it was 0.17 ± 0.07 μl/g. That is, ~83.7% of the [131I]sulfamidase taken up by the brain actually entered the brain parenchyma, demonstrating transfer across the capillary wall formed by the BBB. Approximately 16.3% remained in the capillary fraction 10 minutes after injection in 2-day-old mice.
Figure 4. Distribution volume of [131I]sulfamidase in brain parenchyma and capillary fraction 10 minutes after IV injection in 2-day-old mice.
Each column represents the mean value ± SE from four mice. Simultaneously measured [125I]albumin space was subtracted in each fraction. An asterisk indicates a significant difference between the distribution values in the parenchyma and capillary fraction: *P < 0.05.
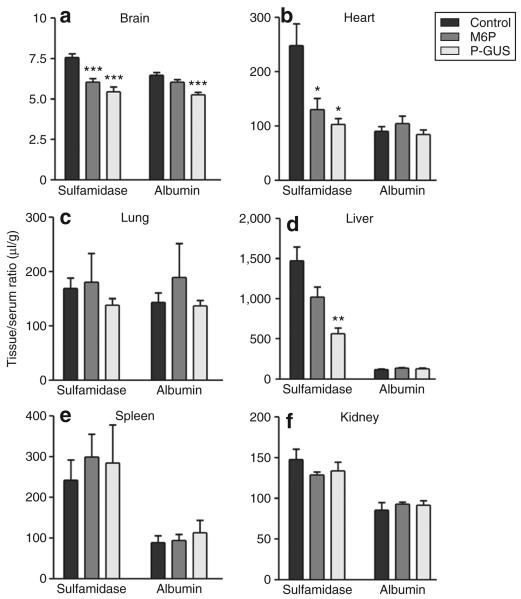
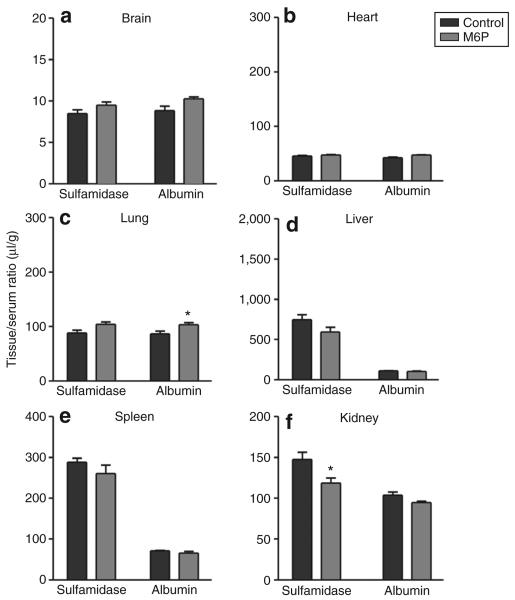
The [131I]sulfamidase uptake in the brain and peripheral tissues was also examined 10 minutes after IV co-injection of M6P (2 μmol) or P-GUS (0.1 nmol; 30 μg) with [131I]sulfamidase in 2-day-old and 8-week-old mice (Figures 5 and 6). M6P reduced the apparent tissue/serum ratios in the brain and heart, and also decreased the apparent uptake in the liver. P-GUS reduced the apparent tissue/serum ratios of [131I]sulfamidase in the brain, heart, and liver in 2-day-old mice, but failed to affect the apparent uptake of [125I]albumin in all the tissues examined, except in the brain. In 8-week-old mice, M6P did not affect the apparent tissue uptake of [131I]sulfamidase in the brain and peripheral tissues, except in the kidney. The vascular spaces, as measured by [125I]albumin, were unchanged by the co-injection of M6P in 2-day-old and 8-week-old mice.
Figure 5. Effects of phosphorylated β-glucuronidase (P-Gus) and mannose 6-phosphate (M6P) on tissue/serum ratios of [131I]sulfamidase and [125I]albumin 10 minutes after IV injection in 2-day-old mice.
The mice received [131I]sulfamidase and [125I]albumin (5.5 × 105 cpm of each) intravenously with P-GUS (30 μg) or M6P (2 μmol). Ten minutes later, the mice were killed, and serum as well as (a) brain, (b) heart, (c) lung, (d) liver, (e) spleen, and (f) kidney were collected. Each column represents the mean value ± SE from four to six mice. Asterisks indicate a significant difference from the control value: *P < 0.05, **P < 0.01, and ***P < 0.001.
Figure 6. Effects of mannose 6-phosphate (M6P) on tissue/serum ratios of [131I]sulfamidase and [125I]albumin 10 minutes after intravenous (IV) injection in 8-week-old mice.
The mice received [131I]sulfamidase and [125I]albumin (5.5 × 105 cpm of each) IV with M6P (2 μmol). Ten minutes later, serum as well as (a) brain, (b) heart, (c) lung, (d) liver, (e) spleen, and (f) kidney were collected. Each column represents the mean value ± SE from four to six mice. An asterisk indicates a significant difference from the control value: *P < 0.05.
Table 2 summarizes the effects of M6P and P-GUS on the net uptake of [131I]sulfamidase in the brain and peripheral tissues 10 minutes after IV injection in 2-day-old and 8-week-old mice. The net uptake in μl/g tissue was calculated by subtracting the vascular space measured by the μl/g of [125I]albumin from the μl/g of [131I]sulfamidase in each tissue. M6P and P-GUS significantly inhibited the net uptake of [131I]sulfamidase in 2-day-old mice (P < 0.001 in the brain and lung, P < 0.01 in the heart). In 8-week-old mice, the net uptake of sulfamidase in the brain was negligible. In the liver, M6P (P < 0.05) and P-GUS (P < 0.01) significantly inhibited the net uptake of [131I]sulfamidase in 2-day-old mice, and M6P tended to decrease the uptake in 8-week-old mice. In the kidney, both agents significantly (P < 0.05) inhibited the uptake in 2-day-old mice, and M6P tended to reduce the uptake in 8-week-old mice. In the spleen, there was no effect of M6P on the uptake, regardless of age.
Table 2.
Effects of M6P and P-GUS on the net uptake of [131I]sulfamidase in the brain and periphery 10 minutes after IV co-injection in 2-day- and 8-week-old mice
| [131I]Sulfamidase uptake (μl/g) |
|||
|---|---|---|---|
| Control | +M6P | +P-GUS | |
| 2-Day-old | |||
| Brain | 1.10 ± 0.16 | 0.06 ± 0.04*** | 0.24 ± 0.116*** |
| Heart | 158.1 ± 31.1 | 25.9 ± 6.7** | 18.9 ± 3.9** |
| Lung | 25.4 ± 2.6 | 2.1 ± 2.0*** | 6.2 ± 4.0*** |
| Liver | 1,355.3 ± 162.2 | 883.5 ± 125.6* | 432.1 ± 65.1** |
| Spleen | 153.4 ± 33.9 | 205.3 ± 41.6 | 171.3 ± 62.6 |
| Kidney | 62.3 ± 7.2 | 35.7 ± 2.2* | 41.9 ± 5.9* |
| 8-Week-old | |||
| Brain | 0.04 ± 0.04 | 0.00 ± 0.20 | n.d. |
| Heart | 2.90 ± 0.41 | 0.54 ± 0.34 | n.d. |
| Lung | 2.54 ± 1.49 | 1.66 ± 1.32 | n.d. |
| Liver | 636.1 ± 60.7 | 491.5 ± 59.1 | n.d. |
| Spleen | 217.3 ± 10.2 | 194.2 ± 22.4 | n.d. |
| Kidney | 43.6 ± 7.82 | 23.9 ± 6.97 | n.d. |
Abbreviations: IV, intravenous; M6P, mannose 6-phosphate; n.d., not determined; P-GUS, phosphorylated β-glucuronidase.
Net tissue uptake of [131I]sulfamidase was calculated by subtracting [125I]albumin space from the value of [131I]sulfamidase in each tissue. Values are the mean ± SE of four to six determinations.
Asterisks indicate a signifcant difference from the control value:
P < 0.05
P < 0.01
P < 0.001
DISCUSSION
The length, density, and inner surface area of the brain capillaries of mice increase after birth, and reach adult levels ~3 weeks of age.14 During this early postnatal growth, the function of the BBB shows numerous developmental changes, such as loss and gain of transport functions for endogenous substances, as well as establishment of tight junctions between capillary endothelial cells that act as a physical barrier. Cerebral microvessels forming the BBB align closely with neurons in the brain. Therefore, once a circulating substance crosses the BBB, it can be immediately available throughout the brain. Tightening of the BBB occurs at the moment of birth, and the barrier function for serum proteins is fully developed early in the postnatal period.12,13,15
The M6P/IGF2 receptor is widely distributed throughout fetal tissues including the brain and is expressed on brain capillaries. Its expression is developmentally regulated, with high prenatal levels followed by a postnatal decrease.16-18 The M6P/IGF2 receptors present at the cell surface of most cells participate in the endocytosis of secreted lysosomal enzymes for subsequent sorting to the lysosome. Recent evidence suggests that they can also mediate transcytosis of lysosomal enzymes across the BBB in the neonatal mouse. This mechanism may provide a major transport pathway for lysosomal enzymes across the BBB in neonates.11
In this study with mice, we observed a significant influx of [131I]sulfamidase into the neonatal brain, but the influx into adult brains was negligible. In the neonatal brain, sulfamidase remained mostly intact after uptake, and was localized primarily in brain parenchyma after IV injection. These results suggest that sulfamidase crosses the BBB from blood to brain in a biologically active form in neonates. From the difference in brain influx rate between [131I]sulfamidase and [125I]albumin, one can estimate the neonatal BBB transport rate for the enzyme. Both M6P and P-GUS inhibited [131I]sulfamidase uptake, suggesting that the transport process is probably identical to the M6P/IGF2 receptor-mediated transcytosis process,11 that has recently been shown to transport P-GUS across the neonatal BBB. It seems plausible, therefore, that all M6P-containing acid hydrolases could use this receptor as a common transport system across the neonatal BBB in vivo. No M6P receptor-dependent uptake of [131I]sulfamidase was detected in the adult brain. These results can explain why ERT was effective in treating CNS manifestations of murine MPS IIIA only if started at birth.10 Just as the transport of P-GUS, transport of sulfamidase across the BBB appears to be developmentally regulated, the M6P receptor-dependent process is present only in the first 2 weeks of life in the mouse. The fact that treatment of adult MPS IIIA mice did not reverse brain storage can be explained by downregulation of the M6P receptor in the neonatal period.
The estimated brain influx rate for [131I]sulfamidase was 0.123 μl/g-min in neonatal mice. The rate is ~58% of the rate measured for P-GUS (0.21 μl/g-min).11 The brain uptake of [131I]sulfamidase 10 minutes after IV injection in neonates (Figures 1 and 3, and Supplementary Figure S2) was also found to be lower than that of P-GUS, and lesser distributions of [131I]sulfamidase were also shown in peripheral tissues including heart, lung, liver, and spleen when compared with those reported for P-GUS. Although both sulfamidase and P-GUS are high-affinity ligands for the M6P/IGF2 receptor,19,20 the difference in the systemic distribution between them can probably be explained by the difference in the number of M6P residues on the two enzymes, which determines the efficiency of their uptake. This is supported by data showing that M6P-inhibitable uptake of sulfamidase by fibroblasts was somewhat lower for sulfamidase than that of P-GUS (data not shown).
Although ERT has been effective in addressing many manifestations of LSDs,21 inability to deliver the enzyme across the BBB after the newborn period remains a challenging obstacle to the treatment of CNS LSDs. For this reason, the development of strategies for enhanced delivery of sulfamidase to the brain is of particular importance. Recently, high-dose ERT in adult MPS VII mice was partially successful in clearing CNS storage.22 The mechanisms of transport across the BBB in the adult when enzymes are given in such large doses, are not yet clear.23
This study provides a second example of the M6P/IGF2 receptor-mediated transport across the BBB in newborn mice at much lower doses, and this is probably a transport process that is available to all phosphorylated lysosomal enzymes. Utilizing this transport system beyond the neonatal period could be of benefit in treating many LSDs that produce CNS storage and neurological disability. Doing so will require discovery of pharmacological methods to restore the receptor-mediated transcytosis activity seen in the neonate to the adult BBB. We recently reported that epinephrine restores M6P receptor-mediated transport of infused P-GUS to levels seen in the neonate.24 This approach could potentially allow delivery of all M6P-containing ligands across the BBB in the adult and greatly improve the prospect for treatment of CNS lysosomal storage in many disorders. However, these promising findings have so far been limited to rodent models. Whether they can be extended to deliver enzymes across the BBB in humans with LSDs is an important question that needs to be answered by further research.
MATERIALS AND METHODS
Construction of mammalian expression vector for human sulfamidase
The complementary DNA for human sulfamidase (Image Clone #5226903, Open Biosystems, Huntsville, AL) was sequenced to confirm the integrity of the complementary DNA and a 1.8-kb fragment containing the entire coding region subcloned into the mammalian expression vector, pCXN (pCXN-hsulfamidase).25
Establishment of a CHO-K1 stable cell line expressing recombinant human sulfamidase
We established a CHO-K1 cell line stably expressing human sulfamidase by electroporation as described earlier 26 and used a highly expressing clone to produce enzyme for purification from 2-day collections in PF CHO medium (Sigma-Aldrich, St. Louis, MO).
Purification of recombinant human sulfamidase
Conditioned medium stored at −20 °C was thawed, pooled, and filtered through a 0.2-μm filter, and assayed using 4-methylumbelliferyl-α-N-sulphoglucosaminide.27 The medium was concentrated by ultrafiltration in an Amicon stirred cell with a BioMax 50, 50,000 MW cutoff membrane, dialyzed against three changes of 50 mmol/l sodium acetate, 0.025% NaN3, pH 4.7, centrifuged to remove a substantial precipitate, and applied to a 25 ml column of SP-Sepharose equilibrated with 50 mmol/l sodium acetate, 0.025% NaN3, pH 4.7. After washing with 10 column volumes of equilibration buffer, the sulfamidase was eluted with a 0–500 mmol/l NaCl gradient in the same buffer. Selected fractions containing sulfamidase activity were pooled and concentrated in multiple Centricon-10 units. The concentrate was applied to a 350 ml Sephacryl S-300 column equilibrated with 50 mmol/l sodium acetate, 150 mmol/l NaCl, 0.025% NaN3, pH 5.0, and eluted using the same buffer. The sulfamidase peak was located by enzyme assay and the selected fractions were pooled and concentrated using Centricon-10 units. Batches were analyzed for sulfamidase activity, protein by micro-Lowry and sodium dodecyl sulfate-polyacrylamide gel electrophoresis + silver stain to determine purity. P-GUS was produced and purified as described earlier.11
Endocytosis of human sulfamidase by human MPS IIIA fibroblasts
Culture dishes (35 mm) of GM-312 human MPS IIIA fibroblasts were incubated with 30 U ± 2 mmol/l M6P for 7 hours at 37 °C, 5% CO2. The dishes were placed on ice and washed 5× with ice-cold saline, and then 0.5 ml of 25 mmol/l Tris, pH 7.2, 140 mmol/l NaCl buffer was added per dish. After being frozen at −20 °C, the plates were thawed and scraped. The extracts were transferred to 1.7-ml microcentrifuge tubes, freeze-thawed twice, and sonicated for 15 seconds in a cup horn sonicator. After centrifugation at 14,000g for 5 minutes, the supernatants were removed and dialyzed against 5 mmol/l sodium acetate, pH 4.0, for 6 hours at 4 °C. The dialyzed homogenates were assayed for sulfamidase27 and protein.28 Uptake values are presented as units of sulfamidase taken up per milligram protein in 7 hours. The data are compared against the data adapted from Bielicki et al.1
Inhibition of P-GUS uptake by purified sulfamidase
Culture dishes (35 mm) of GM-2784 human MPS VII fibroblasts were incubated with 5,000 U (1 μg) of purified P-GUS, either alone or with the addition of 2.5 or 100 μg purified sulfamidase ± 2 mmol/l M6P at 37 °C, 5% CO2 for 2 hours. The cells were washed 5× with ice-cold phosphate-buffered saline, solubilized in 0.5 ml of 1% sodium deoxycholate, and assayed for P-GUS and protein. The data are presented as the percentages of M6P-dependent uptake in the control in the presence or absence of purified sulfamidase.
Radioactive labeling
Sulfamidase was radioactively labeled using the iodobead method (Pierce, Rockford, IL) with [131I]Na (Amersham Pharmacia, Piscataway, NJ), as we reported earlier in the context of labeling GUS.11 The use of a single iodobead allows controlled iodination, so that both enzymatic activity and susceptibility to endocytosis by the M6PR are preserved. Labeled, active enzyme was separated from free iodine on a Sephadex G-10 column. Albumin was labeled with [125I]Na (Amersham Pharmacia) using the chloramine-T method, and purified on a column of Sephadex G-10. Each reagent was freshly prepared on the day of the experiment.
Animals
Male CD-1 mice from our in-house colony were studied at 2 days and 8 weeks of age. The mice had free access to food and water and were maintained on a 12-hour dark/light cycle in a room with controlled temperature (24 ± 1 °C) and humidity (55 ± 5%). The studies were approved by the Saint Louis University Animal Care and Use Committee, and carried out in a facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care.
Drug administration and experimental procedures
Mice were anesthetized with isoflurane before receiving an IV injection of [131I]sulfamidase with [125I]albumin (550,000 cpm of each) into the superficial temporal vein (neonates) or jugular vein (adults). At 1, 2, 3, 4, 5, 7.5, and 10 minutes after the injection, mice were killed under light anesthesia with isoflurane, and the blood, brain, heart, lung, liver, spleen, and kidneys were collected immediately. Sera from mouse blood was isolated by centrifugation. In order to test the M6P receptor dependency of the uptake and tissue distribution of [131I]sulfamidase, unlabeled P-GUS (30 μg) or M6P (2 μmol) was included in the IV injection. The doses of these were determined on the basis of data from our earlier study showing the inhibition of transport of P-GUS mediated through the M6P/IGF2 receptor in the neonatal BBB.11
Multiple-time regression analysis
This method29 was used for calculating the blood-to-brain unidirectional influx rate (Kin) of radiolabeled compounds into the brain. The brain/serum ratios were plotted against exposure time estimated from the equation:
| eq. (1) |
where Am and Cp(t) are the cpm/g of brain and the cpm/μl of serum at time t, respectively. Kin was measured as the slope of the linear portion of the relation between the brain/serum ratios and the respective exposure times. The exposure time was calculated as the area under the serum concentration-time curve (the part of eq. 1 involving the integral sign) divided by the serum concentration at time t. The y-intercept of the line represents Vi, the distribution volume in the brain at t = 0.
Capillary depletion
In order to determine whether [131I]sulfamidase crosses the full width of the cell wall formed by the BBB, capillary depletion was carried out.30 The mice were killed 10 minutes after IV injection of [131I]sulfamidase and [125I]albumin, and the brains were immediately removed and emulsified in a glass homogenizer (8–10 strokes) at 4 °C in a ninefold volume of physiological buffer (10 mmol/l HEPES, 141 mmol/l NaCl, 4 mmol/l KCl, 2.8 mmol/l CaCl2, 1 mmol/l MgSO4, 1 mmol/l NaH2PO4, and 10 mmol/l d-glucose adjusted to pH 7.4). Dextran solution was added to the homogenate to a final concentration of 26%. An aliquot was centrifuged at 5,400g for 15 minutes at 4 °C in a swinging bucket rotor. The pellet containing the brain microvessels and the supernatant containing the brain parenchyma were carefully separated. We confirmed, by γ-glutamyl transferase assay and visualization, that this procedure produces a preparation of highly purified brain capillaries. The results were expressed as capillary/serum and parenchyma/serum ratios, both corrected for vascular space contamination by subtracting the respective ratios for [125I]albumin.
In vivo stability of [131I]sulfamidase in serum and brain
The stability of [131I]sulfamidase in serum and brain was examined using HPLC. Ten minutes after IV injection of [131I]sulfamidase at 5 × 106 cpm in 2-dayold mice, the animals were killed, and the sera (10 μl) were diluted with 90 μl of mobile phase. The brain was homogenized in 200 μl of mobile phase, centrifuged at 20,000g for 15 minutes, and the supernatant was collected. Each sample (100 μl) was injected onto an HPLC (Shimadzu HPLC system LC-10AT, Tokyo, Japan; size exclusion column, BioSep-SEC-S 2000, 7.8φ × 300 mm and guard cartridge system KJO-4282 with AJO-4348, silica, 3.0φ × 4 mm; Phenomenex, Torrance, CA). The extent of degradation of [131I]sulfamidase during the extraction process in the brain was determined by adding [131I]sulfamidase into the brain homogenate. The mobile phase consisted of 25 mmol/l sodium phosphate buffer (pH 6.8). Fractions were collected at 1-minute intervals for 60 minutes at the flow rate of 1.0 ml/min, and the radioactivity in each fraction was detected using a γ counter.
Statistical analysis
The mean values are presented with their SEs and compared by one-way analysis of variance followed by Newman-Keuls multiple comparison test, or by two-tailed paired t-test with Welch's correction for linear regression results using the Prism 4.0 program (GraphPad, San Diego, CA).
Supplementary Material
SDS-PAGE under reducing conditions of two independent preparations of purified recombinant human sulfamidase visualized by silver staining.
Typical radio-HPLC chromatograms in brain and serum 10 minutes after IV injection of [131I]sulfamidase (5 × 106 cpm) in 2-day-old mice.
ACKNOWLEDGMENTS
We gratefully acknowledge Kamelia Markova for technical assistance. We thank Tracey Baird for editorial assistance in the preparation of this manuscript. This study was supported by The Sanfilippo Syndrome Medical Research Foundation (W.A.B. and W.S.S.), VA Merit Review (W.A.B.), R01NS41863 (W.A.B.), and National Institutes of Health grant GM34182 (W.S.S.). This work was done in St. Louis, Missouri in the United States.
REFERENCES
- 1.Bielicki J, Hopwood JJ, Melville EL, Anson DS. Recombinant human sulphamidase: expression, amplification, purification and characterization. Biochem J. 1998;329:145–150. doi: 10.1042/bj3290145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman C, Hopwood JJ. Human liver sulphamate sulphohydrolase. Determinations of native protein and subunit Mr values and influence of substrate agylcone structure on catalytic properties. Biochem J. 1986;234:83–92. doi: 10.1042/bj2340083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott HS, Blanch L, Guo XH, Freeman C, Orsborn A, Baker E, et al. Cloning of the sulphamidase gene and identification of mutations in Sanfilippo A syndrome. Nat Genet. 1995;11:465–467. doi: 10.1038/ng1295-465. [DOI] [PubMed] [Google Scholar]
- 4.Neufeld EF, Meunzer J. The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th edn. McGraw-Hill; New York: 2001. pp. 3421–3452. [Google Scholar]
- 5.Bhaumik M, Muller VJ, Rozaklis T, Johnson L, Dobrenis K, Bhattacharyya R, et al. A mouse model for mucopolysaccharidosis type III A (Sanfilippo syndrome) Glycobiology. 1999;9:1389–1396. doi: 10.1093/glycob/9.12.1389. [DOI] [PubMed] [Google Scholar]
- 6.Perkins KJ, Muller V, Weber B, Hopwood JJ. Prediction of Sanfilippo phenotype severity from immunoquantification of heparan-N-sulfamidase in cultured fibroblasts from mucopolysaccharidosis type IIIA patients. Mol Genet Metab. 2001;73:306–312. doi: 10.1006/mgme.2001.3190. [DOI] [PubMed] [Google Scholar]
- 7.Cleary MA, Wraith JE. Management of mucopolysaccharidosis type III. Arch Dis Child. 1993;69:403–406. doi: 10.1136/adc.69.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gliddon BL, Yogalingam G, Hopwood JJ. Purification and characterization of recombinant murine sulfamidase. Mol Genet Metab. 2004;83:239–245. doi: 10.1016/j.ymgme.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Savas PS, Hemsley KM, Hopwood JJ. Intracerebral injection of sulfamidase delays neuropathology in murine MPS-IIIA. Mol Genet Metab. 2004;82:273–285. doi: 10.1016/j.ymgme.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Gliddon BL, Hopwood JJ. Enzyme-replacement therapy from birth delays the development of behavior and learning problems in mucopolysaccharidosis type IIIA mice. Pediatr Res. 2004;56:65–72. doi: 10.1203/01.PDR.0000129661.40499.12. [DOI] [PubMed] [Google Scholar]
- 11.Urayama A, Grubb JH, Sly WS, Banks WA. Developmentally regulated mannose 6-phosphate receptor-mediated transport of a lysosomal enzyme across the blood-brain barrier. Proc Natl Acad Sci USA. 2004;101:12658–12663. doi: 10.1073/pnas.0405042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moos T, Møllgård K. Cerebrovascular permeability to azo dyes and plasma proteins in rodents of different ages. Neuropathol Appl Neurobiol. 1993;19:120–127. doi: 10.1111/j.1365-2990.1993.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 13.Vorbrodt AW, Dobrogowska DH. Immunocytochemical evaluation of blood-brain barrier to endogenous albumin in adult, newborn and aged mice. Folia Histochem Cytobiol. 1994;32:63–70. [PubMed] [Google Scholar]
- 14.Bar T. The vascular system of the cerebral cortex. Adv Anat Embryol Cell Biol. 1980;59:1–62. doi: 10.1007/978-3-642-67432-7. [DOI] [PubMed] [Google Scholar]
- 15.Stewart PA, Hayakawa EM. Interendothelial junctional changes underlie the developmental “tightening” of the blood-brain barrier. Brain Res. 1987;429:271–281. doi: 10.1016/0165-3806(87)90107-6. [DOI] [PubMed] [Google Scholar]
- 16.Lee SJ, Nathans D. Proliferin secreted by cultured cells binds to mannose 6-phosphate receptors. J Biol Chem. 1988;263:3521–3527. [PubMed] [Google Scholar]
- 17.Sklar MM, Kiess W, Thomas CL, Nissley SP. Developmental expression of the tissue insulin-like growth factor II/mannose 6-phosphate receptor in the rat. Measurement by quantitative immunoblotting. J Biol Chem. 1989;264:16733–16738. [PubMed] [Google Scholar]
- 18.Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu Rev Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh P, Dahms NM, Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol. 2003;4:202–212. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- 20.Hawkes C, Kar S. The insulin-like growth factor-II/mannose-6-phosphate receptor: structure, distribution and function in the central nervous system. Brain Res Brain Res Rev. 2004;44:117–140. doi: 10.1016/j.brainresrev.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Desnick RJ. Enzyme replacement and enhancement therapies for lysosomal diseases. J Inherit Metab Dis. 2004;27:385–410. doi: 10.1023/B:BOLI.0000031101.12838.c6. [DOI] [PubMed] [Google Scholar]
- 22.Vogler C, Levy B, Grubb JH, Galvin N, Tan Y, Kakkis E, et al. Overcoming the blood-brain barrier with high-dose enzyme replacement therapy in murine mucopolysaccharidosis VII. Proc Natl Acad Sci USA. 2005;102:14777–14782. doi: 10.1073/pnas.0506892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banks WA. Are the extracellular [correction of extracelluar] pathways a conduit for the delivery of therapeutics to the brain? Curr Pharm Des. 2004;10:1365–1370. doi: 10.2174/1381612043384862. [DOI] [PubMed] [Google Scholar]
- 24.Urayama A, Grubb JH, Banks WA, Sly WS. Epinephrine enhances lysosomal enzyme delivery across the blood brain barrier by up-regulation of the mannose 6-phosphate receptor. Proc Natl Acad Sci USA. 2007;104:12873–12878. doi: 10.1073/pnas.0705611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orii KO, Grubb JH, Vogler C, Levy B, Tan Y, Markova K, et al. Defining the pathway for Tat-mediated delivery of beta-glucuronidase in cultured cells and MPS VII mice. Mol Ther. 2005;12:345–352. doi: 10.1016/j.ymthe.2005.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LeBowitz JH, Grubb JH, Maga JA, Schmiel DH, Vogler C, Sly WS. Glycosylation-independent targeting enhances enzyme delivery to lysosomes and decreases storage in mucopolysaccharidosis type VII mice. Proc Natl Acad Sci USA. 2004;101:3083–3088. doi: 10.1073/pnas.0308728100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karpova EA, Voznyi Y, Keulemans JL, Hoogeveen AT, Winchester B, Tsvetkova IV, et al. A fluorimetric enzyme assay for the diagnosis of Sanfilippo disease type A (MPS IIIA) J Inherit Metab Dis. 1996;19:278–285. doi: 10.1007/BF01799255. [DOI] [PubMed] [Google Scholar]
- 28.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 29.Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- 30.Triguero D, Buciak J, Pardridge WM. Capillary depletion method for quantification of blood-brain barrier transport of circulating peptides and plasma proteins. J Neurochem. 1990;54:1882–1888. doi: 10.1111/j.1471-4159.1990.tb04886.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDS-PAGE under reducing conditions of two independent preparations of purified recombinant human sulfamidase visualized by silver staining.
Typical radio-HPLC chromatograms in brain and serum 10 minutes after IV injection of [131I]sulfamidase (5 × 106 cpm) in 2-day-old mice.