Abstract
Objective
To assess the evidence for a differential effect of positive prevention interventions among individuals infected and not infected with human immunodeficiency virus (HIV) in developing countries, and to assess the effectiveness of interventions targeted specifically at people living with HIV.
Methods
We conducted a systematic review and meta-analysis of papers on positive prevention behavioural interventions in developing countries published between January 1990 and December 2006. Standardized methods of searching and data abstraction were used. Pooled effect sizes were calculated using random effects models.
Findings
Nineteen studies met the inclusion criteria. In meta-analysis, behavioural interventions had a stronger impact on condom use among HIV-positive (HIV+) individuals (odds ratio, OR: 3.61; 95% confidence interval, CI: 2.61–4.99) than among HIV-negative individuals (OR: 1.32; 95% CI: 0.77–2.26). Interventions specifically targeting HIV+ individuals also showed a positive effect on condom use (OR: 7.84; 95% CI: 2.82–21.79), which was particularly strong among HIV-serodiscordant couples (OR: 67.38; 95% CI: 36.17–125.52). Interventions included in this review were limited both in scope (most were HIV counselling and testing interventions) and in target populations (most were conducted among heterosexual adults or HIV-serodiscordant couples).
Conclusion
Current evidence suggests that interventions targeting people living with HIV in developing countries increase condom use, especially among HIV-serodiscordant couples. Comprehensive positive prevention interventions targeting diverse populations and covering a range of intervention modalities are needed to keep HIV+ individuals physically and mentally healthy, prevent transmission of HIV infection and increase the agency and involvement of people living with HIV.
الملخص
الغرض
تقييم البيّنات الخاصة بفروق تأثير التدخلات الوقائية الإيجابية بين المصابين وغير المصابين بفيروس الإيدز في البلدان النامية، وتقييم فعالية التدخلات التي استهدفت المعايشين لفيروس الإيدز.
الطريقة
أجرى الباحثون مراجعة منهجية وتحليلاً تلوياً للبحوث الخاصة بالتدخلات السلوكية الوقائية الإيجابية في البلدان النامية والتي نُشرت خلال الفترة من كانون الثاني/يناير 1990 وكانون الأول/ديسمبر 2006. واستخدم الباحثون طرقاً معيارية للبحث واستخلاص المعطيات، وحسبوا أحجام التأثير الجماعي باستخدام نماذج التأثيرات المعشاة.
الموجودات
تلاءمت تسع عشرة دراسة مع خصائص الإدراج في المراجعة. وفي التحليل التلوي كان للتدخلات السلوكية تأثير أقوى على استخدام العازل الذكري بين الإيجابيين لفيروس الإيدز (نسبة الأرجحية: 3.61؛ وفاصلة الثقة 95%: 2.61 – 4.99) مقارنة بالسلبيين لفيروس الإيدز (نسبة الأرجحية: 1.32؛ فاصلة الثقة 95%: 0.77 – 2.26). كما أظهرت التدخلات التي استهدفت على وجه الخصوص الإيجابيين للفيروس تأثيراً إيجابياً على استخدام العازل الذكري (نسبة الأرجحية 7.84؛ فاصلة الثقة 95% 2.82 – 21.79)، وكان التأثير أقوى على وجه الخصوص بين الزوجين المختلفين في الحالة المصلية (نسبة الأرجحية: 67.38؛ فاصلة الثقة 95%: 36.17 – 125.52). وكانت التدخلات المدرجة في هذه المراجعة محصورة النطاق (أكثرها كانت التدخلات الخاصة بمشورة واختبار فيروس الإيدز) ومحصورة في الفئات السكانية المستهدفة (أكثرها أجريت بين البالغين المشتهين للجنس المغاير أو الأزواج المختلفين في الحالة المصلية).
الاستنتاج
تشير البيّنات الحالية إلى أن التدخلات التي تستهدف المعايشين لفيروس الإيدز في البلدان النامية أدت إلى زيادة استخدام العازل الذكري، ولاسيما بين الأزواج المختلفين في الحالة المصلية. وهناك حاجة إلى التدخلات الوقائية الإيجابية الشاملة التي تستهدف مختلف الفئات السكانية وتغطي مجالاً من الأنماط الوقائية للحفاظ على الصحة البدنية والنفسية للإيجابيين لفيروس الإيدز، ومنع انتقال العدوى بالفيروس، وزيادة نشاط ومشاركة المعايشين للفيروس.
Résumé
Objectif
Évaluer les éléments probatoires d’un effet différentiel des interventions de prévention efficaces chez les sujets infectés et non infectés par le virus de l'immunodéficience humaine (VIH) dans les pays en développement, et évaluer l’efficacité des interventions s’adressant de manière spécifique aux personnes vivant avec le VIH.
Méthodes
Nous avons conduit une révision systématique et une méta-analyse des articles scientifiques sur les interventions comportementales de prévention efficaces dans les pays en développement publiés entre janvier 1990 et décembre 2006. Des méthodes standardisées de recherche et d'abstraction de données ont été utilisées. La taille des effets globalisés a été calculée en utilisant des modèles à effets aléatoires.
Résultats
Dix-neuf études présentaient les critères d’inclusion. D’après la méta-analyse, les interventions comportementales ont eu une plus forte incidence sur l’utilisation du préservatif chez les individus séropositifs (VIH+) (rapport de cotes, RC: 3,61 ; intervalle de confiance à 95 %, IC: 2,61-4,99) que chez les individus séronégatifs (RC: 1,32; IC à 95 %: 0,77-2,26). Les interventions ciblant spécifiquement les individus VIH+ ont également montré un effet positif sur l’utilisation du préservatif (RC: 7,84; IC à 95 %: 2,82- 21,79), particulièrement élevé parmi les couples sérodifférents (RC: 67,38; IC à 95 %: 36,17-125,52). Les interventions comprises dans cette analyse étaient limitées à la fois dans leur but (la plupart étaient des interventions de conseil et de dépistage du VIH) et dans leurs populations cibles (la plupart ont été réalisées auprès d’adultes hétérosexuels ou de couples sérodifférents).
Conclusion
Les preuves dont nous disposons actuellement suggèrent que les interventions ciblant les personnes vivant avec le VIH dans les pays en développement augmentent l’utilisation du préservatif, notamment chez les couples sérodifférents. Des interventions de prévention positives complètes, ciblant des populations diverses et couvrant un éventail de modalités d’intervention, sont nécessaires pour maintenir les individus VIH+ en bonne santé physique et mentale, prévenir la transmission de l’infection à VIH et augmenter l’action et l’implication des personnes vivant avec le VIH.
Resumen
Objetivo
Evaluar los datos relacionados con el efecto diferencial de las intervenciones favorables en prevención entre individuos infectados y no infectados por el virus de la inmunodeficiencia humana (VIH) en los países en desarrollo y evaluar la eficacia de las intervenciones dirigidas específicamente a las personas que conviven con el VIH.
Métodos
Se llevó a cabo una revisión sistemática y un metanálisis de artículos sobre intervenciones conductuales para la prevención positiva en países en desarrollo, publicados entre enero de 1990 y diciembre de 2006. Se emplearon métodos estandarizados de búsqueda y de extracción de datos. Las magnitudes de los efectos agrupados se calcularon mediante la utilización de modelos de efectos aleatorios.
Resultados
Diecinueve estudios cumplían los criterios de inclusión. Por lo que respecta al metanálisis, las intervenciones conductuales tuvieron un mayor impacto sobre el uso del preservativo entre los individuos VIH-positivos (VIH+) (oportunidad relativa, OR: 3,61; intervalo de confianza del 95%, CI: 2,61 - 4,99) que entre los individuos VIH-negativos (OR: 1,32; CI del 95%: 0,77 - 2,26). Las intervenciones específicas dirigidas a los individuos VIH+ también tuvieron un efecto positivo en el uso del preservativo (OR: 7,84; CI del 95%: 2,82 - 21,79) y, en especial, entre las parejas serodiscordantes al VIH (OR: 67,38; CI del 95%: 36,17 - 125,52). Las intervenciones incluidas en esta revisión estuvieron limitadas tanto por el alcance de las mismas (la mayoría eran intervenciones de asesoramiento y pruebas del VIH) como por las poblaciones diana (la mayoría se llevaron a cabo entre adultos heterosexuales o parejas discordantes al VIH).
Abstract
Conclusión
Los datos actuales sugieren que las intervenciones dirigidas a las personas que conviven con el VIH en los países en desarrollo incrementan el uso del preservativo, especialmente entre parejas serodiscordantes al VIH. Las intervenciones exhaustivas de prevención positiva dirigidas a distintas poblaciones y que abarcan varios tipos de intervenciones son necesarias para mantener la salud física y psíquica de las personas VIH+, prevenir la transmisión de la infección por el VIH y aumentar la capacidad de actuación y de implicación de las personas que conviven con el VIH.
Introduction
Historically, efforts to prevent human immunodeficiency virus (HIV) infection have focused on reducing HIV infection risk among individuals with HIV-negative (HIV−) or unknown serostatus. Initially, this reflected concerns over stigmatization and discrimination associated with interventions targeting HIV-infected (HIV+) individuals and limited availability of HIV testing services.1 Recently, however, there has been a dramatic scale-up of HIV testing, antiretroviral therapy (ART) availability and associated care worldwide. Consequently, many more people living with HIV now know their serostatus and are living longer and healthier lives.2
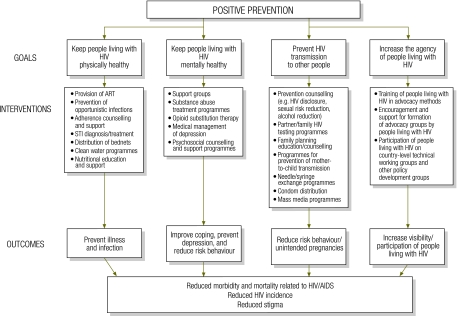
Today, programme planners recognize that continued reliance on general HIV prevention messages may limit the effectiveness and sophistication of prevention strategies.3 It may be more efficient to change behaviour among fewer HIV+ individuals than many HIV− individuals.4 Recent data show that in many sub-Saharan African countries, most new cases of HIV infection occur in HIV-serodiscordant couples, and rates of HIV disclosure and condom use in such couples remain low.4,5 Focusing attention on HIV-serodiscordant couples may therefore be one of the most effective ways of reducing HIV transmission. Efforts to reduce stigma have alleviated some of the concerns regarding prevention programmes aimed at HIV-infected persons.4 As a result, HIV prevention activities increasingly target individuals who know that they are HIV+.6 This strategy is known as positive prevention, although it has also been called prevention for, by or with positives,1,7–11 and, most recently, positive health, dignity and prevention.12 There is no clear consensus on what positive prevention entails, but it generally includes activities centred on four main goals: (i) keeping HIV+ individuals physically healthy; (ii) keeping such persons mentally healthy; (iii) preventing further transmission of HIV; and (iv) involving people living with HIV in prevention activities, leadership and advocacy.4,13 Fig. 1 outlines a conceptual framework that shows how positive prevention goals are related to selected interventions and outcomes. The framework is broad and includes biomedical as well as behavioural interventions. The scope of our review was limited to behavioural interventions, which allowed for a more focused examination of one aspect of positive prevention.
Fig. 1.
Conceptual framework showing goals, selected interventions and outcomes of positive preventiona
AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; HIV, human immunodeficiency virus; STI, sexually transmitted infection.
a “Positive prevention” denotes preventive interventions that target HIV+ individuals.
Three previous reviews have examined behavioural interventions targeting people living with HIV.14–16 However, almost all the included studies had been conducted in the United States of America. There have been no similar reviews of positive prevention interventions in developing country settings. Given the scale-up of HIV testing and treatment in developing countries and the unique social, economic and epidemiologic features of these settings, the purpose of this paper was to assess the efficacy of HIV prevention interventions with HIV+ individuals in developing country settings.
Methods
Objectives
This review is part of a larger series of systematic reviews of HIV-related behavioural interventions in developing countries. Other interventions reviewed include mass media interventions,17 psychosocial support,18 treatment as prevention,19 voluntary counselling and testing20 and peer education.21 We used standardized methods across all reviews and report results according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement.22
People living with HIV may be reached by interventions that target a broad audience of both HIV+ and HIV− individuals or by interventions that target them specifically. Our review therefore had two objectives. The first was to assess the evidence for a differential effect of interventions by serostatus. In other words, do interventions that target both HIV+ and HIV− individuals work differently in these two groups? The second was to assess the effectiveness of interventions targeted specifically at HIV+ individuals.
Inclusion criteria
Studies were included in the review if they met the following criteria: (i) an HIV-specific behavioural intervention was implemented; (ii) the intervention was conducted in a developing country, defined on the basis of The World Bank categories of low-income, lower-middle income or upper-middle income economies23; (iii) the evaluation design compared post-intervention outcomes using either a pre/post or multi-arm study design (including post-only exposure analysis); (iv) behavioural, psychological, social, care or biological outcome(s) related to HIV prevention were presented; (v) pre-post or multi-arm outcomes of interest were stratified by known or clinically suspected HIV serostatus of the participants (objective 1), or the intervention specifically targeted HIV+ individuals (objective 2); and (vi) the article was published in a peer-reviewed journal between January 1990 and December 2006. No language restrictions were applied; English translations were obtained when necessary. If two articles presented data for the same project and target population, the article with the longest follow-up was retained for analysis.
Search strategy
First, we reviewed all articles included in the larger series of systematic reviews of HIV-related behavioural interventions in developing countries to determine whether they met the criteria for positive prevention. Our review encompassed articles previously published and reviews of interventions currently in progress, including condom social marketing, partner notification, free condom distribution, abstinence-based interventions, comprehensive sex education interventions, needle/syringe programmes, family planning for HIV+ women and behavioural counselling.
Second, we searched electronic databases specifically for positive prevention articles. A standard set of search terms (available at: http://www.jhsph.edu/dept/ih/globalhealthresearch/HIVpositiveprevention.pdf) was generated and entered into five electronic databases, all of which covered the full range of included dates: the United States National Library of Medicine’s Gateway system (including Medline), PsycINFO, Sociological Abstracts, Excerpta Medica Database (EMBASE) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL). Links to medical subject heading terms and explosion of terms were used where available.
Third, we hand-searched the tables of contents of four journals: AIDS, AIDS and Behaviour, AIDS Care and AIDS Education and Prevention. We also examined the reference lists of included articles to identify articles we might have missed. This process was iterated until no new articles were found.
Study selection
Initial inclusion/exclusion of studies was based on title and abstract review by a member of the study staff. Remaining citations were then screened by two senior study staff on the basis of the inclusion criteria above. The results were merged for comparison, and discrepancies were discussed to establish consensus. Final inclusion/exclusion of studies was based on a thorough reading of the full-text article.
Data extraction
Each article meeting the inclusion criteria underwent data extraction by two independent reviewers. Data were entered into a systematic coding form that included detailed questions on intervention, study design, methods and outcomes. The two completed coding forms were compared and discrepancies were resolved by a third reviewer.
Rigour score
The rigour of the study design for included articles was assessed by means of an eight-point scale, with one point awarded for each of the following items: (i) prospective cohort; (ii) control or comparison group; (iii) pre-/post-intervention data; (iv) random assignment of participants to the intervention; (v) random selection of subjects for assessment, or assessment of all subjects who participated in the intervention; (vi) follow-up rate of 80% or more; (vii) comparison groups equivalent on socio-demographic measures; and (viii) comparison groups equivalent at baseline on outcome measures.
Meta-analysis
We converted effect size estimates to the common metric of an odds ratio, since all studies compared two groups and reported dichotomous outcomes. We used standard meta-analytic methods to derive standardized effect size estimates24 and used Comprehensive Meta-Analysis V.2.2 (Biostat, Inc., Englewood, United States of America) to conduct statistical analyses. For each outcome, we entered odds ratios (ORs) directly into the program or calculated ORs from data reported in articles. ORs were pooled using random effects models. We attempted to contact authors when published articles provided insufficient information to make these calculations.
Meta-analysis was conducted for outcomes reported in at least three studies. For both study objectives, the only outcome that met this criterion was male condom use. Condom use was defined in terms of the dichotomous proportion of respondents who either: (i) did or did not use condoms, or (ii) did or did not have unprotected sex. When articles presented multiple measures of condom use (e.g. condom use at last sexual encounter, consistent condom use in the last 3 months, condom use with primary/non-primary partners), we calculated an average effect size across measures within each study and used the average effect size estimate in cross-study meta-analysis. When articles presented multiple follow-up times, we used the comparison with the longest follow-up. We also summarize results for outcomes that were common across two studies, although data from these studies were not meta-analysed: contraceptive use, multiple sex partners and HIV serostatus disclosure.
Results
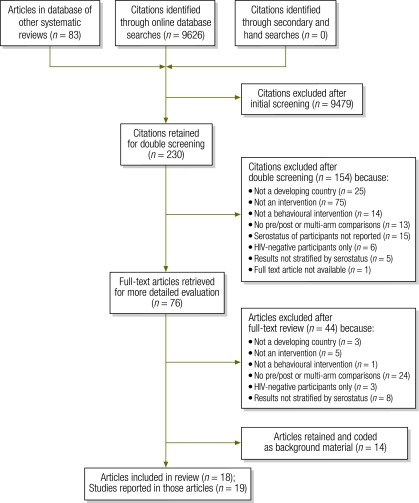
From over 9000 articles identified in the initial search, 230 were determined to be potentially relevant and 18 ultimately met our inclusion criteria (Fig. 2).25–42 These 18 articles reported on 19 studies, as one article described both an individual and a couples-based intervention.32 Of the studies included in the review, 15 were conducted in sub-Saharan African countries, 1 in Asia (China), 1 in South America (Brazil), and 2 (reported in one article) in three countries (Kenya, United Republic of Tanzania and Trinidad and Tobago). Target populations included heterosexual adults in 12 studies; HIV-serodiscordant couples in 5; pregnant women in 1, and commercial sex workers in 1. Most studies (n = 14) were conducted in a clinic setting, 2 in participants’ homes and 2 in both clinic and home settings. One study did not report the setting. Table 1 and Table 2 (available at: http://www.who.int/bulletin/volumes/88/8/09-068213) provide further information on individual study characteristics and rigour scores. On average, studies received 3.9 out of 8 possible points for study design and rigour. There was no clear association between study rigour and results, most likely owing to multiple sources of heterogeneity across studies (in setting, target population, intervention and comparison groups) and to differences in study quality.
Fig. 2.
Disposition of citations during the search and screening process in systematic review of positive preventiona interventions in developing countries
HIV, human immunodeficiency virus.
a “Positive prevention” denotes preventive interventions that target HIV+ individuals.
Table 1. Studies included in systematic review of the literature on behavioural interventions for HIV positive prevention in developing countries.
| Author and year | Setting | Population characteristics | Description | Study design |
|---|---|---|---|---|
| Interventions targeting HIV+ and HIV− individuals | ||||
| Allen et al., 199325 | Kigali, Rwanda | Female paediatric and antenatal care clients Gender: 100% female Agea range: 18–35 |
35-minute educational video and group discussion led by physician and social worker. HIV test results and counselling 3 weeks later. At request of study subjects, male sex partners invited to watch video, attend group sessions and be tested for HIV. Couples could choose to receive test results together. Condoms and spermicide distributed free. | Time series, no comparison arm. Assessments at baseline (n = 1 458), at 12 months (n = 1 254) and at 24 months (n = not reported). Unit of analysis: individual. Participants not randomly selected. |
| Allen et al., 199226 | Kigali, Rwanda | Female paediatric and antenatal care clients Gender: 100% female Mean age: 29 Age range: 20–40 |
35-minute educational video and group discussion led by physician and social worker. HIV test results and counselling 3 weeks later. At request of study subjects, male sex partners invited to watch video, attend group sessions and be tested for HIV. Couples could choose to receive test results together. Condoms and spermicide distributed free. | Cross-sectional, comparing women who were tested for HIV individually with those tested with their partners. Unit of analysis: individual. Participants not randomly selected. |
| Farquhar et al., 200427 | Nairobi, Kenya | Women attending antenatal care and their partners Gender: 100% female Mean age (SD): women tested individually: 23.7 (4.4) women whose partners were tested; results received individually: 24.1 (4.6) women whose partners were tested; results received as couple: 23.8 (4.4) |
Group education about HIV transmission, with encouragement to inform male partners about HIV VCT. Return in 1 week, alone or with partner, for optional VCT, counselling on safe sex during pregnancy and on breastfeeding, and free condoms; return in 2 weeks for more counselling: if HIV−, breastfeeding recommended; if HIV+, other infant feeding options given, along with nevirapine for both mother and infant and counselling on its use at delivery. More counselling and optional infant HIV testing offered 3 and 6 months postpartum. Women with HIV-related symptoms treated and referred to local clinics. | Prospective cohort, no comparison arm. Assessments at baseline (group education, n = 2 836), at 1 week (VCT, n = 2 104) and at 2 weeks (follow-up counselling, n = 1 630). In addition, 122 HIV+ women returned 1 week postpartum. Unit of analysis: individual. Participants not randomly selected. |
| King et al., 199528 | Kigali, Rwanda | HIV+ and HIV− urban women Gender: 100% female Age distribution: 20–24 (4.4%) 25–29 (28.7%) 30–34 (36.7%) 35–39 (25.7%) 40–44 (4.6%) |
15-minute educational video in Kinyarwanda on contraceptive methods and group discussion led by nurse. Oral contraceptives, injectable progestins and Norplant provided free to women enrolled in programme. Other contraceptive methods made available to women and their partners were intrauterine devices, condoms (both before and after intervention), tubal ligation and vasectomy. | Time series, no comparison arm. Assessments at baseline (n = 502) and after intervention (n = 470). Average follow-up time differed by participant and outcome. Unit of analysis: individual. Participants not randomly selected. |
| Machekano et al., 199829 | Harare, Zimbabwe | Male factory workers Gender: 100% male Age: NR |
Pre-test counselling in factories; subjects encouraged to visit project clinic for HIV test results and counselling 2 weeks after blood draw. Free STD diagnostic and treatment services and condoms also available at project clinic. Video on preventing HIV infection shown at all times in waiting area of project clinic. | Time series, no comparison arm. Assessments at baseline (n = 2 414) and after intervention (n = 2060). Mean follow-up time per subject was 1.2 years. Unit of analysis: individual. Participants not randomly selected. |
| Pickering et al., 199330 | The Gambia | Female commercial sex workers Gender: 100% female Mean age: 31.9 |
Sex workers examined, treated for existing STDs and tested for HIV. Given test results and counselled in subsequent visit. All given free condoms and told to return to clinic for treatment or condoms whenever necessary. Free condoms also distributed daily in all bars included in study. | Time series, no comparison group. Assessments at baseline (n = 31), at 1 month (n = 29) and at 2–5 months (n = 31). Unit of analysis: individual. Participants not randomly selected. |
| Roth et al., 200131 | Kigali, Rwanda | Heterosexual males in cohabitating union Gender: 50% male, 50% female Mean age: males: 39 females: 32 |
Male-focused counselling programme with educational video entitled “Responsibility” and small group discussion. During return visit, trained counsellor gave men their HIV test results in individual counselling session and encouraged them to share them with partners; all female partners had already been tested for HIV. | Before/after, no comparison arm. Assessments at baseline (n = 684 couples) and at 12 months (n = 684 couples). Unit of analysis: individual and couple. Participants not randomly selected. |
| VCT Efficacy Group, 2000 Individuals32 | Nairobi, Kenya; Dar es Salaam, United Republic of Tanzania; Port of Spain, Trinidad and Tobago | General population: individuals Gender: 49.2% males, 50.8% females Mean age (SD): intervention males: 28.9 (9.7) intervention females: 28.6 (8.6) control males: 28.1 (9.1) control females: 28.5 (8.8) |
Individuals randomized to HIV VCT or health information. VCT arm: client-centred counselling (personalized risk assessment and risk-reduction plan, role plays and condom use demonstrations); test results available 2 weeks after blood draw. Health information arm: 15-minute video and group discussion about HIV and condom use. | Randomized controlled trial with 1 intervention and 1 control group. Assessments at baseline (n = 3 120), at 7 months (n = 2 550) and at 14 months (n = 2 196). Unit of analysis: individual. Participants not randomly selected. |
| VCT Efficacy Group, 2000 Couples32 | Nairobi, Kenya; Dar es Salaam, United Republic of Tanzania; and Port of Spain, Trinidad and Tobago | General population: couples Gender: 50% males, 50% females Mean age (SD): intervention males: 31.5 (8.4) intervention females: 25.9 (6.6) control males: 32.1 (32.1) control females: 26.7 (7.4) |
Couples randomized to HIV VCT or health information. VCT arm: client-centred counselling (personalized risk assessment and risk-reduction plan, role plays and condom use demonstrations); test results available 2 weeks after blood draw. Health information arm: 15-minute video and group discussion about HIV and condom use; VCT offered at first follow-up. | Randomized controlled trial with 1 intervention and 1 control group. Assessments at baseline (n = 1 174), 7-months (n = 1 001) and at 14months (n = 890). Unit of analysis: individual. Participants not randomly selected. |
| Interventions targeting HIV+ individuals only | ||||
| Allen et al., 200333 | Lusaka, Zambia | HIV serodiscordant couples Gender: 50% male, 50% female Age: NR |
Same-day couples’ VCT service, with free diagnosis and treatment of STDs, condom skills training and free condoms. More counselling provided on request at 3-month intervals and when sexual contacts without protection were reported. | Before/after, no comparison arm. Assessments at baseline (n = 818 couples) and at 12 months (n = 584 couples). Unit of analysis: couple. Participants not randomly selected. |
| Allen et al., 199234 | Kigali, Rwanda | HIV serodiscordant couples Gender: 50% male, 50% female Age: NR |
Educational video and group discussion led by social worker. Free condoms and spermicides offered. At post-test counselling, project counsellor confidentially distributed HIV test results individually, but couples encouraged to receive them together. | Before/after, no comparison arm. Assessments at baseline (n = 60 couples) and at 12 months (n = 53 couples). Unit of analysis: couple. Participants not randomly selected. |
| Balmer et al., 199435 | Nairobi, Kenya | HIV+ individuals Gender: NR Age: NR |
Weekly group counselling sessions for 6 months to initiate and sustain behaviour change and provide psychological support. Sessions based on unified HIV/AIDS counselling theory combining behavioural, psychoanalytical and humanistic axioms. | Randomized controlled trial. Assessments at baseline (n = 20) and at 6 months (n = 20). Unit of analysis: individual. Participants not randomly selected. |
| Bunnell et al., 200636 | Tororo, Uganda | HIV+ adults initiating ART Gender: 25.4% male, 74.6% female Median age: males: 41; females: 37 |
ART delivered weekly at home, with referral as needed for free medical and psychological care. Behavioural intervention with group education on ART at enrolment, testing of cohabitating partners through home-based family VCT and counselling on risk reduction strategies to protect HIV− partners or those with unknown HIV status. Free condoms provided on request. | Before/after, no comparison arm. Assessments at baseline (n = 926) and at 6 months (n = 815). Unit of analysis: individual, couple and aggregated cohort. Participants not randomly selected. |
| Da Silveira et al., 200637 | Pelotas, Brazil | HIV+ women attending HIV outpatient clinic Gender: 100% female Age distribution: 15–19 (5.6%); 20–29 (41.5%); 30–39 (29.4%); 40–49 (17.6%) ≥ 50 (5.9%) |
Educational programme with four educational modules on HIV infection, general health-care measures and condom use delivered by physician during routine medical consultation. Use of flipcharts with graphic displays to reinforce intervention’s main contents. Patients could request as many free condoms as they wished from clinic pharmacy after consultation. | Non-randomized trial with 1 intervention group and 1 control group. Assessments at baseline (n = 340), 30 days (n = 332) and at 60 days (n = 335). Unit of analysis: individual. Participants not randomly selected. |
| Jones et al., 200638 | Lusaka, Zambia | Sexually active HIV+ women Gender: 100% women Mean age: 29 |
Three-session group educational and skills-building intervention with videos and role playing to illustrate sexual barrier products, risk reduction and sexual negotiation strategies. At each visit, participants given1-month supply of male and female condoms and vaginal lubricants and screened and treated for STDs and vaginal infections. | Randomized controlled trial. Assessments at baseline (n = 240), at 6 months (n = 233) and at 12 months (n = 166). Unit of analysis: individual. Participants not randomly selected. |
| Kamenga et al., 199139 | Kinshasa, Democratic Republic of the Congo | HIV serodiscordant couples Gender: 50% male, 50% female Mean age: M+, F− couples: males: 39.8; females: 32.0 M−, F+ couples: males: 37.9; females: 30.7 |
HIV serodiscordant couples invited to special clinic where serostatus confirmed. Each couple was subsequently informed of HIV test results, first individually by counsellor of the same sex and then together as couple by both members of the counselling team. Couples then counselled about STDs, HIV infection and condom use; followed monthly at counselling centre and given condoms and a sexual activity calendar at each visit. | Time series, no comparison arm. Assessments at baseline (n = 168 couples), at 6 months (n = 149 couples) and at 18 months (n = 140 couples). Unit of analysis: couple. Participants not randomly selected. |
| MacNeil et al., 199940 | United Republic of Tanzania | Sexually active HIV+ adults Gender: 34% male, 66% female Mean age: 31.9 |
At least once a month, enhanced care and support consisting of ongoing counselling of HIV+ person on prevention and problem-solving, education of other family members, condom provision and, when necessary, referral for treatment. | Randomized control trial comparing enhanced programme with standard health services. Assessments at baseline (n = 154) and at 6 months (n = 144). Unit of analysis: individual. Participants not randomly selected. |
| Ryder et al., 200041 | Kinshasa, Democratic Republic of the Congo | HIV serodiscordant couples Gender: 50% male, 50% female Mean age: M+, F− couples: males: 40.7; females: 25.4 M−, F+ couples: males: 38.7; females: 31.0 |
Couples tested for HIV and informed of results first individually by counsellor of the same sex and then together as couple; counselled on safe sex, condom use and HIV-associated risks, and followed by monthly counselling sessions to identify difficulties faced and develop corrective strategies. | Time series, no comparison arm. Assessments at baseline (n = 178 couples), at 18 months (n = 167 couples) and at 30 months (n = 139 couples). Unit of analysis: individual and couple. Participants not randomly selected. |
| Yang et al., 200142 | Fuyang City, China | HIV+ individuals and their spouses Gender: 50% male, 50% female Age range: 20–49 |
Couples educated at home on HIV and condom use every other month for 12 months. Emphasis on importance of consistent condom use; free condoms distributed. | Before/after, no comparison arm. Assessments at baseline (n = 90 couples) and at 12 months (n = 84 couples). Unit of analysis: individual and couple. Participants not randomly selected. |
AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; HIV, human immunodeficiency virus; NR, not reported; SD, standard deviation; STD, sexually-transmitted disease; VCT, voluntary counselling and testing.
a Age throughout the table is expressed in years.
Table 2. Quality scoringa of studies included in systematic review of the literature on behavioural interventions for HIV positive prevention in developing countries.
| Author and year | Cohort | Control/comparison group | Pre/post intervention data | Random assignment of participants to intervention | Random selection of participants for assessmentb | Follow-up ≥ 80% | Comparison groups equivalent on socio-demographics | Comparison groups equivalent at baseline on outcome measure | Final score |
|---|---|---|---|---|---|---|---|---|---|
| Interventions targeting both HIV+ and HIV− individuals | |||||||||
| Allen et al., 199325 | 1 | 0 | 1 | 0 | 0 | 1 | NA | NA | 3 |
| Allen et al., 199226 | 0 | 1 | 0 | 0 | 0 | NA | 0 | NA | 1 |
| Farquhar et al., 200427 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | NR | 3 |
| King et al., 199528 | 1 | 0 | 1 | NR | 1 | NR | NA | NA | 3 |
| Machekano et al.,199829 | 1 | 0 | 1 | 0 | 1 | 1 | NA | NA | 4 |
| Pickering et al., 199330 | 1 | 0 | 1 | 0 | 0 | 0 | NA | NA | 2 |
| Roth et al., 200131 | 1 | 0 | 1 | 0 | 0 | NR | NA | NA | 2 |
| VCT Efficacy Group 2000 (individuals)32 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| VCT Efficacy Group 2000 (couples)32 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Interventions targeting only HIV+ individuals | |||||||||
| Allen et al., 200333 | 1 | 0 | 1 | 0 | 0 | 0 | NA | NA | 2 |
| Allen et al.,199234 | 1 | 0 | 1 | 0 | 0 | 1 | NA | NA | 3 |
| Balmer et al., 199435 | 1 | 1 | 1 | 1 | 0 | 1 | NR | 1 | 6 |
| Bunnell et al., 200636 | 1 | 0 | 1 | 0 | 0 | 1 | NA | NA | 3 |
| Da Silveira et al., 200637 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 5 |
| Jones et al., 200638 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Kamenga et al., 199139 | 1 | 0 | 1 | 0 | 1 | 1 | NA | NA | 4 |
| MacNeil et al., 199940 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Ryder et al., 200041 | 1 | 0 | 1 | 0 | 1 | 0 | NA | NA | 3 |
| Yang et al., 200142 | 1 | 0 | 1 | 0 | 0 | 1 | NA | NA | 3 |
NA, not applicable; NR, not reported; VCT, voluntary counselling and testing.
a A score of 1 indicates that the article met the criterion; a score of 0 indicates that it did not. However, studies with mixed designs have been given 1 point in this table.
b If a probability sample was used to select participants, a “1” is in the column. Similarly, if a mixed sampling strategy was used but randomization was conducted in at least one sampling frame, a “1” is in the column. If a census sample of all individuals receiving the intervention was used for assessment, a “1” is also in the column. If a non-probability sample was used, a “0” is in the column.
Differential effect of interventions by serostatus
Nine studies addressed our first objective.25–32 Seven were conducted with heterosexual adults, 1 with pregnant women and 1 with female commercial sex workers. Eight evaluated HIV counselling and testing interventions and 1 evaluated a family planning education programme. Most interventions also included condom distribution. For this objective, 2 outcomes were measured across multiple studies: condom use and contraceptive use.
Condom use
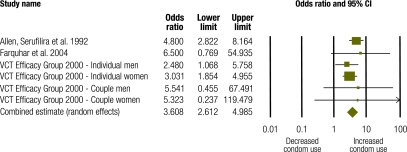
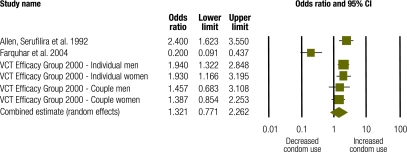
Four studies with a combined study population of 4322 generated 6 discrete effect sizes for condom use among HIV+ and HIV− individuals.26,27,32 Among HIV+ individuals (n = 889), pooled data suggest that interventions had a positive effect on condom use (OR: 3.61; 95% confidence interval, CI: 2.61–4.99) (Fig. 3). The Q statistic of 2.82 showed no statistically significant heterogeneity (P = 0.73; I2 = 0.000). Among HIV− individuals from these same studies (n = 3433), pooled data show no statistically significant intervention effect on condom use (OR: 1.32; 95% CI: 0.77–2.26) (Fig. 4). The Q statistic of 33.14 showed statistically significant heterogeneity (P = 0.0001; I2 = 84.92). Meta-analysis results for HIV+ and HIV− individuals differed significantly (P = 0.002).
Fig. 3.
Meta-analysis of condom use among HIV positive individuals following a behavioural intervention
CI, confidence interval; VCT, voluntary counselling and testing.
Fig. 4.
Meta-analysis of condom use among HIV negative individuals following a behavioural intervention
CI, confidence interval; VCT, voluntary counselling and testing.
The 4 studies that stratified condom use outcomes by serostatus were all evaluations of HIV counselling and testing interventions, and all included comparisons of couples versus individual counselling. Therefore, we conducted meta-analysis comparing couples versus individual counselling for both HIV+ and HIV− individuals. Meta-analysis results showed no difference between couples and individual counselling with respect to condom use among either HIV+ or HIV− individuals (HIV+ pooled effect size: OR: 1.78; 95% CI: 0.48–6.54; Q = 29.15; P = 0.0.0001; I2 = 89.71; HIV− pooled effect size: OR: 0.63; 95% CI: 0.15–2.62; Q = 35.09; P = 0.0001; I2 = 91.45). Meta-analysis results for couples versus individual counselling among HIV+ and HIV− individuals were not significantly different (P = 0.29).
One study27 is an outlier (Fig. 4) with an OR below 1, indicating reduced condom use, probably because of the nature of the comparison group. While other studies employed before–after or intervention–control comparisons, this study compared individuals who received couples counselling with those who received individual counselling. Among HIV− individuals, couples counselling resulted in decreased condom use compared with individual counselling, likely because couples where both partners tested negative felt safe foregoing condom use.
Contraceptive use
Two studies25,26 examined the effect of HIV counselling and testing on contraceptive use, stratified by serostatus. Both studies were conducted by the same research team among women attending antenatal and paediatric clinics in Rwanda. Both showed a limited effect of HIV testing on contraceptive use. In the first study, HIV+ women showed less hormonal contraceptive use over time from baseline to the 12-month follow-up assessment, while HIV− women showed no change in hormonal contraceptive use over time.25 In the second study, HIV+ women were significantly more likely to be using spermicides than HIV− women.26
Interventions targeting HIV+ individuals
Ten studies addressed our second objective: 5 with HIV+ heterosexual adults and 5 with HIV-serodiscordant couples.33–42 All of the latter studies evaluated HIV counselling and testing interventions. Studies with HIV+ heterosexual adults all evaluated counselling and group education interventions, although 2 also included HIV care and treatment.36,40 For this objective, three outcomes were measured across multiple studies: condom use, multiple sex partners and HIV disclosure.
Condom use
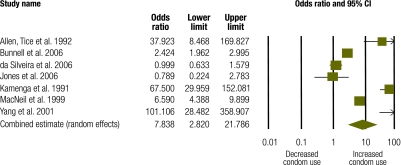
Seven studies with a combined study population of 1801 generated seven discrete effect sizes for condom use.34,36–40,42 Pooled, these data show a strong and significant effect on condom use (OR: 7.84; 95% CI: 2.82–21.79) (Fig. 5). The Q statistic of 141.45 showed statistically significant heterogeneity (P = 0.0001; I2 = 95.76).
Fig. 5.
Meta-analysis of condom use in studies of positive preventiona interventions
CI, confidence interval.
a “Positive prevention” denotes preventive interventions that target HIV+ individuals.
Condom use results were also stratified by target population. Four studies measured condom use following counselling and group education among HIV+ heterosexual adults.36–38,40 Pooled data from these studies (n = 1489) show a trend towards increased condom use associated with the intervention, but this trend did not reach significance (OR: 2.08; 95% CI: 0.93–4.62; P = 0.074). The Q statistic of 40.56 showed statistically significant heterogeneity (P = 0.0001; I2 = 92.60). Three studies measured condom use following HIV counselling and testing among HIV-serodiscordant couples.34,39,42 Pooled data from these studies (n = 312) show a very strong and highly significant intervention effect on condom use (OR: 67.38; 95% CI: 36.17–125.52). The Q statistic of 0.96 showed no statistically significant heterogeneity (P = 0.62; I2 = 0.000) across these three studies. Meta-analysis results for condom use across these two population groups were significantly different (P = 0.002).
Multiple sex partners
Two studies examined the effect of education and counselling among HIV+ heterosexual adults on the outcome “multiple sex partners”, and both suggested a positive although modest intervention effect.38,40 In Zambia, the percentage of participants reporting sexual activity with non-primary partners decreased from 2% at baseline to 0.04% at 6- and 12-month follow-up assessments (significance not reported).38 In the United Republic of Tanzania, the percent of participants reporting sexual activity with non-primary partners decreased from 31.8% at baseline to 21.4% at the 3-month and 18.2% at the 6-month follow-up assessment (baseline to 3-month follow-up, not significant; baseline to 6-month follow-up, P = 0.05).40
HIV status disclosure
Two studies examined disclosure of HIV status as an outcome.40,42 Both evaluated counselling and education interventions with HIV+ heterosexual adults, and both measured disclosure before and after the intervention. Both found a significant increase in HIV status disclosure following the intervention. In the United Republic of Tanzania, HIV status disclosure to anyone increased from 18.8% at baseline to 84.4% at the 12-month follow-up (P < 0.05).40 In China, HIV status disclosure to spouses increased from 3.6% at baseline to 11.9% at follow-up (P = 0.04), but rates remained low.42
Discussion
Of the 19 studies included in our review, 9 targeted both HIV+ and HIV− individuals and stratified results by serostatus. Almost all were HIV counselling and testing interventions which can more easily report results by serostatus than other behavioral interventions. Meta-analysis, though based on limited data, suggests that such interventions may have a stronger impact on condom use among HIV+ participants than among HIV− participants. The remaining 10 studies evaluated behavioural interventions specifically targeting people living with HIV, which were evenly divided between HIV counselling and testing for HIV-serodiscordant couples and group counselling and education for HIV+ adults. Combined, these interventions showed a positive effect on condom use, but this effect was strikingly larger among serodiscordant couples. Together, these findings suggest that positive prevention interventions are effective at changing behaviour in developing country settings and should be expanded.
These results are consistent with those found in the broader literature from both developing and developed country settings. Several previous systematic reviews of voluntary HIV counselling and testing also suggest that such interventions have the strongest impact on behaviour change among HIV+ individuals and serodiscordant couples.20,43–45 Our finding that interventions targeting people living with HIV in developing countries are generally effective is consistent with findings from three previous systematic reviews covering interventions conducted primarily in the United States.14–16
The results of this review should be viewed in the light of its limitations. Unlike other systematic reviews of positive prevention interventions based almost entirely in the United States,14,16 we chose not to limit our inclusion criteria to controlled trials. Instead, we employed broad study design criteria to capture a range of effectiveness data. Given the lack of rigorous trials conducted in developing countries, this strategy allowed us to include more available intervention evaluation data. However, this approach also increases the risk of bias. In particular, self-selection bias and self-reporting bias may have compromised results, as only four studies randomly assigned participants to the intervention, and most outcomes were based on self-reporting. Studies scored an average of only 3.9 out of 8 possible points for study design rigour. Limitations of the available evidence base suggest that future research should use more rigorous designs and measure biological outcomes when appropriate. Nevertheless, although we employed broad study design inclusion criteria, we still required studies to be published in peer-reviewed journals. While our experience has shown that unpublished studies and programme reports tend to be of lower methodological quality, there may be innovative or well designed studies in the grey literature that were not included as evidence in this review.
We were also limited by the lack of consistency of outcome measures across studies and were only able to meta-analyse results for condom use, which is only one of many behaviours for the prevention of HIV infection. In addition, our condom use measure does not fully capture the variety of sexual behaviours, such as oral sex and mutual masturbation, which may pose significantly less risk when engaged in without a condom. Although meta-analysis provides a succinct summary of results from diverse studies, the need to standardize outcome measures can obscure nuances in actual levels of risk across studies and respondents.
The studies included in our review were conducted among a relatively narrow range of target populations. Almost all targeted general adult populations, HIV-serodiscordant couples or general populations of HIV+ adults; only one study was conducted with commercial sex workers. Because we had limited or no data on high-risk populations such as commercial sex workers, injection drug users and men who have sex with men, we were unable to stratify our results by these important populations, and it is unclear to what extent the results can be generalized to them. Further research into positive preventive interventions with such populations is warranted for both ethical and epidemiological reasons. First, they are often at highest risk for both HIV infection and its negative health consequences in both generalized and concentrated HIV epidemics, and they are often underserved by HIV prevention interventions. In addition, sex workers can easily be infected with HIV by clients and then transmit it to their partners, offspring and other clients. Similarly, injection drug users can transmit HIV infection to both sex and drug-sharing partners.
In addition, the 19 studies included in this review represent a relatively narrow range of interventions: 14 HIV counselling and testing interventions and 5 group education and counselling interventions for HIV+ individuals. We found no articles – even in our larger database of 84 articles from previous systematic reviews of HIV behavioural interventions in developing countries – that evaluated interventions such as needle/syringe exchange programmes, condom social marketing, peer education or mass media campaigns or other environmental/structural interventions. In general, the studies in our database either did not target HIV+ individuals or did not assess the serostatus of participants.
Our conceptual model for positive prevention is comprehensive; it covers a broad range of interventions designed to keep people living with HIV physically and mentally healthy, prevent HIV transmission to other people and increase the involvement of HIV+ individuals in prevention activities. Previous World Health Organization (WHO) guidelines for essential prevention and care interventions for HIV+ individuals in resource-limited settings have been similarly comprehensive, although focused on interventions in the health sector.13 While recognizing that not all interventions will be needed or equally appropriate in all countries, the WHO guidelines recommend 13 biomedical and behavioural interventions seen as low in cost and of particular importance for people living with HIV.13 The behavioural interventions identified in this review did not cover the full spectrum of possible behavioural interventions for the prevention of HIV infection, and they were rarely linked with biomedical interventions such as the provision of ART.More comprehensive programming will be necessary to reduce the spread of HIV and achieve the WHO/Joint United Nations Programme on HIV/AIDS (UNAIDS) goal of universal access to comprehensive HIV prevention, treatment , care and support for people living with HIV by 2010.13
Behavioural and biomedical interventions for HIV+ prevention can be conducted either as part of routine HIV care and treatment in medical settings or in community-based settings. As ART treatment for HIV+ individuals becomes increasingly available in developing countries, routine medical visits will provide one practical setting for prevention among such individuals, as they have consistent contact with providers. However, in most developing country settings, ART is not initiated until a patient’s CD4+ lymphocyte count drops below 200 cells/µl.46 A large number of HIV+ individuals do not meet this criterion and therefore have minimal interaction with the health system during the infection’s long latency period. Community-based interventions are needed to reach HIV+ individuals in developing countries who know their serostatus but are not regularly accessing medical care. Such interventions also offer the opportunity for involvement and leadership by people living with HIV. Although current interventions are promising they have the potential to be much more effective if designed and led by people living with HIV themselves. This review included interventions conducted in community settings, but few such interventions were identified; the lack of existing literature in this area limits the usefulness of the review findings. Finally, although great strides have been made in increasing access to HIV testing, the majority of people living with HIV in developing countries remain untested and unaware of their serostatus. Interventions must continue to encourage HIV testing and counselling, especially within couples, as HIV serodiscordance is common4 and rates of HIV status disclosure to sexual partners are low.5
In conclusion, behavioural interventions targeting HIV+ individuals in developing countries appear to be effective, especially among HIV-serodiscordant couples. These findings have several public health implications. First, the global expansion of HIV testing and treatment programmes provides a mechanism for both identifying such individuals and providing HIV prevention messages and services targeted towards them. Efforts should be made to integrate HIV prevention messages and services into HIV care and treatment settings as well as HIV testing and counselling programmes. Moreover, because many HIV+ individuals have limited contact with health care settings, community-based programmes should also provide HIV prevention messages and services to them. Community and clinic-based programmes should be linked to provide comprehensive care to people living with HIV. Comprehensive positive prevention programmes should focus not only on preventing transmission of HIV but also on maintaining the physical and mental health and the dignity of the individual. Although this review focused on behavioural interventions, a full set of behavioural and biomedical interventions should be implemented to stem the spread of HIV and improve the health and quality of life of HIV+ individuals in developing countries.
Acknowledgements
The authors thank Sidney Callahan, Lisa Fiol Powers, Alexandra Melby, Marta Mulawa, Erica Rosser and Lauren Tingey for their assistance with coding and Elena Tuerk for her coordination of the project.
Funding:
The project described here was supported by WHO and by award number R01MH071204 from the United States National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Competing interests:
None declared.
References
- 1.Auerbach JD. Principles of positive prevention. J Acquir Immune Defic Syndr. 2004;37:S122–5. doi: 10.1097/01.qai.0000140611.82134.aa. [DOI] [PubMed] [Google Scholar]
- 2.Report on the global AIDS epidemic. Geneva: Joint United Nations Programme on HIV/AIDS; 2008. Available from: http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp [accessed 21 May 2009].
- 3.Global HIV Prevention Working Group. HIV prevention in the era of expanded treatment access. 2004. Available from: http://www.kff.org/hivaids/loader.cfm?url=/commonspot/security/getfile.cfm&PageID=36967 [accessed 3 December 2007].
- 4.Bunnell R, Mermin J, De Cock KM. HIV prevention for a threatened continent: implementing positive prevention in Africa. JAMA. 2006;296:855–8. doi: 10.1001/jama.296.7.855. [DOI] [PubMed] [Google Scholar]
- 5.Medley A, Garcia-Moreno C, McGill S, Maman S. Rates, barriers and outcomes of HIV serostatus disclosure among women in developing countries: implications for prevention of mother-to-child transmission programmes. Bull World Health Organ. 2004;82:299–307. [PMC free article] [PubMed] [Google Scholar]
- 6.Janssen RS, Valdiserri RO. HIV prevention in the United States: increasing emphasis on working with those living with HIV. J Acquir Immune Defic Syndr. 2004;37:S119–21. doi: 10.1097/01.qai.0000140610.82134.e3. [DOI] [PubMed] [Google Scholar]
- 7.Kalichman S, editor. Positive prevention: reducing HIV transmission among people living with HIV/AIDS. Storrs: Springer; 2005. [Google Scholar]
- 8.Centers for Disease Control and Prevention Advancing HIV prevention: new strategies for a changing epidemic – United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:329–32. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC), Health Resources and Services Administration, National Institutes of Health, HIV Medicine Association of the Infectious Diseases Society of America. Incorporating HIV prevention into the medical care of persons living with HIV. Recommendations of CDC, the Health Resources and Services Administration, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2003;52:1–24. [PubMed] [Google Scholar]
- 10.Collins C, Morin SF, Shriver MD, Coates TJ. Designing primary prevention for people living with HIV/AIDS. San Francisco: Center for AIDS Prevention Studies, University of California San Francisco; 2000. [Google Scholar]
- 11.International H. IV/AIDS Alliance. Positive prevention: prevention strategies for people with HIV/AIDS Brighton: International HIV/AIDS Alliance; 2007. Available from: http://www.aidsalliance.org/includes/Publication/Positive_prevention.pdf [accessed 12 May 2010]. [Google Scholar]
- 12.Global Network of People Living with HIV/AIDS. Moving forward on ‘positive health, dignity and prevention’ – people living with HIV set principles for engagement. Press release. 8 May 2009. Available from: http://www.gnpplus.net/images/stories/20090508_news_release_phdp_final.pdf [accessed 21 May 2009].
- 13.Essential prevention and care interventions for adults and adolescents living with HIV in resource-limited settings. Geneva: World Health Organization; 2007. Available from: http://www.who.int/hiv/topics/prevention_and_care/en/ [accessed 30 April 2009].
- 14.Crepaz N, Lyles CM, Wolitski RJ, Passin WF, Rama SM, Herbst JH, et al. Do prevention interventions reduce HIV risk behaviours among people living with HIV? A meta-analytic review of controlled trials. AIDS. 2006;20:143–57. doi: 10.1097/01.aids.0000196166.48518.a0. [DOI] [PubMed] [Google Scholar]
- 15.Gilliam PP, Straub DM. Prevention with positives: a review of published research, 1998-2008. J Assoc Nurses AIDS Care. 2009;20:92–109. doi: 10.1016/j.jana.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Johnson BT, Carey MP, Chaudoir SR, Reid AE. Sexual risk reduction for persons living with HIV: research synthesis of randomized controlled trials, 1993 to 2004. J Acquir Immune Defic Syndr. 2006;41:642–50. doi: 10.1097/01.qai.0000194495.15309.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertrand JT, O'Reilly K, Denison J, Anhang R, Sweat M. Systematic review of the effectiveness of mass communication programs to change HIV/AIDS-related behaviors in developing countries. Health Educ Res. 2006;21:567–97. doi: 10.1093/her/cyl036. [DOI] [PubMed] [Google Scholar]
- 18.Sweat M, Kennedy C, Medley A, O’Reilly K. Psychosocial support for HIV-infected populations in developing countries: a key yet understudied component of positive prevention. AIDS. 2007;21:1070–1. doi: 10.1097/QAD.0b013e3280f774da. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy C, O’Reilly K, Medley A, Sweat M. The impact of HIV treatment on risk behaviors in developing countries: a systematic review. AIDS Care. 2007;19:707–20. doi: 10.1080/09540120701203261. [DOI] [PubMed] [Google Scholar]
- 20.Denison JA, O’Reilly KR, Schmid GP, Kennedy CE, Sweat MD. HIV voluntary counseling and testing and behavioral risk reduction in developing countries: a meta-analysis, 1990-2005. AIDS Behav. 2008;12:363–73. doi: 10.1007/s10461-007-9349-x. [DOI] [PubMed] [Google Scholar]
- 21.Medley A, Kennedy C, O’Reilly K, Sweat M. Effectiveness of peer education interventions for HIV prevention in developing countries: a systematic review and meta-analysis. AIDS Educ Prev. 2009;21:181–206. doi: 10.1521/aeap.2009.21.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The World Bank. Countries and regions [Internet site]. Available from: http://www.worldbank.org [accessed 17 April 2008].
- 24.Cooper H, Hedges LV, editors. The handbook of research synthesis New York: Russell Sage Foundation; 1994. [Google Scholar]
- 25.Allen S, Serufilira A, Gruber V, Kegeles S, Van de Perre P, Carael M, et al. Pregnancy and contraception use among urban Rwandan women after HIV testing and counseling. Am J Public Health. 1993;83:705–10. doi: 10.2105/AJPH.83.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen S, Serufilira A, Bogaerts J, Van de Perre P, Nsengumuremyi F, Lindan C, et al. Confidential HIV testing and condom promotion in Africa. Impact on HIV and gonorrhea rates. JAMA. 1992;268:3338–43. doi: 10.1001/jama.268.23.3338. [DOI] [PubMed] [Google Scholar]
- 27.Farquhar C, Kiarie JN, Richardson BA, Kabura MN, John FN, Nduati RW, et al. Antenatal couple counseling increases uptake of interventions to prevent HIV-1 transmission. J Acquir Immune Defic Syndr. 2004;37:1620–6. doi: 10.1097/00126334-200412150-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King R, Estey J, Allen S, Kegeles S, Wolf W, Valentine C, et al. A family planning intervention to reduce vertical transmission of HIV in Rwanda. AIDS. 1995;9:S45–51. [PubMed] [Google Scholar]
- 29.Machekano R, McFarland W, Mbizvo MT, Bassett MT, Katzenstein D, Latif AS. Impact of HIV counselling and testing on HIV seroconversion and reported STD incidence among male factory workers in Harare, Zimbabwe. Cent Afr J Med. 1998;44:98–102. [PubMed] [Google Scholar]
- 30.Pickering H, Quigley M, Pépin J, Todd J, Wilkins A. The effects of post-test counselling on condom use among prostitutes in The Gambia. AIDS. 1993;7:271–3. doi: 10.1097/00002030-199302000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Roth DL, Stewart KE, Clay OJ, van der Straten A, Karita E, Allen S. Sexual practices of HIV discordant and concordant couples in Rwanda: effects of a testing and counselling programme for men. Int J STD AIDS. 2001;12:181–8. doi: 10.1258/0956462011916992. [DOI] [PubMed] [Google Scholar]
- 32.The Voluntary HIV-1 Counseling and Testing Efficacy Study Group Efficacy of voluntary HIV-1 counselling and testing in individuals and couples in Kenya, Tanzania, and Trinidad: a randomised trial. Lancet. 2000;356:103–12. doi: 10.1016/S0140-6736(00)02446-6. [DOI] [PubMed] [Google Scholar]
- 33.Allen S, Meinzen-Derr J, Kautzman M, Zulu I, Trask S, Fideli U, et al. Sexual behavior of HIV discordant couples after HIV counseling and testing. AIDS. 2003;17:733–40. doi: 10.1097/00002030-200303280-00012. [DOI] [PubMed] [Google Scholar]
- 34.Allen S, Tice J, Van de Perre P, Serufilira A, Hudes E, Nsengumuremyi F, et al. Effect of serotesting with counselling on condom use and seroconversion among HIV discordant couples in Africa. BMJ. 1992;304:1605–9. doi: 10.1136/bmj.304.6842.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balmer DH. The efficacy of a scientific and ethnographic research design for evaluating AIDS group counselling. Couns Psychol Q. 1994;7:429–40. doi: 10.1080/09515079408254164. [DOI] [Google Scholar]
- 36.Bunnell R, Ekwaru JP, Solberg P, Wamai N, Bikaako-Kajura W, Were W, et al. Changes in sexual behavior and risk of HIV transmission after antiretroviral therapy and prevention interventions in rural Uganda. AIDS. 2006;20:85–92. doi: 10.1097/01.aids.0000196566.40702.28. [DOI] [PubMed] [Google Scholar]
- 37.da Silveira MF, dos Santos IS. Impact of an educational intervention to promote condom use among the male partners of HIV positive women. J Eval Clin Pract. 2006;12:102–11. doi: 10.1111/j.1365-2753.2005.00626.x. [DOI] [PubMed] [Google Scholar]
- 38.Jones DL, Weiss SM, Bhat GJ, Bwalya V. Influencing sexual practices among HIV-positive Zambian women. AIDS Care. 2006;18:629–34. doi: 10.1080/09540120500415371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamenga M, Ryder RW, Jingu M, Mbuyi N, Mbu L, Behets F, et al. Evidence of marked sexual behavior change associated with low HIV-1 seroconversion in 149 married couples with discordant HIV-1 serostatus: experience at an HIV counselling center in Zaire. AIDS. 1991;5:61–7. doi: 10.1097/00002030-199101000-00009. [DOI] [PubMed] [Google Scholar]
- 40.MacNeil JM, Mberesero F, Kilonzo G. Is care and support associated with preventive behaviour among people with HIV? AIDS Care. 1999;11:537–46. doi: 10.1080/09540129947695. [DOI] [PubMed] [Google Scholar]
- 41.Ryder RW, Kamenga C, Jingu M, Mbuyi N, Mbu L, Behets F. Pregnancy and HIV-1 incidence in 178 married couples with discordant HIV-1 serostatus: additional experience at an HIV-1 counselling centre in the Democratic Republic of the Congo. Trop Med Int Health. 2000;5:482–7. doi: 10.1046/j.1365-3156.2000.00582.x. [DOI] [PubMed] [Google Scholar]
- 42.Yang F, Wu Z, Xu C.[Acceptability and feasibility of promoting condom use among families with human immunodeficiency virus infection in rural area of China]. Zhonghua Liu Xing Bing Xue Za Zhi 200122330–3.Chinese [PubMed] [Google Scholar]
- 43.Higgins DL, Galavotti C, O’Reilly KR, Schnell DJ, Moore M, Rugg DL, et al. Evidence for the effects of HIV antibody counseling and testing on risk behaviors. JAMA. 1991;266:2419–29. doi: 10.1001/jama.266.17.2419. [DOI] [PubMed] [Google Scholar]
- 44.Weinhardt LS, Carey MP, Johnson BT, Bickham NL. Effects of HIV counseling and testing on sexual risk behavior: a meta-analytic review of published research, 1985–1997. Am J Public Health. 1999;89:1397–405. doi: 10.2105/AJPH.89.9.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolitski RJ, MacGowan RJ, Higgins DL, Jorgensen CM. The effects of HIV counseling and testing on risk-related practices and help-seeking behavior. AIDS Educ Prev. 1997;9:52–67. [PubMed] [Google Scholar]
- 46.Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach, 2006 revision. Geneva: World Health Organization; 2006. Available from: http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf [accessed 11 May 2009]. [PubMed]