Abstract
Liver is the major organ that eliminates xenobiotics from the body, a process that is accomplished by a series of drug-processing genes (DPGs). These genes encode transporters on both basolateral and apical membranes of hepatocytes, as well as phase I and II enzymes. The current study compares the expression of hepatic DPGs in adult and aged mouse livers and explores the potential effects of energy restriction (ER) on these genes during aging. Of 79 quantified hepatic DPGs, 52 were expressed lower in 24-month-old aged mice than in 12-month-old adult mice. Furthermore, the mRNA expression of multiple xenobiotic-activated transcription factors also decreased with age. Six-month ER exerted less of an effect on the hepatic DPGs than did aging. ER increased the mRNAs of two and decreased the mRNAs of nine DPGs in adult mice. In aged mice, ER increased the mRNAs of 10 and decreased the mRNAs of 5 DPGs. The only mRNA that was increased by both ER and aging was Gstm3. ER increased the mRNAs of Cyp2b10, Ugt1a9, Gsta1, and Oatp1a4 only in adult mice and decreased the mRNAs of Aldh6a1, Pon3, Ugt1a1, Sult1a1, and Atp8b1 only in aged mice. In summary, the reduced mRNA expression of hepatic DPGs in aged mice indicates decreased drug-processing capability, whereas ER did not compensate for the global reduction of hepatic DPG expression in aged mice. The hepatic transcription factors are likely to mediate the changes in hepatic DPG expression during aging and ER.
The geriatric population is rapidly increasing in the world. The proportion of persons aged 60 or older is projected to reach 22% of the global population in 2050, with 33% in more developed regions and 20% in less developed regions (United Nations Population Ageing and Development 2009, http://www.un.org/esa/population/publications/ageing/ageing2009chart.pdf). Health care for this population presents a major medical and economic problem. The elderly population is more susceptible to various diseases, and they often take several drugs at the same time. Liver, the major organ for drug elimination from the body, exhibits decreased volume, diminished hepatobiliary functions, declined phase I drug metabolism capability, and increased or decreased expression of a variety of proteins in the elderly population (Schmucker, 2005).
Liver metabolizes endogenous and exogenous compounds (nutrients and drugs), as well as maintains energy balance in the body. Numerous liver functions are accomplished by proteins encoded by a series of drug-processing genes (DPGs), which include basolateral uptake transporters, phase I and II enzymes, and apical and basolateral efflux transporters.
Hepatocytes have transporters on both their basolateral and apical sides to remove xenobiotics from intestine-derived portal blood and to excrete them or their metabolites into bile or blood. Uptake solute carriers include organic anion-transporting polypeptides (Oatps), sodium/taurocholate-cotransporting polypeptide (Ntcp), organic cation transporters (Octs), organic anion transporters (Oats), and so on. Apical efflux transporters, including the multidrug resistance proteins (Mdrs), multidrug resistance-associated proteins (Mrps), bile salt export pump (Bsep), and breast cancer resistance protein (Bcrp), and so on, transport compounds into bile. Several Mrps, such as Mrp3 and Mrp4, are located on the basolateral membrane of hepatocytes and transport chemicals back into blood.
Phase I enzymes in the liver are mainly cytochromes P450 (P450s), aldehyde dehydrogenases (Aldhs), carboxylesterases (Cess), and paraoxonases (Pons). These enzymes catalyze oxidation, reduction, and hydrolysis reactions. Phase II enzymes often conjugate phase I metabolites to make them more water soluble to be excreted by efflux transporters. In typical phase II reactions, glucuronic acid, sulfate, or glutathione is added to these compounds by UDP-glucuronosyltransferases (Ugts), sulfotransferases (Sults), and glutathione transferases (Gsts), respectively.
The expression of hepatic DPGs is regulated by multiple xenobiotic-activated transcription factors (Klaassen and Slitt, 2005). These transcription factors detect xenobiotics in hepatocytes and accordingly turn on/off the expression of certain DPGs. In general, these changes in gene expression will facilitate the elimination of xenobiotics. Included among these hepatic xenobiotic transcription factors are pregnane X receptor (PXR), constitutive androstane receptor (CAR), peroxisome proliferator-activated receptor α (PPARα), aryl hydrocarbon receptor (AhR), and nuclear factor erythroid 2-related factor 2 (Nrf2).
Energy restriction (ER) has been extensively investigated as an intervention that both extends lifespan and delays age-related diseases in laboratory animals (Anderson and Weindruch, 2007). It has been proposed that caloric restriction might produce beneficial effects by reprogramming energy metabolism (Anderson and Weindruch, 2007). However, the exact effects of ER on hepatic drug metabolism remain unknown. It has been reported that a 35% reduction of dietary calories protects male rats from a lethal dose of thioacetamide as a result of enhanced tissue repair (Ramaiah et al., 1998), although the induction of hepatic microsomal Cyp2e1 by ER led to exaggerated hepatotoxicity at a low dose of thioacetamide (Ramaiah et al., 2001).
The current study is designed to determine the changes of hepatic DPGs during aging, as well as what effects ER has on these liver genes in adult and aged mice. To fulfill this purpose, adult (6-month) and aged (12-month) male C57BL/6 male mice were placed on either a control or energy restricted dietary program for 6 months. The expression of hepatic DPGs, including transporters, phase I and II enzymes, and key xenobiotic transcription factors, was determined. Data generated from mice in this study may provide information for the prediction of drug efficacy, pharmacokinetics, and toxicity in elderly and energy-restricted human populations.
Materials and Methods
Animals.
The 6-month ER feeding procedure was described previously (Colman et al., 2007). In brief, male C57BL/6 mice at 6 months (adult) and 18 months (aged) of age were separated into control (C) and ER groups, respectively. For the control adult and aged groups, the initial energy intake was 90% of the mean ad libitum energy intake and was gradually decreased to 75% of the ad libitum intake by the end of the experimental period. The ER groups were restricted in energy intake to ∼55% of the ad libitum during the 6-month experimental period. AIN-93M diet (TestDiet, Richmond, IN), containing 12.7% protein, 4.1% fat, 5.0% fiber, and 73% carbohydrates, was used as the control diet. A modified AIN-93M diet enriched 67% in vitamins and minerals was given to the adult and aged ER groups to ensure that the ER mice received approximately the same amounts of micronutrients as the control groups (Reeves et al., 1993). By the end of the study, the adult mice were 12 months old and the aged mice were 24 months old. Livers were harvested from 9:00 AM to 12:00 PM, snap-frozen with liquid nitrogen, and stored at −80°C.
RNA Preparation.
Total liver RNA was prepared with RNA-Bee reagent (Tel-Test Inc., Friendswood, TX) from frozen tissue following the manufacturer's instructions. Purified RNA was stored at −80°C. RNA concentrations were quantified with a Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA) and diluted to a final concentration of 50 ng/μl. The quality of RNA was determined by agarose gel electrophoresis. Five individual samples from each group were used for gene expression assays.
Multiplex Suspension Array.
The mRNA levels of hepatic genes were determined by QuantiGene P2.0 technology (Affymetrix Inc., Santa Clara, CA), as described previously (Zhang et al., 2009). In brief, 0.5-μg RNA samples were loaded into 96-well plates that contained X-MAP beads (Affymetrix Inc.) coated with capture probes, label extenders, and blockers. After several incubation and washing steps, streptavidin-conjugated R-phycoerythrin fluorescence was analyzed by a Bio-Plex 200 system array reader interfaced with Bio-Plex data manager software 5.0 (Bio-Rad Laboratories, Hercules, CA). The majority of the genes quantified in the current study have been previously characterized by our laboratory (Cheng et al., 2005; Alnouti and Klaassen, 2006, 2008a,b; Alnouti et al., 2006; Buckley and Klaassen, 2007; Cheng and Klaassen, 2009). Details of the plex sets used in the current study can be found at www.panomics.com with the following numbers: 21073, 21150, 21152, 21153, 21174, and 21175. The mRNA expression level of each gene was presented as a ratio to the loading control gene Rpl13a in the same panel, except for panels 21152 and 21153, for which glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was used as the loading control.
Statistical Analysis.
All the data were analyzed using one-way analysis of variance followed by Duncan's multiple range test (p ≤ 0.05).
Hierarchical Cluster Analysis.
JMP7 (SAS Institute, Cary, NC) was used to distinguish the hepatic gene expression pattern across age-feeding groups. Hierarchical clustering was obtained from DPGs, including phase I enzymes, phase II enzymes, and transporters, or transcription factors. The highest expression of a specific gene is represented by the darkest region of the gray-scale spectrum, whereas the lowest expression is indicated by the lightest region. The spectrum is specific to the expression to each individual gene. The relative intensities of gray scale should not be used to compare the expression levels of different genes.
Results
Effects of Aging and ER on the mRNA Levels of Hepatic Uptake Transporters.
The body composition changes after the 6-month ER in these mice were reported previously (Colman et al., 2007). In brief, the 12-month-old adult control mice weighed 30.5 g with 21.5% body fat; the adult ER mice weighed 24.4 g with 13.4% body fat; the 24-month-old aged control mice weighed 32.1 g with 14.5% body fat; and the aged ER mice weighed 28 g with 12.8% body fat.
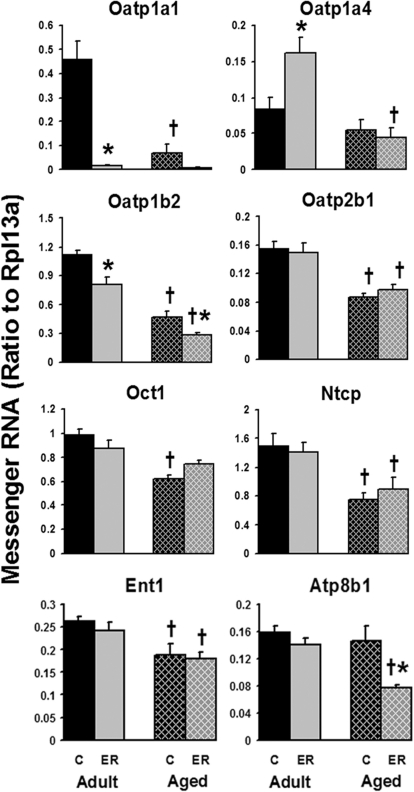
Multiple uptake transporters are expressed on the basolateral membranes of hepatocytes. Aging caused significant reduction in the mRNA expression of Oatp1a1, Oatp1b2, Oatp2b1, Oct1, Ntcp, and equilibrative nucleoside transporter 1 (Ent1) (Fig. 1). Among these uptake transporters, Oatp1a1 was reduced most markedly with age. The 24-month-old mice only expressed approximately 15% of the hepatic Oatp1a1 mRNA as the 12-month-old mice.
Fig. 1.
Effects of aging and ER on the mRNA levels of uptake transporters in mouse livers. Adult groups were 12 months of age, and aged groups were 24 months of age; C, control. Data are expressed as mean ± S.E. of five mice per group. *, statistically different from same-age control group; †, statistically different from adult mice on the same diet. p ≤ 0.05, one-way analysis of variance followed by Duncan's test. The same illustrations are applied to the following figures.
The 6-month ER decreased the mRNA of Oatp1a1 by 96% in adult and 91% in aged mouse livers. In addition, ER also decreased ∼30 to 50% of the hepatic expression of Oatp1b2 mRNA in both age groups and the apical aminophospholipid flippase Atp8b1 mRNA in aged mice. However, ER increased Oatp1a4 mRNA 92% in adult but not in aged mouse livers. In summary, the expression of most hepatic uptake transporters decreased with aging; ER decreased the mRNA levels of Oatp1a1 and Oatp1b2 at both ages and Atp8b1 in aged mice only, whereas it increased Oatp1a4 mRNA expression in adult mice.
Effects of Aging and ER on the mRNA Expression of Phase I Enzymes in Mouse Livers.
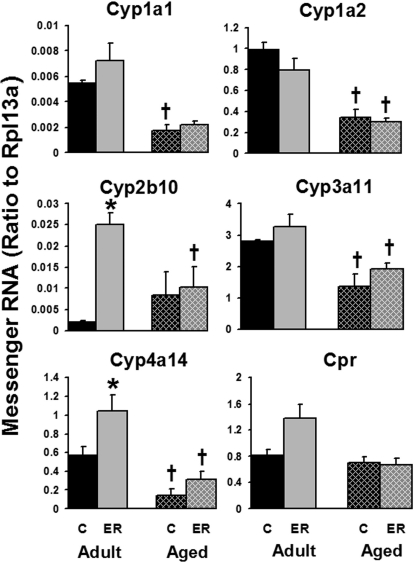
As shown in Fig. 2, mRNA levels of major hepatic drug-metabolizing P450s, including Cyp1a1, Cyp1a2, Cyp3a11, and Cyp4a14, decreased with age, except for Cyp2b10. These P450s decreased 50 to 75% between 12 and 24 months of age. The mRNA expression of P450 reductase, the common electron donor of the P450s, was not changed by aging in mouse livers. Six months of ER increased the mRNA expression of Cyp2b10 and Cyp4a14 by 1140 and 81%, respectively, in adult mouse livers but not in aged mice. The hepatic expression of Cyp3a11 and Cyp4a14 tended to be enhanced by ER in both age groups, but significant induction was only observed in Cyp4a14 mRNA in livers of adult mice. Cyp2e1, another important member of P450s in liver, was not influenced by either aging or ER at the mRNA level in the current study (data not shown). In summary, the majority of hepatic P450s decreased by aging, and ER enhanced the expression of Cyp2b10 and Cyp4a14 in adult mouse livers.
Fig. 2.
Effects of aging and ER on the mRNA expression of P450s in mouse livers.
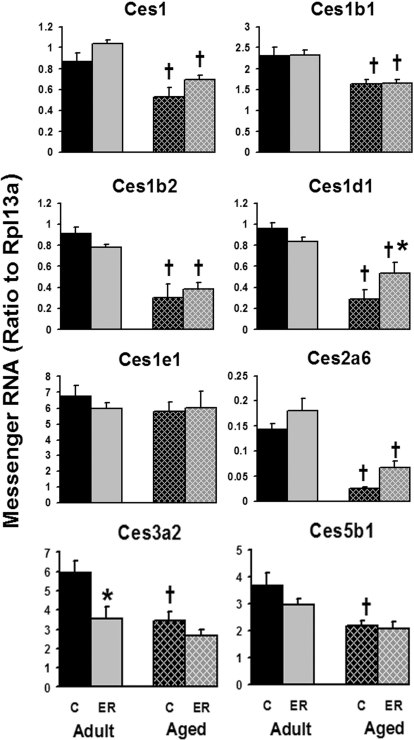
The mRNA expression of most hepatic Cess decreased with age except for Ces1e1 (Fig. 3). For example, the hepatic mRNA of Ces1b2, Ces1d1, and Ces2a6 was reduced 65 to 82%, and Ces1, Ces1b1, Ces3a2, and Ces5b1 were decreased 28 to 42% in aged mice. ER increased the hepatic expression of Ces1d1 (84%) in aged mouse livers and decreased the mRNA level of Ces3a2 (40%) in adult mouse livers. The hepatic expression of other Cess was not significantly influenced by ER in either age group.
Fig. 3.
Effects of aging and ER on the mRNA levels of Cess in mouse livers.
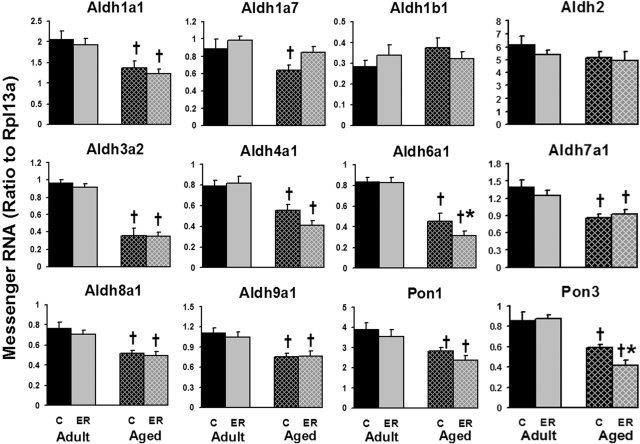
Aldhs were abundantly expressed in mouse liver. Aging decreased the mRNA expression of most of the Aldhs (Fig. 4) but not Aldh1b1 and Aldh2. In aged mouse livers, the mRNA of Aldh3a2 and Aldh6a1 decreased 63 and 45%, respectively, compared with adult mice. Approximately 30% less mRNA of Aldh1a1, Aldh1a7, Aldh4a1, Aldh7a1, Aldh8a1, and Aldh9a1 was expressed in aged mice. Compared with aging, ER had less of an effect on Aldh mRNA levels; however, ER decreased Aldh6a1 mRNA in aged mice. In summary, hepatic Aldh mRNA levels were markedly decreased by aging but less influenced by ER.
Fig. 4.
Effects of aging and ER on the mRNA expression of Aldhs and Pons in mouse livers.
Aging decreased the expression of both Pon1 and Pon3 (∼30%) in the liver as shown in Fig. 4. ER further decreased the Pon3 mRNA 30% in the aged group but did not influence its expression in livers of adult mice.
Effects of Aging and ER on the mRNA Expression of Phase II Enzymes in Mouse Livers.
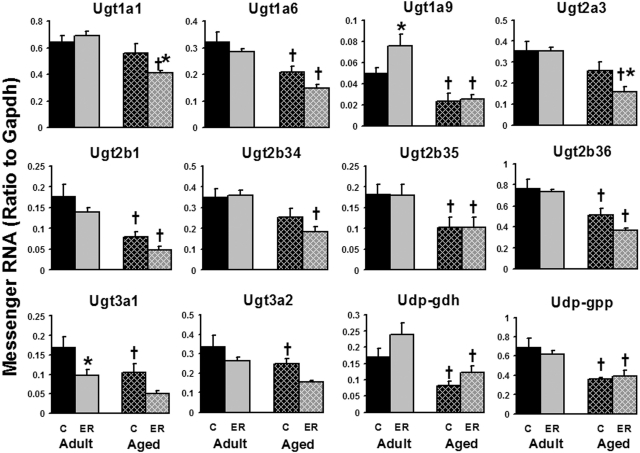
The mRNA levels of major hepatic conjugation enzymes were quantified, including 7 Ugts, UDP-glucose pyrophosphorylase (Udp-gpp), and UDP-glucose dehydrogenase (Udp-gdh); 2 Sults that can be detected in male mouse liver, as well as 2 isoforms of 3′-phosphoadenosine-5′-phosphosulfate synthase (Papss); and 13 Gsts. Compared with adult mice (12 months), aged mice (24 months) expressed 25 to 55% less of the following hepatic enzymes involved in glucuronidation: Ugt1a6, Ugt1a9, Ugt2b1, Ugt2b35, Ugt2b36, Ugt3a1, Ugt3a2, Udp-gdh, and Ugp-gpp (Fig. 5). ER decreased the mRNA levels of Ugt1a1 (27%) and Ugt2a3 (38%) in aged mouse livers and Ugt3a1 (42%) in adult mouse livers, but increased Ugt1a9 (53%) expression in adult mouse livers. In addition, the aged ER group tended to express less Ugt1a6, Ugt2b1, Ugt2b34, Ugt2b36, Ugt3a1, and Ugt3a2 mRNA than the aged control-feeding group, but these differences were not statistically significant. Ugt1a5 was the only Ugt that was not significantly influenced by either aging or ER (data not shown). In summary, aging decreased the mRNA levels of 9 of 13 hepatic glucuronidation enzymes examined in the present study, whereas ER influenced the expression of 4 Ugts in the two age groups.
Fig. 5.
Effects of aging and ER on the mRNA expression of enzymes involved in hepatic glucuronidation in mouse livers.
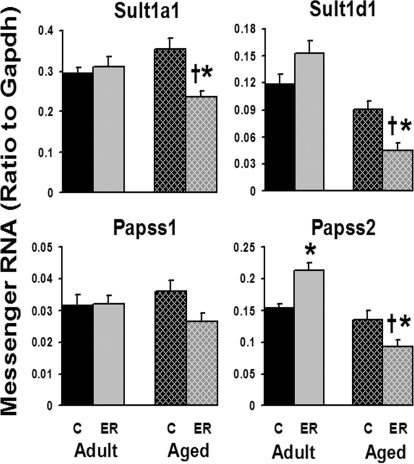
In contrast to most phase I and II enzymes, the hepatic expression of sulfation-related enzymes was not significantly influenced by aging, but it was by ER (Fig. 6). Because of the female-dominant expression of hepatic Sults, only a few Sults were detected in male mouse livers in this study. In aged mice, the 6-month ER markedly decreased mRNAs of Sult1a1 (33%) and Sult1d1 (51%), as well as Papss2 (32%). However, ER induced the expression of Papss2 in adult mouse livers 38%. The hepatic expression of Papss1 was not significantly influenced by either aging or ER. In general, ER had a more predominant effect on the hepatic sulfation enzymes than did aging.
Fig. 6.
Effects of aging and ER on the mRNA expression of Sults and Papss in mouse livers.
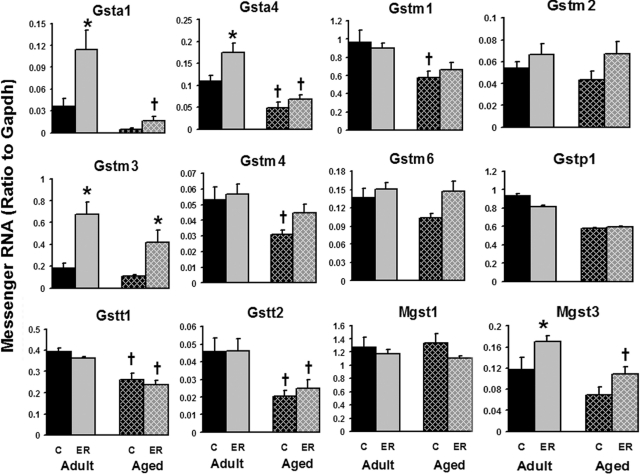
Figure 7 shows the mRNA expression of hepatic Gsts with aging and ER. Aging decreased the mRNA levels of 5 Gsts (Gsta4, Gstm1, Gstm4, Gstt1, and Gstt2) of the 12 quantified in this study. In adult mouse livers, Gsta1, Gsta4, Gstm3, and Mgst3 were induced by ER, whereas in aged mice, only Gstm3 was induced by ER. Among all the Gsts, ER increased Gsta1 and Gstm3 the most in both age groups, which were increased ∼200 and ∼270%, respectively. In contrast, Mgst1 was stably expressed regardless of age and energy intake. In summary, the mRNA of several hepatic Gsts was decreased during aging, whereas ER can increase the expression of some Gsts in adult and aged mouse livers.
Fig. 7.
Effects of aging and ER on the mRNA expression of Gsts in mouse livers.
Effects of Aging and ER on the mRNA Levels of Hepatic Efflux Transporters.
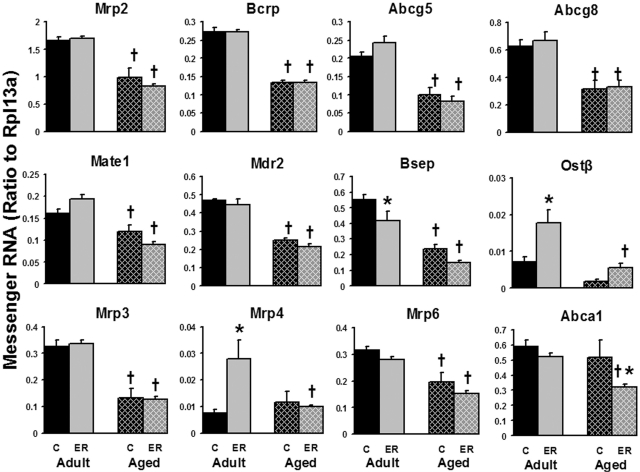
The mRNA expression of most efflux transporters was decreased by aging (Fig. 8). Approximately 50% reduction occurred at the mRNA level of the major apical efflux transporters, including Mrp2, Bcrp, the half cholesterol transporters ATP-binding cassette subfamily G members 5 and 8 (Abcg5 and Abcg8), Mdr2, Bsep, as well as basolateral efflux transporters Mrp3 and Mrp6. The aged mice expressed 26% less multidrug and toxin extrusion transporter mRNA than adult mice. The mRNA of organic solute transporter β (Ostβ) was expressed 74% lower in 24-month-old aged mice than in 12-month-old adult mice, but the difference was not statistically significant. The partner of Ostβ, Ostα, was expressed at a very low level in liver (data not shown). For basolateral transporters, the mRNA levels of Mrp4 and cholesterol efflux transporter Abca1 were not changed during aging. Six months of ER decreased the expression of Bsep but increased that of Ostβ in adult mouse livers. On the basolateral membrane, ER markedly increased the expression of Mrp4 only in adult mice and decreased Abca1 mRNA only in aged mice. In brief, hepatic efflux transporters were expressed less in aged mice, and ER increased the expression of Ostβ and Mrp4 in adult mice but decreased the mRNAs of Bsep and Abca1 in adult and aged mice, respectively.
Fig. 8.
Effects of aging and ER on the mRNA expression of efflux transporters in mouse livers.
Expression of Drug Metabolism-Related Transcription Factors in ER Adult and Aged Mouse Livers.
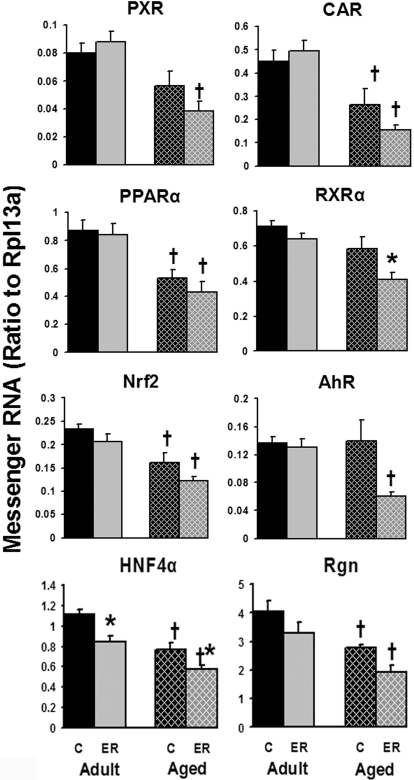
In aged mouse livers, the majority of xenobiotic and drug metabolism-related transcription factors decreased ∼30% compared with adults (Fig. 9). These transcription factors are CAR, PPARα, Nrf2, and hepatocyte nuclear factor 4α (HNF4α). PXR tended to be expressed lower in the livers of aged control or ER mice compared with corresponding adult groups. The senescence marker regucalcin was also decreased ∼30% in 24-month-old aged mouse livers compared with 12-month-old adult mice. Compared with the aforementioned transcription factors, the mRNAs of retinoid X receptor α (RXRα) and AhR were not changed by aging but were decreased 30 and 57% by ER, respectively, only in aged mouse livers. ER also significantly decreased the mRNA expression of HNF4α in both age groups. In summary, the expression of hepatic drug metabolism-related transcription factors was lower in aged than adult mice, whereas long-term ER decreased the mRNAs of HNF4α in adult mice, as well as RXRα and HNF4α in aged mouse livers.
Fig. 9.
Effects of aging and ER on the mRNA expression of drug metabolism-related transcription factors in mouse livers.
Discussion
Aging is a biological process characterized as a time-dependent functional decline in various tissues, including liver. Aged cells lose the capability to withstand external and internal challenges, leading to increased morbidity and mortality (Yu, 1996). ER has been shown to exert various beneficial effects in attenuating aging and age-related diseases (Anderson and Weindruch, 2007; Anderson et al., 2009). However, studies of ER effects on liver functions are either general, such as the whole genome profiling of hepatic gene expression (Cao et al., 2001), or focus on energy metabolism, such as glucose metabolism (Hagopian et al., 2003), or several specific drug-metabolizing enzymes (Agrawal and Shapiro, 2003). The current study was designed to reveal how aging influences the hepatic drug-processing system and whether long-term ER can counteract the changes caused by aging.
A hierarchical analysis clearly shows that the majority of hepatic DPGs decreased at the mRNA level in aged mouse livers compared with adult mice (Fig. 10). These results in mice are consistent with those in aged rats characterized by full-genome Affymetrix Inc. arrays (Lee et al., 2008). Among 79 DPGs examined in the current study, 52 were expressed significantly lower in aged than in adult mouse livers. These genes encode enzymes involved in phase I and II drug metabolism, as well as transporters that function in the uptake and efflux of xenobiotics in hepatocytes. The reduced expression of these fundamental genes in liver provides a molecular explanation for the decreased hepatic drug-processing capability in the elderly population described previously (Klotz, 2009).
Fig. 10.
Hierarchical cluster analysis of effects of aging and ER on mRNA levels of DPGs in mouse livers. Each column represents an age-feeding group, and each row shows a particular gene with its name indicated on the right. The darkness of the region is positively correlated with the expression level. Each spectrum is specific to the scale of mRNA expression for each gene on an individual basis. Adult, 12 months old; aged, 24 months old; C, control. The same illustrations are also applicable to Fig. 11.
The expression of DPGs is regulated by multiple transcription factors, which are considered critical in xenobiotic pharmacokinetics and toxicity. For example, the activation of nuclear receptor CAR by chemicals such as phenobarbital, an anticonvulsant drug, induces various enzymes and transporters, thereby increasing the hepatic activities of drug metabolism and transport (Kodama and Negishi, 2006). CAR target genes include phase I enzymes (e.g., Cyp2b, Cyp2c, and Cyp3a), phase II enzymes (e.g., Sults, Ugts, and Gsts) (Ueda et al., 2002), and transporters such as Mrp2 and Mrp4 (Kast et al., 2002; Assem et al., 2004). Similar to CAR, PXR (Timsit and Negishi, 2007), AhR (Nebert et al., 2004), Nrf2 (Nguyen et al., 2003), PPARα (Barbier et al., 2004), HNF4α (Chiang, 2009), and RXRα, the common heterodimer partner for many nuclear receptors (Brtko, 2007), can also be activated by certain drugs and accordingly regulate the expression of a battery of hepatic DPGs. PPARα can be activated by a large class of drugs, such as fibrates (Barbier et al., 2004). The knockout mouse model revealed that PPARα is important in maintaining longevity, delaying various non-neoplastic spontaneous aging lesions, and preventing the onset of hepatocellular carcinomas (Howroyd et al., 2004).
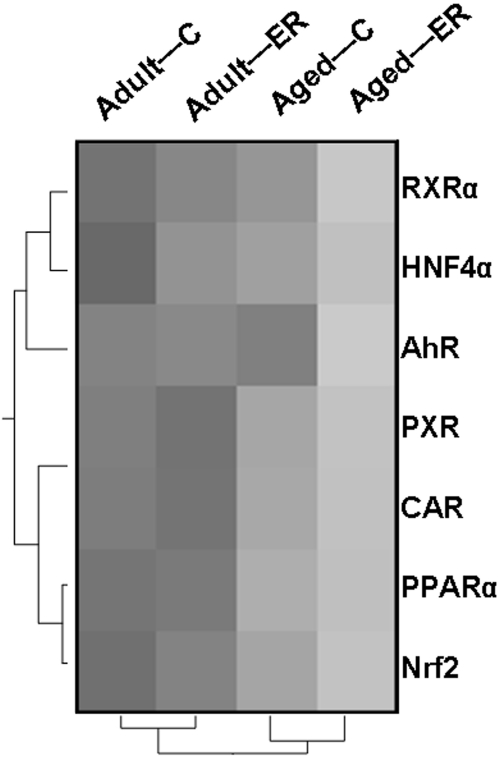
As shown by hierarchical analysis (Fig. 11), the mRNA expression of these transcription factors, except for AhR, decreases in aged mouse livers, which might account for the decreased basal expression of hepatic DPGs in aged mice. Given the fact that numerous therapeutic drugs and xenobiotics can activate these transcription factors, the decreased expression of these transcription factors might lead to altered drug response and processing capability in aged mice.
Fig. 11.
Hierarchical cluster analysis of effects of aging and ER on the mRNA expression of hepatic transcription factors related to drug metabolism.
Because of its well known antiaging function, the effect of ER on these hepatic DPGs was investigated in aged mice. We were surprised to find that the 6-month ER only influenced very few DPGs (11 of 79 DPGs) in aged mouse liver. More interestingly, the majority (9 of 11) of the changed genes were decreased instead of being increased by ER. These genes encode the apical aminophospholipid flippase Atp8b1, phase I enzymes Aldh6a1 and Pon3, phase II enzymes Ugt1a1, Ugt2a3, Sult1a1, Sult1d1, and Papss2, as well as basolateral efflux transporter Mrp6. Of note, mRNAs of three of the four quantified sulfation-related enzymes were decreased by ER, suggesting that ER might reduce hepatic sulfation capability in aged mouse livers. The only two genes that were increased by ER at the mRNA level in aged mice were the phase I enzyme Ces1d1 and the phase II enzyme Gstm3. Based on these data, ER is not capable to compensate for the age-induced decrease in hepatic DPG expression, at least at the mRNA level.
In adult mice, ER increased the mRNA levels of 10 hepatic DPGs and decreased 5 others. Of note, except for Gstm3, the hepatic DPGs changed by ER in adult mice are different from those in aged mice. ER enhanced the expression of phase I enzymes Cyp2b10 and Cyp4a14 but suppressed Ces3a2 in adult mice. ER might enhance glutathione conjugation in adult mice because the mRNA expression of several Gsts (Gsta1, Gsta4, Gstm3, and Mgst3) increased after 6-month ER but had less of an effect on sulfation (increased Papss2) and glucuronidation (decreased Ugt3a1) in adult mouse livers. For basolateral transporters, ER increased the mRNA of uptake transporter Oatp1a4 and efflux transporter Mrp4 but decreased those of Oatp1a1 and Oatp1b2. For apical transporters, the bile acid efflux transporters Bsep and Ostβ were decreased and increased by ER, respectively. Because of the important physiological roles of these hepatic transporters (Kusuhara and Sugiyama, 2009), altered disposition of endobiotics and xenobiotics is expected in adult mice undergoing ER. In summary, ER has a significant influence on some hepatic DPGs and could influence hepatic drug metabolism in adult mice.
Hepatic transcription factors might play a role in different ER-induced expression profiles of the aforementioned hepatic DPGs in adult and aged mice. For example, the mRNA of Cyp2b10, the prototypical CAR target gene, is increased by ER only in adult mice but not in aged mouse livers (Fig. 2), which corresponds with high and low expression levels of CAR in adult and aged mice, respectively (Fig. 9). CAR was previously reported to be activated by fasting and ER in mice, and prevented ER-induced weight loss by decreasing circulating thyroid hormone (Maglich et al., 2004). Other transcription factors, such as PPARα (Corton et al., 2004), which responds to both energy status and drugs in the liver, could also mediate the ER-induced changes in hepatic DPGs. Although the transcription factors themselves were not significantly influenced by ER at the mRNA level (Fig. 9), ER could influence hepatic drug metabolism through the activation of numerous hepatic transcription factors.
In addition to the changes of mRNA expression of hepatic drug metabolism genes revealed in mice by the current study, investigators have shown protein expression and activities of various hepatic enzymes and transporters in aged and/or ER rats. Although little reduction was observed in the activity of most P450 isoforms in aged male rats, the male-dominant Cyp2c11 isoform decreased ∼70% in mRNA, protein, and catalytic activity between 5 and 23 months of age (Agrawal and Shapiro, 2003). Because Cyp2c11 comprises approximately 50% of total hepatic P450s in male rats, a dramatic decrease in drug-processing capacity would be expected in aged male rats. In humans, age-associated decrease in the predominant human isoform CYP3A4/5 may account for the decrease in the metabolism of many drugs in the elderly population (Hunt et al., 1992).
Exposure to certain xenobiotics is known to induce numerous hepatic DPGs. Low-dose phenobarbital treatments (1 and 10 mg/kg) induced the expression of Cyp2b1, Cyp2b2, and Cyp3a1 to the same degree in young and aged rats (Agrawal and Shapiro, 2003). However, exposure to toluene (1 g/kg) decreased the transcription of more phase II enzymes and efflux transporters in 24-month-old rats than in 4-month-old rats (Lee et al., 2008). Long-term caloric restriction induced several P450 isoforms in rats (Leakey et al., 1989b) and attenuated the age-related decrease in Gst activity toward 1,2-dichloro-4-nitrobenzene, but had no effect on age-related changes in Ugt and Sult activities toward hydroxysteroids in aged rats (Leakey et al., 1989a). These studies suggest that aging and ER might alter the hepatic metabolism of some chemicals but not others.
Taken together, aging reduces the mRNAs of the majority of hepatic DPGs and xenobiotic-related transcription factors, which might lead to decreased hepatic drug-processing capability in aged mice. In comparison, ER only increases or decreases the expression of a small portion of hepatic enzymes and transporters in an age-dependent manner. Thus, ER is not sufficient to counteract the effects of aging on hepatic drug metabolism gene expression. However, ER could influence the disposition of certain drugs in mice through its influences on the expression of certain hepatic DPGs. Results generated by this study suggest that age and dietary habit can both influence the hepatic drug-processing system in mice. Hypothesizing that similar influences occur in humans, then age and dietary habit should be taken into consideration for clinical drug prescriptions.
Acknowledgments.
We thank Dr. Rachel Chennault for technical assistance with the QuantiGene Bioplex assay.
This work was supported in part by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK-081461]; National Institutes of Health National Institute of Environmental Health Sciences [Grants ES-009716, ES-009649, ES-013714]; National Institutes of Health National Center for Research Resources [Grant RR-021940]; National Institutes of Health National Institute on Aging [Grant AG-00908]; and the University of Wisconsin Comprehensive Cancer Center.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.032599.
- DPG
- drug-processing gene
- Oatp
- organic anion-transporting polypeptide
- Ntcp
- sodium/taurocholate-cotransporting polypeptide
- Oct
- organic cation transporter
- Oat
- organic anion transporter
- Mdr
- multidrug resistance protein
- Mrp
- multidrug resistance-associated protein
- Bsep
- bile salt export pump
- Bcrp
- breast cancer resistance protein
- P450
- cytochrome P450
- Aldh
- aldehyde dehydrogenase
- Ces
- carboxylesterase
- Pon
- paraoxonase
- Ugt
- UDP-glucuronosyltransferase
- Sult
- sulfotransferase
- Gst
- glutathione transferase
- PXR
- pregnane X receptor
- CAR
- constitutive androstane receptor
- PPARα
- peroxisome proliferator-activated receptor α
- AhR
- aryl hydrocarbon receptor
- Nrf2
- nuclear factor erythroid 2-related factor 2
- ER
- energy restriction
- Gapdh
- glyceraldehyde-3-phosphate dehydrogenase
- Ent1
- equilibrative nucleoside transporter 1
- Udp-gpp
- UDP-glucose pyrophosphorylase
- Udp-gdh
- UDP-glucose dehydrogenase
- Papss
- 3′-phosphoadenosine-5′-phosphosulfate synthase
- Abc
- ATP-binding cassette
- Ost
- organic solute transporter
- HNF4α
- hepatocyte nuclear factor 4α
- RXRα
- retinoid X receptor α.
References
- Agrawal AK, Shapiro BH. (2003) Constitutive and inducible hepatic cytochrome P450 isoforms in senescent male and female rats and response to low-dose phenobarbital. Drug Metab Dispos 31:612–619 [DOI] [PubMed] [Google Scholar]
- Alnouti Y, Klaassen CD. (2006) Tissue distribution and ontogeny of sulfotransferase enzymes in mice. Toxicol Sci 93:242–255 [DOI] [PubMed] [Google Scholar]
- Alnouti Y, Klaassen CD. (2008a) Regulation of sulfotransferase enzymes by prototypical microsomal enzyme inducers in mice. J Pharmacol Exp Ther 324:612–621 [DOI] [PubMed] [Google Scholar]
- Alnouti Y, Klaassen CD. (2008b) Tissue distribution, ontogeny, and regulation of aldehyde dehydrogenase (Aldh) enzymes mRNA by prototypical microsomal enzyme inducers in mice. Toxicol Sci 101:51–64 [DOI] [PubMed] [Google Scholar]
- Alnouti Y, Petrick JS, Klaassen CD. (2006) Tissue distribution and ontogeny of organic cation transporters in mice. Drug Metab Dispos 34:477–482 [DOI] [PubMed] [Google Scholar]
- Anderson RM, Shanmuganayagam D, Weindruch R. (2009) Caloric restriction and aging: studies in mice and monkeys. Toxicol Pathol 37:47–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Weindruch R. (2007) Metabolic reprogramming in dietary restriction. Interdiscip Top Gerontol 35:18–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assem M, Schuetz EG, Leggas M, Sun D, Yasuda K, Reid G, Zelcer N, Adachi M, Strom S, Evans RM, et al. (2004) Interactions between hepatic Mrp4 and Sult2a as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J Biol Chem 279:22250–22257 [DOI] [PubMed] [Google Scholar]
- Barbier O, Fontaine C, Fruchart JC, Staels B. (2004) Genomic and non-genomic interactions of PPARalpha with xenobiotic-metabolizing enzymes. Trends Endocrinol Metab 15:324–330 [DOI] [PubMed] [Google Scholar]
- Brtko J. (2007) Retinoids, rexinoids and their cognate nuclear receptors: character and their role in chemoprevention of selected malignant diseases. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 151:187–194 [DOI] [PubMed] [Google Scholar]
- Buckley DB, Klaassen CD. (2007) Tissue- and gender-specific mRNA expression of UDP-glucuronosyltransferases (UGTs) in mice. Drug Metab Dispos 35:121–127 [DOI] [PubMed] [Google Scholar]
- Cao SX, Dhahbi JM, Mote PL, Spindler SR. (2001) Genomic profiling of short- and long-term caloric restriction effects in the liver of aging mice. Proc Natl Acad Sci USA 98:10630–10635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Klaassen CD. (2009) Tissue distribution, ontogeny, and hormonal regulation of xenobiotic transporters in mouse kidneys. Drug Metab Dispos 37:2178–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Maher J, Dieter MZ, Klaassen CD. (2005) Regulation of mouse organic anion-transporting polypeptides (Oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways. Drug Metab Dispos 33:1276–1282 [DOI] [PubMed] [Google Scholar]
- Chiang JY. (2009) Hepatocyte nuclear factor 4alpha regulation of bile acid and drug metabolism. Expert Opin Drug Metab Toxicol 5:137–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Nam G, Huchthausen L, Mulligan JD, Saupe KW. (2007) Energy restriction-induced changes in body composition are age specific in mice. J Nutr 137:2247–2251 [DOI] [PubMed] [Google Scholar]
- Corton JC, Apte U, Anderson SP, Limaye P, Yoon L, Latendresse J, Dunn C, Everitt JI, Voss KA, Swanson C, et al. (2004) Mimetics of caloric restriction include agonists of lipid-activated nuclear receptors. J Biol Chem 279:46204–46212 [DOI] [PubMed] [Google Scholar]
- Hagopian K, Ramsey JJ, Weindruch R. (2003) Caloric restriction increases gluconeogenic and transaminase enzyme activities in mouse liver. Exp Gerontol 38:267–278 [DOI] [PubMed] [Google Scholar]
- Howroyd P, Swanson C, Dunn C, Cattley RC, Corton JC. (2004) Decreased longevity and enhancement of age-dependent lesions in mice lacking the nuclear receptor peroxisome proliferator-activated receptor α (PPARα). Toxicol Pathol 32:591–599 [DOI] [PubMed] [Google Scholar]
- Hunt CM, Westerkam WR, Stave GM. (1992) Effect of age and gender on the activity of human hepatic CYP3A. Biochem Pharmacol 44:275–283 [DOI] [PubMed] [Google Scholar]
- Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA. (2002) Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem 277:2908–2915 [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Slitt AL. (2005) Regulation of hepatic transporters by xenobiotic receptors. Curr Drug Metab 6:309–328 [DOI] [PubMed] [Google Scholar]
- Klotz U. (2009) Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev 41: 67–76 [DOI] [PubMed] [Google Scholar]
- Kodama S, Negishi M. (2006) Phenobarbital confers its diverse effects by activating the orphan nuclear receptor car. Drug Metab Rev 38:75–87 [DOI] [PubMed] [Google Scholar]
- Kusuhara H, Sugiyama Y. (2009) In vitro-in vivo extrapolation of transporter-mediated clearance in the liver and kidney. Drug Metab Pharmacokinet 24:37–52 [DOI] [PubMed] [Google Scholar]
- Leakey JA, Cunny HC, Bazare J, Jr, Webb PJ, Lipscomb JC, Slikker W, Jr, Feuers RJ, Duffy PH, Hart RW. (1989a) Effects of aging and caloric restriction on hepatic drug metabolizing enzymes in the Fischer 344 rat. II: Effects on conjugating enzymes. Mech Ageing Dev 48:157–166 [DOI] [PubMed] [Google Scholar]
- Leakey JE, Cunny HC, Bazare J, Jr, Webb PJ, Feuers RJ, Duffy PH, Hart RW. (1989b) Effects of aging and caloric restriction on hepatic drug metabolizing enzymes in the Fischer 344 rat. I: The cytochrome P-450 dependent monooxygenase system. Mech Ageing Dev 48:145–155 [DOI] [PubMed] [Google Scholar]
- Lee JS, Ward WO, Wolf DC, Allen JW, Mills C, DeVito MJ, Corton JC. (2008) Coordinated changes in xenobiotic metabolizing enzyme gene expression in aging male rats. Toxicol Sci 106:263–283 [DOI] [PubMed] [Google Scholar]
- Maglich JM, Watson J, McMillen PJ, Goodwin B, Willson TM, Moore JT. (2004) The nuclear receptor CAR is a regulator of thyroid hormone metabolism during caloric restriction. J Biol Chem 279:19832–19838 [DOI] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. (2004) Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem 279:23847–23850 [DOI] [PubMed] [Google Scholar]
- Nguyen T, Sherratt PJ, Pickett CB. (2003) Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol 43:233–260 [DOI] [PubMed] [Google Scholar]
- Ramaiah SK, Apte U, Mehendale HM. (2001) Cytochrome P4502E1 induction increases thioacetamide liver injury in diet-restricted rats. Drug Metab Dispos 29:1088–1095 [PubMed] [Google Scholar]
- Ramaiah SK, Soni MG, Bucci TJ, Mehendale HM. (1998) Diet restriction enhances compensatory liver tissue repair and survival following administration of lethal dose of thioacetamide. Toxicol Appl Pharmacol 150:12–21 [DOI] [PubMed] [Google Scholar]
- Reeves PG, Rossow KL, Lindlauf J. (1993) Development and testing of the AIN-93 purified diets for rodents: results on growth, kidney calcification and bone mineralization in rats and mice. J Nutr 123:1923–1931 [DOI] [PubMed] [Google Scholar]
- Schmucker DL. (2005) Age-related changes in liver structure and function: implications for disease? Exp Gerontol 40:650–659 [DOI] [PubMed] [Google Scholar]
- Timsit YE, Negishi M. (2007) CAR and PXR: the xenobiotic-sensing receptors. Steroids 72:231–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Hamadeh HK, Webb HK, Yamamoto Y, Sueyoshi T, Afshari CA, Lehmann JM, Negishi M. (2002) Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol Pharmacol 61:1–6 [DOI] [PubMed] [Google Scholar]
- Yu BP. (1996) Aging and oxidative stress: modulation by dietary restriction. Free Radic Biol Med 21:651–668 [DOI] [PubMed] [Google Scholar]
- Zhang YK, Yeager RL, Klaassen CD. (2009) Circadian expression profiles of drug-processing genes and transcription factors in mouse liver. Drug Metab Dispos 37:106–115 [DOI] [PMC free article] [PubMed] [Google Scholar]