Abstract
Background
Dental amalgam is a widely used restorative material containing 50% elemental mercury that emits mercury vapor. No randomized clinical trials have determined whether there are adverse immunologic effects associated with this low-level mercury exposure in children. The objective of this study was to evaluate a sub-population of the New England Children’s Amalgam Trial (NECAT) for in vitro manifestations of immunotoxic effects of dental amalgam.
Methods
A randomized clinical trial in which children requiring dental restorative treatment were randomized to either amalgam for posterior restorations or resin composite. A total of 66 children, aged 6–10 years, were assessed for total white cell numbers, T-cell, B-cell, neutrophil and monocyte responsiveness over a five-year period. Owing to the small number of participants, the study is exploratory in nature with limited statistical power.
Results
The mean number of tooth surfaces restored during the five-year period was 7.8 for the amalgam group and 10.1 for composite group. In the amalgam group there was a slight, but not statistically significant, decline in responsiveness of T-cells and monocytes at 5–7 days post treatment; no differences were consistently observed at 6, 12 or 60 months.
Conclusions
This study confirms that treatment of children with dental amalgams leads to increased, albeit low level, exposure to mercury. In this exploratory analysis of immune function, amalgam exposure did not cause overt immune deficits, although small transient effects were observed 5–7 days post restoration.
Clinical implications
These findings suggest that immunotoxic effects of amalgam restorations in children need not be a concern when choosing this restorative dental material.
Keywords: dental amalgam, mercury, immunotoxicity
INTRODUCTION
Exposure to mercury compounds is widespread in the U. S. population as well as throughout the world. It is well known that the toxic effects of mercury are directly related to its chemical form, dose and route of exposure.1–3 Concerns regarding mercury exposures have generally focused on neurodevelopment and nephrotoxicity.4 More recently there has been significant interest in the potential immunotoxic effects of mercuric compounds, with particular concern focused on the impact of chronic exposure to low levels of mercury.5–12, 12–18 Such immunotoxic effects could perturb the immune system leading to immune suppression, a decline in immune competence and possibly autoimmune destruction or immune stimulation contributing to hypersensitivity reactions.
The potential of mercuric compounds for inducing immunotoxicity has led to concern that dental amalgams may have similar adverse affects. Conventional dental amalgam is an alloy that consists of approximately 50% mercury which may be released as mercury vapor or corrosion products such as Hg++. While the total mercury body burden is derived from multiple sources, dental amalgams are the largest single source of systemic inorganic mercury exposure in the general population. 19 It is estimated that more than 70 million dental amalgam restorations are placed annually in the United States20; however, the health risks posed by the chronic release of metallic mercury vapor from amalgams remain unclear.
Studies on adult populations where amalgams were considered the primary source of mercury have not found significant associations between neuropsychological function and various amalgam exposure indices including urinary mercury level, number of amalgam restorations, total number of amalgam surfaces and number of occlusal amalgam surfaces. 21–26 Other studies suggest associations between dental amalgams and neurodegenerative disorders such as Alzheimer Disease and multiple sclerosis. 27, 28 There is also no clear evidence that dental amalgams contribute to adverse effects on the immune system of adults such as hyperreactivity and cytogenetic alterations.
Amalgam restorations in a child’s mouth are associated with increased exposure to mercury, as determined by significantly elevated urinary mercury levels.29–32 It is a concern that there is a lack of data on the possible immunotoxic effects of mercury from dental amalgams in children. Bellinger et al 33 and DeRouen et al 34 recently reported results from two separate randomized clinical trials that found no statistically significant differences in adverse neuropsychological or renal effects observed over a 5–7 year period in children whose caries were restored using amalgam or composite materials. We now report on a substudy of the New England Childrens Amalgam Trial (NECAT) in which the immune cells of these children were evaluated in vitro for manifestations of immunotoxic effects of dental amalgam.
METHODS
Study Design and Participants
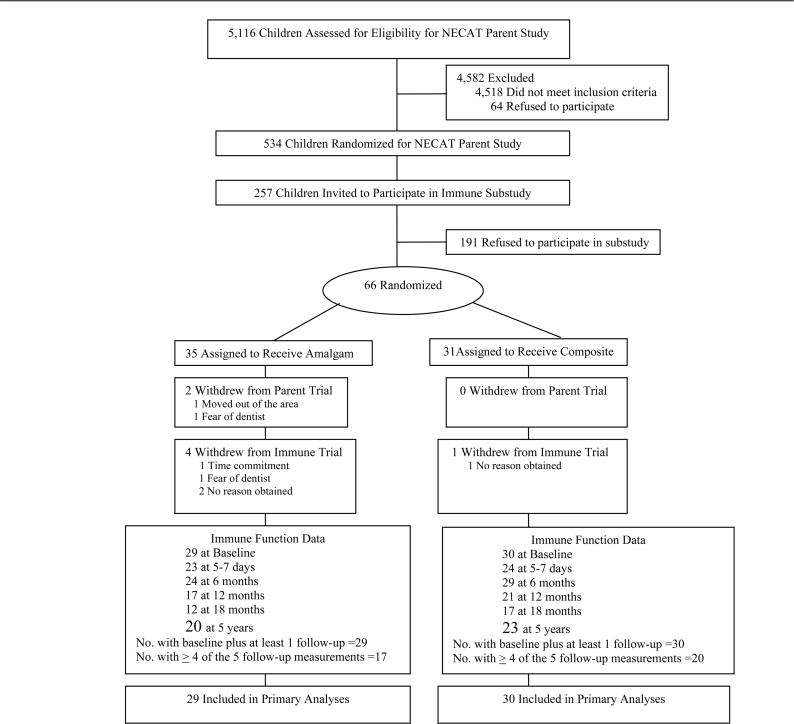
Participants were a subsample of children in NECAT equally sampled from each treatment group (Figure 1). The main trial and the immune function substudy were approved by the institutional review boards of all participating sites. Children were eligible if they were 6 to 10 years of age at last birthday, fluent in English, had no known prior or existing amalgam restorations, had two or more posterior teeth with dental caries such that restoration would include the occlusal surfaces, and, by parent report, had no physician-diagnosed psychological, behavioral, neurologic, immunologic, or renal disorders. A total of 5,116 children were screened for NECAT eligibility, of which 598 were confirmed eligible and 534 provided parental consent and child assent for participation in the main trial. A detailed description of both the main trial and the immune function substudy protocols is provided elsewhere.31, 33
Figure 1.
Profile of recruitment, randomization, and follow-up in the New England Children’s Amalgam Trial immune function substudy. Recruitment period ran from September 1997 through September 1999, with follow-up ending March 2005. The primary reason for refusal to participate in the substudy was fear of blood draws, mentioned by 75% of invited participants, followed by time commitment, mentioned by 40%.
For the immune function substudy, the goal was to recruit 80 subjects, 40 in each treatment group. Of the 257 children invited to participate in the substudy, 66 (25%) provided consent/assent (35 children in the amalgam treatment group and 31 children in the composite group). The primary reason for refusal to participate in the substudy was fear of blood draws, mentioned by 75% of invited participants, followed by time commitment, mentioned by 40%. Both baseline and follow-up immune function data, which were required for inclusion in this analysis, were available for 59 children (29 amalgam group, 30 composite group).
Interventions and Follow-up
For children assigned to the amalgam arm, a dispersed phase amalgam (Dispersally, Denstply Caulk; Milford, DE) was used to restore all posterior teeth with caries at baseline and to restore incident caries during the 5-year trial period. For children assigned to the composite arm, resin-based composite material (Dyract, Dentsply Caulk on permanent dentition or Z100: 3M ESPE; St. Paul, MN on primary dentition) was used for all restorations. Following standard clinical practice, however, for both groups composite material was used to restore caries in the front teeth.
Children in both groups had semi-annual dental examinations and restorative dental visits, where pertinent dental data were documented. At the annual visits, anthropometric measurements were made and a urine sample collected. Initially, attempts were made to collect timed overnight urine samples but, mid-trial, a switch was made to spot samples. Hair samples were collected biennially. Blood samples were collected on all subjects at baseline (pre-randomization), 5–7 days after completion of baseline dental treatment, and 6, 12, and 60 months post-randomization.
Mercury measurements
Details on the measurement methods for total urinary mercury (U-Hg), hair Hg, and blood lead have previously been published.31, 34 The detection limit for U-Hg, initially 1.5 ng/mL, was reduced to 0.45 ng/mL after February 1, 2000 as a result of increasing the volume of urine analyzed from each child. This altered detection limit prevents the direct comparison of U-Hg values from samples taken before and after February 2000. For this reason, only urine data from years 3–5 are considered in this analysis. Non-detectable concentrations (<0.45 ng/ml) were imputed as 0.45/√2.35 U-Hg is expressed as creatinine-corrected U-Hg (μg/g).36, 37
Immunological outcome measures
Heparinized blood samples were sent to a central laboratory (University of Pennsylvania), where all assays were performed. In order to maintain the sample tubes at room temperature they were shipped overnight in insulated containers with pre-warmed (30°C) gel packs. Immune parameters that were assessed fall into four catgories: white blood cell enumeration (WBC), assessment of T- and B-cell responsiveness and analysis of neutrophil and monocyte responsiveness. Assays were optimized and checked for reproducibility. Fluorescent calibration beads were employed to standardize the flow cytometer from day to day; this enabled comparison of absolute results (i.e. fluorescence intensity) rather than just percentages of positive cells. Test samples were run to ensure reproducibility, particularly with respect to blood stability under shipping conditions. Blood samples were coded to ensure that laboratory technicians were blinded to subject treatment group.
Total WBC enumeration were performed using Wright’s stain and hemocytometer. Distribution of neutrophils, monocytes, T cells, B cells, NK cells as well as CD4 and CD8 subtypes was determined by flow cytometry using the IMK+ Simultest kit (Becton Dickinson). Functional analysis of T cells following mitogenic activation employed two approaches: analysis of activation markers and cell cycle distribution. T-cells were incubated with phytohemagglutinin [(PHA); 5 μg/ml] for 24 hrs in order to assess expression of activation markers; CD69 and CD25 expression was determined by immunofluorescence using flow cytometry as previously described.14 Cell cycle distribution was assessed after 72 hr incubation in the presence of PHA.38 B cell activation was monitored by analyzing expression of CD69 and increased expression of CD23 following stimulation with pokeweed mitogen [(PWM); 10 μg/ml].
The functional status of neutrophils and monocytes was determined by monitoring phorbol myristate acetate (PMA); 0.5 μg/ml]-induced oxidative burst. The fluorescent probes dihydroethidium and dihydrorhodmine were employed to assess both O2−· and H2O2 generation, respectively; fluorescence was determined by flow cytometry 30 min after cell activation.39
Sample Size and Power Calculations
As this is an exploratory study of immune function, there was little information on which to base power calculations. With the 59 children in the final main analysis, there is approximately 67% power to detect an effect size of 0.63, or 80% power to detect an effect size of 0.74 which is a measure of the strength of the relationship between two variables (an effect size of 0.2 is considered small, 0.5 medium and 0.8 large). Considering the limited power and lack of existing knowledge on the subject, our emphasis during interpretation of the results is on the observation of trends rather than statistical significance.
Statistical Analysis
The primary analysis compared each of the immune function markers by treatment assignment. Time trends in means for each outcome were depicted graphically by treatment group. Repeated measures analysis of covariance (ANCOVA) models were used to test the difference over time in each outcome. Because the profiles of the means for amalgam and composite groups were similar over time (i.e., there was no interaction between time and treatment), models included only main effects, adjusted for baseline corresponding immune function measurement, age, gender, socioeconomic status, hair mercury, and blood lead level. Socioeconomic status was calculated using the method of Green.40
Secondary analyses compared immune outcomes by urinary-Hg excretion at year 5 with ANCOVA, restricted to participants with both immune function measures and U-Hg measures at year 5 (n=20 amalgam group, n=23 composite group). Regardless of treatment assignment, children were categorized as having low (0–0.49 μg/g), medium (0.50–0.99 μg/g) or high (1.0–2.2 μg/g) U-Hg, using categories based on the distribution of U-Hg level among all participants at year 5. All statistical tests were 2-sided, performed at an alpha level of 0.05, and conducted using SAS v.9.1 (Cary, North Carolina).
RESULTS
Baseline characteristics of substudy participants
Substudy participants in the two treatment groups were similar in terms of most baseline characteristics including age, race, household income, education of primary caregiver, full-scale IQ, hair and urinary mercury concentrations (Table 1). Moreover, the immune function substudy population reflected the baseline characteristics of the larger NECAT population. Two differences were noted, however. First, the gender distribution was less balanced across treatment groups in the substudy, with more boys (65.5%) in the amalgam group and more girls (63.3%) in the composite group. Second, the mean number of carious surfaces was lower in the amalgam group than in the composite (7.8 vs 10.1 surfaces) in the substudy. These differences were not statistically significant and were likely due to small numbers.
Table 1.
Baseline characteristics of immune function substudy participants and all NECAT participants, by treatment group
| Immune Function Substudy Participants | All NECAT Participants* | |||
|---|---|---|---|---|
| N=59 | N=534 | |||
| Amalgam | Composite | Amalgam | Composite | |
| n=29 | n=30 | n=267 | n=267 | |
| Site, N (%) | ||||
| Boston | 11 (37.9) | 14 (46.7) | 144 (53.9) | 147 (55.1) |
| Maine | 18 62.1) | 16 (53.3) | 123 (46.1) | 120 (44.9) |
| Carious surfaces, mean (SD) [range] | 7.8 (5.2) [2–17] | 10.1 (6.3) [2–27] | 9.8 (6.9) [2–39] | 9.3 (6.2) [2–36] |
| Age (mean, SD) | 8.1 (1.3) | 8.0 (1.4)] | 7.9 (1.3) | 7.9 (1.4) |
| Gender, N (%) | ||||
| Female | 10 (34.5) | 19 (63.3) | 131 (49.1) | 156 (58.4) |
| Male | 19 (65.5) | 11 (36.7) | 136 (50.9) | 111 (41.6) |
| Race†, N (%) | ||||
| Non-Hispanic White | 26 (89.7) | 27 (90.0) | 165 (64.0) | 158 (60.3) |
| Non-Hispanic Black | 1 (3.5) | 1 (3.3) | 49 (19.0) | 49 (18.7) |
| Hispanic | 0 (0) | 0 (0) | 15 (5.8) | 23 (8.8) |
| Other | 2 (6.9) | 2 (6.7) | 29 (11.2) | 32 (12.2) |
| Household income, N (%) | ||||
| ≤ $20,000 | 5 (18.5) | 10 (33.3) | 74 (29.2) | 86 (33.1) |
| $20,001 – $40,000 | 14 (51.9) | 13 (43.3) | 113 (44.7) | 109 (41.9) |
| > $40,000 | 8 (29.6) | 7 (23.3) | 66 (26.1) | 65 (25.0) |
| Education of primary caretaker, N (%) | ||||
| < High school | 5 (17.2) | 7 23.3) | 34 (13.2) | 38 (14.6) |
| High school graduate | 23 (79.3) | 22 73.3) | 197 (76.4) | 194 (74.3) |
| College graduate | 1 (3.5) | 1 (3.3) | 27 (10.4) | 29 (11.1) |
| Ever had asthma, N (%) | ||||
| Yes | 6 (20.7) | 5 (16.7) | 50 (19.4) | 33 (12.6) |
| No | 23 (79.3) | 25 (83.3) | 208 (80.6) | 229 (87.4) |
| Ever had allergies, N (%) | ||||
| Yes | 4 (13.8) | 5 (16.7) | 38 (14.7) | 31 (11.8) |
| No | 25 (86.2) | 25 (83.3) | 220 (85.3) | 231 (88.2) |
| Hair mercury concentration, mean (SD) [range], ng/mg hair | 0.4 (0.3) [0.1–1.1] | 0.4 (0.5) [0.1–1.1] | 0.4 (0.5) [0.1–4.4] | 0.4 (0.5) [0.1–4.5] |
| Blood lead concentration, mean (SD) [range], μg/dl | 1.9 (1.4) [1–7] | 2.2 (1.4) [1–6] | 2.4 (1.9) [1–13] | 2.3 (1.5) [1–11] |
The number for all NECAT participants includes those who later withdrew (85 of 534). For race, lead, asthma and allergy, data were available for 520 participants; for income, 513; for education and hairy mercury, 519.
Race was self-reported by the parents of the children. The other category included individuals who identified themselves as Asian, Pacific Islander, Native American, biracial or other, which they were asked to specify.
Four categories of baseline immune status are displayed in Table 2: WBC number/distribution, lymphocyte function, monocyte function and neutrophil function. Total WBC as well as the distribution of lymphocytes, monocytes and neutrophils did not differ between the two treatment groups at baseline. T-cells obtained from both treatment groups exhibited similar levels of mitogenic responsiveness at baseline; 83.9% and 77% of T-cells from the amalgam group expressed CD69 and CD25, respectively, 24 hr following activation with PHA compared to 82.2% and 74.7% for the composite group. Baseline values for B-cells from the two treatment groups were similar as well. In addition, the percentage of monocytes exhibiting an oxidative burst in response to PMA was similar in both treatment groups with respect to the production of both O2−· (ethidium fluorescence) and H2O2 (rhodamine fluorescence); 69.7% (O2−·) and 55.7% (H2O2) monocytes for the amalgam group compared with 65.6% (O2−·) and 54.2% (H2O2) for the composite group. Neutrophil oxidative burst at baseline also did not differ between treatment groups; 92.2% (O2−·) and 87.6% (H2O2) for the amalgam group compared with 88.9% (O2−·) and 82.4% (H2O2) for the composite group. It should also be noted that the amount of free radicals produced as measured as a function of the mean channel fluorescence did not vary significantly between the two groups (data not shown).
Table 2.
Baseline Immune Data
| Amalgam | Composite | |||
|---|---|---|---|---|
| Variable | N | Mean | N | Mean |
| Total WBC | 31 | 11.6±3.3 | 30 | 1.8±4.4 |
| % Lymphocytes | 30 | 39.2±11.9 | 30 | 41±11.9 |
| % Granulocyes | 30 | 52.8±11.8 | 30 | 52.0±11.4 |
| % Monocytes | 30 | 6.6±1.7 | 30 | 6.0±1.7 |
| T-cell function: | ||||
| %CD69+ (control) | 30 | 3.5±2.4 | 30 | 3.0±2.6 |
| %CD69+ (PHA) | 30 | 83.9±9.5 | 30 | 82.2±16.0 |
| %CD25+ (control) | 30 | 5.2±3.2 | 30 | 4.5±3.1 |
| %CD25+ (PHA) | 30 | 77.0±16.3 | 30 | 74.7±21.9 |
| B-cell function: | ||||
| %CD69+ (control) | 30 | 13.6±7.6 | 30 | 15.4±8.6 |
| %CD69+ (PWM) | 30 | 75.6±13.9 | 30 | 71.5±14.9 |
| %CD23+ (control) | 30 | 10.3±7.7 | 30 | 11.6±8.3 |
| %CD23+ (PHA) | 30 | 47.5±18.3 | 30 | 39.3±20.7 |
| Monocyte function: | ||||
| % Eth+(control) | 30 | 6.6±4.0 | 29 | 5.9±3.6 |
| % Eth+(PMA) | 30 | 69.7±15.8 | 29 | 65.6±19.8 |
| % Rho+(control) | 30 | 5.6±7.1 | 29 | 5.7±10.0 |
| % Rho+(PMA) | 30 | 55.7±21.5 | 29 | 54.2±22.4 |
| Neutrophil function: | ||||
| % Eth+(control) | 30 | 5.2±3.4 | 30 | 4.6±2.8 |
| % Eth+(PMA) | 30 | 92.2±7.5 | 30 | 88.9±16.9 |
| % Rho+(control) | 30 | 5.9±15.2 | 30 | 2.8±2.5 |
| % Rho+(PMA) | 30 | 87.6±17.8 | 30 | 82.4±19.7 |
Exposure to dental amalgam
The cumulative number of surfaces restored over the course of the trial was 16.9 for children in the composite group and 13.5 for children in the amalgam group with an average of 10.6 amalgam-filled surfaces in the amalgam group and none in the composite group. Numbers of restored surfaces were greatest shortly after entry into the study. While fillings placed in primary teeth at baseline were often lost over the course of the trial, children had recurrent treatment needs averaging approximately 1 additional filled surface per year. The mean number of restored surfaces present in each child’s mouth at the end of the five years was 5.9 in the composite group and 5.3 in the amalgam group (of which 4.2 were restored with amalgam).
Urinary mercury excretion
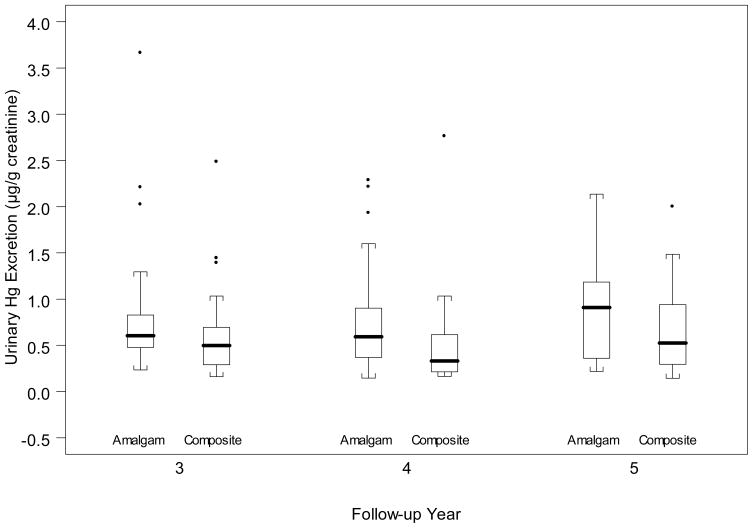
Children assigned to the amalgam group had significantly higher mean urinary mercury excretion levels than did children assigned to the composite group (Figure 2). Mean urinary mercury excretion in the amalgam group was 0.89, 0.81 and 0.85 μg/g creatinine at years 3, 4 and 5, respectively. This compares to 0.64, 0.50 and 0.68 μg mercury/g creatinine for the composite group. Urinary mercury was detectable in 65% (year 3), 48% (year 4) and 61% (year 5) of the amalgam group and in 24% (year 3), 24% (year 4) and 42% (year 5) of the composite group.
Figure 2.
Urinary mercury excretion by year and treatment group. Boxes indicate upper and lower quartiles, and error bars indicate 2.5% and 97.5% values with points for outliers. P=0.07 for the difference between amalgam and composite groups at year 3; P=0.03 at year 4; and P=0.20 at year 5.
Immunologic function
Changes in immunologic measurements from baseline were assessed at 5–7 days post-treatment, 6 months, 12 months and 60 months post-treatment (Table 3). The distribution of lymphocytes, monocytes and neutrophils fluctuated over time, both within and between treatment groups. No consistent or statistically significant differences were observed between the two treatment groups in graphical evaluations of trends or in the ANCOVA models.
Table 3.
Changes in Immune Status from Baseline Values
| 5–7 days post | 6 months post | 12 months post | 60 months post | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amalgam | Composite | Amalgam | Composite | Amalgam | Composite | Amalgam | Composite | |||||||||
| Variable | N | Mean | N | Mean | N | Mean | N | Mean | N | Mean | N | Mean | N | Mean | N | Mean |
| Total WBC | 23 | −0 ±3.6 | 24 | .4±7.2 | 24 | 0.6±3.5 | 29 | 0.7±3.8 | 17 | 1.2±5.8 | 21 | −0.4±3.6 | 20 | −1.0±4.0 | 23 | −1.7±5.5 |
| % Lymphocytes | 22 | 1.5±10.7 | 24 | .71±11.9 | 24 | 6.8±12.1 | 28 | 3.6±14.9 | 17 | 10.4±20.2 | 21 | 7.5±14.6 | 20 | −5.1±8.5 | 23 | −8.7±13.3 |
| % Granulocyes | 22 | −1.6 ±11.1 | 24 | −1.2±11.8 | 24 | −6.7±2.5 | 28 | −0.1±3.3 | 17 | −9.9±20.6 | 21 | −7.7±15.2 | 20 | 1.5±8.5 | 23 | 4.4±12.7 |
| % Monocytes | 22 | −.0 ±1.3 | 24 | .3±1.6 | 24 | −0.2±2.0 | 28 | 0.1±1.3 | 17 | −2.1±2.4 | 21 | −1.4±1.4 | 20 | −0.2±1.3 | 23 | −0.8±1.8 |
| T-cell function: | ||||||||||||||||
| %CD69+ (ctrl) | 23 | −.2±3.8 | 24 | −.0±2.4 | 24 | 0.6±2.9 | 29 | 2.5±4.2 | 17 | 1.3±3.6 | 21 | 0.6±2.8 | 20 | 1.7±5.5 | 23 | 7.2±19.5 |
| %CD69+ (PHA) | 23 | −6.5±23.6 | 24 | 4.2±20.8 | 24 | −1.5±26.3 | 28 | 4.5±20.6 | 17 | 5.7±9.6 | 21 | 5.5±28.4 | 20 | 0.9±17.0 | 23 | 4.0±17.9 |
| %CD25+ (ctl) | 23 | .3±4.4 | 24 | −.0±3.0 | 24 | 0.5±4.4 | 28 | 2.3±4.4 | 17 | 1.1±4.2 | 21 | 0.4±3.7 | 20 | 3.9±7.9 | 23 | 4.6±6.6 |
| %CD25+ (PHA) | 23 | −6.0±25.3 | 24 | 1.3±28.1 | 24 | 2.0±31.4 | 28 | 4.7±36.3 | 17 | 13.8±18.6 | 21 | 13.6±30.7 | 20 | 14.8±16.3 | 23 | 14.0±24.4 |
| B-cell function: | ||||||||||||||||
| %CD69+ (ctl) | 23 | −.9±11.3 | 24 | −2.5±10.4 | 24 | 2.9±14.2 | 28 | 4.0±16.2 | 17 | 2.1±10.8 | 21 | −3.4±9.8 | 20 | −3.8±22.0 | 23 | −6.6±11.5 |
| %CD69+ (PWM) | 23 | −5.2±16.8 | 24 | −2.2±21.6 | 24 | −0.4±24.9 | 28 | 5.2±21.9 | 17 | −5.9±22.3 | 21 | −1.3±26.9 | 20 | −8.4±24.9 | 23 | 1.8±14.1 |
| %CD23+ (ctl) | 23 | 0.1±9.9 | 24 | −1.2±8.9 | 24 | 3.0±10.8 | 28 | 6.5±16.3 | 17 | 2.5±14.5 | 21 | −1.5±11.8 | 20 | −1.6±14.3 | 23 | −3.0±11.6 |
| %CD23+ (PHA) | 23 | 2.5±12.5 | 24 | 1.5±21.7 | 24 | 9.8±25.7 | 28 | 13.0±28.4 | 17 | −1.3±27.7 | 21 | 3.8±30.7 | 20 | −3.3±26.9 | 23 | 10.9±23.5 |
| Monocyte function: | ||||||||||||||||
| % Eth+(ctl) | 23 | 0.7±4.6 | 24 | 0.9±4.9 | 24 | 1.0±5.5 | 27 | 1.1±5.2 | 17 | 0.4±6.1 | 21 | 1.4±5.2 | 20 | 1.7±5.7 | 22 | 2.1±4.3 |
| % Eth+(PMA) | 23 | −7.8±26.4 | 24 | 5.7±19.6 | 24 | −6.2±19.9 | 27 | −4.9±30.1 | 17 | −30.7±22.7 | 21 | −18.4±26.1 | 20 | 6.3±21.1 | 22 | 3.1±26.8 |
| % Rho+(ctl) | 23 | −.7±7.0 | 24 | −2.3±11.0 | 24 | −1.8±6.2 | 27 | −2.8±11.3 | 17 | −1.0±3.5 | 21 | −0.4±3.5 | 20 | −0.8±6.1 | 22 | −2.3±11.4 |
| % Rho+(PMA) | 23 | −8.4±30.2 | 24 | 0.4±29.2 | 24 | −5.6±27.7 | 27 | −2.1±29.7 | 17 | −22±20.8 | 21 | −15.3±26.7 | 20 | 7.8±24.5 | 22 | 8.8±28.7 |
| Neutrophil function | ||||||||||||||||
| % Eth+(ctl) | 23 | 0.1±4.5 | 24 | 0.1±3.1 | 24 | −0.6±3.8 | 28 | 0.3±5.5 | 17 | −0.1±4.0 | 21 | −0.9±5.4 | 20 | −2.1±2.4 | 23 | −0.4±6.5 |
| % Eth+(PMA) | 23 | −6.5±20.4 | 24 | 3.1±21.1 | 24 | −8.3±24.9 | 28 | −9.8±34.6 | 17 | −14.5±23.6 | 21 | −13.4±36.6 | 20 | 2.3±8.1 | 23 | 6.1±19.6 |
| % Rho+(ctl) | 23 | −.4±22.9 | 24 | −.5±1.8 | 24 | −3.8±17.1 | 28 | 0.3±4.2 | 17 | 0.1±3.5 | 21 | 0.6±4.2 | 20 | −5.4±18.8 | 23 | −1.2±3.2 |
| % Rho+(PMA) | 23 | −8.0±19.5 | 24 | 7.2±24.5 | 24 | −5.0±29.7 | 28 | −0.5±26.3 | 17 | −7.3±31.1 | 21 | −2.0±25.4 | 20 | 1.8±13.0 | 23 | 9.3±25.4 |
In the amalgam group there was a slight, but not statistically significant, decline in responsiveness of T-cells (measured by expression of the activation markers CD69 and CD25 in response to PHA) at 5–7 days post treatment; however, no differences were consistently observed at 6, 12 or 60 months. No differences between treatment groups were noted for proliferative responses of T-cells (data not shown) or B-cell function (measured by expression of CD69 and CD23) over time.
The functional status of monocytes and neutrophils were monitored by measuring the generation of free radicals, O2−· (ethidium fluorescence) and H2O2 (dihydrorhodmine fluorescence), following activation by PMA. Compared to the composite group, monocytes from the amalgam population exhibited reduced responsiveness within 5–7 days in terms of both O2−· (7.8%) and H2O2 (8.4%); this trend did not persist beyond this time point. Neutrophil responses exhibited fluctuation within and between both treatment groups however none of these were statistically significant.
In secondary analyses considering urinary mercury excretion, no consistent trends or statistically significant differences in immune function measures were observed comparing children with low, medium, or high levels of urinary mercury excretion (data not shown).
DISCUSSION
The aim of this randomized study was to ascertain whether exposure to low levels of mercury released by dental amalgams caused adverse effects on host defense systems in children. Specifically, we assessed lymphocytes, monocytes and neutrophils, obtained from children who were randomized to receive either amalgam or composite restorations, for in vitro manifestations of immunotoxicity. As there was little existing knowledge on this subject and limited power, this was an exploratory study; therefore, results and interpretations are based upon the observation of trends rather than statistical significance. Considering these limitations, results should be viewed with caution. The study was strengthened by its eligibility criteria of at least two posterior teeth with caries and no prior amalgam restorations, which ensured equivalence of treatment groups at baseline as well as high restoration rates to study potential adverse effects of dental amalgam. Indeed, the dental amalgam treatment group presented with small but persistent statistically significant elevations in urinary mercury 3–5 years following treatment. However, there were no consistent or statistically significant differences between the two treatment groups in terms of lymphocyte (both B and T-cell), monocyte and neutrophil blood distribution.
This is the first study to report on the immune status of children following treatment with dental amalgam. These findings are strengthened by the randomized trial design which ensures that differences among the treatment groups are not due to possible confounding factors. The range of cell functions that could be assessed was limited by the size and frequency of blood samples and limited knowledge of potential in vivo effects of both dental amalgams and mercury on the immune status of children. In vitro studies suggest that mercury exposure can lead to immunotoxicity culminating in lymphocyte and monocyte death.14, 15, 41, 42 Thus, it was important for us to assess both number and distribution of WBCs; any reduction in cell number could be interpreted as evidence of chronic exposure to high levels of mercury. Our results indicate that dental amalgams, compared to composite restorations, failed to alter either the absolute number or distribution of B and T-cells (and their subsets), monocytes and neutrophils in the blood of the treated children. This is not surprising inasmuch as amalgam treated children had levels of urinary mercury excretion that were low relative to established background population levels.4 Similarly, Herrstrom et al43 did not detect any evidence of amalgam mercury-induced cytogenetic damage to lymphocytes; it is noteworthy that they did observe cytogenetic damage in cells from individuals treated with composite fillings.
Several investigators have demonstrated that low levels of both inorganic and organic mercury can lead to altered immune function. Indeed, we and others have shown that in vitro exposure of lymphocytes and monocytes to 10–500 ng/ml of mercury impairs cell responsiveness.14, 42, 44–48 Furthermore, Petruccioli et al 49 demonstrated that monkeys exposed to mercuric chloride for periods up to 120 days manifested reduced levels of serum IgG and IgM. Consistent with these observations we did observe a small transient, but not statistically significant decline in lymphocyte and monocyte responsiveness during the 5–7 day period following amalgam treatment. It is interesting to note that Henderson et al 50 also observed decreased lymphocyte responsiveness in some individuals receiving dental amalgam. Moreover, Osorio et al 51 observed transient effects of amalgam exposure on the distribution of subsets of T-cells.
The slight decline in T-cell responsiveness that we observed was reflected in activation marker expression following in vitro mitogenic stimulation. Furthermore, the reductions were transient and not present at 6, 12 or 60 months following treatment. There was a similar transient decline in monocyte responsiveness measured in terms of the cells ability to mount an oxidative burst in response to PMA in vitro. No differences between treatment groups were noted for neutrophils. The magnitude and duration of these fluctuations lead us to conclude that they are unlikely to be of clinical significance and might be attributable to sampling variation since the sample size is small and statistical significance is lacking. Nonetheless, the possibility still exist that dental amalgams may have transient untoward effects on host defense and is worthy of further investigation.
It should be noted that it has been suggested that certain individuals may exhibit heightened sensitivity to toxic substances such as mercury due to genetic abnormalities. For instance, Yoshida et al52,53 recently demonstrated that metallothionein-null mice were more sensitive to mercury toxicity than wild-type mice. The NECAT study was not designed to evaluate the role of genetic polymorphism in the safety of amalgam restorations. For this reason, the current analysis is unable to provide information on the role metallothionein deficiency or other genetic polymorphisms in amalgam safety.
In conclusion, our randomized study confirms that treatment of children with dental amalgams leads to increased, albeit low level, exposure to mercury. Within our small sample size this exposure did not cause overt immune deficits, although we did observe a transient decline in certain aspects of both lymphocyte and monocyte activation that warrants further investigation. Given the exclusion criteria and exploratory nature of this analysis, these results do not support a move at this time to discontinue the use of mercury amalgam as the standard of care for restoration of caries. This is especially important since the safety of many mercury-free alternatives has not been thoroughly tested. For example, Herrstrom et al 50 have reported evidence of immunotoxicity associated with the use of acrylate-containing tooth fillings and none associated with mercury containing amalgam fillings. Indeed, the lack of evidence for immunotoxic, neuropsychological or renal effects of dental amalgam together with factors such as low cost, improved longevity and expertise in handling, support the continuation of amalgam materials for restorative dentistry.33, 34, 54, 55.
Acknowledgments
The authors would like to acknowledge Ali Zekavat, Rose Espiritu and the SDM Flow Cytometry for their technical expertise and contribution to the execution of this study. This study was supported by USPHS grant N01 DE 72622.
Footnotes
Publisher's Disclaimer: Please note that this is not the final published version of the manuscript. The final published manuscript is available at the JADA website free of charge: http://jada.ada.org/cgi/reprint/139/11/1496.
References
- 1.Atchison WD, Hare MF. Mechanisms of methylmercury-induced neurotoxicity. FASEB J. 1994;8:622–9. doi: 10.1096/fasebj.8.9.7516300. [DOI] [PubMed] [Google Scholar]
- 2.Clarkson TW. The toxicology of mercury. Critical Reviews in Clinical Laboratory Sciences. 1997;34(3):369–403. doi: 10.3109/10408369708998098. [DOI] [PubMed] [Google Scholar]
- 3.Harada M. Minamata disease: Methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25(1):1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Concise international chemical assessment document 50: Elemental mercury and inorganic mercury compounds: human health aspects. Geneva, Switzerland: WHO; 2003. [Google Scholar]
- 5.Dantas DCM, Queiroz MLS. Immunoglobulin E and autoantibodies in mercury-exposed workers. Immunopharm Immunotox. 1997;19(3):383–92. doi: 10.3109/08923979709046983. [DOI] [PubMed] [Google Scholar]
- 6.Dieter MP, Luster MI, Boorman GA, Jameson CW, Dean JH, Cox JW. Immunological and biochemical responses in mice treated with mercuricchloride. Toxicol Appl Pharmacol. 1983;68:218–28. doi: 10.1016/0041-008x(83)90006-6. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence D. Heavy metal modulation of lymphocyte activation. I. In vitro effects of heavy metals on primary humoral immune responses. Toxicol Appl Pharmacol. 1981;57:439–51. doi: 10.1016/0041-008x(81)90241-6. [DOI] [PubMed] [Google Scholar]
- 8.Stejskal VDM, Cederbrant K, Lindvall A, Forsbeck M. Melisa-an in vitro tool for the study of metal allergy. Toxic in Vitro. 1994;8(5):991–1000. doi: 10.1016/0887-2333(94)90233-x. [DOI] [PubMed] [Google Scholar]
- 9.Stejskal VDM, Forsbeck M, Cederbrant K, Asteman O. Mercury-specific lymphocytes: an indication of mercury allergy in man. J Clin Immunol. 1996;16(1):31–40. doi: 10.1007/BF01540970. [DOI] [PubMed] [Google Scholar]
- 10.Warfvinge G, Larsson Å. Contact stomatitis to mercury associated with spontaneous mononuclear cell infiltrates in Brown Norway (BN) rats with HgCl2-induced autoimmunity. J Oral Pathol Med. 1994;23(10):441–5. doi: 10.1111/j.1600-0714.1994.tb00441.x. [DOI] [PubMed] [Google Scholar]
- 11.Gopinath C. Pathology of toxic effects on the immune system. Inflamm Res. 1996;45(Supplement 2):S74–S78. [PubMed] [Google Scholar]
- 12.Koropatnick J, Zalups RK. Effect of non-toxic mercury, zinc or cadmium pretreatment on the capacity of human monocytes to undergo lipopolysaccharide-induced activation. British Journal of Pharmacology. 1997;120(5):797–806. doi: 10.1038/sj.bjp.0700975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollard KM, Hultman P. Effects of mecury on the immune system. Metal Ions in Biological Systems. 1997;34:421–40. [PubMed] [Google Scholar]
- 14.Shenker BJ, Rooney C, Vitale LA, Shapiro IM. Immunotoxic effects of mercuric compounds on human lymphocytes and monocytes. I. Suppression of T-cell activation. Immunopharm Immunotox. 1992;14(3):539–53. doi: 10.3109/08923979209005410. [DOI] [PubMed] [Google Scholar]
- 15.Shenker BJ, Datar S, Mansfield K, Shapiro IM. Induction of apoptosis in human T-cells by organomercuric compounds: A flow cytometric analysis. Toxicol Appl Pharmacol. 1997;143:397–406. doi: 10.1006/taap.1997.8111. [DOI] [PubMed] [Google Scholar]
- 16.Shenker BJ, Guo TL, Shapiro IM. Low-level metylmercury exposure causes human T-cells to undergo apoptosis: evidence of mitochondrial dysfunction. Environ Res. 1998;77:149–59. doi: 10.1006/enrs.1997.3816. [DOI] [PubMed] [Google Scholar]
- 17.Shenker BJ, Guo TL, OI, Shapiro IM. Induction of apoptosis in human T-cells by methyl mercury: temporal relationship between mitochondrial dysfunction and loss of reductive reserve. Toxicol Appl Pharmacol. 1999;157:23–35. doi: 10.1006/taap.1999.8652. [DOI] [PubMed] [Google Scholar]
- 18.Via C, Nguyen P, Niculescu F, Papadimitriou J, Hoover D, Silbergeld EK. Low-dose exposure to iorganic mercury accelerates disease and mortality in acquired murine lupus. Environ Health Pers. 2003;111(10):1273–7. doi: 10.1289/ehp.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cederbrant K, Gunnarsson L-G, Hultman P, Norda R, Tibbling-Grahn L. In vitro lymphoproliferative assays with HgCl2 cannot identify patients with systemic symptoms attributed to dental amalgam. J Dent Res. 1999;78(8):1450–8. doi: 10.1177/00220345990780081101. [DOI] [PubMed] [Google Scholar]
- 20.American Dental Association. 1999 Survey of Dental Services Rendered. Chicago, IL: ADA Survey Center; 2002. [Google Scholar]
- 21.Kingman A, Albers J, Arezzo J, Garabrant D, Michalek J. Amalgam exposure and neurological function. Neuro Toxicology. 2005;26:241–55. doi: 10.1016/j.neuro.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Factor-Litvak P, Hasselgren G, Jacobs D, et al. Mercury derived from dental amalgams and neuropsychologic function. Environ Health Pers. 2003;111(5):719–23. doi: 10.1289/ehp.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalen K, Lygre G, Kove H, Gjerdet N, Askevold E. Memory functions in persons with dental amalgam. J Dent Res. 2003;31:487–92. doi: 10.1016/s0300-5712(03)00086-1. [DOI] [PubMed] [Google Scholar]
- 24.Nitschke I, Muller F, Smith J, Hopfenmuller W. Amalgam fillings and cognitive abilities in a representative sample of the elderly population. Gerontology. 2000;17:39–44. doi: 10.1111/j.1741-2358.2000.00039.x. [DOI] [PubMed] [Google Scholar]
- 25.Bjorkman L, Pedersen N, Lichtenstein P. Physical and mental health related to dental amalgam fillings in Swedish twins. Community Dent Oral Epidemiol. 1966;24:260–7. doi: 10.1111/j.1600-0528.1996.tb00856.x. [DOI] [PubMed] [Google Scholar]
- 26.Saxe S, Snowdon D, Wekstein M, et al. Dental amalgam and cognitive function in older women: findings from the Nun study. J Am Dent Assoc. 1995;126:1495–501. doi: 10.14219/jada.archive.1995.0078. [DOI] [PubMed] [Google Scholar]
- 27.Mutter J, Naumann J, Sadaghiani C, Schneider R, Walach H. Alzheimer disease: mercury as pathogenetic factor and apolipoprotein E as a moderator. Neuroendocrinol Lett. 2004;25:331–9. [PubMed] [Google Scholar]
- 28.Bates M, Fawcett J, Garrett N, Cutress T, Kjellstrom T. Health effects of dental amalgam exposure: a retrospective cohort study. Int J Epidemiol. 2004;33(894):902. doi: 10.1093/ije/dyh164. [DOI] [PubMed] [Google Scholar]
- 29.Trepka M, Heinrich J, Krause C, et al. Factors affecting internal mercury burdens among eastern German children. Arch Environ Health. 1997;52(2):134–8. doi: 10.1080/00039899709602877. [DOI] [PubMed] [Google Scholar]
- 30.Gabrio T, Benedikt T, Broser G, et al. 10 years of obsevation by public health offices in Baden-Wurttemberg--assessment of human biomonitoring for mercury due to dental amalgam fillings and other sources [In German] Gesundheitswesen. 2003;65(5):327–35. doi: 10.1055/s-2003-39541. [DOI] [PubMed] [Google Scholar]
- 31.Maserejian N, Trachtenberg F, Assmann S, Barregard L. Dental amalgam exposure measures and urinary mercury levels in the New England Children’s Amalgam Trial. Environmental Health Perspectives. 2008 doi: 10.1289/ehp.10440. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy M, Schwartz S, Dijak M, Weber J-P, Tardif R, Rouah F. Childhood urine mercury excretion: dental amalgam and fish consumption as exposure factors. Environ Res. 2004;94:283–90. doi: 10.1016/j.envres.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Bellinger D, Daniel D, Trachtenerg F, Tavares M, McKinlay S. Dental amalgam restorations and children’s neurosychological function: the New England Children’s Amalgam Trial. Environ Health Pers. 2006;115(3):440–6. doi: 10.1289/ehp.9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeRouen T, Martin MD, Leroux B, et al. Neurobehavioral effects of dental amalgam in children. JAMA. 2006;295:1784–92. doi: 10.1001/jama.295.15.1784. [DOI] [PubMed] [Google Scholar]
- 35.Hornung R, Reed L. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- 36.Barber T, Wallis G. Correction of urinary mercury concentraton by specific gravity, osmolality and creatinine. J Occup Med. 1986;28:354–9. [PubMed] [Google Scholar]
- 37.Barregard L. Mercury from dental amalgam: looking beyond the average. Occupation Environ Med. 2005;62:352–3. doi: 10.1136/oem.2004.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shenker BJ, Besack D, McKay TL, Pankoski L, Zekavat A, Demuth DR. Induction of cell cycle arrest in lymphocytes by Actinobacillus actinomycetemcomitans cytolethal distending toxin rquires three subunits formaximum activity. J Immunol. 2005;174:2228–34. doi: 10.4049/jimmunol.174.4.2228. [DOI] [PubMed] [Google Scholar]
- 39.OI, Datar S, Koch CJ, Shapiro IM, Shenker BJ. Mercuric compounds inhibit human monocyte function by inducing apoptosis: evidence for formation of reactive oxygen species, development of mitochondrial membrane permeability transiton and loss of reductive reserve. Toxicol. 1997;124:211–24. doi: 10.1016/s0300-483x(97)00153-4. [DOI] [PubMed] [Google Scholar]
- 40.Green L. Manual for scoring socioeconomic status for research on health behavior. Public Health Rep. 1970;85:815–27. [PMC free article] [PubMed] [Google Scholar]
- 41.Shenker BJ, Berthold P, Decker S, et al. Immunotoxic effects of mercuric compounds on human lymphocytes and monocytes. II. Alterations in cell viability. Immunopharm Immunotox. 1992;14(3):555–77. doi: 10.3109/08923979209005411. [DOI] [PubMed] [Google Scholar]
- 42.Shenker BJ, Berthold P, Rooney C, Vitale LA, DeBolt K, Shapiro IM. Immunotoxic effects of mercuric compounds on human lymphocytes and monocytes. III. Alterations in B-cell function and viability. Immunopharm Immunotox. 1993;15(1):87–112. doi: 10.3109/08923979309066936. [DOI] [PubMed] [Google Scholar]
- 43.Herrstrom P, Bratt I, Holmen A, Hogstedt B. Micronuclei in lymphocyte subsets in relation to plasma mercury, dental amalgam and acrylate-containing tooth fillings. Science of the Total Environment. 2003;309:253–5. doi: 10.1016/S0048-9697(03)00022-6. [DOI] [PubMed] [Google Scholar]
- 44.Shenker BJ, Mayro JS, Rooney C, Vitale LA, Shapiro IM. Immunotoxic effects of mercuric compounds on human lymphocytes and monocytes. IV. Alterations in cellular glutathione content. Immunopharm Immunotox. 1993;15(2 & 3):273–90. doi: 10.3109/08923979309025999. [DOI] [PubMed] [Google Scholar]
- 45.Nakatsuru S, Oohashi J, Nozaki H, Nakada S, Imura N. Effect of mercurials on lymphocyte functions. Toxicology. 1985;36:297–305. doi: 10.1016/0300-483x(85)90032-0. [DOI] [PubMed] [Google Scholar]
- 46.Nordlind K. Inhibition of lymphoid cell DNA synthesis by metal allergens at various concentrations. Int Archs Allergy Appl Immun. 1983;70:191–2. doi: 10.1159/000233321. [DOI] [PubMed] [Google Scholar]
- 47.Castranova V, Bowman L, Reasor M, Miles P. Effects of heavy metal ions on selected oxidative metabolic processes in rat alveolar macrophages. Toxicol Appl Pharmacol. 1980;53:13–23. doi: 10.1016/0041-008x(80)90375-0. [DOI] [PubMed] [Google Scholar]
- 48.Malamud D, Dietrich S, Shapiro IM. Low levels of mercury inhibit the respiratory burst in human polymorphonuclear leukocytes. Biochem Biophys Res Comm. 1985;128:1145–51. doi: 10.1016/0006-291x(85)91060-5. [DOI] [PubMed] [Google Scholar]
- 49.Petruccioli L, Turillazzi P. Serum immunoglobulin levels in monkeys treated with methylmercury. Drug Chem Toxicol. 1990;13:297–307. doi: 10.3109/01480549009032288. [DOI] [PubMed] [Google Scholar]
- 50.Henderson D, Clifford R, Young D. Mercury-reactive lymphocytes in peripheral blood are not a marker for dental amalgam associated disease. J Dentistry. 2001;29:469–74. doi: 10.1016/s0300-5712(01)00025-2. [DOI] [PubMed] [Google Scholar]
- 51.Osorio E, Toledano M, Bravo M, Osorio R. Short-term changes in lymphocytes after placement of silver amalgam restorations in healthy subjects. Dent Mater. 1995;11:323–6. doi: 10.1016/0109-5641(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida M, Watanabe C, Horie K, Satoh M, Sawada M, Shimada A. Neurobehavioral changes in metallothionein-null mice prenatally exposed to mercury vapor. Toxicology Let. 2005;155:361–8. doi: 10.1016/j.toxlet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Yoshida M, Watanabe C, Kishimoto M, Yasutake A, Satoh M, Sawada M, Akama Y. Behavioral changes in metallothionein-null mice after the cessation of long-term, low-level exposure to mercury vapor. Toxicology Let. 2006;161:210–18. doi: 10.1016/j.toxlet.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 54.Soncini J, Maserejian NTF, Tavares M, Hayes C. The longevity of amalgam versus compomer/composite restorations in posterior primary and permanent teeth: findings from the New England Children’s Amalgam Trial. J Am Dent Assoc. 2007;138(6):763–72. doi: 10.14219/jada.archive.2007.0264. [DOI] [PubMed] [Google Scholar]
- 55.Bernardo M, Martin LH, Martin MD, Leroux BG, Rue T, Leitao J, DeRouen TA. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. J Am Dent Assoc. 2007;138(6):775–83. doi: 10.14219/jada.archive.2007.0265. [DOI] [PubMed] [Google Scholar]