Abstract
Biologically active recombinant human Flt3 ligand was expressed and isolated from transgenic barley seeds. Its expression is under the control of a tissue specific promoter that confines accumulation of the recombinant protein to the endosperm tissue of the seed. The recombinant Flt3 ligand variant expressed in the seeds contains a HQ-tag for affinity purification on IMAC resin. Using sandwich enzyme-linked immunosorbent assay seventy-three individual barley lines were analyzed for expression. Seventeen lines accumulated threshold amount of the protein in the seeds. The tagged protein was purified from seed extract to near homogeneity using sequential chromatography on IMAC affinity resin and cation exchange resin. The barley expressed recombinant Flt3 ligand migrates as two bands of 20 kDa and 22 kDa in SDS-PAGE which is slightly bigger than the 19 kDa predicted from the sequence. This indicates that post-translational modifications occur in the plant. We show that the recombinant Flt3 ligand protein is a glycoprotein containing α-1,3-fucose and α-1,2-xylose. The HQ-tagged Flt3 ligand variant exhibits comparable biological activity to commercial Flt3 ligand. This is the first report showing expression and accumulation of recombinant human growth factor in barley seeds with a yield of active protein similar to bacterial expression system. The present results demonstrate that plant molecular farming is viable approach for the bioproduction of human derived growth factors.
Keywords: Animal-free, expression system, growth factors, molecular farming, recombinant human Flt3 ligand
Introduction
Growth factors play a major role in medical research as components of cell culture media used for proliferation and differentiation of various cells. Their use is expected to increase dramatically as progress is made in stem cell research, tissue engineering and regenerative medicine. Furthermore, their application as biopharmaceuticals for human therapeutic use is becoming increasingly important.
Recombinant growth factors are commercially produced in different host expression systems such as bacteria, yeast, insect and mammalian cells. While these systems can be efficient, there is still a need for new production systems that are serum- and animal-free, more economical, and void of endotoxins and other contaminating components, such as pyrogenic and pro-inflammatory agents that may affect their use in biological systems and assays. A number of different plant systems are in development for the efficient production of protein biopharmaceuticals such as growth factors (for review see [1]). Barley seeds and other cereals are plant systems that have been explored with good results for molecular farming [2, 3]. Using barley seeds for heterologous protein production is an attractive option because they are a cost-saving alternative that are easy to grow and with well-established post-harvest handling and processing methods [4, 5]. The majority of human proteins that have been produced in plants show significant structural, biochemical and functional similarity to proteins from animal cell systems [6]. Furthermore, barley as a host system features genetic self-containment, as barley is a self-pollinating crop plant. Barley is Generally Regarded As Safe (G.R.A.S.) by the FDA with low secondary metabolite content, such as phenolics (unpublished data). Presently, most plant produced biopharmaceuticals and other recombinant proteins have been expressed in tobacco which is known for the presence of alkaloids and other compounds potentially toxic to humans [7]. Accumulating heterologous proteins in seeds presents many advantages as seeds are natural long-term storage vehicles of proteins within an inert environment. They can be stored for years without affecting the quality of the protein [4, 8] and they have a relatively simple protein profile with the major part belonging to few and dominant polypeptide classes that can be separated with relative ease from the target protein, thus facilitating downstream procedures considerably [9].
Flt3 ligand is an essential hematopoietic growth factor involved in proliferation and differentiation of stem cells and in the development of NK cells, B cells, T cells, monocytes/macrophages and dendritic cells. Flt3 ligand may be of clinical value as it is, together with other growth factors and cytokines, considered a stimulant of the immune system through its effect on both dendritic and natural killer cells production (reviewed by [10]). Recombinant human Flt3 ligand is commercially available from bacterial source and CHO cells. In the present paper, we describe the production of recombinant human Flt3 ligand in barley grain using the Orfeus expression system developed by ORF Genetics for production of recombinant proteins termed ISOkines.
To our knowledge, this is the first report describing expression and purification of a recombinant human growth factor from cereal seeds with biological activity comparable to commercially available counterparts. This is also the first publication on expression of Flt3 ligand in a plant system. The results demonstrate that barley is a proficient system for the production of active recombinant growth factors for cell culture, media preparation and medical application.
Methods and materials
Expression cassette
The cDNAs for human Flt3 ligand (Genebank Accession No. NP 001450), with N-terminal HQ6-tag, were codon-optimized according to barley codon usage (GeneArt, Germany) and subsequently used to prepare an expression cassette under the control of the 0.45 kb D-hordein promoter from barley (unpublished; Sörensen et al. 1996; Genebank Accession No. X84368).
Barley transformation and selection of transformants
Hordeum vulgare L. Cv Golden Promise plants were grown vegetatively for about 65–85 days or until approximately 8 to 14 days post anthesis. Barley heads were selected and the seeds sterilized with 70% ethanol and 3% sodium hypochlorite for 40 min in rotary shaker and placed overnight at 4°C. The next day the immature embryos were removed from the seeds, the embryonic axis excised and dispatched from the scutellum which was placed on a regeneration media essentially as described by Tingay et. al. [11]. A. tumefaciens culture, harboring the Flt3 ligand binary vector pb22g1-12, with the cDNA for human Flt3 ligand under the control of the 0.45 kb D-hordein promoter from barley, was pipetted onto each explant. After removing excess A. tumefaciens the explants were transferred to fresh regeneration media plates and placed in dark cabinet at 24°C. After three days the explants were transferred to a fresh regeneration media supplemented with 50μg/ml hygromycin B (Duchefa Biochemie BV) and left there for four to six weeks. To regenerate shoots, the calli were transferred to shoot-induction media (SIM) and surviving callus and regenerating shoots transferred to fresh SIM every two weeks until small plantlets were formed. The plantlets were then transferred to root-induction media (RIM) with 20μg/ml hygromycin B and surviving plants potted in soil. All transformants analyzed were from the T0 generation.
Human Flt3 ligand accumulation analysis in first generation seeds
A total of 73 independent transgenic barley lines were selected for analysis of Flt3 ligand accumulation in seeds. From each transgenic line two seeds from first seed generation (T1 seeds) were randomly selected for analysis. The seeds were initially crushed by mechanical force prior to milling in a Retsch MM301 bead mill (Qiagen) in a 96 deep-well microplate format. Water-soluble proteins were extracted from the pulverized seeds by extraction in 500 μL of 50 mM potassium phosphate, pH 7.0. Extraction was performed for 1 hour at room temperature by mixing in the bead mill and the crude protein extract clarified by centrifugation at 3,000g for 5 min. at 4°C. Clarified extract was collected and used for quantitative analysis of the target protein Flt3 ligand. The target protein accumulation in the seeds was analyzed using a Flt3 ligand sandwich enzyme-linked immunosorbent assay (sandwich ELISA) kit (DY308, R&D systems, Minneapolis, MN) according to the manufacturer’s instructions.
Purification of barley seed-expressed human Flt3 ligand
Second generation seeds (T2) were harvested during the full ripeness stage of grain development and air dried in Grainman seed drier for 24 hours at 35°C before dehusking. Six hundred grams of threshed and dehusked seeds were milled by Retsch ZM200 rotor mill (Retsch, Germany) and extraction performed for 1 hour in 3 L extraction solution, containing 50 mM potassium phosphate, pH 7.0. Homogenate was clarified by centrifugation at 20,000g for 60 min. at 4°C. Initial batch mode affinity step was performed using a nickel-based affinity resin provided by Upfront, Denmark. The resin was mixed with the extraction solution for 1 hour at 4°C and subsequently allowed to settle. The resin was washed repeatedly with equilibration buffer (50 mM potassium phosphate, 0.5 M NaCl, 50 mM imidazole, pH 7.4) to remove weakly bound endogenous seed proteins. The remaining protein was eluted from the resin with elution buffer containing 50 mM potassium phosphate, 0.5 M NaCl, 500 mM imidazole, pH 7.4. The eluted fraction was collected and buffer exchange performed by using HiPrep 26/10 desalting column (GE Healthcare). The protein was eluted in 30 mM sodium citrate, 0.5 M NaCl pH 4.5. As the final step in the purification the buffer exchanged protein-solution was applied to a 1 mL SP FF ion exchange column (GE Healthcare). The column was washed with 30 mM sodium acetate, 0.5 M NaCl, pH 4.5 to remove remaining endogenous seed proteins. Bound Flt3 ligand was then eluted with 30 mM sodium acetate, 1 M NaCl, pH 4.5. Protein concentration of the eluted protein was determined using Bradford protein assay (Biorad, Hercules, CA) using bovine serum albumin as standard.
Purified Flt3 ligand was prepared for biological activity measurement by buffer exchange using HiTrap desalting column (GE Healthcare). The protein was eluted in 10 mM acetic acid pH 5.0 and subsequently freeze-dried.
Immunoblotting
Proteins were fractionated by SDS-PAGE [12] and transferred to a polyvinylideneflouride blotting membrane (Immobilon-P, Millipore, Boston, MA) using wet blot transfer (BioRad). Transfer buffer was 20 mM Tris, 150 mM glycine containing 20% (v/v) methanol. Rabbit antiserum against human Flt3 ligand (ABC509) was obtained from Autogen-Bioclear, UK. Immunodetection was carried out by using horseradish peroxidase labeled secondary antibody and 3,3′,5,5′-tetramethylbenzidine stabilized substrate for colorometric analysis.
Proteolytic digestion and mass spectrometry
For quality analyses, the recombinant product was loaded and run on SDS-PAGE, followed by Coomassie staining. Relevant band(s) were excised and treated for in-gel digestion, essentially as described [13]. Briefly, the stain was removed and the gel band was dried using neat acetonitrile. Trypsin (modified, sequence grade porcine from Promega (Madison, WI, USA) was introduced and proteolysis was conducted at 30 °C overnight. After acidification, the generated peptides were analyzed by MALDI-TOF-MS (matrix assisted laser desorption/ionization time of flight mass spectrometry), on an Ultraflex III TOF/TOF instrument (Bruker, Bremen, Germany). The matrix was alpha-cyano-4-hydroxycinnamic acid and the instrument was optimized for analytes up to 4000 Da and operated according to the manufacturer. Internal calibration was performed using tryptic autolytic fragment.
The generated peptide mass list was used to search for identity with known proteins in the NCBInr database, using the search engine ProFound (http://prowl.rockefeller.edu/prowl-cg). Alternatively, the target protein (i.e. Flt3 ligand) was inserted in GPMAW (Lighthouse Data, Odense, Denmark) and analyzed with regard to the obtained peptide masses.
Glycosylation analysis of Isokine FLT3 ligand
Glycosylation of FLT3 ligand was analyzed by specific antibodies to α-1,3-fucose (AS07 268 Agrisera, Vännäs, Sweden) and β-1,2-xylose (AS07 267 Agrisera). Two hundred fifty nanogram of Isokine Flt3 ligand and commercially available E. coli produced recombinant Flt3 ligand were loaded on a SDS-PAGE gel. Immunoblotting was performed as described above.
Biological assay in cultures of hematopoietic precusors
The biological activity of ISOkine Flt3 ligand was measured by the growth and differentiation of hematopoietic precursors isolated from human midgestation livers. Fetal livers were obtained from elective abortions at the University of California San Francisco and used with the approval of the University’s Committee for Human Research. The gestational age of the tissue was estimated based on the foot-length of the fetus. Two populations of cells were studied which were isolated by negative selection using immunomagnetic beads, density fractionation and fluorescence-activated cell sorting. A detailed protocol describing the isolation procedure has been published [14]. Briefly, cells were sorted based on their high expression of CD34 (CD34++) and lack of expression of a panel of mature blood cell antigens (CD3, CD14, CD19, CD20, CD56 and CD235a). The two cell populations isolated differed in their expression of CD38, with CD38−CD34++ cells being highly enriched for hematopoietic stem cells and CD38+CD34++ cells representing a population of hematopoietic progenitors in the early stages of differentiation.
The effects of Flt3 ligand on the growth and differentiation of hematopoietic precursors was determined in cultures using serum-deprived medium formulated as previously described [15]. CD38−CD34++ and CD38+CD34++ cells were seeded at 3 × 102 and 2 × 103 cells/well, respectively, in 96-well V-bottom tissue culture plates (Corning Incorporated, Corning, NY) in a total volume of 200 μl/well. 4–6 replicate wells were established for each concentration of Flt3 ligand and 8–12 replicates were used for control cultures without any source of Flt3 ligand added. The effects of Flt3 ligand were tested over a range of 1.6 to 200 ng/ml. E. coli produced recombinant human Flt3 ligand purchased from a commercial source was used as a control. The growth of CD38−CD34++ cells was supported by 20 ng/ml recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) and 20 ng/ml recombinant human interleukin (IL)-15 (R&D Systems) with or without Flt3 ligand. CD38+CD34++ cells were cultured with recombinant human IL-7 (R&D Systems) and IL-15, each used at 20 ng/ml, and the indicated concentrations or Flt3 ligand. Cells were grown for 13 days at 37°C in a fully humidified atmosphere.
The lineage composition of cells generated in liquid cultures was analyzed based on the expression of cell surface markers using an LSR II flow-cytometer (BD Biosciences, San Jose, CA). The cultured cells were pelleted by centrifugation and resuspended in PBS with 5% normal mouse serum (Gemini Bio-Products, Inc., Woodland, CA) and 0.01% NaN3 (Sigma Chemical Co., St. Louis, MO). Cells were incubated with saturating levels of labeled monoclonal antibodies (mAb) for 30 minutes on ice. mAbs used were CD1a-phycoerythrin (PE) (clone BL6, Beckman Coulter, Inc., Miami, FL), CD19-PE (clone SJ25-C1, Invitrogen, Carlsbad, CA), CD34-allophycocyanin (APC) (clone 581, Invitrogen) and CD56-FITC (Clone C5.9, Exalpha, Boston, MA). Fluorochrome-labeled isotype-matched antibodies were purchased from Invitrogen and used to negative controls measure background staining of cultured cells. After staining, the cells were washed with PBS containing 0.5% bovine serum-albumin (Roche Applied Science, Indianapolis, IN) and 0.01% NaN3 and suspended in the same buffer supplemented with 2 μg/ml propidium iodide (PI) (Invitrogen) [14].
Flow cytometry was used to determine both the frequencies of mAb staining and to determine the relative number of cells in each sample by collecting samples at the same flow rate and over the same time period. The number of PI− events collected was thus proportional to the number of live cells generated. Relative live-cell numbers were converted to actual cell numbers by measuring the fraction of PI− events collected from a sample with a known number of live cells based on counting using a hemocytometer and trypan blue exclusion staining. Preliminary studies have shown a linear relationship between hemocytometer and flow cytometric cell-counts (M.O.M., unpublished data). Flow cytometric data was parsed using FlowJo software v. 8.7 (Tree Star, Inc.; Ashland, OR). The significance of differences in cell numbers between two groups of replicate cultures was determined using a two-tailed unpaired t-test, with P-value of ≤0.05 considered to represent a significant difference.
Cell proliferation assay using AML5 cells
The biological activity of ISOkine Flt3 ligand was determined with cell proliferation assay using human acute myeloid leukemia cells (OCI/AML5) and commercial Flt3 ligand as standard. The cells were maintained in assay free medium supplemented with fetal bovine serum. The cell proliferation was analyzed by incubating the cells with serial dilutions of Flt3 ligand for 70 hours prior to colorimetric determination of cell growth using Promega substrate cell titer 96 aqueous one solution reagent and optical density reading at 490 nm. The assay was performed by SBH Sciences, MA.
Results & discussions
Transgenic barley seeds accumulate Flt3 ligand
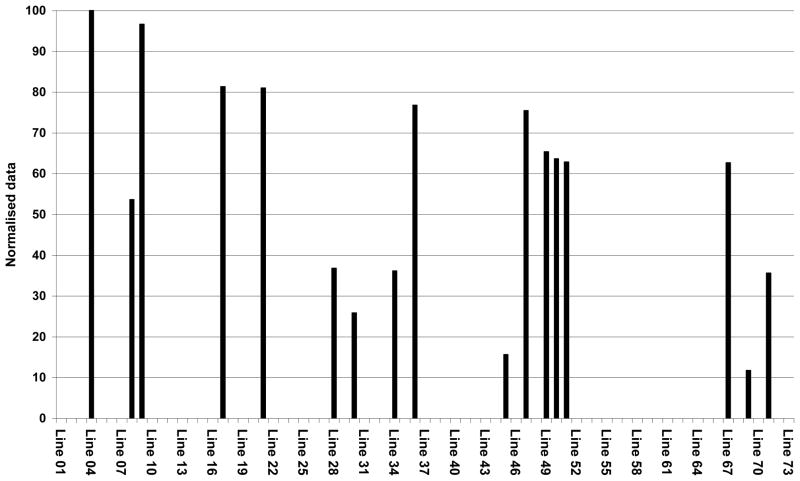
Seventy-three transgenic barley lines were selected for quantitative analysis of ISOkine Flt3 ligand protein in T1 seeds by sandwich ELISA. Seventeen lines (23%) accumulated detectable amount of the protein under the regulation of the D-hordein promoter from barley (Fig. 1). The data in Figure 1 is normalized to the highest OD450 value obtained for a barley line in the sandwich ELISA assay. Variable levels of Flt3 ligand accumulation were observed in seed extracts from individual barley lines. The highest accumulation was up to 60 mg recombinant protein/Kg seeds. The actual level of accumulated protein is probably higher then this value as indicated by the substantial amount of the Flt3 ligand observed in re-extracted pellet (data not shown). The detection of elite barley lines based on the sandwich ELISA results was confirmed with western blot analysis using anti-human Flt3 ligand antibodies (ABC509; Autogen-Bioclear, UK) and specific anti-tag antibodies (Agrisera, Sweden) (data not shown). The elite T1 generation barley lines identified were used for propagation of T2 seeds in greenhouse.
Figure 1.
Selection of elite barley lines. Transgenic barley lines were screened for Flt3 ligand content by sandwich ELISA. The accumulation level of the protein in seeds was determined and is shown in percentage of highest value obtained for the barley lines.
Purification and characterization of barley produced Flt3 ligand
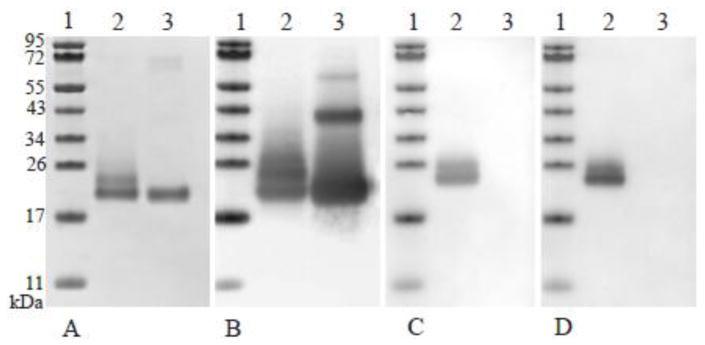
T2 seeds were used for protein purification and characterization of the Flt3 ligand. The protein was purified to near homogeneity (>98% purity) by affinity chromatography and IEX chromatography steps as described above. The yield of Flt3 ligand expressed in barley was close to 12 mg of purified protein per Kg of seeds—a figure which compares quite well to other expression systems. Theoretically, the 155 amino acid residues protein has a calculated mass of 19.9 kDa. However, the Flt3 ligand purified from barley shows bands of two different sizes in a Coomassie brilliant blue-stained SDS-PAGE gel (Fig. 2-A, Lane 2). The identity of the two bands was established by western blot analysis with a specific anti-Flt3 ligand antibodies (Fig. 2-B, Lane 1) as well as by proteolytic digestion of the protein followed by peptide mass fingerprinting on MALDI-ToF-MS (data not shown). Both techniques confirmed that the two bands observed are composed of Flt3 ligand polypeptides. The peptide coverage was quite limited (20–25%), but the lack of arginine and lysine residues in some stretches of the protein results in rather long tryptic fragments difficult to analyze. However, the main reason for the limited peptide coverage as well as the discrepancy between calculated and observed molecular weight resides likely in posttranslational modifications, especially glycosylation. Flt3 ligand contains five potential sites for glycosylation in eukaryotic systems, one N-linked and four Ser/Thr/Pro rich regions that may be substrates for O-linked glycosylation, according to the NetNGlyc 1.0 and NetOGlyc 3.1 protein glycosylation server (Center for biological sequence analysis, Technical University of Denmark), respectively. Glycosylation at one or more of these sites in the protein would affect its mobility on the gel, explaining the higher molecular weight protein bands observed on the gel. Furthermore, heterogeneous glycosylation has been observed for expressed growth factors in other plant systems like tobacco [16] and rice seeds [17]. In contrast, E. coli produced Flt3 ligand presents a single band in a Coomassie brilliant blue-stained SDS-PAGE (Fig. 2-A, Lane 3) as well as in western blot analysis with anti-Flt3 ligand antibodies. In addition, a larger band around 60 kDa is observed (Fig. 2-B, Lane 1). Analysis of the glycosylation of Isokine Flt3 ligand, using specific antibodies, demonstrated that the Isokine Flt3 ligand contains α-1,3-fucose and α-1,2-xylose derivatives (Fig. 2-C,-D). The E. coli produced recombinant Flt3 ligand showed no reactivity to these antibodies as expected. It has been shown that glycosylation is an important element of many growth factors and cytokines which can enhance their activity and/or stability in biological systems [18].
Figure 2.
Comparison of ISOkine Flt3 ligand and an E. coli produced Flt3 ligand from commercial source. Lane 1 contains a molecular weight marker, lane 2, purified ISOkine Flt3 ligand and in lane 3 E. coli produced Flt3 ligand from commercial source. Figure A shows Coomassie blue stained SDS-PAGE gel. Six hundred nanogram was loaded on the gel. Figure B–D show Western blot analysis using polyclonal anti-Flt3 ligand antibodies (B), anti-α-1,3-fucose specific antibody (C) and anti-β-1,2-xylose specific antibody (D). Two hundred and fifty nanogram of protein was loaded on the gel for the Western blot application.
Barley produced Flt3 ligand is biologically active
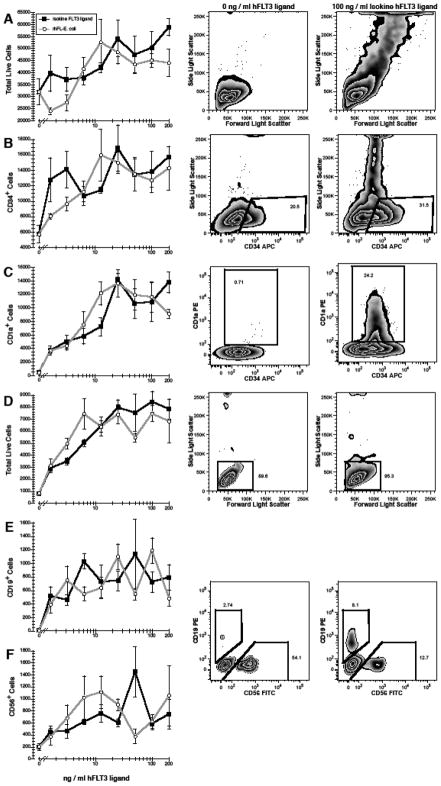
The biological activity of ISOkine Flt3 ligand was tested in cultures of purified hematopoietic precursors. CD38−CD34++ fetal liver cells represent a population of primitive progenitors that are enriched for hematopoietic stem cells and are known to express Flt3 (CD135) and grow in response to Flt3 ligand [19, 20]. CD38−CD34++ cells were cultured with different concentrations of Flt3 ligand in the presence of GM-CSF and IL-15. GM-CSF was used since this cytokine is known to support the growth of primitive progenitors in synergism with Flt3 ligand [19]. IL-15 was added to support the growth of dendritic cells in combination with Flt3 ligand and GM-CSF [20–22]. Addition of ISOkine Flt3 ligand yielded a dose-dependent 1.8-fold increase in cell numbers (Fig. 3-A). Whereas 19-fold expansion in CD34++/+ progenitors was measured in cultures supported by GM-CSF+IL-15 (Fig. 3-B), addition of 25 ng/ml ISOkine Flt3 ligand resulted in a 56-fold increase in CD34++/+ cells (P<0.001). The generation of dendritic cells, identified by their expression of CD1a [23, 24], was also significantly increased in a dose-dependant manner by Flt3 ligand (Fig. 3-C). Fewer than 4.8 × 102 CD1a+ cells were enumerated in control cultures, whereas a mean 1.4 × 104 CD1a+ cells were generated in the presence of 25 ng/ml ISOkine Flt3 ligand (P<0.001). The presence of a large population of cells with a high side-light scatter in cultures containing Flt3 ligand was also indicative of the presence of dendritic cells (Fig. 3-A) [23].
Figure 3.
Effects of ISOkine Flt3 ligand on the growth and differentiation of hematopoietic stem cells and progenitors. 3 × 102 purified CD38−CD34++ (A–C) and 2 × 103 CD38+CD34++ (D–F) cells were cultured with ≤200 ng/ml Flt3 ligand from barley or bacterial source. In addition, all CD38−CD34++ cultures contained GM-CSF+IL-15 and lymphopoiesis was supported in all CD38+CD34++ cultures by IL-7+IL-15. Data in the line plots represent the mean±standard error. Representative flow cytometric plots are shown for cultures without Flt3 ligand (center column) and with 100 ng/ml ISOkine Flt3 ligand (right column). All flow cytometric plots are gated to show only live (PI−) cells (not shown). Note the presence of high side-light scatter cells in cultures with ISOkine Flt3 ligand in row A. Polygons in rows B and C represent the electronic gates used to define the cell populations shown in the corresponding line plots. B- and NK-cells were defined by a low side-light scatter as shown indicated by the rectangular gat in row D and by polygonal gates used to define CD19+ and CD56+ cells as indicated in rows E–F. Numbers indicate the percentage of events that fall within the corresponding gate.
The capacity of Flt3 ligand to support the development of B- and NK-lymphocytes was assessed in cultures of CD38+CD34++ cells, a mixed population of early progenitors including lymphoid progenitors. B- and NK-cell growth and differentiation was supported by IL-7 and IL-15, respectively [22, 25]. A dose dependant increase in cell numbers was observed with the addition of Flt3 ligand (Fig. 3-D), ranging from mean 8.2 × 102 cells recovered in cultures without Flt3 ligand to a mean 8.4 × 103 cells in the presence of 100 ng/ml ISOkine Flt3 ligand (P<0.001). Addition of Flt3 ligand increased the production of CD19+ B-cells and CD56+ NK-cells. At 50 ng/ml Flt3 ligand, 126-fold more B-cells (P=0.003) and 7-fold more NK-cells (P<0.001) were generated than in its absence (Fig. 3-E,-F).
These data demonstrate that ISOkine Flt3 ligand is capable of supporting the growth of primitive hematopoietic progenitors in combination with another early-acting hematopoietic growth factor, GM-CSF. This observation is consistent with Flt3 ligand being a growth factor for stem cells and early progenitors found among CD38−CD34++ fetal liver cells [19]. The pleiotropic effects of Flt3 ligand include the capacity to support the production of dendritic cells [26], which was also evident in our cultures. Additionally, Flt3 ligand is known to support the early stages of lymphopoiesis when combined with growth factors such as IL-7 and IL-15 [27–29]. ISOkine Flt3 ligand increased the number of NK-cells and, in particular, the number of B-cells generated in cultures of CD38+CD34++ immature progenitors. In each of the measures of Flt3 ligand bioactivity, barley produced Flt3 ligand performed comparably to a commercially available Flt3 ligand from bacterial source (Fig. 3). Thus, ISOkine Flt3 ligand represents a functionally active form of soluble Flt3 ligand.
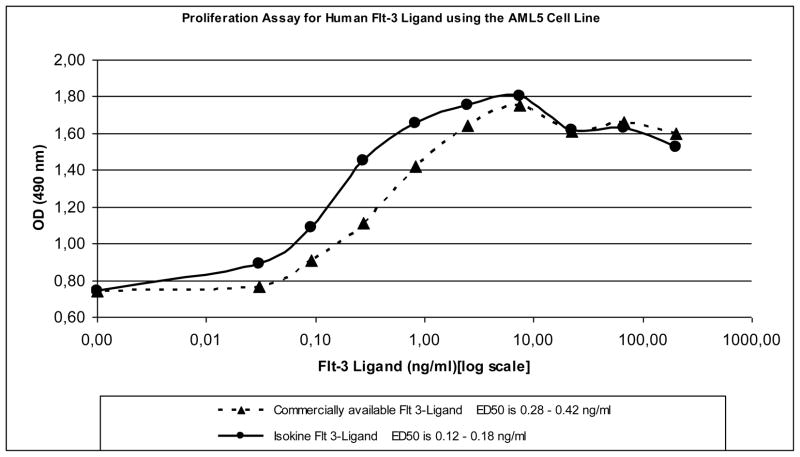
The biological activity of purified ISOkine Flt3 ligand from transgenic barley seeds was also determined with cell proliferation assay using AML5 cells that require Flt3 ligand for normal growth. The protein was found to be biologically active with an ED50 of 0.15 ng/ml and a calculated net OD450 for ED50 0.518 and maximum cell density level of OD450 1.06 (Fig. 4). For comparison, commercially available Flt3 ligand had an ED50 of 0.35 ng/ml and a calculated net OD450 for ED50 0.456 and maximum cell density level of OD450 1.01. Comparable chromatography fraction from barley seeds that do not produce Flt3 ligand showed no biological activity in the assay (data not shown). Thus, purified Flt3 ligand from barley seeds is functionally active and can be used for proliferation of Flt3 ligand responsive AML5 cells.
Figure 4.
Effect of Flt3 ligand on AML5 cell proliferation. The number of viable cells was assayed after incubation with different concentrations of ISOkine Flt3 ligand and E. coli produced recombinant Flt3 ligand.
Concluding remarks
In this study, we have demonstrated for the first time a high expression of biologically active Flt3 ligand in a plant system. The growth factor was specifically expressed in barley seeds—a strategy that presents many advantages such as self containment, ease of processing and storage as well as a relatively simple protein profile. Screening for recombinant protein in T1 seeds showed a high level of transformation rate and high accumulation of the protein in the barley seeds. The protein was purified to homogeneity by conventional chromatography prior to biological assay analysis. The plant derived Flt3 ligand is biologically as active as counterpart expressed in bacteria. In addition the yield, which used to be one of the drawback of plant-based expression system, compare well to prokaryotic production. In conclusion, we show that barley is an excellent platform to produce functional and animal-free recombinant growth factors and cytokines in large amount.
Acknowledgments
M.O.M. was supported by NIH grant DK068441 and Blood Systems Inc. The authors would like to thank Bergrós Þorgrímsdóttir, Helga Sigurđardóttir and Sören Nagel for technical assistance.
References
- 1.Liénard D, Sourrouille C, Gomord V, Faye L. Pharming and transgenic plants. Biotechnol Annu Rev. 2007;13:115–47. doi: 10.1016/S1387-2656(07)13006-4. [DOI] [PubMed] [Google Scholar]
- 2.Horvath H, Huang J, Wong O, Kohl E, Okita T, Kannangara CG, Von Wettstein D. The production of recombinant proteins in transgenic barley grains. PNAS. 2000;97(4):1914–1919. doi: 10.1073/pnas.030527497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhong G-Y, Peterson D, Delaney DE, Bailey M, Witcher DR, Register JC, III, Bond D, Li C-P, Marshall L, Kulisek E, Ritland D, Meyer T, Hood EE, Howard JA. Commercial production of aprotinin in transgenic maize seeds. Mol Breed. 1999;5:345–356. [Google Scholar]
- 4.Stöger E, Vaquero C, Torres E, Sack M, Nicholson L, Drossard J, Williams S, Keen D, Perrin Y, Christou P, Fischer R. Cereal crops as viable production and storage systems for pharmaceutical scFv antibodies. Plant Mol Biol. 2000;42(4):583–90. doi: 10.1023/a:1006301519427. [DOI] [PubMed] [Google Scholar]
- 5.Twyman RM, Stoger E, Schillberg S, Christou P, Fischer R. Molecular farming in plants: host systems and expression technology. Trends Biotechnol. 2003;21(12):570–8. doi: 10.1016/j.tibtech.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Cramer CL, Boothe JG, Oishi KK. Transgenic plants for therapeutic proteins: Linking upstream and downstream strategies. Currr Topics Microbiol Immunol. 1999;240:95–118. doi: 10.1007/978-3-642-60234-4_5. [DOI] [PubMed] [Google Scholar]
- 7.Gutiérrez-Ortega A, Sandoval-Montes C, de Olivera-Flores TJ, Santos-Argumedo L, Gómez-Lim MA. Expression of functional interleukin-12 from mouse in transgenic tomato plants. Transgenic Res. 2005;14(6):877–85. doi: 10.1007/s11248-005-1464-8. [DOI] [PubMed] [Google Scholar]
- 8.Kusnadi AR, Evangelista RL, Hood EE, Howard JA, Nikolov ZL. Processing of transgenic corn seed and its effect on the recovery of recombinant beta-glucuronidase. Biotechnol Bioeng. 1998;60(1):44–52. doi: 10.1002/(sici)1097-0290(19981005)60:1<44::aid-bit5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Shewry PR. Barley Seed Proteins. In: MacGregor J, Bhatty R, editors. Barley: Chemistry and Technology. AACC; St. Paul Minnesota, U.S.A: 1993. pp. 131–197. [Google Scholar]
- 10.Lyman SD. Biologic effects and potential clinical applications of Flt3 ligand. Curr Opin Hematol. 1998;5(3):192–6. doi: 10.1097/00062752-199805000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Tingey S, McElroy D, Kalla R, Fieg S, Wang M, Thornton S, Brettell R. Agrobacterium tumefaciens-mediated barley transformation. Plant J. 1997;11:1369–1376. [Google Scholar]
- 12.Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of protein in the range of 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 13.Hellman U. Peptide mapping using MALDI-TOFMS. In: Silberring J, Ekman R, editors. Mass spectrometry and hyphenated techniques in neuropeptide research. John Wiley & Sons, Inc; 2002. pp. 259–275. [Google Scholar]
- 14.Muench MO, Suskind DL, Bárcena A. Isolation, growth and identification of colony-forming cells with erythroid, myeloid, dendritic cell and NK-cell potential from human fetal liver. Biological Procedures Online. 2002;4:10–23. doi: 10.1251/bpo29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muench MO, Humeau L, Paek B, Ohkubo T, Lanier LL, Albanese CT, Bárcena A. Differential effects of interleukin-3, interleukin-7, interleukin 15, and granulocyte-macrophage colony-stimulating factor in the generation of natural killer and B cells from primitive human fetal liver progenitors. Exp Hematol. 2000;28:961–973. doi: 10.1016/s0301-472x(00)00490-2. [DOI] [PubMed] [Google Scholar]
- 16.James EA, Wang C, Wang Z, Reeves R, Shin JH, Magnuson NS, Lee JM. Production and characterization of biologically active human GM-CSF secreted by genetically modified plant cells. Protein Expr Purif. 2000;19(1):131–8. doi: 10.1006/prep.2000.1232. [DOI] [PubMed] [Google Scholar]
- 17.Sardana R, Dudani AK, Tackaberry E, Alli Z, Porter S, Rowlandson K, Ganz P, Altosaar I. Biologically active human GM-CSF produced in the seeds of transgenic rice plants. Transgenic Res. 2007;16(6):713–21. doi: 10.1007/s11248-006-9062-y. [DOI] [PubMed] [Google Scholar]
- 18.Opdenakker G, Rudd PM, Wormald M, Dwek RA, van Damme J. Cells regulate the activities of cytokines by glycosylation. The FASEB Journal. 1995;9:453–457. doi: 10.1096/fasebj.9.5.7896019. [DOI] [PubMed] [Google Scholar]
- 19.Muench MO, Roncarolo MG, Rosnet O, Birnbaum D, Namikawa R. Colony-forming cells expressing high levels of CD34 are the main targets for granulocyte colony-stimulating factor and macrophage colony-stimulating factor in the human fetal liver. Exp Hematol. 1997;25:277–287. [PubMed] [Google Scholar]
- 20.Muench MO, Roncarolo MG, Menon S, Xu Y, Kastelein R, Zurawski S, Hannum CH, Culpepper J, Lee F, Namikawa R. FLK-2/FLT ligand regulates the growth of early myeloid progenitors isolated from human fetal liver. Blood. 1995;85:963–972. [PubMed] [Google Scholar]
- 21.Siena S, Di Nicola M, Bregni M, Mortarini R, Anichini A, Lombardi L, Ravagnani F, Parmiani G, Gianni AM. Massive ex vivo generation of functional dendritic cells from mobilized CD34+ blood progenitors for anticancer therapy. Exp Hematol. 1995;23:1463–1471. [PubMed] [Google Scholar]
- 22.Bykovskaia SN, Buffo M, Zhang H, Bunker M, Levitt ML, Agha M, Marks S, Evans C, Ellis P, Shurin MR, Shogan J. The generation of human dendritic and NK cells from hemopoietic progenitors induced by interleukin-15. J Leukoc Biol. 1999;66:659–666. doi: 10.1002/jlb.66.4.659. [DOI] [PubMed] [Google Scholar]
- 23.Muench MO, Barcena A. Broad distribution of colony-forming cells with erythroid, myeloid, dendritic cell, and NK cell potential among CD34(++) fetal liver cells. J Immunol. 2001;167:4902–4909. doi: 10.4049/jimmunol.167.9.4902. [DOI] [PubMed] [Google Scholar]
- 24.Dougan SK, Kaser A, Blumberg RS. CD1 expression on antigen-presenting cells. Curr Top Microbiol Immunol. 2007;314:113–141. doi: 10.1007/978-3-540-69511-0_5. [DOI] [PubMed] [Google Scholar]
- 25.Dittel BN, LeBien TW. The growth response to IL-7 during normal human B cell ontogeny is restricted to B-lineage cells expressing CD34. J Immunol. 1995;154:58–67. [PubMed] [Google Scholar]
- 26.Dong J, McPherson CM, Stambrook PJ. FLT ligand: a potent dendritic cell stimulator and novel antitumor agent. Cancer Biol Ther. 2002;1:486–489. doi: 10.4161/cbt.1.5.161. [DOI] [PubMed] [Google Scholar]
- 27.Yu H, Fehniger TA, Fuchshuber P, Thiel KS, Vivier E, Carson WE, Caligiuri MA. Flt3 ligand promotes the generation of a distinct CD34(+) human natural killer cell progenitor that responds to interleukin-15. Blood. 1998;92:3647–3657. [PubMed] [Google Scholar]
- 28.Namikawa R, Muench MO, de Vries JE, Roncarolo MG. The FLK2/FLT3 ligand synergizes with interleukin-7 in promoting stromal-cell-independent expansion and differentiation of human fetal pro-B cells in vitro. Blood. 1996;87:1881–1890. [PubMed] [Google Scholar]
- 29.Muench MO, Humeau L, Paek B, Ohkubo T, Lanier LL, Albanese CT, Barcena A. Differential effects of interleukin-3, interleukin-7, interleukin 15, and granulocyte-macrophage colony-stimulating factor in the generation of natural killer and B cells from primitive human fetal liver progenitors. Exp Hematol. 2000;28:961–973. doi: 10.1016/s0301-472x(00)00490-2. [DOI] [PubMed] [Google Scholar]