Abstract
Many studies have used pilocarpine to stimulate accommodation in both humans and monkeys. However, the concentrations of pilocarpine used and the methods of administration vary. In this study, three different methods of pilocarpine administration are evaluated for their effectiveness in stimulating accommodation in rhesus monkeys. Experiments were performed in 17 iridectomized, anesthetized rhesus monkeys aged 4–16 years. Maximum accommodation was stimulated in all these monkeys with a 2% pilocarpine solution maintained on the cornea for at least 30 min in a specially designed perfusion lens. In subsequent topical pilocarpine experiments, baseline refraction was measured with a Hartinger coincidence refractometer and then while the monkeys were upright and facing forward, commercially available pilocarpine (2, 4, or 6%) was applied topically to the cornea as 2 or 4 drops in two applications or 6 drops in three applications over a five minute period with the eyelids closed between applications. Alternatively, while supine, 10–12 drops of pilocarpine were maintained on the cornea in a scleral cup for 5 min. Refraction measurements were begun 5 min after the second application of pilocarpine and continued for at least 30 min after initial administration until no further change in refraction occurred. In intravenous experiments, pilocarpine was given either as boluses ranging from 0.1 mg/kg to 2 mg/kg or boluses followed by a constant infusion at rates between 3.06 mg/kg/h and 11.6 mg/kg/h. Constant 2% pilocarpine solution on the eye in the perfusion lens produced 10.88 ± 2.73 D (mean ± SD) of accommodation. Topically applied pilocarpine produced 3.81 D ± 2.41, 5.49 D ± 4.08, and 5.55 D ± 3.27 using 2%, 4%, and 6% solutions respectively. When expressed as a percentage of the accommodative response amplitude obtained in the same monkey with constant 2% pilocarpine solution on the eye, the responses were 34.7% for 2% pilocarpine, 48.4% for 4% pilocarpine, and 44.6% for 6% pilocarpine. Topical 4% and 6% pilocarpine achieved similar, variable accommodative responses, but neither achieved maximum accommodation. IV boluses of pilocarpine achieved near maximal levels of accommodation at least ten times faster than topical methods. Doses effective for producing maximum accommodation ranged from 0.25 mg/kg to 1.0 mg/kg. IV pilocarpine boluses caused an anterior movement of the anterior lens surface, a posterior movement of the posterior lens surface, and a slight net anterior movement of the entire lens. Considerable variability in response amplitude occurred and maximum accommodative amplitude was rarely achieved with topical application of a variety of concentrations of commercially available pilocarpine. Intravenous infusion of pilocarpine was a rapid and reliable method of producing a nearly maximal accommodative response and maintaining accommodation when desired.
Keywords: accommodation, lens, monkey, pilocarpine
1. Introduction
Accommodation is a dioptric change in optical power of the eye to change focus from far to near. The mechanism of accommodation has been studied extensively in both humans and monkeys. Rhesus monkeys are a long and widely used animal model for human accommodation and presbyopia because rhesus monkeys have high accommodative amplitudes (Bito et al., 1982; Croft et al., 2006), the anatomy of the ocular accommodative structures (Lütjen-Drecoll et al., 1988a, b) and the accommodative mechanism (Glasser and Kaufman, 1999) are similar to that in humans and rhesus monkeys develop presbyopia with a similar age course as humans (Bito et al., 1982, 1987). Age related changes in the rhesus monkey eye are similar in all respects to that in humans and rhesus monkeys are an excellent animal model for studies of human accommodation and presbyopia (Koretz et al., 1987b). Other animal species either do not accommodate or if they do, they have accommodative mechanisms and accommodative anatomy quite unlike that of the human eye and therefore cannot be used for studies of accommodation in general and are inappropriate animal models for the study of human accommodation (Glasser, 2003; Glasser et al., 1994, 1995; Glasser and Howland, 1995; Rohen et al., 1989; Samuelson, 1996). Mammals such as mice and pigs have extremely diminutive ciliary muscles (Samuelson, 1996) and considerably more spherical lenses (Campbell and Hughes, 1981; Vakkur and Bishop, 1963; Vilupuru and Glasser, 2001) than primates rendering them unsuitable as animal models of human accommodation. No animal species other than primates have an accommodative mechanism similar to humans, an accommodative anatomy similar to humans and develop presbyopia with the same relative age course as humans. The applicability of rhesus monkeys to humans with regard to accommodation and presbyopia and the absence of other appropriate animal models means that rhesus monkeys are the only animal model in which to study the mechanisms of accommodation and presbyopia and experimental surgical procedures to restore accommodation to the presbyopic eye (Koopmans et al., 2006). The study of aspects of accommodation and presbyopia in rhesus monkeys relies on the ability to induce accommodation. While this can be accomplished behaviorally in awake monkeys (Bossong et al., 2009), the ability to scrutinize and image different aspects of the accommodative movements in the eyes often relies on contact imaging techniques such as ultrasound biomicroscopy (Croft et al., 2009) and gonioscopy (Ostrin and Glasser, 2007; Rosales et al., 2008; Wendt et al., 2008) that require working on anesthetized monkeys. It is therefore necessary to be able to induce accommodation in anesthetized monkeys in a reliable and reproducible manner. While this can be accomplished with Edinger-Westphal (EW) stimulation of the brain, for example, this complex neuro-physiological procedure is not widely used or generally accessible and further, EW stimulation necessarily results in a rapid and strong convergence eye movement because of the proximity of the EW nucleus to the ocular-motor nucleus in the brain. Therefore, this study was undertaken to establish how well intravenous (i.v.) pilocarpine infusion can be used to induce rapid and, if desired, sustained natural accommodative responses in anesthetized monkeys without contact with the eye and without inducing systematic convergence eye movements. The ability to achieve this will permit other ocular imaging techniques to be used on rhesus monkeys that may otherwise be compromised by eye movements. This may further help to elucidate aspects of the accommodative mechanism and the cause of presbyopia to facilitate the growing effort directed at restoring the accommodative ability to the presbyopic eye.
In conscious humans, accommodation is typically stimulated either with a visual stimulus or pharmacologically with topically applied pilocarpine. Accommodation has been studied in anesthetized monkeys by stimulating the Edinger-Westphal (EW) nucleus in the brain (Crawford et al., 1989; Glasser et al., 2006; Rosales et al., 2008) or using pharmacological stimulation with pilocarpine or carbachol (Ostrin and Glasser, 2005; Wendt et al., 2008). Carbachol and pilocarpine are cholinergic agonists which bind at the cholinergic receptors to cause an accommodative response by directly stimulating a contraction of the ciliary muscle.
A variety of different methods have been used to apply drugs to the eye to induce accommodation in anesthetized monkeys including: topical application of eye-drops (Koopmans et al., 2006; Nishi and Nishi, 1998); injection into the anterior chamber (Haefliger and Parel, 1994; Tornqvist, 1964; Tornqvist, 1966); topical application to the apex of the cornea of very small volumes (1–10 μl) of highly concentrated solutions (Bito et al.,1982; Haefliger and Parel, 1994; Millar and Kaufman, 1995); maintaining pilocarpine on the cornea with purpose built perfusion lens (Glasser and Kaufman, 1999; Wendt et al., 2008); and iontophoresis in the case of carbachol (Koretz et al., 1987a; Vilupuru and Glasser, 2002).
Application of commercially available muscarinic agonists to the eye may be the most readily available method of stimulating accommodation. Topical administration of carbachol or pilocarpine can be problematic for a number of reasons. This can include low absorption of the drugs through the cornea and systemic effects when high concentrations are used (Asseff et al., 1973; Harris, 1968; Janes and Stiles, 1959; Kiland et al., 1997). Efforts have been made to ensure that the drug enters the eye only through the cornea and does not come in contact with the limbus which may lead to a systemic reaction (Rohen et al., 1989). In humans, topical pilocarpine induced accommodative responses have been shown to vary among similar aged individuals, dependent on iris color and absorption of the drug by the ocular pigment epithelium and the amplitudes achieved do not always compare well with maximal accommodative response amplitudes elicited with visual stimulation (Ostrin and Glasser, 2004; Wold et al., 2003). The time-course of the topically induced accommodative response is slow and variable and the accommodative response achieved will therefore depend on the time after topical application at which the response is measured. A number of different investigators have used 4% pilocarpine eye drops topically to stimulate accommodation in monkeys (Koopmans et al., 2006; Nishi and Nishi, 1998). Although topical application of pilocarpine is relatively widely used to stimulate accommodation in anesthetized monkeys, no study has systematically evaluated the efficacy of stimulating accommodation in monkeys in this way, the effects of different concentrations, the time-course of the accommodative response or the amplitudes achieved.
Pilocarpine can also be administered topically using constant topical perfusion of the cornea with a 2% pilocarpine solution for 30–60 min in a purpose designed perfusion lens. This produced a stable level of accommodation which was assumed to be the maximal accommodative response amplitude (Wendt et al., 2008). Using this method, pilocarpine appears to penetrate the cornea at a similar rate to topical pilocarpine drops. However, constant exposure to the pilocarpine ensures that the eye achieves a stable maximum level of accommodation. The accommodative response achieved in this way is slow. This slow response time means that only a single stimulation can be performed per experiment using this method. Other drawbacks of constant perfusion derive from the presence of the perfusion lens in front of the eye. Many commonly used accommodation measurement techniques cannot be used in conjunction with the perfusion lens and the slow response times involved. These include goniovideography, refractometry, intraocular pressure measurements, magnetic resonance imaging, and continuous ultrasound biometry. Finally, when imaging methods can be used in conjunction with the perfusion lens (Glasser et al., 2006; Wendt et al., 2008) the manipulations of the eye which are required to switch from saline to pilocarpine solution can cause discontinuities in data recording.
In addition to topical application, pilocarpine has been used to stimulate accommodation in anesthetized monkeys by intramuscular (i.m.) injection (Millar and Kaufman, 1995; Peterson et al., 1999; Tornqvist, 1964) or by intravenous infusion (Hubbard et al., 1996; Tornqvist, 1964). However, high doses can produce systemic side effects in addition to a paradoxical reduction in accommodative amplitude due to a systemic decrease in blood pressure (Tornqvist, 1964, 1967). Protective i.m. low doses of atropine in the range of 0.05–0.1 mg/kg given prior to pilocarpine administration completely prevent any systemic side effects while allowing ocular effects to occur (Haefliger and Parel, 1994; Hubbard et al., 1996; Tornqvist, 1967). Although i.v. pilocarpine has been used to induce accommodation in anesthetized monkeys (Hubbard et al., 1996; Kaufman and Bárány, 1976; Tornqvist, 1964), only the studies by Tornquist from the 1960s have been specifically directed at characterizing the accommodative response. However, Tornquist’s studies did not include systematic analysis of the time-course of the i.v. pilocarpine induced accommodative response, the effects of a bolus delivery versus a constant infusion or what is required to achieve a sustained accommodative response.
Studies in monkeys have shown that carbachol iontophoresis induced accommodation differs from EW stimulated accommodation (Ostrin and Glasser, 2005). Similarly, in humans, accommodation stimulated with topical pilocarpine differs from the visual stimulus induced response (Koeppl et al., 2005). In particular, pharmacological induced contraction of the ciliary muscle produces a net forward movement of the lens that does not appear to occur when the stimulus to accommodate comes from the brain (Bolz et al., 2007; Drexler et al., 1997; Vilupuru and Glasser, 2005). This difference could be because topical drug stimulation results in far higher concentrations of drug reaching the ciliary neuron-muscle receptors to cause a supra-maximal contraction of the muscle compared to the amount of neurotransmitter release at neuromuscular junctions when the stimulus to accommodate comes from the brain (Crawford et al., 1990; Ostrin and Glasser, 2005). Intravenous pilocarpine delivery rates and concentrations can be rigorously controlled relative to topical drug administration and just-sufficient doses, as opposed to supra-maximal doses, can be administered to avoid side effects. Therefore, it is possible that i.v. pilocarpine may produce an accommodative response that is more similar to the neuronally elicited response than the topical pharmacologically stimulated response. This aspect of the i.v. pilocarpine induced accommodative response has not previously been studied.
Here, several methods of producing a pilocarpine stimulated accommodative response in rhesus monkeys were studied to evaluate their ability to produce a rapid, repeatable, and sustained accommodative response. Prior studies have not documented a method to achieve a rapid and a sustained, natural accommodative response in monkey eyes with pharmacological stimulation. The methods considered in this study were constant topical administration with a perfusion lens to maintain pilocarpine solution in contact with the cornea, topical application of pilocarpine eye drops of various concentrations, and bolus and constant i.v. infusion of various pilocarpine concentrations.
2. Methods
2.1. Animal preparation
All experiments conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were performed in accordance with animal protocols approved by the University of Houston Institutional Animal Care and Use Committee. Experiments were performed on the eyes of 17 rhesus monkeys (Macaca mulatta) ranging in age from 4 to 16 years. Each monkey had previously undergone total iridectomy and was housed in rooms away from bright lighting. Monkeys were initially anesthetized with intramuscular 15 mg/kg ketamine and 0.5 mg/kg acepromazine (Phoenix Pharmaceutical, St. Joseph, MO). Experiments were performed under intravenous propofol (PropoFlo, Abbott Laboratories, North Chicago, IL) anesthesia with an initial bolus of 1.5 mg/kg and a continuous infusion at a rate of 0.5 mg/kg/min. Throughout all experiments, pulse rate, SpO2, and temperature were monitored and the monkey was wrapped in a 37 °C water heated pad to maintain body temperature. In addition to propofol, some monkeys received 0.05 mg/kg i.m. medetomidine (Pfizer) to stabilize eye movements. In these cases, monkeys were intubated following propofol anesthesia and were respirated either periodically, manually with an Ambu bag or constantly with a Harvard pump with O2 at a rate of 1 L per minute. After these experiments, medetomidine was reversed with 0.25 mg/kg i.m. apitamezole (Pfizer). Due to logistical reasons, not all of the 17 monkeys were used in each type of experiment. Table 1 shows the different monkeys and the experiments in which they were used.
Table 1.
Table showing monkeys and the experiments performed. The age of the monkey shows the age range from the first experiment to the last experiment performed. The numbers in the table indicate the number of times the monkey was used in a particular experiment.
| Monkey | Age | Perfusion lens 2% |
Eye drops |
Scleral Cup |
IV Pilocarpine bolus only |
Sustained IV pilocarpine Infusion |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2% | 4% | 6% | 2% | 4% | 6% | |||||
| 4 | 15.1–16.6 | 1 | 1 | |||||||
| 8 | 10.2–11.4 | 1 | 1 | |||||||
| 34 | 15.6–16.5 | 1 | 1 | 1 | ||||||
| 38 | 8.3–11.0 | 1 | 1 | 1 | 1 | |||||
| 50 | 4.5–6.7 | 1 | 1 | 2 | 2 | 3 | ||||
| 54 | 9.4–10.5 | 1 | 1 | 1 | ||||||
| 58 | 9.3–10.6 | 1 | 1 | |||||||
| 64 | 7.2–8.6 | 1 | 1 | |||||||
| 66 | 11.3–12.6 | 1 | 1 | 1 | ||||||
| 70 | 8.3–10.0 | 1 | 2 | |||||||
| 73 | 6.0–7.5 | 1 | 1 | |||||||
| 99 | 4.8–6.6 | 1 | 1 | |||||||
| 109 | 6.9–8.4 | 1 | 1 | |||||||
| 111 | 7.25–8.6 | 1 | 2 | 1 | 1 | |||||
| 112 | 7.3–8.6 | 1 | 1 | |||||||
| 114 | 7.2–8.6 | 1 | 1 | |||||||
| 115 | 8.1–9.6 | 1 | 1 | |||||||
2.2. Accommodative measurements
Static measurements of accommodation were made using a Hartinger coincidence refractometer (Carl Zeiss Meditec, Jena, Germany). Photorefraction was used for dynamic accommodation measurements similar to that described previously (Vilupuru and Glasser, 2002). An infrared sensitive CCD camera (Cohu, San Diego, CA) was used which was fitted with a bank of infrared light emitting diodes. The camera was fixed at a distance of 0.3 m from the monkey’s eye. Video images were recorded to digital videotape and simultaneously analyzed on-line in a Matlab (The MathWorks, Inc., Natick, MA) program once every five seconds. Low frequency recordings were used because of the long duration of the experiments. The Matlab program measures vertical luminance profile of the central 40% of the eye and then returns the slope of a linear fit to the profile. To convert the measured slopes to accommodation, a calibration was initially performed with trial lenses of known power held in front of the eye. For each trial lens power, 30 video frames were analyzed and a mean calculated. The mean slopes obtained with each lens were used to develop a calibration curve for all subsequent measurements. On the contra-lateral eye, dynamic biometric (anterior chamber depth and lens thickness) accommodative changes were measured with continuous high-resolution A-scan ultrasound biometry (CUB) (Beers and van der Heijde, 1994; Vilupuru and Glasser, 2005). Biometric measurements were recorded to computer at 0.4 Hz, using a 10-MHz transducer. The transducer was aligned along the optical axis of the eye with a manipulator and maintained in contact with the cornea through ultrasound transmission gel (Liquasonic Ultrasound gel; Chester Laboratories, Inc., Cincinnati, OH). Distances between A-scan peaks representing the anterior and posterior cornea surfaces, anterior and posterior lens surfaces, and the retina were converted to distances using standard sound velocities of 1532 m/s for the anterior and vitreous chamber and 1641 m/s for the lens.
2.3. Topical pilocarpine perfusion
In the initial topical pilocarpine experiments, monkeys were prone on a table with the head in a head holder, upright and facing forward. A clear, plano contact lens was placed on the cornea to prevent dehydration and preserve optical quality. The baseline refraction was measured with the Hartinger. The contact lens was then removed and either saline or pilocarpine solution was maintained in contact with the cornea in a specially designed perfusion lens which was placed over the cornea and held in place beneath the eyelid (Wendt et al., 2008). As the eye accommodates, equatorial lens diameter decreases linearly with accommodation. Lens diameter was therefore used as a measure of the accommodative progression (Glasser et al., 2006). Images of the lens diameter in the iridectomized eyes were captured through the perfusion lens once every 10 seconds by a video camera attached to a slit-lamp microscope. Lens diameters were measured using a custom written Matlab program. The first solution in the perfusion lens was a balanced salt solution (BSS, Alcon, Ft Worth, TX) for baseline lens diameter measurements. This solution was replaced by a 2% pilocarpine solution to stimulate accommodation. Lens diameter measurements proceeded until no further decrease in lens diameter was observed in the on-line recordings. Since lens diameter has been shown to be linearly related to accommodation over the full range of the accommodative response (Glasser et al., 2006), the eye was assumed to have reached maximum accommodation at this point. The time required to achieve a stable minimum lens diameter varied from 30 min to an hour. The perfusion lens was then removed from the eye and the contact lens was placed back on the cornea. Finally, Hartinger measurements were made to determine the accommodative change in refraction.
2.4. Topical pilocarpine eye drops
In the topical pilocarpine experiments, the monkeys were held upright and facing forward in a head holder and baseline refraction was measured with a Hartinger coincidence refractometer with and without a contact lens. Commercially available pilocarpine (Pilocarpine Hydrochloride Ophthalmic Solution: 2% and 4% from Falcon Pharmaceuticals; 6% from Bausch & Lomb) was applied to the surface of the cornea using a pipetter in volumes of either 25 μl (1 drop) or 50 μl (2 drops). Monkeys received 2 or 4 drops in two applications or 6 drops in three applications over a 5-min period with the eyelids closed between applications. Alternatively, while supine, 10–12 drops of pilocarpine created a fluid pool which was maintained on the cornea in a scleral cup for 5 min. After pilocarpine administration, the eyelids remained closed except for brief periods when they were opened for Hartinger refraction measurements. Refraction measurements were begun 5 min after pilocarpine administration was complete. Measurements continued without a contact lens until no further change in refraction was observed for three 5-min intervals. At that point, a contact lens was placed back on the cornea and measurements continued until no further change was confirmed. Measurements typically continued for at least 30 min after initial pilocarpine administration
2.5. Intravenous pilocarpine
Monkeys were prone on a table with the head held in a head holder, upright and facing forward. A clear plano contact lens was placed on the cornea and baseline refraction was measured using the Hartinger. A photorefraction calibration was performed as described above and subsequently, the refractive state of the eye was monitored dynamically using infrared photorefraction. Eye movements were prevented either with sutures tied beneath the lateral and medial rectus muscles or with medetomidine supplementing the propofol anesthesia. In some experiments, dynamic biometric measurements were made with the CUB on one eye while photorefraction was being recorded in the other eye. Baseline measurements (photorefraction and CUB) were started and recorded for 10 min. A 0.05 mg/kg atropine (Phoenix Pharmaceutical, Inc., St. Louis) dose was then given intra-muscularly (i.m.) to provide protection against systemic effects of the pilocarpine and baseline measurements proceeded for 10 min more prior to pilocarpine administration. Pilocarpine solutions were then given i.v. in either a bolus of approximately 3 ml or in a bolus immediately followed by a continuous infusion. Boluses were given by syringe injection into the i.v. anesthetic line over 60 s. Bolus pilocarpine doses delivered were 0.1, 0.25, 0.5, 1.0, and 2.0 mg/kg. Continuous infusions were delivered by a syringe pump (KD Scientific, Inc., Boston) which was controlled by a Matlab program. Continuous infusion rates ranged from 3.06 to 11.6 mg/kg/h. At the end of these experiments, after the pilocarpine infusion was stopped, a 0.5 mg/kg i.v. dose of atropine was given to reverse the effects of the pilocarpine. In one or two instances, pilocarpine administration resulted in mildly increased salivation and mildly sweaty palms, and in one instance a mild cough occurred for a few seconds coincident with the pilocarpine administration, but no other systemic side effects were observed in any of the experiments or for any of the doses used.
3. Results
Experiments were performed in 14 of the 17 monkeys using constant perfusion of 2% pilocarpine in the perfusion lens (Fig. 1). The pilocarpine solution was maintained on the cornea until no further change in lens diameter occurred. This was assumed to have achieved maximum accommodation. The accommodative responses to 2% pilocarpine in the perfusion lens showed a decrease with age which was not statistically significant (p = 0.0589). In subsequent experiments, monkeys received topical applications of 2%, 4%, or 6% pilocarpine either as eye drops or in a scleral cup. As expected, constant perfusion application of pilocarpine achieved the greatest accommodative response in each monkey with an average of 11.59 D ± 2.07 (mean ± SD). The accommodative responses to topical applications showed considerable variability with some achieving levels close to the maximum and others significantly less than maximum. The data shown in Fig. 1 for monkeys #50 and #111 indicate the mean and standard deviations from two topical application experiments each.
Fig. 1.

Maximum accommodative responses were obtained from the 14 monkeys using constant perfusion of 2% pilocarpine. Subsequently, monkeys received topical applications of 2%, 4%, or 6% pilocarpine. Empty spaces in the graph indicate where monkeys were not treated with a particular concentration of pilocarpine.
The topical treatment results from each monkey tested were expressed as a percentage of the maximum accommodation achieved in that monkey using constant pilocarpine perfusion in the perfusion lens (Fig. 2). The mean responses were 34.7% for 2% pilocarpine, 48.4% for 4% pilocarpine, and 44.6% for 6% pilocarpine On average, topical 4% and 6% pilocarpine achieved similar accommodative responses which were higher than those with 2% pilocarpine, but did not approach maximum levels of accommodation. There were no significant differences between any of the three concentrations (p = 0.4200 between 2% and 4%, p = 0.4308 between 2% and 6%, p = 0.7954 between 4% and 6%). Overall, the accommodative responses to topical pilocarpine did not change significantly with age either directly (p = 0.6998) or as a percent of maximum achieved with the perfusion lens (p = 0. 7539).
Fig. 2.

The results from each topical treatment of each monkey were expressed as a percentage of the maximum accommodation achieved in that monkey using constant pilocarpine perfusion in the perfusion lens. The mean responses were 34.7% for 2% pilocarpine, 48.4% for 4% pilocarpine, and 44.6% for 6% pilocarpine.
Pilocarpine experiments using eye drops varied in the number of drops given and the concentrations used. This was because of the inability to achieve the maximum accommodative responses achieved with 2% pilocarpine in the perfusion lens. Fig. 3A and B show the responses of monkeys to the two most frequently used combinations. As before, these responses are expressed as a percentage of the maximum accommodation achieved in that monkey using constant pilocarpine perfusion. Responses showed a high degree of variability and rarely approached the maximum.
Fig. 3.
Inter-individual variability in accommodative responses to identical treatments with pilocarpine. Responses are expressed as a percentage of the maximum accommodative response obtained in each individual monkey with the perfusion lens. (A) Four monkeys which were treated with a total of 4 drops of 4% pilocarpine applied over 5 min had responses from 13.0% to 95.8% of the maximum accommodation achieved with the perfusion lens. (B) Three monkeys were treated with 4 drops of 6% pilocarpine over 5 min. One of these monkeys (#111) received this treatment on two different occasions. The responses varied from 18.0% to 46.2% of maximum accommodation.
When eye drops and topical perfusion in a scleral cup were analyzed separately (Fig. 4), no concentration dependent trend was evident when pilocarpine was applied as topical drops. The number of drops had no consistent effect for any of the concentrations. With 6% pilocarpine, changing the number of drops had little effect on the same monkey (#50). The lower number of drops actually produced more accommodation in monkeys #58 and # 34 using 2% pilocarpine. When pilocarpine was allowed to bathe the cornea in a scleral cup for 5 min, 6% pilocarpine produced an average of 10.3 D ± 3.8 compared to 2.6 D ± 0.3 for 4% and 1.9 D ± 1.8 for 2% pilocarpine.
Fig. 4.
The results from Fig. 2 are separated by method of application. Responses are expressed as a percentage of the maximum accommodative response obtained in each individual monkey with the perfusion lens. The individual monkeys are identified above each bar. The number of drops applied is shown at the base of each bar. (A) Eye drops applied topically to the cornea followed by closing the eyelids resulted in highly variable responses for all concentrations with 6% producing relatively low responses. (B) Experiments where 10–12 drops of pilocarpine were held on the eye with an eyecup for 5 min show a higher accommodative response with 6% pilocarpine, but still considerable variation. In both methods, in only one monkey was the full 100% accommodative response achieved.
In Fig. 5, single time courses are shown for two monkeys to three different methods of pilocarpine administration on different days. Initially, accommodation appeared to increase at similar rates for both topical eye drops and constant perfusion. However, accommodation typically leveled off earlier and at lower amplitude with topical eye drops. The time to reach maximum accommodation with constant perfusion was about 60 min compared to approximately 20 min for topical eye drops. The accommodative response to an intravenous bolus of 1 mg/kg pilocarpine is rapid in comparison to the other two methods with the maximum response occurring within 90 s. In general, intravenous infusion was more than ten times faster than topical methods.
Fig. 5.
Two sample time-course graphs of accommodative responses to pilocarpine are shown for three different methods: topical drops, constant perfusion, and intravenous bolus infusion of 1 mg/kg. The results in each graph are from three different experiments performed on the same monkey on three different days.
Results of a typical accommodation experiment with 5 increasing bolus doses of i.v. pilocarpine administration are shown in Fig. 6. The numbers in the graph indicate each pilocarpine bolus in mg/kg. A clear dose response can be seen as the accommodative amplitude rises with increasing pilocarpine. In addition, the response duration increases with subsequent increasing doses. A possible cumulative effect of systemic pilocarpine may account for some of these dose dependent increases, although the time-course of the initial boluses is relatively short. The maximum accommodation achieved was 14.0 D in response to a 2.0 mg/kg dose of pilocarpine. Accommodation had declined by only about 5 D when an i.v. bolus of 0.5 mg/kg atropine was given 52 min after this last pilocarpine bolus dose. The fluctuations in refraction are due to periodic eye movements that still occur under propofol anesthesia.
Fig. 6.

Accommodative responses to increasing boluses of intravenous pilocarpine are shown as a function of time. The bolus doses used were 0.1, 0.25, 0.5, 1.0, and 2.0 mg/kg pilocarpine. The accommodative fluctuations are due to slow eye movements that sometimes occur under propofol anesthesia.
A series of boluses was given to five monkeys and repeated in one monkey. When plotted together, dose responses show a typical dose response pattern rising toward a plateau (Fig. 7A). When these are normalized to the maximum accommodative response amplitude achieved in each experiment, the dose required to achieve maximum accommodation is seen to range from 0.25 mg/kg to 1.0 mg/kg (Fig. 7B). In two repeated experiments, the maximal dose for monkey #50 varied between 0.5 mg/kg and 1.0 mg/kg. Maximum accommodation for each monkey achieved with various doses of intravenous infusion averaged 78.9 ± 23.2% of the maximum accommodation achieved in the same monkey using constant pilocarpine perfusion in the perfusion lens.
Fig. 7.
Accommodative responses to pilocarpine boluses of increasing dosage were obtained in five monkeys and repeated in one monkey. (A) Total accommodation in diopters is shown plotted as function of pilocarpine dosage. (B) Accommodative levels are normalized to the maximum accommodation achieved during the experimental session and plotted against pilocarpine dosage.
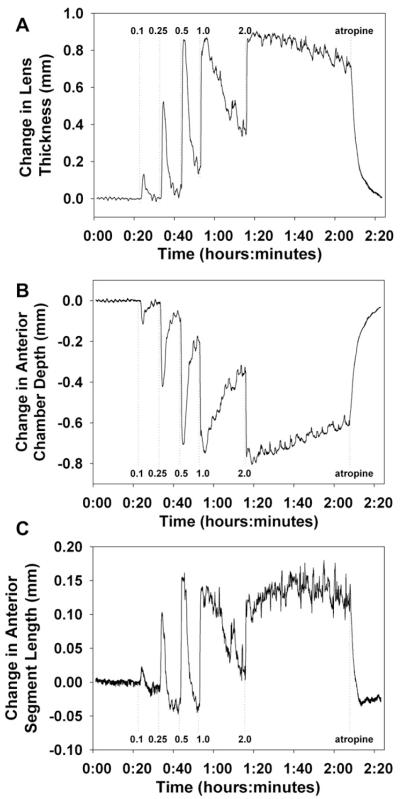
In three bolus i.v. pilocarpine experiments, biometry was measured continuously with the CUB on the right eye while photorefraction was simultaneously measured continuously on the left eye. Fig. 8A shows the change in lens thickness recorded during one experiment. These data were obtained in the right eye concurrent with the photorefraction data from the left eye shown in Fig. 6. The response increased in both amplitude and duration with increasing pilocarpine dosage. Lens thickness increased by a maximum of 0.9 mm. As the lens thickness increased, anterior chamber depth decreased by a maximum of 0.8 mm (Fig. 8B). The change in anterior segment length, the sum of anterior chamber depth and lens thickness, increased with increasing doses to a maximum of 0.18 mm (Fig. 8C).
Fig. 8.
CUB measurements were made on the contra-lateral eye during the same experiment as shown in Fig. 6. Measurements are shown here over time for (A) lens thickness, (B) anterior chamber depth, and (C) anterior segment depth (lens thickness + anterior chamber depth).
There was a consistent increase in lens thickness with an anterior movement of the anterior lens surface and a posterior movement of the posterior lens surface in response to each bolus of pilocarpine. However, the anterior movement of the anterior lens surface was greater as indicated by the net anterior shift of the lens center (half the distance from the anterior surface to the posterior surface). After the initial accommodative phase of the response, the lens tended to shift anteriorly prior to returning to the unaccommodated state (Fig. 9). In two monkeys (4 and 38), the posterior surface of the lens shifted to a position anterior to its starting position.
Fig. 9.
Movements of the anterior lens surface (solid black line), lens center (solid gray line), and the posterior lens surface (dashed black line) are plotted against time for pilocarpine bolus experiments in monkeys (A) #50, (B) #38, and (C) #4. Negative values indicate the anterior direction and positive values indicate the posterior direction. Movements are calculated as changes from the average baseline value during the first 2 min. In A, five increasing bolus doses of 0.1, 0.25, 0.5, 1.0, 2.0 mg/kg were given. In B, 4 bolus doses of 0.25, 0.5, 1.0, 2.0 mg/kg were given. In C bolus doses of 0.1, 0.25, 0.5 mg/kg were in most cases, boluses were given before accommodation had returned to baseline from the prior doses.
The i.v. pilocarpine bolus responses shown in Fig. 9 show a short period of rapid accommodative change followed by either a steady state of accommodation or a slow return to an unaccommodated state. In Fig. 10, lens biometric movements from the periods of rapid accommodative change were plotted against accommodation measured concurrently in the contra-lateral eye with photorefraction. Since the CUB transducer covers the eye, it is not possible to measure the biometry and the refraction in the same eye. The accommodative response is not necessarily identical in the two eyes as indicated in Fig. 11. The data in Fig. 11 were obtained by performing photorefraction measurements on both eyes of a monkey simultaneously during i.v. pilocarpine stimulation. Despite the fact that refraction is measured in one eye and biometry in the other and the response is not identical in the two eyes, there still exists a consistent relationship between the change in lens thickness in one eye and change in accommodation in the other eye. These data show an anterior movement of the anterior lens surface, a posterior movement of the posterior lens surface, and a slight net anterior movement of the lens center during the accommodative phase of the response to pilocarpine boluses.
Fig. 10.
In three experiments, CUB measurements were made on one eye at the same time that photorefraction was measured on the other. Combined accommodative data from multiple periods of rapid accommodation are plotted here against concurrent changes in lens surface position for monkeys (A) #50, (B) #38, and (C) #4. The data show an anterior movement of the anterior lens surface and a posterior movement of the posterior lens surface during accommodation.
Fig. 11.

Photorefraction accommodative responses measured simultaneously in both eyes (left eye: black; right eye: gray) in Monkey #50 in response to boluses of 0.1, 0.25, 0.5, and 1.0 mg/kg pilocarpine followed by 0.25 mg/kg atropine.
Efforts to achieve a sustained sub-maximal accommodative response were attempted with 3 monkeys over 6 experimental sessions. Boluses ranged from 0.5 mg/kg to 2 mg/kg and constant infusion rates varied from 3.06 to 11.6 mg/kg/h. In Fig. 12A, a stable maximal level of accommodation was achieved after the initial bolus followed by constant infusion. In five experiments (Fig. 12 B–F), the accommodative response decreased after the initial bolus. Accommodation increased rapidly to a stable maximum level after supplemental boluses followed by increased constant infusion in three of these experiments (Fig. 12B–D). In the other two experiments, accommodation increased gradually to a maximum level without any supplemental bolus or change in infusion rate. Constant i.v. pilocarpine infusions achieved sustained accommodation for more than 45 min that were between 64% and 132% of maximum accommodation achieved for the same monkey using constant topical pilocarpine perfusion.
Fig. 12.
Photorefraction measured accommodative refractive responses to bolus doses followed immediately by constant i.v. infusion of pilocarpine recorded in three monkeys. Experiments were repeated once in monkey #70 and twice in monkey #50. Constant doses of pilocarpine are shown in black below each accommodative response and bolus amounts of pilocarpine (black) and atropine (gray) are specifically labeled.
Biometric measurements on the contra-lateral eye in each of these experiments are shown in Fig. 13. Missing segments of data in B, E, & F occurred when the eye moved off the CUB transducer during certain portions of the experiment. During the initial accommodative phase of all six experiments, the anterior surface of the lens moved anteriorly, the posterior surface moved posteriorly, and the center of the lens moved slightly anteriorly. Following the initial phase, the lens position remained relatively stable in 3 cases (A–C) and slowly moved anteriorly in the other three cases (D-F).
Fig. 13.
Biometric measurements on the contra-lateral eye in each of the experiments shown in Fig. 12. Constant doses of pilocarpine are shown in black below each accommodative response and bolus amounts of pilocarpine (black) and atropine (gray) are specifically labeled.
Analysis of maximum accommodative responses to i.v. pilocarpine as a function of age show a decrease in accommodation of −0.4725 D/year, although this is not statistically significant (p = 0.0853, n = 10). IV pilocarpine infusion experiments were repeated in 2 monkeys. When accommodation was expressed as a percentage of maximum accommodation, there is no significant relationship with age (p = 0.7876).
4. Discussion
With topical application of commercially available pilocarpine drops, considerable variability in response amplitude occurred and maximum accommodative amplitude (as determined from the constant corneal perfusion experiments) was rarely achieved. This was true for drops of all concentrations tested. When pilocarpine bathed the cornea in a scleral cup for 5 min, the accommodative response was still variable, and low for both 2% and 4% pilocarpine solutions. Accommodation stopped increasing within 20 min after topical application, whereas accommodation continued to increase over 60 min in some cases with constant corneal perfusion. This is consistent with previous studies which have shown that only about 1–3% of the pilocarpine applied topically as eye drops penetrates the eye and that the maximum concentration occurs within 15 min of application (Asseff et al., 1973; Harris, 1968).
Intravenous boluses of pilocarpine produce rapid, dose dependent accommodative responses. The accommodative responses were of surprisingly short duration for sub-maximal doses, but lasted longer with increasing doses. Due to the rapid response time and the relatively short durations from lower pilocarpine concentrations, a sub-maximal dose could be repeated several times to produce repeated responses using intravenous infusion of pilocarpine. Tornqvist (1964) reported that i.v. pilocarpine gave essentially the same results as i.m. pilocarpine in that both produced protracted accommodation and pupil constriction for higher doses in the same range as those used here. The only systemic time-course data shown by Tornqvist (1964), were from intramuscular injection, and indicate elevated accommodative levels 5–15 min after pilocarpine injection. This contrasts with the i.v. infusion results from the current study where accommodation decreased to near baseline within 5 min after initial sub-maximal doses. Hubbard et al. reported administering 2 mg/kg i.v. pilocarpine and waiting 10 min for the response to stabilize, although no systematic accommodation time-course data are shown in that study. An i.v. dose of 2 mg/kg would be supra-maximal in every monkey tested in the present study. In the current study, supra-maximal doses continued to produce elevated, although not constant, levels of accommodation 40 min after administration, at which point monkeys were given 0.5 mg/kg atropine to end the experiment.
On six occasions, pilocarpine was administered by constant i.v. infusion following initial bolus administration. The goal of these experiments was to sustain accommodation at a constant level over an extended period of time. In these experiments, accommodation was maintained for the entire period of time that pilocarpine infusion continued – between 40 min and an hour. The level of accommodation tended to drop after an initial bolus and then increase with subsequent constant infusion with or without subsequent boluses. Once high systemic levels of pilocarpine were attained, accommodation continued at near constant levels and decreased relatively slowly after constant pilocarpine was stopped.
Previous studies indicate that topical pilocarpine in humans produces an “unphysiological” response in which the posterior lens surface moves anteriorly during accommodation (Koeppl et al., 2005). During carbachol stimulated accommodation in monkeys, the posterior lens surface initially moves in the posterior direction and then shifts anteriorly during the later phase of accommodation (Ostrin and Glasser, 2005). Stimulation of the Edinger-Westphal nucleus in monkeys causes lens thickness to increase in both the anterior and posterior directions with a slight net anterior movement of the lens center (Ostrin and Glasser, 2005; Vilupuru and Glasser, 2005). The results from i.v. stimulation in this present study show a clear anterior shift of the anterior lens surface and a posterior shift of the posterior lens surface during accommodation with a slight net anterior movement of the lens center during most of the accommodative response. Thus, the i.v. pilocarpine stimulated accommodative response is strikingly similar to the EW stimulated accommodative response, and differs from the carbachol iontophoretically stimulated accommodative response. New infusions of pilocarpine boluses clearly cause rapid movements of the posterior lens to positions posterior of the starting point. However, this is then followed by an anterior movement of the posterior lens surface as soon as accommodation stops and the accommodative response begins to decline again. This anterior movement of the posterior lens surface during relaxation of accommodation often continues until the posterior lens surface is anterior to its starting point. Evidence of this behavior was seen in all three bolus experiments. In contrast to bolus infusions, slow constant infusion of pilocarpine caused inconsistent results with regard to this anterior movement of the posterior lens surface. It is not clear why the accommodative response differs with different drug stimulation protocols, but it could be due to the differing rates at which the drug reaches different parts of the ciliary muscle dependent on the different administration methods. It is clear, however, that the i.v. pilocarpine administered accommodative response is characteristic of the normal brain induced accommodative response, although the disaccommodative phase shows differences.
The results show that i.v. pilocarpine administration in anesthetized monkeys is a safe and effective method to produce a reproducible and rapidly occurring, natural, accommodative response without systemic side-effects and without systematic convergent eye movements associated with brain stimulated accommodation. This method of stimulating accommodation in anesthetized monkeys will be useful in conjunction with imaging techniques that require rapid or sustained accommodation and ocular stability to better understand the accommodative mechanism and the progression of presbyopia.
Acknowledgements
Thanks to Chris Kuether for making the perfusion lens. This work was funded in part by a grant from AMO to AG and by NEI Core Grant P30 EY007551 to the University of Houston College of Optometry.
References
- Asseff CF, Weisman RL, Podos SM, Becker B. Ocular penetration of pilocarpine in primates. Am. J. Ophthalmol. 1973;75:212–215. doi: 10.1016/0002-9394(73)91015-5. [DOI] [PubMed] [Google Scholar]
- Beers APA, van der Heijde GL. In vivo determination of the biomechanical properties of the component elements of the accommodative mechanism. Vision Res. 1994;34:2897–2905. doi: 10.1016/0042-6989(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Bito LZ, DeRousseau CJ, Kaufman PL, Bito JW. Age-dependent loss of accommodative amplitude in rhesus monkeys: an animal model for presbyopia. Invest. Ophthalmol. Vis. Sci. 1982;23:23–31. [PubMed] [Google Scholar]
- Bito LZ, Kaufman PL, DeRousseau CJ, Koretz J. Presbyopia: an animal model and experimental approaches for the study of the mechanism of accommodation and ocular aging. Eye. 1987;1(2):222–230. doi: 10.1038/eye.1987.41. [DOI] [PubMed] [Google Scholar]
- Bolz M, Prinz A, Drexler W, Findl O. Linear relationship of refractive and biometric lenticular changes during accommodation in emmetropic and myopic eyes. Br. J. Ophthalmol. 2007;91:360–365. doi: 10.1136/bjo.2006.099879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossong H, Swann M, Glasser A, Das VE. Applicability of infrared photorefraction for measurement of accommodation in awake-behaving normal and strabismic monkeys. Invest Ophthalmol. Vis. Sci. 2009;50:966–973. doi: 10.1167/iovs.08-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MCW, Hughes A. An analytic, gradient index schematic lens and eye for the rat which predicts aberrations for finite pupils. Vision Res. 1981;21:1129–1148. doi: 10.1016/0042-6989(81)90016-x. [DOI] [PubMed] [Google Scholar]
- Crawford K, Terasawa E, Kaufman PL. Reproducible stimulation of ciliary muscle contraction in the cynomolgus monkey via a permanent indwelling midbrain electrode. Brain Res. 1989;503:265–272. doi: 10.1016/0006-8993(89)91673-9. [DOI] [PubMed] [Google Scholar]
- Crawford KS, Kaufman PL, Bito LZ. The role of the iris in accommodation of rhesus monkeys. Invest. Ophthalmol. Vis. Sci. 1990;31:2185–2190. [PubMed] [Google Scholar]
- Croft MA, Glasser A, Heatley G, McDonald J, Ebbert T, Dahl DB, Nadkarni NV, Kaufman PL. Accommodative ciliary body and lens function in rhesus monkeys, I: normal lens, zonule and ciliary process configuration in the iridectomized eye. Invest. Ophthalmol. Vis. Sci. 2006;47:1076–1086. doi: 10.1167/iovs.04-1523. [DOI] [PubMed] [Google Scholar]
- Croft MA, McDonald JP, Nadkarni NV, Lin TL, Kaufman PL. Age-related changes in centripetal ciliary body movement relative to centripetal lens movement in monkeys. Exp. Eye Res. 2009;89:824–832. doi: 10.1016/j.exer.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler W, Baumgartner A, Findl O, Hitzenberger CK, Fercher AF. Biometric investigation of changes in the anterior eye segment during accommodation. Vision Res. 1997;37:2789–2800. doi: 10.1016/s0042-6989(97)00066-7. [DOI] [PubMed] [Google Scholar]
- Glasser A. How other species accommodate. In: Guthoff R, Ludwig K, editors. Current Aspects of Human Accommodation II. Kaden Verlag; Heidelberg: 2003. pp. 13–37. [Google Scholar]
- Glasser A, Howland HC. In vitro changes in back vertex distance of chick and pigeon lenses: species differences and the effects of aging. Vision Res. 1995;35:1813–1824. doi: 10.1016/0042-6989(94)00292-t. [DOI] [PubMed] [Google Scholar]
- Glasser A, Kaufman PL. The mechanism of accommodation in primates. Ophthalmology. 1999;106:863–872. doi: 10.1016/S0161-6420(99)00502-3. [DOI] [PubMed] [Google Scholar]
- Glasser A, Murphy CJ, Troilo D, Howland HC. The mechanism of lenticular accommodation in the chick eye. Vision Res. 1995;35:1525–1540. doi: 10.1016/0042-6989(94)00211-4. [DOI] [PubMed] [Google Scholar]
- Glasser A, Troilo D, Howland HC. The mechanism of corneal accommodation in chicks. Vision Res. 1994;34:1549–1566. doi: 10.1016/0042-6989(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Glasser A, Wendt M, Ostrin L. Accommodative changes in lens diameter in rhesus monkeys. Invest. Ophthalmol. Vis. Sci. 2006;47:278–286. doi: 10.1167/iovs.05-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefliger E, Parel JM. Accommodation of an endocapsular silicone lens (Phaco-Ersatz) in the aging rhesus monkey. J. Refract. Corneal Surg. 1994;10:550–555. [PubMed] [Google Scholar]
- Harris JE. Problems in drug penetration. In: Leopold IH, editor. Symposium on Ocular Therapy. CV Mosby Co; St Louis, MO, USA: 1968. pp. 96–105. [Google Scholar]
- Hubbard WC, Kee C, Kaufman PL. Aceclidine effects on outflow facility after ciliary muscle disinsertion. Ophthalmologica. 1996;210:303–307. doi: 10.1159/000310729. [DOI] [PubMed] [Google Scholar]
- Janes RG, Stiles JF. The penetration of C-14 labeled atropine into the eye. Arch. Ophthalmol. 1959;62:97–102. doi: 10.1001/archopht.1959.04220010073007. [DOI] [PubMed] [Google Scholar]
- Kaufman PL, Bárány EH. Loss of acute pilocarpine effect on outflow facility following surgical disinsertion and retrodisplacement of the ciliary muscle from the scleral spur in cynomolgus monkey. Invest. Ophthalmol. Vis. Sci. 1976;15(10):793–807. [PubMed] [Google Scholar]
- Kiland JA, Peterson JA, Gabelt BT, Kaufman PL. Effect of DMSO and exchange volume on outflow resistance washout and response to pilocarpine during anterior chamber perfusion in monkeys. Curr. Eye Res. 1997;16:1215–1220. doi: 10.1076/ceyr.16.12.1215.5026. [DOI] [PubMed] [Google Scholar]
- Koeppl C, Findl O, Kriechbaum K, Drexler W. Comparison of pilocarpine-induced and stimulus-driven accommodation in phakic eyes. Exp. Eye Res. 2005;80:795–800. doi: 10.1016/j.exer.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Koopmans SA, Terwee T, Glasser A, Wendt M, Vilipuru AS, van Kooten TG, Norrby S, Haitjema HJ, Kooijman AC. Accommodative lens refilling in rhesus monkeys. Invest. Ophthalmol. Vis. Sci. 2006;47:2976–2984. doi: 10.1167/iovs.05-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koretz JF, Bertasso AM, Neider MW, True-Gabelt B, Kaufman PL. Slit-lamp studies of the rhesus monkey eye. II Changes in crystalline lens shape, thickness and position during accommodation and aging. Exp. Eye Res. 1987a;45:317–326. doi: 10.1016/s0014-4835(87)80153-7. [DOI] [PubMed] [Google Scholar]
- Koretz JF, Neider MW, Kaufman PL, Bertasso AM, DeRousseau CJ, Bito LZ. Slit-lamp studies of the rhesus monkey eye. I. Survey of the anterior segment. Exp. Eye Res. 1987b;44:307–318. doi: 10.1016/s0014-4835(87)80014-3. [DOI] [PubMed] [Google Scholar]
- Lütjen-Drecoll E, Tamm E, Kaufman PL. Age changes in rhesus monkey ciliary muscle: light and electron microscopy. Exp. Eye Res. 1988a;47:885–899. doi: 10.1016/0014-4835(88)90070-x. [DOI] [PubMed] [Google Scholar]
- Lütjen-Drecoll E, Tamm E, Kaufman PL. Age-related loss of morphologic responses to pilocarpine in rhesus monkey ciliary muscle. Arch. Ophthalmol. 1988b;106:1591–1598. doi: 10.1001/archopht.1988.01060140759051. [DOI] [PubMed] [Google Scholar]
- Millar JC, Kaufman PL. PGF2 alpha/pilocarpine interactions on IOP and accommodation in monkeys. Exp. Eye Res. 1995;61:677–683. doi: 10.1016/s0014-4835(05)80018-1. [DOI] [PubMed] [Google Scholar]
- Nishi O, Nishi K. Accommodation amplitude after lens refilling with injectable silicone by sealing the capsule with a plug in primates. Arch. Ophthalmol. 1998;116:1358–1361. doi: 10.1001/archopht.116.10.1358. [DOI] [PubMed] [Google Scholar]
- Ostrin LA, Glasser A. Comparisons between pharmacologically and Edinger-Westphal-stimulated accommodation in rhesus monkeys. Invest. Ophthalmol. Vis. Sci. 2005;46:609–617. doi: 10.1167/iovs.04-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrin LA, Glasser A. Accommodation measurements in a prepresbyopic and presbyopic population. J. Cataract Refract. Surg. 2004;30:1435–1444. doi: 10.1016/j.jcrs.2003.12.045. [DOI] [PubMed] [Google Scholar]
- Ostrin LA, Glasser A. Edinger-Westphal and pharmacologically stimulated accommodative refractive changes and lens and ciliary process movements in rhesus monkeys. Exp. Eye Res. 2007;84:302–313. doi: 10.1016/j.exer.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JA, Tian B, Geiger B, Kaufman PL. Latrunculin-A causes mydriasis and cycloplegia in the cynomolgus monkey. Invest. Ophthalmol. Vis. Sci. 1999;40:631–638. [PubMed] [Google Scholar]
- Rohen JW, Kaufman PL, Eichhorn M, Goeckner PA, Bito LZ. Functional morphology of accommodation in raccoon. Exp. Eye Res. 1989;48:523–537. doi: 10.1016/0014-4835(89)90035-3. [DOI] [PubMed] [Google Scholar]
- Rosales P, Wendt M, Marcos S, Glasser A. Changes in crystalline lens radii of curvature and lens tilt and decentration during dynamic accommodation in rhesus monkeys. J. Vis. 2008;8:18–22. doi: 10.1167/8.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson DA. A reevaluation of the comparative anatomy of the eutherian iridocorneal angle and associated ciliary body musculature. Vet. Comp. Ophthalmol. 1996;6:153–172. [Google Scholar]
- Tornqvist G. Effect of topical carbachol on the pupil and refraction in young and presbyopic monkeys. Invest. Ophthalmol. Vis. Sci. 1966;5(2):186–195. [Google Scholar]
- Tornqvist G. Accommodation in monkeys: some pharmacological and physiological aspects. Acta. Ophthalmol. 1967;45:429–460. doi: 10.1111/j.1755-3768.1967.tb06508.x. [DOI] [PubMed] [Google Scholar]
- Tornqvist G. Comparative studies of the effect of pilocarpine on the pupil and on the refraction in two species of monkey (Cercopithecus ethiops and Macaca irus) Invest. Ophthalmol. Vis. Sci. 1964;3(4):388–398. [PubMed] [Google Scholar]
- Vakkur GJ, Bishop PO. The schematic eye in the cat. Vis. Res. 1963;3:357–381. doi: 10.1016/0042-6989(63)90009-9. [DOI] [PubMed] [Google Scholar]
- Vilupuru AS, Glasser A. Optical and biometric relationships of the isolated pig crystalline lens. Ophthalmic. Physiol. Opt. 2001;21:296–311. doi: 10.1046/j.1475-1313.2001.00593.x. [DOI] [PubMed] [Google Scholar]
- Vilupuru AS, Glasser A. Dynamic accommodation in rhesus monkeys. Vis. Res. 2002;42:125–141. doi: 10.1016/s0042-6989(01)00260-7. [DOI] [PubMed] [Google Scholar]
- Vilupuru AS, Glasser A. The relationship between refractive and biometric changes during Edinger-Westphal stimulated accommodation in rhesus monkeys. Exp. Eye Res. 2005;80:349–360. doi: 10.1016/j.exer.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt M, Croft MA, McDonald J, Kaufman PL, Glasser A. Lens diameter and thickness as a function of age and pharmacologically stimulated accommodation in rhesus monkeys. Exp. Eye Res. 2008;86:746–752. doi: 10.1016/j.exer.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold JE, Hu A, Chen S, Glasser A. Subjective and objective measurement of human accommodative amplitude. J. Cataract Refract. Surg. 2003;29:1878–1888. doi: 10.1016/s0886-3350(03)00667-9. [DOI] [PubMed] [Google Scholar]