Summary
Cortical dysplasia is often associated with intractable seizures. Studies in animal models have described changes in inhibitory and excitatory synaptic function that contribute to hyperexcitability. The role of changes in intrinsic excitability and abnormal dendritic properties has received less attention. Changes in hyperpolarization-activated non-selective cation (HCN) channels have been implicated in several models of epilepsy. Here we review evidence for alterations in HCN channels and dendritic morphology in the rat freeze-lesion model of cortical dysplasia. Immunocytochemical HCN1 staining, typically seen in the apical dendrites of layer V pyramidal cells in normal cortex, was greatly reduced in the region adjacent to the freeze-induced microgyrus. Although staining was preserved in layer I, fewer dendrites were stained in upper cortical layers. Deeper cortical layers were virtually devoid of immunoreactivity. Examination of biocytin-labeled pyramidal cells revealed markedly altered dendritic trees in the lesioned animals. In addition, resting membrane properties were altered and a subpopulation of neurons with abnormal dendritic arbors was present. These changes are likely to interact with the previously reported synaptic changes in this model of cortical dysplasia. HCN channel alterations are a potentially important cellular mechanism underlying hyperexcitability in cortical dysplasia.
Keywords: Epilepsy, HCN channels, cortical dysplasia, dendritic excitability
Introduction
Cortical dysplasias, microgyria, and heterotopias are associated with intractable seizure disorders in humans (Sisodiya 2004). Up to 43% of patients receiving surgical treatment for intractable seizures have some sort of cortical microdysgenesis (Finardi et al. 2006; Leventer et al., 2008). Anticonvulsant drug therapy is often ineffective in patients with cortical malformations (Sisodiya, 2004). Studies in vitro of brain slices prepared from human dysplastic neocortex have demonstrated that this tissue displays intrinsic hyperexcitability (Mattia et al. 1995; Avoli et al. 1999; Cepeda et al. 2003). Many of the electrophysiological and anatomical features of human cortical dysplasia are reproduced in the rat freeze-lesion model where hyperexcitability is seen predominantly in the area adjacent (0.5 to 2.5 mm lateral) to the microsulcus (Jacobs et al. 1996; Hablitz and DeFazio 1998). Thalamic afferents abnormally innervate the hyperexcitable zone in the freeze lesion model (Rosen et al., 2000). The possibility of aberrant inputs from the microgyral region or immediately adjacent cortex has not been examined. Malformed neurons in this area with aberrant connections could contribute to altered excitability.
Neocortical layer V pyramidal cells have long apical dendrites extending vertically for millimeters as they send their apical tufts into layer I. Immunocytochemical studies have shown that hyperpolarization-activated non-selective cation (HCN) channels are highly expressed in the apical dendrites of these neurons (Lorincz et al., 2002) where HCN-mediated Ih currents regulate dendritic excitability (Berger et al., 2001). Impairments in neocortical Ih have been reported in several models of epilepsy (Chen et al., 2002; Wahl-Schott and Biel, 2009) although changes in cortical dysplasia have not been investigated. Here we explore the possible role of changes in two factors on increased excitability in the freeze-lesion model of cortical dysplasia, namely, (1) altered morphological features of pyramidal cells in dysplastic cortex and (2) changes in immunohistochemical staining for HCN1, the principal HCN subunit expressed in neocortex. HCN1 staining appears to be markedly reduced in this model and a subpopulation of pyramidal cells with markedly distorted apical dendrites has been identified.
Neuronal features in the experimental freeze lesion
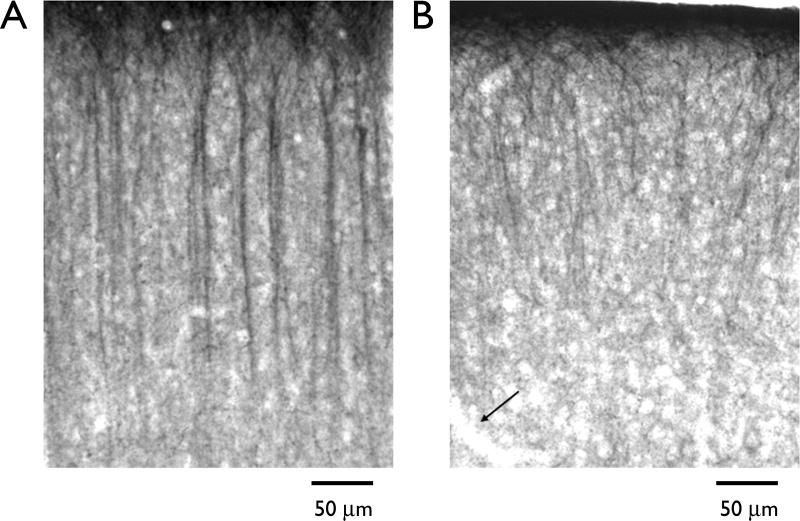
Focal freeze lesions were induced in postnatal day 1 Sprague-Dawley rats as described previously (Jacobs et al., 1996; Hablitz and DeFazio, 19987). Freeze-lesioned and sham-operated rats were allowed to recover for 19–23 days. As described previously for rat neocortex (Lorincz et al., 2002), prominent HCN1 staining in seen in the apical dendrites of layer V pyramidal cells in normal cortex (Fig. 1A). Apical tufts located in layer I also are well-stained. In contrast, staining in sections from animals who had received freeze lesions on postnatal day 1 was greatly reduced in the region adjacent to the microgyrus (Fig. 1B). Although staining was preserved in layer I, fewer dendrites were stained in upper cortical layers. Deeper cortical layers were virtually devoid of immunoreactivity.
Figure 1.
Photomicrographs of HCN1 immunoreactivity in rat neocortex. (A) Section stained with an antibody to HCN1. Sections were incubated in an anti-HCN1 antibody (Chemicon; diluted 1:2500) overnight at room temperature. After washing, sections were incubated in a biotinylated secondary antibody for 1 h and then transferred to a avidin-biotin-peroxidase solution for 90 min. Visualization was completed using the cobalt/nickel DAB method. The apical dendrites of layer V neurons were well stained whereas cell bodies and axons were largely immunonegative. (B) Section from lesioned animal processed in parallel with section shown in A. HCN1 immunoreactivity is markedly reduced and dendrites that are immunoreactive show abnormal organization and branching. Arrow indicates border of microgyrus.
The HCN immunoreactivity results suggested that Ih should be reduced or absent in neurons near the microgyrus. Since Ih is active at rest in neocortical pyramidal cells (Berger et al., 2001), neurons near the lesion should have more negative resting potentials and larger somatic input resistance than neurons in sham operated animals. Complex interactions between intrinsic properties, synaptic and voltage-dependent currents will determine the net effect on excitability resulting from the changes observed here. (Dyhrfjeld et al., 2009)
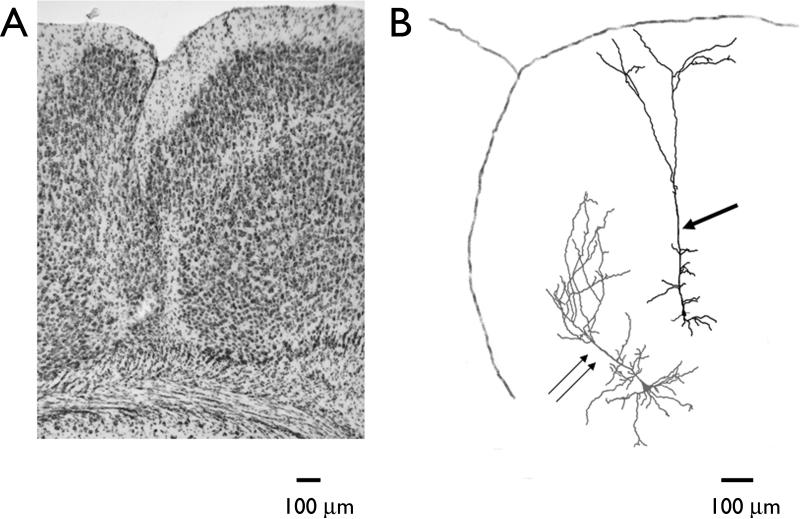
Previous studies of pyramidal neurons in the hyperexcitable zone adjacent to the microsulcus have reported that neuronal morphology was well preserved (Jacobs et al., 1996; Luhmann et al., 1998. We observed a small population of neurons with markedly altered dendritic branching patterns. Fig. 2A shows a photomicrograph of a typical freeze-induced microgyrus. When neurons were labeled in the hyperexcitable zone adjacent to the microgyrus, deeper layer pyramidal cells were found to have normal appearing apical dendrites which ascended and branched in layer 1 (Fig. 2B arrow). In contrast, neurons located immediately below the lesion had a markedly altered dendritic tree (Fig. 2B, double arrows). The apical dendrite begins to ascend vertically but then abruptly turned to the right as it approached the lesion. This type of morphology was seen in approximately 10% of the cells examined (n =30).
Figure 2.
Morphological appearance of freeze-induced microgyrus and pyramidal neurons. (A) Nissl-stained section showing characteristic features of a freeze-induced microgyrus. (B) Camera lucida reconstruction of two pyramidal neurons, one located in the hyperexcitable zone (single arrow) and a second in the vicinity of the microgyrus (double arrow). A tracing of the pial surface and infolding of the microsulcus is shown. Normal dendritic patterns are seen in the neuron located in the hyperexcitable zone whereas aberrant dendritic orientation is seen in the cell close to the microgyrus.
Cortical dysplasia induced in rats by freeze lesions in the PN 0-1 period has been extensively studied. Alterations in excitatory and inhibitory synaptic transmission have been reported. However, there has been little indication that cortical dysplasia was associated with changes in intrinsic membrane excitability or neuronal morphology, aside from disruptions of cortical lamination. Our recent findings described above demonstrates that HCN1 expression, as visualized by immunocytochemistry, is decreased in the vicinity of the freeze-induced microgyrus, in resting membrane properties are altered and a subpopulation of neurons with abnormal dendritic arbors is present. These changes are likely to interact with the previously reported synaptic changes to produce the hyperexcitability seen in this model.
Hyperexcitability in freeze-induced dysplastic cortex is thought to be due to increases in innervation which result in a shift in the balance between inhibition and excitation (Jacobs and Prince, 2005). This hyperinnervation has been postulated to be due to redirection of afferents originally destined to the microgyral region to the hyperexcitable zone (Zsombok and Jacobs, 2007). Another possible source of excessive innervation could be the atypical pyramidal cells identified here. The extensive dendritic fields and lack of HCN1 expression could make these cells hyperexcitable and possible initiators of epileptiform activity. We are currently reconstructing the collateral axonal projections of these cells to determine if they target cells in the hyperexcitable zone.
Discussion – the potential effects of HCN abnormalities
HCN-mediated Ih currents are potent regulators of single-cell and network excitability (Chen et al., 2002). The exact mechanisms underlying the effects of alterations in Ih on neuron and circuit activity are unclear. Further studies on intrinsic excitability and synaptic integration are needed to determine if our observed effects could contribute to hyperexcitability in the freeze-lesion model. It will also be important to test for Ih changes in interneurons. Neocortical interneurons have robust, ZD7288-sensitive, Ih currents (Wu and Hablitz, 2005). Interneuron Ih changes in epilepsy have received little attention.
The direction of HCN expression change in epilepsy is varied. Febrile seizures in rats are associated with an upregulation of Ih (Dyhrfjeld-Johnsen et al., 2008) whereas bidirectional changes are seen in status epilepticus models (Shin et al., 2008). In a rat model of absence epilepsy there is a rapid decline in HCN1 expression that precedes seizure onset (Kole et al., 2007). This loss can be prevented by environmental manipulations early in development resulting in decreased seizure activity (Schridde et al., 2006), suggesting that targeting Ih may have therapeutic value.
Acknowledgements
This work was supported by National Institutes of Health grants NS22373, P30-NS47466, P30-HD38985 and P30-NS57098. We thank K. Alison Margolies for technical assistance.
Footnotes
Disclosure
None of the authors has any conflict of interest to disclose.
References
- Avoli M, Bernasconi A, Mattia D, Oliveira A, Hwa GGC. Epileptiform discharges in the human dysplastic neocortex: In vitro physiology and pharmacology. Ann Neurol. 1999;46:816–26. doi: 10.1002/1531-8249(199912)46:6<816::aid-ana3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Berger T, Larkum ME, Lüscher H-R. High Ih channel density in the distal apical dendrite of layer V pyramidal cells increases bidirectional attenuation of EPSPs. J Neurophysiol. 2001;85:855–68. doi: 10.1152/jn.2001.85.2.855. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Hurst RS, Flores-Hernandez J, Hernandez-Echeagaray E, Klapstein GJ, Boylan MK, Calvert CR, Jocoy EL, Nguyen OK, Andre VM, Vinters HV, Ariano MA, Levine MS, Mathern GW. Morphological and electrophysiological characterization of abnormal cell types in pediatric cortical dysplasia. Journal of Neuroscience Research. 2003;72:472–86. doi: 10.1002/jnr.10604. [DOI] [PubMed] [Google Scholar]
- Chen K, Aradi I, Santhakumar V, Soltesz I. H-channels in epilepsy: new targets for seizure control? Trends in Pharmacological Sciences. 2002;23:552–557. doi: 10.1016/s0165-6147(02)02110-7. [DOI] [PubMed] [Google Scholar]
- Dyhrfjeld-Johnsen J, Morgan R, Foldy C, Soltesz I. Upregulated h-current in hyperexcitable CA1 dendrites after febrile seizures. Frontiers Cellular Neuroscience. 2008;2:2. doi: 10.3389/neuro.03.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyhrfjeld-Johnsen J, Morgan R, Soltesz I. Double trouble? Potential for hyperexcitability following both channelopathic up- and downregulation of Ih in epilepsy. Frontiers in Neuroscience. 2009;3:25–33. doi: 10.3389/neuro.01.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finardi A, Gardoni F, Bassanini S, et al. NMDA receptor compostion differs among anatomically diverse malformations of cortical development. J Neuropath Exp Neurol. 2006;65:883–93. doi: 10.1097/01.jnen.0000235117.67558.6d. [DOI] [PubMed] [Google Scholar]
- Hablitz JJ, DeFazio T. Excitability changes in freeze-induced neocortical microgyria. Epilepsy Res. 1998;32:75–82. doi: 10.1016/s0920-1211(98)00041-2. [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Prince DA. Excitatory and inhibitory postsynaptic currents in a rat model of epileptogenic microgyria. J Neurophysiol. 2005;93:687–96. doi: 10.1152/jn.00288.2004. [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Gutnick MJ, Prince DA. Hyperexcitability in a model of cortical maldevelopment. Cereb Cortex. 1996;6:514–23. doi: 10.1093/cercor/6.3.514. [DOI] [PubMed] [Google Scholar]
- Kole MHP, Brauer AU, Stuart GJ. Inherited cortical HCN1 channel loss amplifies dendritic calcium electrogenesis and burst firing in a rat absence epilepsy model. Journal of Physiology (Lond) 2007;578:507–25. doi: 10.1113/jphysiol.2006.122028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventer RJ, Guerrini R, Dobyns WB. Malformations of cortical development and epilepsy. Dialogues in Clinical Neuoscience. 2008;10:47–62. doi: 10.31887/DCNS.2008.10.1/rjleventer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz A, Notomi T, Tamas G, Shigemoto R, Nusser Z. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci. 2002;5:1185–93. doi: 10.1038/nn962. [DOI] [PubMed] [Google Scholar]
- Luhmann HJ, Karpuk N, Qü M, Zilles K. Characterization of neuronal migration disorders in neocortical structures.II.Intracellular in vitro recordings. J Neurophysiol. 1998;80:92–102. doi: 10.1152/jn.1998.80.1.92. [DOI] [PubMed] [Google Scholar]
- Mattia D, Olivier A, Avoli M. Seizure-like discharges recorded in human dysplastic neocortex maintained in vitro. Neurology. 1995;45:1391–5. doi: 10.1212/wnl.45.7.1391. [DOI] [PubMed] [Google Scholar]
- Rosen GD, Burstein D, Galaburda AM. Changes in efferent and afferent connectivity in rats with induced cerebrocortical microgyria. J Comp Neurol. 2000;418:423–40. [PubMed] [Google Scholar]
- Schridde U, Strauss U, Brauer AU, van Luijtelaar G. Environmental manipulations early in development alter seizure activity, Ih and HCN1 protein expression later in life. Eur J Neurosci. 2006;23:3346–58. doi: 10.1111/j.1460-9568.2006.04865.x. [DOI] [PubMed] [Google Scholar]
- Shin M, Brager D, Jaramillo TC, Johnston D, Chetkovich DM. Mislocalization of h channel subunits underlies h channelopathy in temporal lobe epilepsy. Neurobiology of Disease. 2008;32:26–36. doi: 10.1016/j.nbd.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisodiya SM. Malformations of cortical development: burdens and insights from important causes of human epilepsy. Lancet Neurology. 2004;3:29–38. doi: 10.1016/s1474-4422(03)00620-3. [DOI] [PubMed] [Google Scholar]
- Wahl-Schott C, Biel M. HCN channels:Structure, cellular regulation and physiological function. Cellular and Molecular Life Sciences. 2009;66:470–94. doi: 10.1007/s00018-008-8525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Hablitz JJ. Cooperative activation of D1 and D2 dopamine receptors enhances a hyperpolarization-activated inward current in layer I interneurons. J Neurosci. 2005;25:6322–8. doi: 10.1523/JNEUROSCI.1405-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsombok A, Jacobs KM. Postsynaptic currents prior to onset of epileptiform activity in rat microgyria. J Neurophysiol. 2007;98:178–86. doi: 10.1152/jn.00106.2007. [DOI] [PubMed] [Google Scholar]