Abstract
Periventricular leukomalacia (PVL) is a lesion of the immature cerebral white matter in the perinatal period and associated predominantly with prematurity and cerebral ischemia/reperfusion as well as inflammation due to maternofetal infection. It consists of focal necrosis in the periventricular region and diffuse gliosis with microglial activation and premyelinating oligodendrocyte (pre-OL) injury in the surrounding white matter. We previously showed nitrotyrosine in pre-OLs in PVL, suggesting involvement of nitrosative stress in this disorder. Here we hypothesize that inducible nitric oxide synthase (iNOS) expression is increased in PVL relative to controls. Using immunocytochemistry in human archival tissue, the density of iNOS-expressing cells was determined in the cerebral white matter of 15 PVL cases [29–51 postconceptional (PC) weeks] and 16 control cases (20–144 PC weeks). Using a standardization score of 0–3, the density of iNOS-positive cells was significantly increased in the diffuse component of PVL (score of 1.8 ± 0.3) cases compared to controls (score of 0.7 ± 0.3) (P = 0.01). Intense iNOS expression occurred in reactive astrocytes in acute through chronic stages and in activated microglia primarily in the acute stage, suggesting an early role for microglial iNOS in PVL’s pathogenesis. This study supports an important role for iNOS-induced nitrosative stress in the reactive/inflammatory component of PVL.
Keywords: Nitrotyrosine, Oxidative stress, Astrocytes, Microglia
Introduction
Periventricular leukomalacia (PVL), the predominant pathological substrate underlying cerebral palsy and potentially cognitive deficits in long-term survivors of prematurity, is a lesion of the immature cerebral white matter typically associated with cerebral hypoxia-ischemia with additional contribution from maternofetal infection in some cases [2, 7, 13, 20]. Pathologically, the PVL lesion is composed of a focally necrotic component in the deep periventricular white matter that is characterized by macrophages and axonal spheroids as well as a diffuse component in the surrounding white matter characterized by reactive astrogliosis and activated microglia. Evidence suggests that there is damage to pre-OLs in the diffuse component [14, 38, 41, 42] which likely results in the myelin deficits in long-term survivors of PVL [6, 8, 10, 19, 28]. In addition to OLs, damage has now been shown to include diffuse axonal injury throughout the white matter [16] and gray matter lesions (neuronal loss and/or gliosis) preferentially in the thalamus, basal ganglia, hippocampus, and brainstem [21, 34].
The causes of PVL are complex and likely include cerebral ischemia/reperfusion and maternal/neonatal infection with fetal/neonatal systemic inflammation [7, 13, 20, 38, 41, 42]. Because of the complexity of the pathogenesis, identifying common sources of damage is critical to formulate potential therapeutic treatments. Common to both ischemia/reperfusion and infection/inflammation is the production of reactive nitrogen species, in particular nitric oxide (NO). NO production in excess can be detrimental, particularly in the presence of reactive oxygen species, which are known to be associated with PVL [2, 12, 14]. When NO is produced in the setting of oxidative stress, the highly reactive peroxynitrite is formed, leading to macromolecule damage and the formation of the protein adduct, nitrotyrosine (NT). This adduct formation has been identified by our group in PVL in developing oligodendrocytes [14], white matter neurons [12], and Cajal-Retzius cells of layer I in the cerebral cortex [12]. One of the most potent sources of NO is inducible nitric oxide synthase (iNOS), the synthetic enzyme that produces NO from the terminal nitrogen of l-arginine. This enzyme is known to be upregulated following hypoxic injury in animal models [18, 24] as well as exposure to inflammatory cytokines [4], both thought to play a role in the pathogenesis of PVL [7, 13, 41].
In the following study, we hypothesized that the density of iNOS-immunopositive cells is significantly increased in PVL compared to controls adjusted for age. While we report on findings in both the focal and diffuse lesion, we focused predominantly on the diffuse component rather than the focal necrotic component, because it results in widespread damage, and is therefore the likely basis of the global neurological deficits in long-term survivors of PVL. Using single- and double-label immunocytochemistry (ICC), we found clusters of iNOS-positive macrophages in the periventricular focal necrosis. Within the diffuse component, we found an increase in the density of reactive astrocytes expressing iNOS and a transient increase in activated microglia with iNOS in the diffuse lesion associated with the earliest, acute stage of PVL. These data support an important role for iNOS-induced nitrosative stress as well as a deleterious role of inflammatory cells in the pathogenesis of PVL and subsequent damage to pre-OLs of the white matter.
Materials and methods
Clinicopathologic database information
Cases were collected from the autopsy services of the Departments of Pathology, Children’s Hospital Boston and Brigham and Women’s Hospital, Boston, with parental permission according to Institutional Review Board protocols. Archival, paraformaldehyde-fixed, paraffin-embedded, brain tissue from either the parietal, occipital, or frontal lobes of 15 PVL cases and 16 control cases (i.e., lacking PVL) was used throughout the study. All cases were classified as PVL or control (non-PVL) by the systematic examination of standardized microscopic sections, stained with conventional hematoxylin-and-eosin/Luxol-fast-blue, from each brain and spinal cord by the study neuropathologists (HCK and RDF). As in all studies from our laboratory, PVL was defined as a lesion of the immature cerebral white matter with focal and diffuse components in combination (see above) [3, 14, 16]. We subclassified PVL into three temporal stages based upon histopathologic features of the periventricular focal necrosis: acute PVL was defined as focal periventricular necrosis characterized by coagulative necrosis, cytoplasmic hypereosinophilia and nuclear pyknosis of all tissue elements, and hypereosinophilic axonal spheroids; organizing or subacute PVL with the focal periventricular necrosis characterized by infiltrating macrophages; and chronic PVL characterized by focal cavitation or scar formation with or without mineralization of axons. As determined in the PVL studies from this laboratory [3, 12, 14, 16], the control cases were defined as perinatal deaths in which neuropathologic examination did not reveal PVL; in these cases, there was no or minimal neuropathologic changes in the white and gray matter.
We analyzed iNOS expression in the cerebral white matter of 15 cases of PVL and 16 control cases without PVL. The PVL cases ranged in age from 29 to 51 PC weeks (median of 39 PC weeks) and 0–15 PN weeks (median of 3 PN weeks); the controls ranged from 20 to 144 PC weeks (median of 38 PC weeks) and 0–104 PN weeks (median of 0 PN weeks). The primary causes of death in the PVL group were: complications of prematurity n = 3; congenital heart disease n = 4; Potter sequence n = 2; primary pulmonary hypertension n = 1; urea cycle defect n = 1; disseminated cytomegalic inclusion (CMV)infection n = 1; undiagnosed inborn error of metabolism n = 1; skeletal dysplasia n = 1; and central congenital hypoventilation syndrome n = 1. The causes of death in the control group included: complications of prematurity n = 2; congenital heart disease n = 2; unexplained n = 2; chromosomal anomalies n = 2; pulmonary hypoplasia n = 1; congenital renal dysplasia n = 1; spinal muscular atrophy n = 1; Potter’s sequence n = 1; multiple congenital anomalies n = 1; neonatal immune thrombocytopenia n = 1; primary pulmonary hypertension n = 1; and neonatal liver disease n = 1. Stillbirth cases comprised 7% (1/15) of these PVL cases, i.e., the case with disseminated CMV, and 19% (3/16) of the control cases, i.e., one case each of chromosomal anomalies, congenital heart abnormalities, and unexplained stillbirth; in addition, one case of elective termination (1/16, 6%) (for multiple congenital anomalies) was present in the latter group. The postmortem interval (PMI) ranged from 11 to 24 h for the PVL cases with an outlier at 89 h, and 6–24 h for the control cases with outliers at 36, 48, and 132 h. ANCOVA showed no effect of PMI (P = 0.18) in a model that controlled for diagnosis.
Single-label ICC in paraformaldehyde-fixed, paraffin-embedded tissue
Standard methods in deparaffinized tissue sections (5 μm) were performed as previously reported [14]. A rabbit polyclonal antibody made against a synthetic peptide corresponding to the C-terminus of human iNOS was used at a concentration of 1:250 (Calbiochem, San Diego, CA, USA) [30, 43]. Specificity of this antibody was tested using a peptide synthesized by Department of Biological Chemistry and Molecular Pharmacology Biopolymers Laboratory, Harvard Medical School (Boston, MA, USA) with a sequence corresponding to that which the antibody was made against (YRASLEMSAL). A peptide excess of ten times that of the antibody concentration successfully blocked the antibody staining of tissue. Negative controls omitted the primary antibody.
Grading method for single-label ICC
Grading of the cell density (positive cells/high power field) of single-labeled tissue sections was performed in both PVL and control tissue, as previously reported by us [11, 12, 14]. After a survey of the entire section, immunopositive cells per high power field (hpf) (×40 magnification) were counted in three fields within the most intensely stained area of tissue within the periventricular white matter. The three fields were averaged and given an overall standardization score as follows: 0, no cell staining; 1/2, 0–1 immunopositive cell/hpf; 1, 2–10 cells/hpf; 2, 11–20 immunopositive cells/hpf; and 3, >20 cells/hpf. Only cells with a well-defined nucleus and iNOS-stained cytoplasm were counted as positive. In PVL cases, the quantitated fields were in the diffuse component only; the focal necrotic lesion was not included in any scoring. Two observers (RLH and HCK) scored each case without knowledge of case age. Given the obvious nature of white matter pathology in PVL, it was not possible to score iNOS-immunopositive cells blinded to diagnosis.
Double-label ICC
Tissue was deparaffinized, blocked in PBS containing 5% goat serum and 0.1% Triton X-100 for 1 h at room temperature, and incubated overnight at 4°C in blocking solution containing antibodies to iNOS (1:100) (Calbiochem, San Diego, CA, USA) and either astrocytic marker, glial fibrillary acidic protein (GFAP) (1:8,000) (Sternberger Monoclonal, Lutherville, MD, USA) or reactive microglial marker, CD68 (1:25) (Cell Marque, Austin, TX, USA). After a series of washes, the tissue was incubated in blocking solution containing Alexa Fluor goat anti-rabbit 488 and Alexa Fluor goat anti-mouse 590, both at a concentration of 1:1,000 (Molecular Probes, Eugene, OR, USA).
Quantitation of double-label ICC
Tissue sections from a subset of PVL cases and controls were double-labeled with iNOS and GFAP. After a survey of all fields, double-label images in three different fields within the most intensely stained region of periventricular white matter were taken at x20 power. In each field, red images (GFAP) and green images (iNOS) were merged and printed. From the photographic images GFAP-positive/iNOS-positive co-labeled astrocytes were counted. This number was then divided by counts for all GFAP-positive astrocytes to determine the percentage of reactive astrocytes that were iNOS positive. In PVL cases, only the diffuse lesion was analyzed; no analysis was done in the focal necrotic lesion.
Statistical analysis
Age-adjusted differences in PVL relative to controls were accessed using analysis of covariance (ANCOVA) adjusting for postconceptional (PC), postnatal (PN), and gestational age (GA). To assess diagnostic differences in the percentage of astrocytes that are iNOS positive, a t test was done examining PVL and control cases of overlapping ages (35–54 weeks). Using ANCOVA, we also examined whether there was an effect of age on the percentage of astrocytes that are iNOS positive in PVL and control cases.
Results
Neuropathology of PVL
In the PVL cases, the stage and degree of severity varied, reflecting the typical spectrum of this pathology in our autopsy service. Fifty-three percent (8/15) of the PVL cases had recognizable focal periventricular necrosis at the time of brain cutting. Fifty percent of these lesions (4/8) were described as cystic, ranging in size from 0.75 to 2.2 cm. The remaining 50% of the macroscopic lesions were described as chalky-white foci of necrosis, i.e., the classic so-called “white spots” of PVL, and were typically 2–3 mm in diameter. In the remaining 47% of the total PVL cases in this series (7/15), the necrotic foci were detected only microscopically and varied in size up to approximately 2 mm. Thus, approximately one-half of the necrotic foci were detected upon macroscopic examination at autopsy in this modern day of neonatal care, and one-half were detected microscopically, potentially below the limits of detection by conventional neuroimaging. Germinal matrix hemorrhages of variable severity were identified in 27% (4/15) of the PVL cases and 19% (3/16) of controls, one in the latter group with widespread dissemination throughout the ventricular system.
Expression of iNOS in PVL: focal and diffuse components
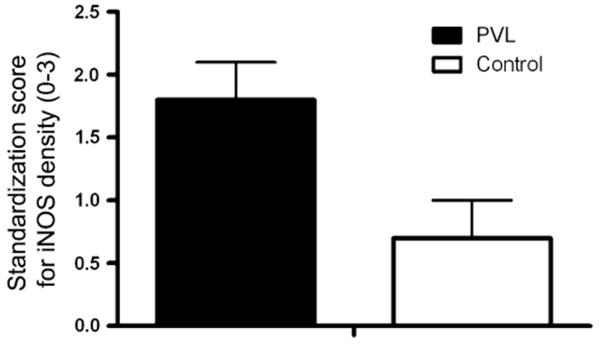
We found iNOS expression in both the focally necrotic and diffusely gliotic components of PVL. In the subacute necrosis with organization, there were clusters of iNOS-positive cells with the morphology of macrophages, i.e., large, round cells with eccentric nuclei (Fig. 1a, b). In the diffuse component, there was extensive iNOS expression in glial cells (Fig. 1c). Using a 0–3 semiquantitative scoring system, in the diffuse component of PVL we found a significant increase in iNOS-positive glia in PVL cases (standardization score of 1.8 ± 0.3) compared to controls (standardization score of 0.7 ± 0.3) controlling for PC age (P = 0.01) (Fig. 2). Results were similar when controlling for PN age or GA.
Fig. 1.
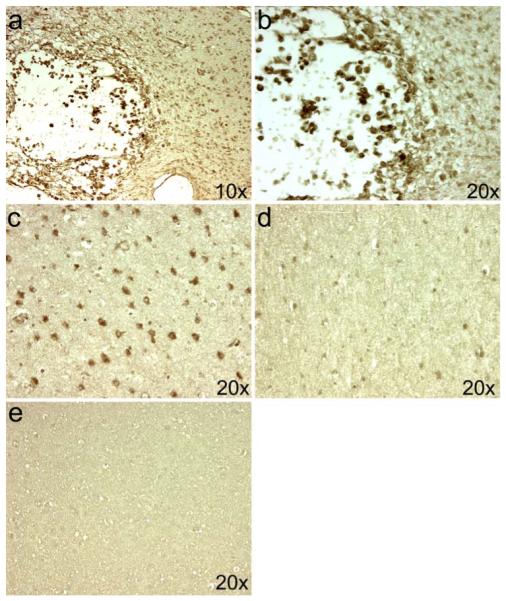
Localization of iNOS expression in PVL. The expression of iNOS is seen in macrophages of the focal necrosis of a subacute PVL lesion in a 39-PC week infant (a, b). In this same case, iNOS expression is seen in the diffusely gliotic white matter surrounding the focal necrosis in cells morphologically identified as reactive astrocytes (c). The number of iNOS-expressing cells is significantly reduced in a control case at 37 PC weeks (d). Binding of the iNOS antibody was blocked in the PVL case from above using an excess of the peptide from which the antibody was produced (e)
Fig. 2.
The expression of iNOS in PVL and controls. The standardization score of iNOS-expressing cells adjusted for PC age is significantly increased in PVL relative to controls (P = 0.01). The score of iNOS-expressing cells adjusted for PN age as well as GA is also significantly increased in PVL relative to controls (data not shown)
Cell-specific expression of iNOS in PVL: diffuse only
To examine cell-specific expression of iNOS, double-label ICC was performed in representative cases of control tissue (n = 11) and PVL tissue with lesions of various ages (n = 12; 5 acute, 6 organizing, 1 chronic). In the diffuse component of PVL, we observed iNOS-positive activated microglia and reactive astrocytes (Fig. 3). We observed that iNOS-positive microglia were predominant in the diffuse component associated with acute lesions [three out five cases (60%) with iNOS-positive microglia] whereas iNOS-negative microglia were predominant in the diffuse component associated with subacute (organizing) lesions [four out of six cases (66%) with iNOS-negative microglia] (Fig. 3). We also observed that iNOS-positive reactive astrocytes were present at all stages of PVL (Fig. 3) and in control tissue to a much lesser degree (see below). To further characterize the colocalization of iNOS and GFAP in PVL and control tissue, we determined the percentage of GFAP-positive reactive astrocytes that colocalize with iNOS in 5 PVL cases (35–51 PC weeks) and 11 controls (23–90 PC weeks) (Fig. 4). In the cases with overlapping PC age (35–51 PC weeks), we found no difference in the percentage of reactive astrocytes expressing iNOS in the PVL cases (86.4% ± 3.17) compared to controls (89.0% ± 7.7) (P = 0.60) (Fig. 4). However, when all cases were examined, there was a significant quadratic effect of age in controls (P < 0.001) with control cases younger than 35 PC weeks (n = 3) and older than 54 PC weeks (n = 1) showing a decreased percentage of astrocytes that were iNOS-positive. It is important to note that while there was no difference in the percentage of reactive astrocytes that were iNOS positive between PVL and controls in the age range of 35–54 PC weeks, there is a significant increase in the number of reactive astrocytes in PVL relative to controls (data previously shown [14]).
Fig. 3.
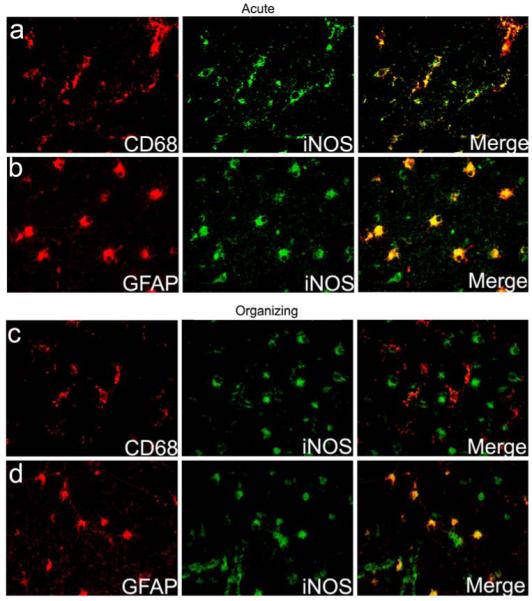
Cell-specific expression of iNOS in the diffuse component of PVL. The expression of iNOS is seen in CD68-positive microglia (a) as well as in GFAP-positive reactive astrocytes (b) in the diffuse component of an acute PVL case at 40 PC weeks. A PVL case of the subacute (organizing) phase at 39 PC weeks shows no iNOS expression in CD68-positive microglia (c), but continues to show strong iNOS expression in GFAP-positive reactive astrocytes (d)
Fig. 4.
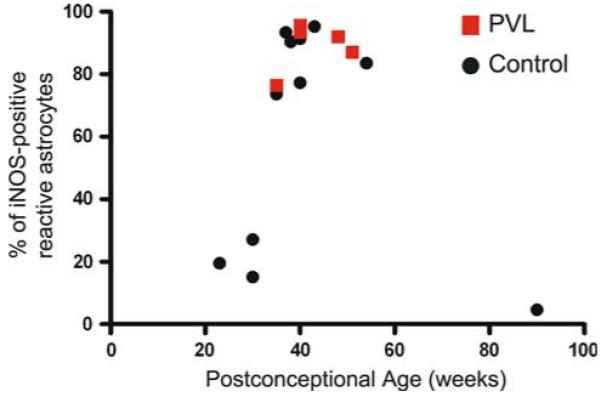
Astrocytic expression of iNOS in PVL and controls. The percentage of GFAP-positive reactive astrocytes that express iNOS was determined in the diffuse component of PVL and in the corresponding periventricular white matter of control cases. In the overlapping ages of 35–54 PC weeks, there is no significant difference in the percentage of GFAP-positive astrocytes that express iNOS, although the overall density of iNOS positive glia (Fig. 2) as well as GFAP positive astrocytes [16] in control tissue is significantly less than in PVL
Discussion
Our major finding in this study is that the density of iNOS-positive cells is increased primarily in reactive astrocytes throughout all temporal stages of PVL in the diffuse component relative to controls. Our finding supports reports of increased iNOS mRNA in PVL [22]. Yet, it expands upon the mRNA finding by demonstrating a significant upregulation of protein which is important given the evidence of post-transcriptional regulation of human iNOS mRNA stability [25, 33]. In addition, our data provide evidence of a temporal cell-specific pattern of iNOS, which appears to be associated with the specific stage of PVL and implicates iNOS-expressing activated microglia early in development of the PVL lesion. Below we discuss the significance of each of our findings with an emphasis upon the relevance to pre-OL nitrosative injury previously reported by us.
Increase in iNOS expression in PVL
In our study, iNOS expression was found in macrophages of the focal necrotic lesion as well as astrocytes and activated microglia in the diffuse component. Upregulation of iNOS protein in each of these cell types has been reported previously in animal models of hypoxia [18, 24], ischemic infarcts in the developing human brain [1] as well as other diverse adult neurological disorders such as multiple sclerosis [27, 32] and Alzheimer’s disease [23, 44]. The expression of iNOS in the diffuse region surrounding the focal lesion in PVL is consistent with the presence of NT adducts found by us in this same region [14]. In our previous study, we found NT adducts in the pre-OLs of the diffuse white matter injury, indicating nitrosative damage, which could potentially result in OL damage, loss, or dysfunction, particularly given the vulnerability of pre-OLs to peroxynitrite, the reaction product of NO and superoxide anion [26]. Our finding of iNOS upregulation in PVL in reactive astrocytes, macrophages, and activated microglia suggests that iNOS is the source of nitrosative stress in PVL and specifically the source of nitrosative damage in pre-OLs.
Cell-specific expression of iNOS in PVL
In the PVL cases, we examined stages of lesion progression characterized by sequential histopathologic features. Interestingly, we found differences in the cell-specific expression of iNOS in the diffuse component depending on the associated age of the focal lesion. Regardless of the stage of the lesion, reactive astrocytes expressed iNOS. In the earliest stage of the lesion, the acute stage, activated microglia were predominantly iNOS-positive. This differed from PVL cases of an organizing or subacute stage in which activated microglia were predominantly iNOS-negative. These findings suggest two possibilities: (1) microglial activation with iNOS upregulation in the absence of astrogliosis may represent the earliest response to an insult and possibly the earliest lesion of PVL; and (2) in PVL, there exist two waves of iNOS expression with an early and transient expression of iNOS upregulation by activated microglia, followed by a second, more stable wave of expression by reactive astrocytes. A feature of human pathological research is that we only see “snap-shots” of the disease process, i.e., the analysis of ongoing events at precisely timed intervals is rarely possible. However, animal models allow for elucidation of the timing and are necessary to address these questions. In support of the idea that microglial activation represents the earliest lesion, an animal model of cerebral hypoxia-ischemia in the immature rat shows the appearance of activated microglia within 4 h of hypoxic insult followed by the appearance of reactive astrocytes at 24 h [39]. In support of the transient expression of iNOS in microglia is a report in a postnatal rat model of iNOS expression appearing within 3 h of hypoxia and then undetectable by 15 h [45]. This finding differs from that of astrocytic expression of iNOS, which has been observed in models of transient ischemia from 3 days to 1 month following the initial insult [9, 31]. These considerations raise the possibility that microglial activation in the absence of reactive astrocytes represents the earliest pathological change in the white matter surrounding early coagulation of tissue in the focal component of PVL.
Expression of iNOS in reactive astrocytes
We detected iNOS predominantly in reactive astrocytes of the diffuse lesion in association with all ages of focal necrosis as well as in the periventricular white matter of controls, although to a much lesser degree. When we examine the percentage of iNOS-positive, GFAP-expressing astrocytes in both PVL and controls at an age spanning the period of term birth, we see no difference between PVL and controls, with approximately 87% of astrocytes expressing iNOS in both groups. Of note, we have previously shown that GFAP-expressing astrocytes are significantly increased in PVL relative to controls in the diffuse lesion [14]. Thus, while a majority of reactive astrocytes at term express iNOS, there is an overall significant increase in reactive astrocytes in PVL also expressing iNOS. As a consequence, overall levels of iNOS expression in PVL appear to be significantly greater than control levels of iNOS in astrocytes in the developing white matter.
The question arises as to the significance and role of the physiological presence of reactive astrocytes and of iNOS expression in reactive astrocytes in normal development given that reactive astrocytes are present in our control cases, albeit to a much lesser degree than our PVL cases, and that 86% of astrocytes expressed iNOS from 35 to 54 PC weeks, a critical period of brain growth and maturation. While our control tissue presents the caveats listed below, the presence and significance of physiological as opposed to pathological gliosis, both defined here by the increased expression of GFAP in human development and pathology remains to be determined. There is evidence in developing kittens, however, of a high density of GFAP-positive astrocytes in the white matter during the first three postnatal weeks which diminishes thereafter [29, 36, 37]. It was suggested that these astrocytes play a role in the development and guidance of axons to their specific cortical destination in this animal model [36]. In this regard, NO plays a stimulatory role in neurite outgrowth [17] as well as in growth cone development [5, 35, 40]. While speculative, a potential role for astrocytic iNOS expression and subsequent NO production in axonal growth in human development is supported by the fact that axonal growth, as determined by the expression of the growth cone protein GAP43, continues through the period of term birth and decreases thereafter [15], a pattern similar to astrocytic iNOS expression. The precise equivalent time-periods, however, between the developing kitten and human infant are unknown, thereby warranting caution when comparing the kitten data to human development.
Potential limitation of the study
An unavoidable limitation of this study involves the nature of control brain tissue from autopsied infants in the perinatal brain. All infants dying in the preterm age range have disease processes that involve terminal hypoxia-ischemia that may affect the brain. Despite the complexity in systemic disease processes and agonal conditions in our control population, there was no significant cerebral white matter pathology on standard histologic examination underscoring the appropriateness of their use for baseline analysis.
Conclusion
Our data provide evidence of not only a pathologic source of nitrosative stress in PVL, i.e., reactive astrocytes and activated microglia, but also a potential physiological role for iNOS-produced NO in normative development. The concept that physiological levels of iNOS and NO play a role in development and that this role switches to a detrimental one when produced in excess in an adverse environment, triggered in the case of PVL by ischemia/reperfusion, the presence of oxidative stress, and subsequent peroxynitrite and nitrotyrosine production, is a major underlying theme in perinatal brain damage.
Acknowledgments
The authors thank Mr. Richard A. Belliveau for assistance in this study. This research was supported by grants from the National Institute of Neurological Diseases and Stroke (PO1-NS38475) (HCK, JJV), National Institute of Child Health and Development (Children’s Hospital Developmental Disabilities Research Center) (P30-HD18655), and the William Randolph Hearst Award.
Abbreviations
- ANCOVA
Analysis of covariance
- GA
Gestational
- GFAP
Glial fibrillary acidic protein
- ICC
Immunocytochemisty
- iNOS
Inducible nitric oxide synthase
- NO
Nitric oxide
- NT
Nitrotyrosine
- PC
Postconceptional
- PMI
Postmortem interval
- PN
Postnatal
- pre-OL
Premyelinating oligodendrocyte
- PVL
Periventricular leukomalacia
Contributor Information
Robin L. Haynes, Departments of Pathology, Children’s Hospital Boston, 300 Longwood Ave, Enders 1109, Boston, MA 02115, USA; Harvard Medical School, Boston, MA 02115, USA
Rebecca D. Folkerth, Departments of Pathology, Children’s Hospital Boston, 300 Longwood Ave, Enders 1109, Boston, MA 02115, USA; Brigham and Women’s Hospital, Boston, MA 02115, USA
Felicia L. Trachtenberg, New England Research Institutes, Watertown, MA 02472, USA
Joseph J. Volpe, Harvard Medical School, Department of Neurology, Children’s Hospital Boston, Boston, MA 02115, USA
Hannah C. Kinney, Departments of Pathology, Children’s Hospital Boston, 300 Longwood Ave, Enders 1109, Boston, MA 02115, USA; Harvard Medical School, Boston, MA 02115, USA
References
- 1.Askalan R, Deveber G, Ho M, Ma J, Hawkins C. Astrocytic-inducible nitric oxide synthase in the ischemic developing human brain. Pediatr Res. 2006;60:687–692. doi: 10.1203/01.pdr.0000246226.89215.a6. doi:10.1203/01.pdr.0000246226.89215.a6. [DOI] [PubMed] [Google Scholar]
- 2.Back SA, Luo NL, Mallinson RA, et al. Selective vulnerability of preterm white matter to oxidative damage defined by F2-isoprostanes. Ann Neurol. 2005;58:108–120. doi: 10.1002/ana.20530. doi:10.1002/ana.20530. [DOI] [PubMed] [Google Scholar]
- 3.Billiards SS, Haynes RL, Folkerth RD, et al. Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathol. 2008;18:153–163. doi: 10.1111/j.1750-3639.2007.00107.x. doi:10.1111/j.1750-3639.2007.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown GC. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem Soc Trans. 2007;35:1119–1121. doi: 10.1042/BST0351119. doi:10.1042/BST0351166. [DOI] [PubMed] [Google Scholar]
- 5.Contestabile A. Roles of NMDA receptor activity and nitric oxide production in brain development. Brain Res Brain Res Rev. 2000;32:476–509. doi: 10.1016/s0165-0173(00)00018-7. doi:10.1016/S0165-0173(00)00018-7. [DOI] [PubMed] [Google Scholar]
- 6.Counsell SJ, Allsop JM, Harrison MC, et al. Diffusion-weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics. 2003;112:1–7. doi: 10.1542/peds.112.1.1. doi:10.1542/peds.112.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Dammann O, Kuban KC, Leviton A. Perinatal infection, fetal inflammatory response, white matter damage, and cognitive limitations in children born preterm. Ment Retard Dev Disabil Res Rev. 2002;8:46–50. doi: 10.1002/mrdd.10005. doi:10.1002/mrdd.10005. [DOI] [PubMed] [Google Scholar]
- 8.De Vries LS, Connell JA, Dubowitz LM, Oozeer RC, Dubowitz V, Pennock JM. Neurological, electrophysiological and MRI abnormalities in infants with extensive cystic leukomalacia. Neuropediatrics. 1987;18:61–66. doi: 10.1055/s-2008-1052453. doi:10.1055/s-2008-1052453. [DOI] [PubMed] [Google Scholar]
- 9.Endoh M, Maiese K, Wagner J. Expression of the inducible form of nitric oxide synthase by reactive astrocytes after transient global ischemia. Brain Res. 1994;651:92–100. doi: 10.1016/0006-8993(94)90683-1. doi:10.1016/0006-8993(94)90683-1. [DOI] [PubMed] [Google Scholar]
- 10.Flodmark O, Lupton B, Li D, et al. MR imaging of periventricular leukomalacia in childhood. AJR Am J Roentgenol. 1989;152:583–590. doi: 10.2214/ajr.152.3.583. [DOI] [PubMed] [Google Scholar]
- 11.Folkerth RD, Keefe RJ, Haynes RL, Trachtenberg FL, Volpe JJ, Kinney HC. Interferon-gamma expression in periventricular leukomalacia in the human brain. Brain Pathol. 2004;14:265–274. doi: 10.1111/j.1750-3639.2004.tb00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folkerth RD, Trachtenberg FL, Haynes RL. Oxidative injury in the cerebral cortex and subplate neurons in periventricular leukomalacia. J Neuropathol Exp Neurol. 2008;67:677–686. doi: 10.1097/NEN.0b013e31817e5c5e. doi:10.1097/NEN.0b013e31817e5c5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. MRDD Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 14.Haynes RL, Folkerth RD, Keefe RJ, et al. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol. 2003;62:441–450. doi: 10.1093/jnen/62.5.441. [DOI] [PubMed] [Google Scholar]
- 15.Haynes RL, Borenstein NS, Desilva TM, et al. Axonal development in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2005;484:156–167. doi: 10.1002/cne.20453. doi:10.1002/cne.20453. [DOI] [PubMed] [Google Scholar]
- 16.Haynes RL, Billiards SS, Borenstein NS, Volpe JJ, Kinney HC. Diffuse axonal injury in periventricular leukomalacia as determined by apoptotic marker fractin. Pediatr Res. 2008;63:656–661. doi: 10.1203/PDR.0b013e31816c825c. doi:10.1203/PDR.0b013e31816c825c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hindley S, Juurlink BH, Gysbers JW, Middlemiss PJ, Herman MA, Rathbone MP. Nitric oxide donors enhance neurotrophin-induced neurite outgrowth through a cGMP-dependent mechanism. J Neurosci Res. 1997;47:427–439. doi:10.1002/(SICI)1097-4547(19970215)47:4<427::AID-JNR8>3.0.CO;2-G. [PubMed] [Google Scholar]
- 18.Ikeno S, Nagata N, Yoshida S, Takahashi H, Kigawa J, Terakawa N. Immature brain injury via peroxynitrite production induced by inducible nitric oxide synthase after hypoxia-ischemia in rats. J Obstet Gynaecol Res. 2000;26:227–234. doi: 10.1111/j.1447-0756.2000.tb01316.x. [DOI] [PubMed] [Google Scholar]
- 19.Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr. 2003;143:171–179. doi: 10.1067/S0022-3476(03)00357-3. doi:10.1067/S0022-3476(03)00357-3. [DOI] [PubMed] [Google Scholar]
- 20.Kadhim H, Tabarki B, Verellen G, De Prez C, Rona A-M, Sebire G. Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology. 2001;56:1278–1284. doi: 10.1212/wnl.56.10.1278. [DOI] [PubMed] [Google Scholar]
- 21.Kadhim H, Tabarki B, De Prez C, Sebire G. Cytokine immunoreactivity in cortical and subcortical neurons in periventricular leukomalacia: are cytokines implicated in neuronal dysfunction in cerebral palsy? Acta Neuropathol. 2003;105:209–216. doi: 10.1007/s00401-002-0633-6. [DOI] [PubMed] [Google Scholar]
- 22.Kadhim H, Khalifa M, Deltenre P, Casimir G, Sebire G. Molecular mechanisms of cell death in periventricular leukomalacia. Neurology. 2006;67:293–299. doi: 10.1212/01.wnl.0000224754.63593.c4. doi:10.1212/01.wnl.0000224754.63593.c4. [DOI] [PubMed] [Google Scholar]
- 23.Katsuse O, Iseki E, Kosaka K. Immunohistochemical study of the expression of cytokines and nitric oxide synthases in brains of patients with dementia with Lewy bodies. Neuropathology. 2003;23:9–15. doi: 10.1046/j.1440-1789.2003.00483.x. doi:10.1046/j.1440-1789.2003.00483.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaur C, Sivakumar V, Ang LS, Sundaresan A. Hypoxic damage to the periventricular white matter in neonatal brain: role of vascular endothelial growth factor, nitric oxide and excitotoxicity. J Neurochem. 2006;98:1200–1216. doi: 10.1111/j.1471-4159.2006.03964.x. doi:10.1111/j.1471-4159.2006.03964.x. [DOI] [PubMed] [Google Scholar]
- 25.Korhonen R, Linker K, Pautz A, Forstermann U, Moilanen E, Kleinert H. Post-transcriptional regulation of human inducible nitric-oxide synthase expression by the Jun N-terminal kinase. Mol Pharmacol. 2007;71:1427–1434. doi: 10.1124/mol.106.033449. doi:10.1124/mol.106.033449. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci USA. 2005;102:9936–9941. doi: 10.1073/pnas.0502552102. doi:10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu JS, Zhao ML, Brosnan CF, Lee SC. Expression of inducible nitric oxide synthase and nitrotyrosine in multiple sclerosis lesions. Am J Pathol. 2001;158:2057–2066. doi: 10.1016/S0002-9440(10)64677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maalouf EF, Duggan PJ, Counsell S, et al. Comparison of findings on cranial ultrasound and magnetic resonance imaging in preterm infants. Pediatrics. 2001;107:719–727. doi: 10.1542/peds.107.4.719. doi:10.1542/peds.107.4.719. [DOI] [PubMed] [Google Scholar]
- 29.Muller CM. Astrocytes in cat visual cortex studied by GFAP and S-100 immunocytochemistry during postnatal development. J Comp Neurol. 1992;317:309–323. doi: 10.1002/cne.903170308. doi:10.1002/cne.903170308. [DOI] [PubMed] [Google Scholar]
- 30.Nicholson S, Bonecini-Almeida Mda G, Lapa e Silva JR, et al. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996;183:2293–2302. doi: 10.1084/jem.183.5.2293. doi:10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niwa M, Inao S, Takayasu M, et al. Time course of expression of three nitric oxide synthase isoforms after transient middle cerebral artery occlusion in rats. Neurol Med Chir (Tokyo) 2001;41:63–73. doi: 10.2176/nmc.41.63. doi:10.2176/nmc.41.63. [DOI] [PubMed] [Google Scholar]
- 32.Oleszak EL, Zaczynska E, Bhattacharjee M, Butunoi C, Legido A, Katsetos CD. Inducible nitric oxide synthase and nitrotyrosine are found in monocytes/macrophages and/or astrocytes in acute, but not in chronic, multiple sclerosis. Clin Diagn Lab Immunol. 1998;5:438–445. doi: 10.1128/cdli.5.4.438-445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pautz A, Linker K, Hubrich T, Korhonen R, Altenhofer S, Kleinert H. The polypyrimidine tract-binding protein (PTB) is involved in the post-transcriptional regulation of human inducible nitric oxide synthase expression. J Biol Chem. 2006;281:32294–32302. doi: 10.1074/jbc.M603915200. doi:10.1074/jbc.M603915200. [DOI] [PubMed] [Google Scholar]
- 34.Pierson CR, Folkerth RD, Billiards SS, et al. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. 2007;114:619–631. doi: 10.1007/s00401-007-0295-5. doi:10.1007/s00401-007-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renteria RC, Constantine-Paton M. Exogenous nitric oxide causes collapse of retinal ganglion cell axonal growth cones in vitro. J Neurobiol. 1996;29:415–428. doi: 10.1002/(SICI)1097-4695(199604)29:4<415::AID-NEU1>3.0.CO;2-B. doi:10.1002/(SICI)1097-4695(199604)29:4<415::AID-NEU1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 36.Rochefort N, Quenech’du N, Watroba L, Mallat M, Giaume C, Milleret C. Microglia and astrocytes may participate in the shaping of visual callosal projections during postnatal development. J Physiol (Paris) 2002;96:183–192. doi: 10.1016/s0928-4257(02)00005-0. doi:10.1016/S0928-4257(02)00005-0. [DOI] [PubMed] [Google Scholar]
- 37.Rochefort N, Quenech’du N, Ezan P, Giaume C, Milleret C. Postnatal development of GFAP, connexin43 and connexin30 in cat visual cortex. Brain Res Dev Brain Res. 2005;160:252–264. doi: 10.1016/j.devbrainres.2005.09.011. doi:10.1016/j.devbrainres.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 38.Shah DK, Doyle LW, Anderson PJ, et al. Adverse neuro-development in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008;153:170–175. doi: 10.1016/j.jpeds.2008.02.033. doi:10.1016/j.jpeds.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 39.Towfighi J, Zec N, Yager J, Housman C, Vannucci RC. Temporal evolution of neuropathologic changes in an immature rat model of cerebral hypoxia: a light microscopic study. Acta Neuropathol. 1995;90:375–386. doi: 10.1007/BF00315011. doi:10.1007/BF00315011. [DOI] [PubMed] [Google Scholar]
- 40.Van Wagenen S, Rehder V. Regulation of neuronal growth cone filopodia by nitric oxide depends on soluble guanylyl cyclase. J Neurobiol. 2001;46:206–219. doi: 10.1002/1097-4695(20010215)46:3<206::aid-neu1003>3.3.co;2-j. doi:10.1002/1097-4695(20010215)46:3<206::AID-NEU1003>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 41.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553–562. doi: 10.1203/00006450-200111000-00003. doi:10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Volpe JJ. Postnatal sepsis, necrotizing entercolitis, and the critical role of systemic inflammation in white matter injury in premature infants. J Pediatr. 2008;153:160–163. doi: 10.1016/j.jpeds.2008.04.057. doi:10.1016/j.jpeds.2008.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang MX, Murrell DF, Szabo C, Warren RF, Sarris M, Murrell GA. Nitric oxide in skeletal muscle: inhibition of nitric oxide synthase inhibits walking speed in rats. Nitric Oxide. 2001;5:219–232. doi: 10.1006/niox.2001.0348. doi:10.1006/niox.2001.0348. [DOI] [PubMed] [Google Scholar]
- 44.Wong A, Luth HJ, Deuther-Conrad W, et al. Advanced glycation endproducts co-localize with inducible nitric oxide synthase in Alzheimer’s disease. Brain Res. 2001;920:32–40. doi: 10.1016/s0006-8993(01)02872-4. doi:10.1016/S0006-8993(01)02872-4. [DOI] [PubMed] [Google Scholar]
- 45.You Y, Kaur C. Expression of induced nitric oxide synthase in amoeboid microglia in postnatal rats following an exposure to hypoxia. Neurosci Lett. 2000;279:101–104. doi: 10.1016/s0304-3940(99)00967-2. doi:10.1016/S0304-3940(99)00967-2. [DOI] [PubMed] [Google Scholar]