Abstract
Infantile spasms (IS) is a devastating epilepsy syndrome of childhood. They occur in 3-12 month old infants and are characterized by spasms, interictal EEG hypsarrhythmia and profound mental retardation. Hormonal therapy (ACTH, corticosteroids) are frequently used, their efficacy is however tainted by severe side effects. For research of novel therapies, a validated animal model of IS is required. We propose the model of spastic seizures triggered by NMDA in infant rats prenatally exposed to betamethasone. The spasms have remarkable similarity to human IS, including motor flexion spasms, ictal EEG electrodecrement, and responsiveness to ACTH. Interestingly, the spasms do not involve the hippocampus. Autoradiographic metabolic mapping as well as tagging the areas of neuronal excitation with c-fos indicate a strong involvement of hypothalamic structures such as the arcuate nucleus, which has significant bilateral connections with other hypothalamic nuclei as well as with the brainstem.
Infantile spasms (IS) represent one of the most devastating epileptic syndromes of infancy. IS are characterized by brief spasms, specific ictal and interictal EEG patterns, and with frequent subsequent cognitive deterioration (Dulac, et al. 2002). IS usually appear in clusters (Kellaway, et al. 1979, Plouin, et al. 1993), involving flexion of the neck and upper body, with upper limb adduction; in some cases also extension or mixed spasms of flexor/extensor spasms occur. In the EEG, the spasms are usually associated with a generalized voltage attenuation (i.e., electrodecremental response), sometimes with an epileptic afterdischarge (Frost & Hrachovy 2005). Between the spasms (interictally) the EEG pattern of hypsarrhythmia consists of high-voltage, disorganized discharges with superimposed multifocal spikes and slow waves (Dulac, et al. 2002).
IS develop between 3-12 months of age with peak occurrence at 6 months. The incidence is approximately 1 case per 3225 live births with a slight predominance (60%) in males (Hrachovy & Frost 2003). This translated into global numbers means that every year in the USA alone, there are about 1400 NEW cases of IS, i.e., children who newly require diagnosis and treatment. Worldwide, there are more than 45 000 new cases of IS per year. Clinical studies show that although the IS may spontaneously remit between 12-24 months of age (Hrachovy, et al. 1991), about 85% of patients become mentally retarded and 67% suffer from intractable epilepsy despite the treatment (Dulac, et al. 2002, Hrachovy & Frost 2003) with emergence of new seizure types (including partial seizures without or with secondary generalization) (Caplan, et al. 2002, Riikonen 1982). IS are associated with a significant risk of mortality. A large follow-up study (Riikonen 1982) has demonstrated that about 31% of the patients died during the follow-up period, many in the first 3 years of life. Moreover, 45% of the survivors were mentally retarded.
IS can be classified into symptomatic or idiopathic/cryptogenic groups. Symptomatic IS are considered a consequence of a known CNS disorder while in the idiopathic/cryptogenic group brain imaging does not show any abnormalities (Okumura, et al. 1998). Control of cryptogenic IS may be sometimes obtained with medication using ACTH or vigabatrin (Baram, et al. 1996, Sankar 2004). However, these drugs have serious side effects sometimes even resulting in death. Side effects as well as the enormous cost burden of the therapy by ACTH are significant. The majority of children on ACTH develops cushingoid obesity, becomes very irritable, has arterial hypertension, electrolyte imbalance, gastric ulcer, growth retardation, cardiomyopathy, and immunosuppression. These serious side effects may occur in up to 43% of treated children (Riikonen & Donner 1979). About 30% of IS patients, who died by age 3, died of complications of ACTH therapy (Riikonen 2001). Some patients with cryptogenic IS may be refractory to ACTH treatment; alternative treatments include corticosteroids (prednisone), benzodiazepines, and valproate, yet all these are less effective compared to ACTH (Baram, et al. 1996, Lux, et al. 2005, Mackay, et al. 2004). These drugs have also significant side effects.
For discovery and development of new effective therapeutic approaches with fewer and less severe side effects, appropriate animal models of IS should be employed. However, animal models of the human IS have been especially difficult to generate because of multiple etiologies of IS (Stafstrom, et al. 2006). In our studies we focused on the development of a model of cryptogenic IS.
Since 1992, when we described age-specific flexion spasms in infant rats after systemic administration of N-methyl-D-aspartic acid (NMDA) (Mareš & Velíšek 1992), we have been working on the development of a model of IS. That study and our further work (Kábová, et al. 1999) demonstrated that NMDA-triggered spasms have the following features: (a) Flexion spasms occurring in clusters. Each of the spasms lasts several seconds and the spasms are separated by periods of normal or almost normal behavior such as walking. Clustered flexion spasms represent the most common seizure type in human IS. (b) Age-specific occurrence equivalent to rat’s infancy. By creating the NMDA dose response, we were able to induce flexion spasms in 7-18 day old rats (P7-18). The spasms were extremely rarely seen in P25 rats and never seen in older rats, yet the rats developed other seizure types following the NMDA administration (Mareš & Velíšek 1992). Thus, similarly to human IS (in older children, the spasms change to another seizure type), there is a switch in the NMDA-induced seizure phenotype after the early developmental period. (c) Ictal EEG electrodecrement associated with the occurrence of flexion spasms. Additionally, between the spasms, large-amplitude asynchronous (chaotic) waves were recorded in the EEG that may represent a rat type of interictal hypsarrhythmia. (d) We (Kábová, et al. 1999) and others (Stafstrom & Sasaki-Adams 2003) have shown learning deterioration in rats experiencing infantile NMDA flexion spasms, correlating with the mental retardation in human IS.
However, while examining the efficacy of antiepileptic treatments used in human IS, we found that only pyridoxine (vitamin B6) in very high doses and valproate (only in P18 rats) were effective against flexion spasms (Kábová, et al. 1999). ACTH, hydrocortisone or clonazepam were without any effect (Kábová, et al. 1999, Velíšek, et al. 2007), thus arguing significantly against the validity of this original model, especially for testing new anticonvulsant strategies against IS.
Based on the hypothesis that infants with IS may exhibit malfunction of the HPA axis - supported by clinical findings of decreased ACTH and cortisol in the CSF as well as by ACTH efficacy (Baram, et al. 1995, Nagamitsu, et al. 2001), we developed a new model of IS using postnatal NMDA administration in rats with pre-existing compromised HPA axis function (Velíšek, et al. 2007). Indeed, prenatal exposure to synthetic corticosteroids (dexamethasone or betamethasone) during the last week of rat pregnancy is associated with altered expression of glucocorticoid receptors in the brain (Velíšek 2005, Welberg, et al. 2001). Additionally, prenatal betamethasone exposure in guinea pigs decreases postnatal ACTH and corticosterone levels (Owen & Matthews 2003) replicating thus findings in children with IS.
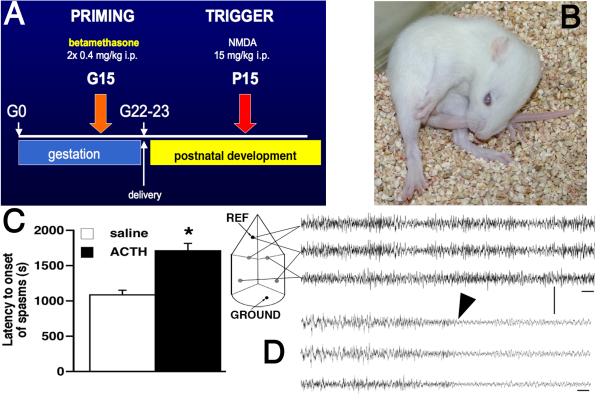
Therefore, we exposed pregnant rats to two doses of betamethasone on gestational day 15 (G15; 2× 0.4 mg/kg i.p. 10 hours apart). The offspring were challenged with NMDA during the appropriate developmental period (P10-P15, most frequently P15; Figure 1A). This combination of prenatal brain impairment and postnatal trigger resulted in a novel model of cryptogenic/idiopathic IS (Velíšek, et al. 2007) with the following unique features:
Similarly to the original model, the P15 rats developed flexion spasms consisting of hyperflexion of both hind and front limbs, head and neck, often including the tail (Figure 1B). Each spasm lasted several seconds and the spasms occurred repeatedly. The period in between spasms was filled with excessive orientation behaviors involving sniffing and exploration, walking, or aimless running in the cage.
In contrast to the original model, the spasms became sensitive to treatment with ACTH. After acute i.p. pretreatment with 0.1 mg/kg of ACTH (15-20 IU/kg), there was a significant delay to onset of NMDA-induced spasms compared to rats receiving saline instead of ACTH (Figure 1C). It should be noted that ACTH treatment did not alter the character of spasms as illustrated in Figure 1B.
-
We reproduced our EEG findings in younger, P12 rats. The EEG during baseline recordings contained lots of fast activity (Figure 1D). During the spasms there were invariably very low amplitude waveforms consistent with ictal EEG electrodecrement (Figure 1D).
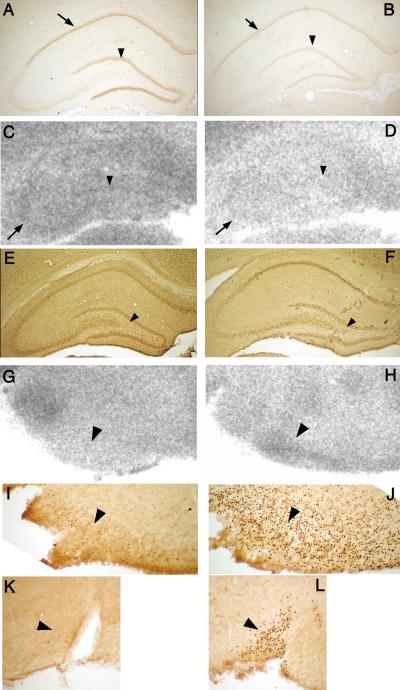
We were further interested, which brain structures are involved in the generation of NMDA-induced flexion spasms in the prenatally compromised brain. For this we determined glucose uptake using metabolic mapping with [14C]2-deoxyglucose (2DG) autoradiography and investigated patterns of neuronal excitation marked by c-fos expression (determined by immunohistochemistry). Because our initial studies indicated that compared to prenatal saline exposure (Figure 2A) prenatal priming with betamethasone decreased hippocampal expression of glucocorticoid receptors (GR; Figure 2B), we investigated 2DG uptake in the hippocampus (Velíšek, et al. 2007). In the following studies, only rats prenatally exposed to betamethasone were used. These rats were either experiencing 45 min of NMDA-triggered spasms or were injected with saline instead of NMDA and were investigated at matched time points (controls).
NMDA spasms did not facilitate glucose uptake in the dorsal hippocampus. Compared to betamethasone-exposed saline-injected controls (Figure 2C), we found significantly decreased hippocampal 2DG uptake after NMDA-induced spasms in betamethasone-exposed rats (Figure 2D).
This finding was confirmed by c-fos expression: In controls (betamethasone exposure, saline injection; Figure 2E), there were some c-fos immunopositive cells especially in the hippocampal hilus. Two hours after NMDA-induced spasms in betamethasone-exposed rats, there was almost no c-fos immunopositivity in the hippocampus (Figure 2F) consistent with decreased activity of hippocampal neurons.
Therefore, we visually inspected 2DG autoradiograms for possible increases in 2DG uptake. We found that hypothalamic structures were very responsive to NMDA-induced spasms. We identified that in the arcuate nucleus of the hypothalamus compared to controls (Figure 2G), there was a significant increase in 2DG uptake after NMDA-induced spasms (Figure 2H).
This was supported by a c-fos study. In contrast to control rats with only several c-fos positive cells in the arcuate nucleus (Figure 2I), the rats after NMDA-induced spasms had the arcuate nucleus packed with c-fos immunopositive cells (Figure 2J) suggesting excessive excitation within this structure.
Additional hypothalamic structures were also involved as further suggested by c-fos expression. For example, the supraoptic nucleus in controls contained no c-fos immunopositive cells (Figure 2K), while two hours after NMDA-spasms, this structure contained many c-fos positive cells (Figure 2L).
Figure 1.
(A) Schedule of the prenatal priming with betamethasone and postnatal triggering of the spasms with NMDA during rat infancy. (B) Phenotype of the spasms in a P15 rat. All limbs are in hyperflexion, including head and neck, as well as the tail. Each spasm lasts for several seconds and the spasms occur in clusters. (C) The model of flexion spasms in prenatally betamethasone-primed brain is sensitive to ACTH treatment: Administration of 0.1 mg/kg ACTH (15-20 IU/kg) one hour prior to the NMDA challenge significantly delayed the onset of spasms triggered by NMDA compared to saline injection. No such effect of ACTH was observed in prenatally saline-exposed rats. (D) EEG recording in an infant, P12, rat after prenatal priming with betamethasone. The scheme on the left indicates the electrode arrangements. Top three traces are baseline recordings prior to the NMDA injection. Bottom three traces show electrodecrement during a flexion spasm (onset marked with an arrowhead) after NMDA trigger. Time base 1 s, calibration 200 μV; band pass filter at 3-70 Hz.
Figure 2.
A In controls prenatally exposed to saline, there is significant expression of glucocorticoid receptors in the neurons of hippocampal CA1 (arrow) and dentate gyrus granule cells (arrowhead). (B) After prenatal betamethasone exposure, there is a significant decrease in the glucocorticoid receptor expression similar to previous findings after prenatal dexamethasone (Welberg, et al. 2001). (C) 2DG uptake in the hippocampus in prenatally betamethasone-exposed infant rats without experience of the NMDA-triggered spasms (saline-injected controls). CA3 pyramidal neuron bend (arrow) can be clearly distinguished as well as the buried blade of the dentate gyrus granule cells (arrowhead). (D) The 2DG uptake in the hippocampus after prenatal betamethasone exposure at 45 min after the NMDA-triggered spasms. There is general decrease in 2DG uptake indicative of decreased metabolic activity. While the CA3 bend can still be distinguished, granule cells are blending with the background. (E) There are few c-fos immunopositive cells in hilus of the hippocampus (arrowhead) after prenatal betamethasone exposure in a rat not experiencing the spasms (injected with saline). (F) In a prenatally betamethasone exposed rat, two hours after the NMDA triggered spasms, there are no c-fos immunopositive cells in the area, speaking thus against excitation in the hippocampus. (G) Hypothalamic arcuate nucleus in a prenatally betamethasone exposed rat without spasms (saline control) does not show any prominent 2DG uptake. (H) However, 45 minutes after the spasms triggered by NMDA, there is a significant increase in 2DG uptake in the entire arcuate nucleus suggesting an increased metabolic activity. (I) Several c-fos immunopositive cells were found in the arcuate nucleus after betamethasone exposure without spasms (in controls). (J) Two hours after the NMDA triggered spasms, arcuate nucleus was full of c-fos positive cells indicating significant neuronal excitation in this hypothalamic structure associated with the HPA axis. (K) Example of another HPA controlling structure, the supraoptic nucleus of the hypothalamus after prenatal betamethasone exposure without postnatal spasms (control). There were no c-fos positive cells. (L) In contrast, supraoptic nucleus 2 hours after NMDA triggered spasms was full of c-fos positive cells suggesting strong excitatory processes.
Thus we established a new model of cryptogenic IS, which reproduced important symptoms of this devastating epileptic syndrome. The spasms can be elicited throughout the infancy of the rat while they evolve into other seizure types (clonic-tonic) before adolescence (Mareš & Velíšek 1992). Spasms elicited in the corticosteroid-primed brain become responsive to ACTH, the drug of choice for human IS. While the effects of prenatal priming can be detected in the hippocampus in terms of decreased expression of glucocorticoid receptors (Velíšek 2005), the hippocampus itself does not seem to be involved in the spasms. Metabolic studies along with the investigation of neuronal excitation support the notion that several hypothalamic structures are involved, namely the arcuate nucleus. This nucleus represents the upstream control center for the HPA axis and has significant intrahypothalamic connections as well as bilateral connections with the brain stem nuclei such as dorsal raphe, nucleus tractus solitarius, and the parabrachial nucleus of the brain stem (Chronwall 1985). Therefore, it seems that IS may be the result of impaired subcortical/extrahippocampal circuitry.
Acknowledgments
Supported by the grants NS-041366, NS-059504 and NS-56093 from NIH, and by Research Grant No. 6-FY08-214 from the March of Dimes Foundation.
REFERENCES
- Baram TZ, Mitchell WG, Hanson RA, Snead OC, Horton EJ. Cerebrospinal fluid corticotropin and cortisol are reduced in infantile spasms. Pediatr Neurol. 1995;13:108–110. doi: 10.1016/0887-8994(95)00121-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Mitchell WG, Tournay A, Snead OC, Hanson RA, Horton EJ. High-dose corticotropin (ACTH) versus prednisone for infantile spasms: a prospective, randomized, blinded study. Pediatrics. 1996;97:375–379. [PMC free article] [PubMed] [Google Scholar]
- Caplan R, Siddarth P, Mathern G, Vinters H, Curtiss S, Levitt J, Asarnow R, Shields WD. Developmental outcome with and without successful intervention. Int Rev Neurobiol. 2002;49:269–284. doi: 10.1016/s0074-7742(02)49017-4. [DOI] [PubMed] [Google Scholar]
- Chronwall BM. Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides. 1985;6(Suppl 2):1–11. doi: 10.1016/0196-9781(85)90128-7. [DOI] [PubMed] [Google Scholar]
- Dulac O, Soufflet C, Chiron C, Kaminska A. What is West syndrome? Int Rev Neurobiol. 2002;49:1–22. doi: 10.1016/s0074-7742(02)49003-4. [DOI] [PubMed] [Google Scholar]
- Frost JD, Jr., Hrachovy RA. Pathogenesis of infantile spasms: a model based on developmental desynchronization. J Clin Neurophysiol. 2005;22:25–36. doi: 10.1097/01.wnp.0000149893.12678.44. [DOI] [PubMed] [Google Scholar]
- Hrachovy RA, Frost JD., Jr. Infantile epileptic encephalopathy with hypsarrhythmia (infantile spasms/West syndrome) J Clin Neurophysiol. 2003;20:408–425. doi: 10.1097/00004691-200311000-00004. [DOI] [PubMed] [Google Scholar]
- Hrachovy RA, Glaze DG, Frost JD., Jr. A retrospective study of spontaneous remission and long-term outcome in patients with infantile spasms. Epilepsia. 1991;32:212–214. doi: 10.1111/j.1528-1157.1991.tb05246.x. [DOI] [PubMed] [Google Scholar]
- Kábová R, Liptáková S, Šlamberová R, Pometlová M, Velíšek L. Age-specific N-methyl-D-aspartate-induced seizures: perspectives for the West syndrome model. Epilepsia. 1999;40:1357–1369. doi: 10.1111/j.1528-1157.1999.tb02006.x. [DOI] [PubMed] [Google Scholar]
- Kellaway P, Hrachovy RA, Frost JD, Jr., Zion T. Precise characterization and quantification of infantile spasms. Ann Neurol. 1979;6:214–218. doi: 10.1002/ana.410060306. [DOI] [PubMed] [Google Scholar]
- Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, O’Callaghan FJ, Verity CM, Osborne JP. The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: a multicentre randomised trial. Lancet Neurol. 2005;4:712–717. doi: 10.1016/S1474-4422(05)70199-X. [DOI] [PubMed] [Google Scholar]
- Mackay MT, Weiss SK, Adams-Webber T, Ashwal S, Stephens D, Ballaban-Gill K, Baram TZ, Duchowny M, Hirtz D, Pellock JM, Shields WD, Shinnar S, Wyllie E, Snead OC., 3rd Practice parameter: medical treatment of infantile spasms: report of the American Academy of Neurology and the Child Neurology Society. Neurology. 2004;62:1668–1681. doi: 10.1212/01.wnl.0000127773.72699.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mareš P, Velášek L. N-methyl-D-aspartate (NMDA)-induced seizures in developing rats. Brain Res Dev Brain Res. 1992;65:185–189. doi: 10.1016/0165-3806(92)90178-y. [DOI] [PubMed] [Google Scholar]
- Nagamitsu S, Matsuishi T, Yamashita Y, Shimizu T, Iwanaga R, Murakami Y, Miyazaki M, Hashimoto T, Kato H. Decreased cerebrospinal fluid levels of beta-endorphin and ACTH in children with infantile spasms. J Neural Transm. 2001;108:363–371. doi: 10.1007/s007020170081. [DOI] [PubMed] [Google Scholar]
- Okumura A, Watanabe K, Negoro T, Aso K, Natsume J, Kubota T, Matsumoto A, Miura K, Furune J, Nomura K, Hayakawa F, Kato T. Evolutional changes and outcome of West syndrome: correlation with magnetic resonance imaging findings. Epilepsia. 1998;39(Suppl 5):46–49. doi: 10.1111/j.1528-1157.1998.tb05150.x. [DOI] [PubMed] [Google Scholar]
- Owen D, Matthews SG. Glucocorticoids and sex-dependent development of brain glucocorticoid and mineralocorticoid receptors. Endocrinology. 2003;144:2775–2784. doi: 10.1210/en.2002-0145. [DOI] [PubMed] [Google Scholar]
- Plouin P, Dulac O, Jalin C, Chiron C. Twenty-four-hour ambulatory EEG monitoring in infantile spasms. Epilepsia. 1993;34:686–691. doi: 10.1111/j.1528-1157.1993.tb00447.x. [DOI] [PubMed] [Google Scholar]
- Riikonen R. A long-term follow-up study of 214 children with the syndrome of infantile spasms. Neuropediatrics. 1982;13:14–23. doi: 10.1055/s-2008-1059590. [DOI] [PubMed] [Google Scholar]
- Riikonen R. Long-term outcome of patients with West syndrome. Brain Dev. 2001;23:683–687. doi: 10.1016/s0387-7604(01)00307-2. [DOI] [PubMed] [Google Scholar]
- Riikonen R, Donner M. Incidence and aetiology of infantile spasms from 1960 to 1976: a population study in Finland. Dev Med Child Neurol. 1979;21:333–343. doi: 10.1111/j.1469-8749.1979.tb01625.x. [DOI] [PubMed] [Google Scholar]
- Sankar R. Initial treatment of epilepsy with antiepileptic drugs: pediatric issues. Neurology. 2004;63:S30–39. doi: 10.1212/wnl.63.10_suppl_4.s30. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Moshé SL, Swann JW, Nehlig A, Jacobs MP, Schwartzkroin PA. Models of pediatric epilepsies: strategies and opportunities. Epilepsia. 2006;47:1407–1414. doi: 10.1111/j.1528-1167.2006.00674_1.x. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Sasaki-Adams DM. NMDA-induced seizures in developing rats cause long-term learning impairment and increased seizure susceptibility. Epilepsy Res. 2003;53:129–137. doi: 10.1016/s0920-1211(02)00258-9. [DOI] [PubMed] [Google Scholar]
- Velíšek L. Prenatal corticosteroid impact on hippocampus: Implications for postnatal outcomes. Epilepsy Behav. 2005;7:57–67. doi: 10.1016/j.yebeh.2005.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velíšek L, Jehle K, Asche S, Velíšková J. Model of infantile spasms induced by N-methyl-D-aspartic acid in prenatally impaired brain. Ann Neurol. 2007;61:109–119. doi: 10.1002/ana.21082. [DOI] [PubMed] [Google Scholar]
- Welberg LAM, Seckl JR, Holmes MC. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience. 2001;104:71–79. doi: 10.1016/s0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]