Summary
The endocannabinoid system plays a central role in retrograde synaptic communication, and controls both glutamatergic and GABAergic transmission via type 1 cannabinoid receptors (CB1). Both in sclerotic human hippocampi and in the chronic phase of pilocarpine-induced epilepsy in mice with sclerosis, CB1 receptor-positive interneuron somata were preserved both in the dentate gyrus and in the CA1 area, and the density of CB1 immunostained fibers increased considerably in the dentate molecular layer. This suggests that, while CB1 receptors are known to be reduced in density on glutamatergic axons, the CB1 receptor-expressing GABAergic axons sprout, or there is an increase of CB1 receptor levels on these fibers. The changes of CB1 immunostaining in association with the GABAergic inhibitory system appears to correlate with the severity of pyramidal cell loss in the CA1 subfield. These results confirm the involvement of the endocannabinoid system associated with GABAergic transmission in human TLE, as well as in the chronic phase of the pilocarpine model in mice. Pharmacotherapy aimed at the modulation of endocannabinoid-mediated retrograde synaptic signaling should take into account the opposite change in CB1 receptor expression observed on glutamatergic versus GABAergic axon terminals.
Keywords: epilepsy, CB1 receptor, sprouting, human hippocampus, pilocarpine model
Introduction
The endocannabinoid system plays a central role in retrograde synaptic communication (Freund, et al. 2003, Mackie 2008, Mackie and Stella 2006). Endocannabinoids are produced and released from postsynaptic neurons in an activity dependent manner, bind to presynaptic CB1 receptors (CB1) located on certain types of axon terminals and suppress neurotransmitter release (Hajos, et al. 2001, Katona, et al. 1999, Mackie 2005).
Cannabinoids have been reported to exert anti-convulsant effects in in vivo models (Shafaroodi, et al. 2004, Wallace, et al. 2003). It was shown that CB1 receptors expressed on excitatory glutamatergic axon terminals are responsible for the anti-convulsant effect (Marsicano, et al. 2003, Monory, et al. 2006) in specific models.
In a previous study, aimed to elucidate potential chronic changes in the activity of the endocannabinoid system in human temporal lobe epilepsy (TLE), we measured the expression level of several genes linked to the cannabinoid signaling system (Ludanyi, et al. 2008). Quantitative real-time PCR experiments provided evidence that mRNA levels of the CB1 receptor is decreased in epileptic hippocampal tissue. Immunostaining for CB1 receptors (using an antibody which labels CB1 receptors both on excitatory and inhibitory terminals) confirmed this reduction. Furthermore, we found a significant reduction in the level of diacylglycerol lipase alpha, the main enzyme responsible for the synthesis of the endocannabinoid 2-arachidonoyl-glycerol (2-AG), as well as in the level of cannabinoid receptor interacting protein 1a, a glutamatergic cell-specific anchoring protein of CB1 (Niehaus, et al. 2007). In contrast, we did not observe significant alterations in the level of the 2-AG degrading enzyme, monoacyl-glycerol lipase (Ludanyi, et al. 2008).
Thus, several molecular elements involved in 2-AG-mediated endocannabinoid signaling at glutamatergic synapses are selectively reduced in the epileptic human hippocampus, which may contribute to the induction of epileptic seizures and cell loss in the hippocampus. However, alterations of CB1 receptors located on inhibitory terminals (Katona, et al. 1999, Katona, et al. 2000), which may well be affected to a similar extent as those on glutamate terminal, but having an opposite effect on seizure susceptibility, have not yet been investigated in TLE.
In this study we focused on the long term changes in CB1 receptor expression and distribution in epilepsy. We have examined the expression pattern of CB1 receptors on hippocampal GABAergic axon terminals (Katona, et al. 1999, Katona, et al. 2000) by immunostaining in CD1 mice in the phase of chronic recurrent seizures and in temporal lobe epileptic patients.
Reorganization of CB1 receptor expressing GABAergic fibers in temporal lobe epileptic patients
The expression pattern of CB1-receptors associated with inhibitory synapses was studied by immunocytochemistry in epileptic hippocampal tissue derived from intractable TLE patients (N=34). Control brains were removed 2 hours after death, and processed for immersion fixation and immunostaining. Strictly the same fixation and staining protocol was followed in the processing of epileptic and control samples, as described earlier (Katona, et al. 2000, Magloczky, et al. 1997).
In the hippocampi of human TLE patients the pattern of cell loss was analyzed by light microscope in sections immunostained for different neurochemical markers labeling principal and non-principal cells. Two types of pathology have been distinguished in the epileptic samples: i) Non-sclerotic type, characterized by mild pyramidal cell loss and damage to interneurons and ii) sclerotic type with nearly total CA1 pyramidal cell loss and profound damage of sensitive interneuron types (Toth, et al. 2007, Wittner, et al. 2005). The distribution and localization of CB1-immunoreactive elements was studied in controls and in the two types of epileptic cases.
Immunostaining revealed numerous CB1-positive cell bodies of interneurons scattered in all hippocampal subfields. Dendrites remained unstained, but a dense meshwork of CB1-immunoreactive axons covered the entire hippocampal formation. The strongest axonal labeling was found in stratum moleculare of the dentate gyrus, and in stratum pyramidale of CA1-CA3 (Katona, et al. 2000).
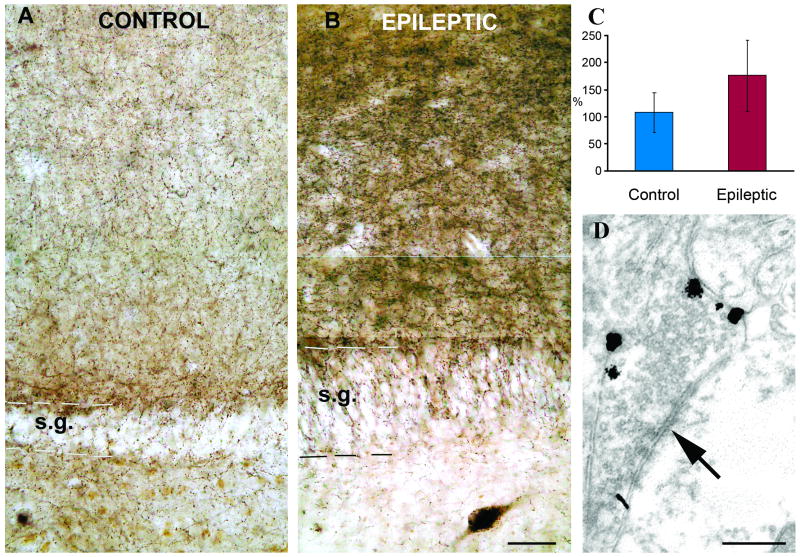
In the non-sclerotic cases, the distribution of CB1 receptors in the dentate gyrus did not show any major changes compared to the normal post mortem controls. In contrast, a strong increase in CB1 receptor immunostaining was found in the dentate gyrus of epileptic patients with CA1 sclerosis. Immunopositive interneuron somata were present both in the dentate gyrus and in the CA1 area. The density of immunostained fibers increased in the dentate molecular layer (Fig. 1) and became inhomogeneous in the hilus forming dense arrays of boutons around the surviving mossy cells and interneurons. Qualitative analysis revealed an increased denisty of immunostained axonal meshworks in the sclerotic stratum moleculare compared to the control or non-sclerotic cases. To quantify this increase, sections with fluorescent immunostaining were processed in 3 control and 10 sclerotic cases, and the density of immunolabeling was measured by confocal laser scanning microscopy. The results showed that the density of fibers has significantly increased in epileptic cases (Fig. 1 C).
Figure 1.
CB1 immunostaining in control and sclerotic epileptic human dentate gyrus. A) Low power light micrograph showing the distribution of CB1-positive profiles in the control human dentate gyrus. Granule cells are always negative for CB1. B) Sclerotic epileptic patients show remarkable differences from the controls. The density and staining intensity of CB1-positive fibers is considerably increased in the stratum moleculare. C) Density of CB1 receptor immunopositive fibers in control (N=3) and sclerotic epileptic patients (N=10) revealed by confocal laeser scanning microscope. The intensity of CB1 receptor staining is elevated in epileptic samples. The difference between control and epileptic samples was highly significant (p<0,05; Student t test). D) A CB1 receptor-positiv terminal from the stratum moleculare of an epileptic patient labelled by immunogold technique establishes symmetric synapse on a dendrite. All of the terminals stained by this CB1 antibody established symmetric synapses. Scales: A,B:50 μm; D:0,5 μm
Target distribution of CB1-immunopositive elements was studied in the stratum moleculare of the dentate gyrus in control and sclerotic TLE subjects, where the highest fiber density was observed. Electron microscopic examination of CB1 receptor immunostaining confirmed earlier conclusions that the antibody did not label glial elements. Examination of immunogold terminals showed that CB1 receptors were localized in the membrane, outside the synaptic active zone in epileptic patients, as it was shown previously in the control human hippocampus (Katona, et al. 2000) (Fig. 1D). Both in controls (N=105, two subjects) and in epileptic patients (N=175, three subjects) the CB1-immunopositive terminals established symmetric synapses mostly on dendrites (75 v.72,5 %, spines 13,2 v. 15,5 %, and cell bodies 11,8 v. 13 %, in control and epileptic subjects, respectively).
Therefore, we can conclude that the target distribution was not changed, although the density of fibers has been elevated.
Changes of CB1-receptor immunostained fibers in a model of temporal lobe epilepsy
We wanted to check whether the density of CB1 receptors increased in epilepsy models as well, or it was a phenomenon specific for human patients.
In the animal model we used intraperitoneal pilocarpine (340 mg/kg) injection to induce status epilepticus in 20-25 g male CD1 mice. Scopolamine was injected (5 mg/kg) in advance to prevent peripheral effects of pilocarpine. Based on the behavioral signs of the acute seizures after the pilocarpine-induced status epilepticus, animals were classified as “weakly” or “strongly” epileptic using the modified Racine scale (Racine, 1972, Turski, et al. 1984). Animals were observed for two hours after the pilocarpine injection, and the behavioral signs of seizures were monitored. In the weak group there were only a few mild seizures, whereas in the strong group the seizures were frequent with intense motor symptoms. After 1-2 month survival, the animals were perfused, and the pattern and degree of cell loss determined. We found that in the weak group none of the hippocampi showed sclerosis, however, in the strong group (Racine 4-5), the CA1 subield in most mice were considerably shrunken, with hardly any pyramidal cells surviving.
We examined the expression pattern of CB1 in the hippocampi of both strong and weak CD1 mice at a survival time of 4-8 weeks (chronic phase).
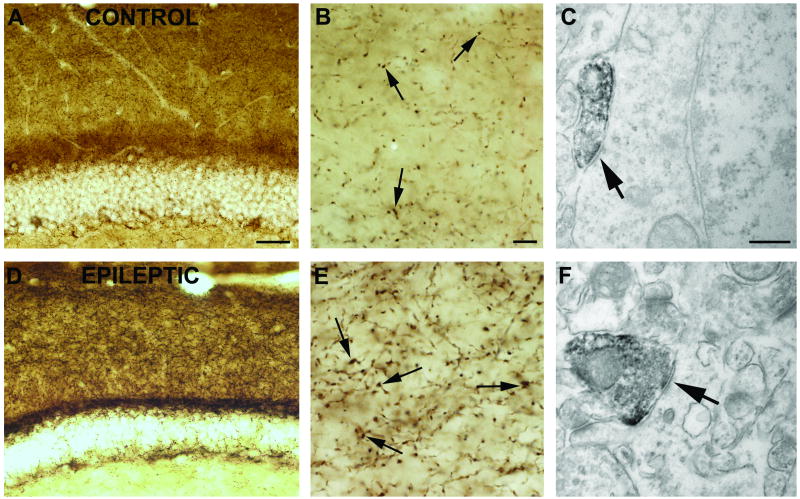
In control samples the most intense staining of CB1-positive fibers was found in the molecular layer of the dentate gyrus (DG), in stratum pyramidale of the cornu Ammonis (CA), and in the subiculum. In contrast, faint labeling was observed in the strata radiatum and granulosum. CB1-immunostaining showed no change in the hippocampi of weak animals compared to controls. In the sclerotic samples the general CB1-immunostaining was much stronger throughout the hippocampus. The most striking difference was found in the strata moleculare and granulosum of the DG (Fig. 2), in the strata pyramidale and radiatum of the CA1.
Figure 2.
CB1 immunostaining in the dentate gyrus of a control (A, B, C) and a “strong” epileptic (D, E, F) mouse. In control samples (A,B), dense staining of CB1-positive fibers can be found in the molecular layer of the DG. In the stratum moleculare of sclerotic animals (D, E) the intensity of CB1 immunostaining is elevated. The most frequent postsynaptic targets of CB1 receptor-positive terminals establishing symmetric synapses (arrows) are cell bodies (C) and dendrites (F) Scales: A,D: 200 μm; B,E: 50 μm; C,F:1 μm.
Electron microscopic examination confirmed that the antibody used in this study visualized CB1 receptors only on terminals giving symmetric synapses (Fig. 2). The cellular and subcellular localization of CB1 receptors was similar in the epileptic and control cases. CB1-positive terminals form synapses mostly on dendrites and somata of granule cells in control and epileptic animals. Glial cell labeling was never observed in our samples using this antibody.
Detailed light microscopic examination of fibers showed that the density of immunostained axons is higher in the sclerotic epileptic samples than in control or non-sclerotic animals (Fig. 2B,E).
Significance of the rearrangement of CB1-receptor expressing GABAergic fibers in epilepsy
Endocannabinoids as retrograde signal molecules are generated by large intracellular Ca2+ transients, complex-spike burst-firing, and/or phospholipase C activation via metabotropic receptors in neurons (Freund, et al. 2003). They bind to presynaptic CB1 receptors located, among others, on GABAergic axon terminals, and thereby decrease transmitter release from inhibitory boutons arriving primarily onto the same neurons (Hajos, et al. 2000). Importantly, seizure activity generates ideal conditions for endocannabinoid synthesis and release from bursting neurons, which likely reduces inhibition of the same cells, and thereby may aggravate epileptic bursting.
Both in sclerotic human hippocampi and in the chronic phase of pilocarpine-induced epilepsy in mice with sclerosis, CB1 receptor-positive cell bodies were preserved in the dentate gyrus and in the CA1 area, and the density of CB1 immunostained fibers increased considerably in the dentate molecular layer suggesting a sprouting of the CB1 receptor-expressing axons. This is in sharp contrast to the observed loss of CB1 mRNA and protein associated with glutamatergic transmission (Ludanyi, et al. 2008). However, in the present study, CB1 receptors expressed by GABAergic terminals were examined separately. In other studies, where the employed CB1 antibody labels both excitatory and the inhibitory fibers (Falenski, et al. 2007, Ludanyi, et al. 2008), the large amount of excitatory terminals, although they stain weaker, may mask changes in the less abundant inhibitory fibers. The changes of CB1 immunostaining in association with the GABAergic inhibitory system appears to correlate with the severity of pyramidal cell loss in the CA1 subfield. Since a strong increase in CB1 receptor-immunostaining was found both in the hippocampi of epileptic patients as well as in mice with CA1 sclerosis, the question arises whether the enhanced endocannabinoid-mediated reduction of GABA release contributes to seizure generation and maintenance, or it should rather be considered a neuroprotective reaction of the network leading to the sprouting of CB1-positive GABAergic interneuron axons (Magloczky and Freund 2005).
An alternative explanation for the enhancement of CB1-receptor immunostaining could be an increase of CB1 receptor levels on GABAergic fibers that brings some of them above detection threshold. Similar changes have been observed by Chen et al. (Chen, et al. 2003) showing a chronic increase of CB1 receptors on axons of cholecystokinin-containing inhibitory cells following febrile seizure-like events.
The sprouting of fibers of CB1 receptor-expressing interneurons and/or the elevation of the level of CB1 receptors both in chronic models and in human patients highlights the significance of the involvement of the endocannabinoid system associated with GABAergic transmission in the chronic phase of TLE. However, any pharmacotherapy aimed at the modulation of endocannabinoid-mediated retrograde synaptic signaling should take into account the opposite change in CB1 receptor expression observed on glutamatergic versus GABAergic axon terminals (Falenski, et al. 2007, Ludanyi, et al. 2008, Marsicano, et al. 2003). Further studies are required to identify causal relationships between epileptic activity and the observed changes in cell type-specific CB1 expression, as well as to determine the time course of events.
Acknowledgments
The authors thank Drs. M. Palkovits, P. Sótonyi and Zs. Borostyánkői /Semmelweis University, Budapest/ for providing control human tissue. The excellent technical assistance of Ms. E. Simon, K. Lengyel, K. Iványi and Mr. Gy. Goda is also acknowledged. This study was supported by grants from EPICURE FP6 EC LSH-CT-2006-037315, NIH DA11322&DA21696, NS 030549, MH 54671, the Howard Hughes Medical Institute, and the Hungarian Scientific Research Fund (NKTH-OTKA CNK 77793).
Footnotes
Disclosure: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. None of the authors has any conflict of interest to disclose.
References
- Chen K, Ratzliff A, Hilgenberg L, Gulyas A, Freund TF, Smith M, Dinh TP, Piomelli D, Mackie K, Soltesz I. Long-term plasticity of endocannabinoid signaling induced by developmental febrile seizures. Neuron. 2003;39:599–611. doi: 10.1016/s0896-6273(03)00499-9. [DOI] [PubMed] [Google Scholar]
- Falenski KW, Blair RE, Sim-Selley LJ, Martin BR, DeLorenzo RJ. Status epilepticus causes a long-lasting redistribution of hippocampal cannabinoid type 1 receptor expression and function in the rat pilocarpine model of acquired epilepsy. Neuroscience. 2007;146:1232–1244. doi: 10.1016/j.neuroscience.2007.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiological Reviews. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Hajos N, Katona I, Naiem SS, MacKie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Magloczky Z, Santha E, Kofalvi A, Czirjak S, Mackie K, Vizi ES, Freund TF. GABAergic interneurons are the targets of cannabinoid actions in the human hippocampus. Neuroscience. 2000;100:797–804. doi: 10.1016/s0306-4522(00)00286-4. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludanyi A, Eross L, Czirjak S, Vajda J, Halasz P, Watanabe M, Palkovits M, Magloczky Z, Freund TF, Katona I. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. The Journal of Neuroscience. 2008;28:2976–2990. doi: 10.1523/JNEUROSCI.4465-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handbook of Experimental Pharmacology. 2005:299–325. doi: 10.1007/3-540-26573-2_10. [DOI] [PubMed] [Google Scholar]
- Mackie K. Signaling via CNS cannabinoid receptors. Molecular and cellular endocrinology. 2008;286:S60–65. doi: 10.1016/j.mce.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Stella N. Cannabinoid receptors and endocannabinoids: evidence for new players. The AAPS Journal. 2006;8:E298–306. doi: 10.1007/BF02854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magloczky Z, Freund TF. Impaired and repaired inhibitory circuits in the epileptic human hippocampus. Trends Neurosci. 2005;28:334–340. doi: 10.1016/j.tins.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Magloczky Z, Halasz P, Vajda J, Czirjak S, Freund TF. Loss of Calbindin-D28K immunoreactivity from dentate granule cells in human temporal lobe epilepsy. Neuroscience. 1997;76:377–385. doi: 10.1016/s0306-4522(96)00440-x. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutierrez SO, van der Stelt M, Lopez-Rodriguez ML, Casanova E, Schutz G, Zieglgansberger W, Di Marzo V, Behl C, Lutz B. Science. Vol. 302. New York, N.Y: 2003. CB1 cannabinoid receptors and on-demand defense against excitotoxicity; pp. 84–88. [DOI] [PubMed] [Google Scholar]
- Monory K, Massa F, Egertova M, Eder M, Blaudzun H, Westenbroek R, Kelsch W, Jacob W, Marsch R, Ekker M, Long J, Rubenstein JL, Goebbels S, Nave KA, During M, Klugmann M, Wolfel B, Dodt HU, Zieglgansberger W, Wotjak CT, Mackie K, Elphick MR, Marsicano G, Lutz B. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51:455–466. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehaus JL, Liu Y, Wallis KT, Egertova M, Bhartur SG, Mukhopadhyay S, Shi S, He H, Selley DE, Howlett AC, Elphick MR, Lewis DL. CB1 cannabinoid receptor activity is modulated by the cannabinoid receptor interacting protein CRIP 1a. Mol Pharmacol. 2007;72:1557–1566. doi: 10.1124/mol.107.039263. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Shafaroodi H, Samini M, Moezi L, Homayoun H, Sadeghipour H, Tavakoli S, Hajrasouliha AR, Dehpour AR. The interaction of cannabinoids and opioids on pentylenetetrazole-induced seizure threshold in mice. Neuropharmacology. 2004;47:390–400. doi: 10.1016/j.neuropharm.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Toth K, Wittner L, Urban Z, Doyle WK, Buzsaki G, Shigemoto R, Freund TF, Magloczky Z. Morphology and synaptic input of substance P receptor-immunoreactive interneurons in control and epileptic human hippocampus. Neuroscience. 2007;144:495–508. doi: 10.1016/j.neuroscience.2006.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Bortolotto ZA, Mello LM, Schwarz M, Turski L. Seizures produced by pilocarpine in mice: a behavioral, electroencephalographic and morphological analysis. Brain Research. 1984;321:237–253. doi: 10.1016/0006-8993(84)90177-x. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, Blair RE, Falenski KW, Martin BR, DeLorenzo RJ. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. The Journal of Pharmacology and Experimental Therapeutics. 2003;307:129–137. doi: 10.1124/jpet.103.051920. [DOI] [PubMed] [Google Scholar]
- Wittner L, Eross L, Czirjak S, Halasz P, Freund TF, Magloczky Z. Surviving CA1 pyramidal cells receive intact perisomatic inhibitory input in the human epileptic hippocampus. Brain. 2005;128:138–52. doi: 10.1093/brain/awh339. [DOI] [PubMed] [Google Scholar]