Summary
One of the major challenges in developing novel therapeutics for human epileptic disorders comes from the wide range of brain abnormalities capable of producing epilepsy. In children and adults that undergo epilepsy surgery for treatment of refractory seizures, these abnormalities range from developmental defects, to injuries, infections, tumors, and ischemia. Given the many molecular mechanisms likely involved in each of these, finding common therapeutic targets seems a futile task. However, patients undergoing surgery for neocortical seizures have surprisingly similar electrophysiological abnormalities that consist of the synchronous firing of large neuronal populations. Surgical removal of these regions is the only means at present time to permanently reduce or eliminate seizures. The precise locations of these hyersynchronous firing regions that produce seizures can be revealed using long-term subdural electrical high density recordings. This therapeutic strategy not only can dramatically reduce seizures, but also offers the potential to generate molecular and cellular information that can be used to ask why certain regions of the cortex become and remain epileptic. We have taken advantage of these detailed clinical and electrophysiological human studies by taking a ‘systems biology’ approach to identify novel biomarkers and drug targets in neocortical human epilepsy. In this paper, our multidisciplinary systems approach will be described that utilizes a relational database to interrelate clinical, quantitative electrophysiological, pathological, and gene expression profiling data together as a means to identify and validate new biomarkers and potential drug targets for human epilepsy.
Keywords: Systems biology, genomics, tissue banking, computational biology, therapeutics, epileptogenesis
Introduction
Epilepsy is a disabling neurological disorder of recurrent seizures affecting up to 1% of the population (Annegers 1993). While single gene defects in ion channels or neurotransmitter receptors are associated with some inherited forms of epilepsy (Berkovic & Steinlein 1999; Noebels 2003; Steinlein 2004; Reid et al. 2009), these mutations cannot account for the majority of patients with epilepsy. In most patients with partial epilepsy, seizures start in focal brain regions in response to a wide variety of brain insults often with no clear histopathological abnormalities (Babb & Pretorius 1993). Patients who fail to respond to anti-epileptic medications can greatly benefit from surgery to remove brain regions where seizures originate. This suggests that these focal epileptic brain regions are both necessary and sufficient to produce clinical seizures. While in young children, epileptic foci are mostly in the neocortex, in adults they frequently involve the hippocampus (Annegers 1993). Little is known about how these often normal-appearing brain regions become and remain epileptic. Regardless of the original brain insult, neocortical epileptic foci show a remarkably similar electrophysiological pattern of localized, abnormal electrical discharges that can become rhythmic and spread to widespread brain regions to produce clinical seizures. Between seizures, and far more frequent than seizures, these focal brain regions generate localized “interictal” discharges that can be used to help identify regions of seizure onset(Asano et al. 2003; Asano et al. 2009).
Since normal neuronal activity is a critical force that shapes nervous system development and plasticity(Katz & Shatz 1996; McAllister et al. 1999; Chen et al. 2001), it seems likely that ongoing ictal and interictal epileptic activity influence the functional and structural changes that could lead to and maintain hyperexcitability and hyperconnectivity. Consistently, genes encoding neurotransmitter receptors, ion channels, transcription factors and neurotrophic factors have been found to be differentially expressed in various animal models of epilepsy and in human epileptic brain tissues (Mody 1998; Coulter 2001; French et al. 2001; Doherty & Dingledine 2002; Becker et al. 2003; Baybis et al. 2004; Najm et al. 2004; Rakhade et al. 2005; Rakhade et al. 2007; Rakhade & Loeb 2008).
One thing that has become clear for a variety of both cortical malformations as well as other secondary lesions is that the structural abnormalities may not themselves be the main epileptic generator (Chugani et al. 1998; Asano et al. 2000). This is not surprising when one considers that lesions composed of tumor cells, infarcts, or glia scars are not likely to be intrinsically, electrically active. Given that most neocortical epileptic foci do not have any pathologically-demonstrated malformations, it would not be unreasonable to hypothesize that epilepsy can develop in relatively normal neocortex adjacent to any brain lesion. It also raises the possibility that the final common pathway for epileptogenesis can be entirely independent of the nature of the inciting lesion.
One of the hypotheses that our laboratory has been testing for a number of years is that focal regions of neocortex that display spontaneous ictal and interictal activities are maintained in an epileptic state through the local expression of specific genes and proteins and that by comparing these abnormal electrical regions in human cortex to nearby less abnormal regions we can identify molecular pathways that maintain the epileptic state. Our work using this approach in human neocortex has thus far has identified a number of robust biomarkers of human epileptic activity that appear to be independent of the ‘cause’ of epilepsy and should make it possible to determine the functional as well as spatial relationship of these activity-dependent genes within“normal” appearing 6-layered neocortex and in adjacent cortical lesions (Rakhade et al. 2005) (Beaumont et. al, unpublished data).
A systems biology approach to generate unbiased drug targets
Systems biology can be variously defined, but one appealing definition is the ability to obtain, integrate and analyze complex data from multiple experimental sources using interdisciplinary tools (Parker et al. 2009). The goal of this seemingly diffuse approach is to find ways to condense and focus diverse and highly variant types of data into meaningful insights. This can be achieved by developing focused hypotheses and testing each with carefully designed studies that have sufficient biological and technical replicates to generate statistically meaningful results. The most highly utilized high-throughput method today involves gene expression profiling of known coding genes through microarray studies. MIcroarrays are high-density arrays of small sequences of DNA that are ‘spotted’ onto glass slides or other surfaces so that a single microscope slide with thousands of these spots that can probe a majority of genes spanning the entire human genome (Loeb & Beaumont 2009). These slides can be quite costly. As a result many microarray experiments are severely underpowered statistically and produce both false positive and false-negative results. Even more problematic are study designs that examine too many variables simultaneously making it nearly impossible to identify those gene expression changes are truly due to the variable under study.
For our epilepsy work, we have been comparing relative gene expression between two or more nearby regions of human neocortex from the same patient. Our goal has been to reduce the number of variables we are measuring and ask the simple question of what is different between neocortex that is more and less epileptic. All samples we take, including our nearby ‘control’ tissues, have been subjected to long-term surface recordings. An alternative experimental design that has been used more extensively compares epileptic tissue to ‘control’ tissue taken from a non-epileptic patient with another disorder such as acute trauma or a brain tumor (Colantuoni et al. 2001; Becker et al. 2003; Arion et al. 2006; Crino 2007). While this study design may also pick up important transcriptional differences due to epilepsy, it will also pick up many other differences to other variables including: 1) Genetic background differences between the two individuals; 2) Effects of different medications (such as anticonvulsants); 3) Differences in tissue processing times and techniques; and, perhaps most importantly, 4) Not knowing whether the ‘control’ tissue is truly electrically quiet, since the tissue is not electrically mapped with subdural recordings. Having too many gene expression changes creates a ‘needle in the haystack’ problem that makes it nearly impossible to differentiate ‘epilepsy genes’ from hundreds or thousands of other genes changed by these other variables.
Keeping track of different types of data
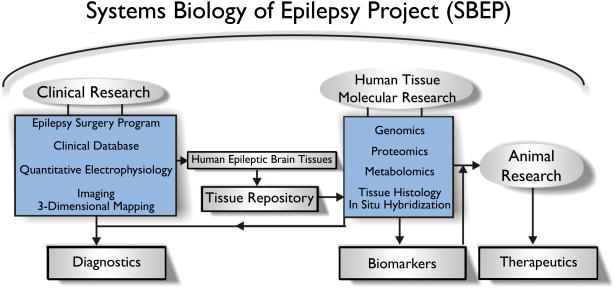
One of the greatest challenges for high throughput experimentation is keeping track of many different types of data that range from gene expression changes to what medications the patient was on at the time of surgery (See Figure 1). This is no small task for human epilepsy work where the electrophysiological data alone consists of often 100 different electrode recording sites producing up to a week of continuous data each. As part of our systems biology approach, we have developed a database that incorporates many data types including clinical, electrophysiological, tissue, and gene data into a relational database. Some of the data we collect are patient-specific such as age, sex, and seizure medications. Other types of data are electrode-specific and include spike frequency, location, and, if the tissue was removed, histology, concentrations of RNA and protein extracts, and gene expression levels. Since the neocortical anatomy of each patient is different, a critical component of this database is placing each of the electrodes into a 3-dimensional brain rendering for each patient as shown in Figure 2 (Hua et al. 2004).
Figure 1. Systems biology of epilepsy project (SBEP).
From our epilepsy surgery program, we have developed a relational database that allows the integration of various types of data starting with clinical information specific to a given patients undergoing surgery and leading the identification of biomarkers (diagnostics) and drug targets. All of our patients undergo a 2-stage surgery where subdural recording electrodes are placed directly on the neocortex in the first stage, and tissue is resected to treat the seizures based on those recordings in the second stage. All of the clinical information is stored together with electrode location specific data including electrophysiology for all electrodes, and histopathology and high-throughput molecular data such as genomic, proteomics, and metabolomics for electrodes under which tissue was resected. A major goal of this work is to test potential drug targets identified from these systems in animal models in order to develop new therapeutics.
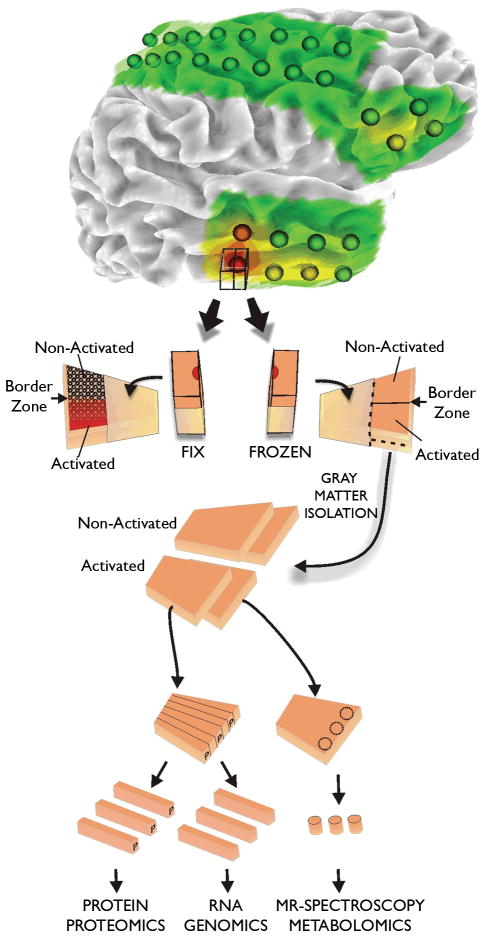
Figure 2. Tissue processing of human epileptic neocortical tissues.
All tissue samples are first identified on 3-dimensional surface maps made for each patients brain from MRI studies. Superimposed on each map are the recording electrode locations. In addition, color coded heat maps indicating the location of specific electrical parameters are placed on the rendering, such as interictal spike frequency shown here. Tissue samples underlying specific electrodes are first subdivided with half placed in paraformaldehyde for tissue sectioning and the other half stored frozen. This frozen piece is later subdivided into strips for preparation of RNA for genomics, protein for proteomics, and samples for metabolomics. In this way, many different systems can be analyzed from the same electrode location or from ‘activated’ subregions identified from the fixed tissues.
The development of a user-friendly and clinically helpful web-based interface to the database is also important. When an interface is helpful only for research, but not clinically useful, it makes it likely that busy clinicians will not take the extra time needed to enter data. Creating such an infrastructure for this system biology approach requires coordination of clinical as well as computational experts, but can create powerful new way to identify both biomarkers and drug targets for epilepsy (Figure 1).
Role of ictal versus interictal activity
Both the spatial and temporal aspects of epileptic activities are likely to have important roles in both epileptogenesis and the generation of clinical seizures. Quite simply, an epileptic discharge is an abnormal, hypersynchronous firing of very large populations of neurons in the brain. Epileptic discharges can occur acutely after brain injuries, as well as chronically in patients with recurrent epileptic seizures (Staley et al. 2005). Although clinical symptoms of seizures are most readily apparent, long term brain recordings show that seizures are relatively infrequent compared to smaller, isolated or grouped epileptic discharges often referred to as interictal spikes (Tao et al. 2005). Despite its relatively higher frequency than seizures, the roles that interictal spiking plays in epileptogenesis are not clear and their clinical significance is controversial. While the identification and removal of regions of seizure onset have beneficial effects, the importance of removing additional regions with these frequent “interictal” spikes is less clear. For example, while interictal spikes have been qualitatively associated with increased seizures (Overweg et al. 1987), other studies suggest that because increased interictal spiking can be seen immediately after seizures that they may actually prevent seizures (Gotman 1991). Nonetheless, studies have shown that resection of regions with frequent interictal spike activity in the temporal lobe is associated with a good surgical outcome (McBride et al. 1991; Kanazawa et al. 1996; Bautista et al. 1999). Given these clinical findings, it is not surprising that there is a growing interest in the molecular roles of interictal spiking in epilepsy (Staley et al. 2005). In fact, interictal spiking and seizure activity have been shown to arise from the same neuronal networks in hippocampal-entorhinal cortex slices (Dzhala & Staley 2003).
Many of the genes we identified at zones of seizure onset are induced in all patients we have examined, regardless of the underlying ‘cause’ of the epilepsy, making them excellent epileptic biomarkers. We recently asked whether the expression levels of these genes correlate with ictal versus interictal activity (Rakhade et al. 2005; Rakhade et al. 2007). Surprisingly, we found no clear correlation between a patient’s seizures frequency and the expression levels of 4 genes (EGR1, EGR2, CFOS, and DUSP6). Specifically, patients with over 10 seizures per day did not necessarily have higher gene expression levels than those with 1 seizure or less per week. In contrast, we found that these same genes correlate precisely to the degree of interictal spiking at each electrode location suggesting a close association or perhaps even a causal relationship between interictal spiking and genes that may keep these focal regions epileptic. These findings also highlight a lack of understanding of the relationship between interictal and ictal activities. This relationship will need to be further elucidated in both humans and animal models to determine whether interictal spiking is a prerequisite for seizures or simply a separate but related form of abnormal hypersynchronous activity and thus whether a biomarker or drug target that affects interictal spiking is also a useful biomarker for seizures.
Quantitative in vivo electrophysiology
Because of the tremendous amount of data generated from subdural electrical recordings, improved, automated methods for identification and analysis of both ictal and interictal activities are needed. This is critical since one of our central goals is to relate these activities to the molecular, cellular, and synaptic basis of these abnormal electrical potentials. In addition to identifying regions of seizure onset and seizure spread, we have developed methods to identify and quantify interictal spiking and then determine their frequency, amplitude, and duration (Rakhade et al. 2007) (Barkmeier, manuscript in preparation). These data are stored in our database and used to generate detailed 3-dimensional electrophysiological heat maps applied to each patient’s anatomical brain surface rendering (Figure 2, top).
Tissue collection and processing
It is important to emphasize that in no way does the research program influence the clinical decisions about what tissues should be removed; and that each patient has signed a consent form indicating that any tissues that are removed for their treatment could be used for research. As there are a number of steps and individuals involved in the identification and removal of epileptic tissues during epilepsy surgery, it is critical that we are certain that a particular tissue we record from is the same tissue that we use in subsequent experiments after the tissue is removed. To be certain of this, we take digital photographs in the operating room with the grids in place and after grid removal. These images are then placed along side our 3-dimensional renderings. At each electrode location a block of tissue is removed and divided in half (Figure 2, bottom). One piece is fixed in 4% paraformaldehyde, sectioned, and stained for histology and other markers. The orientation of the opposing piece is maintained and stored frozen at −80°C. After we identify regions from the fixed tissues that we are interested in, we go back to the frozen piece to prepare various extracts for genomics (RNA), proteomics (protein fractions), and metabolomics (frozen tissues) (Rakhade et al. 2005; Rakhade & Loeb 2008). This method allows us to obtain both histological and molecular information from each electrode recording location. While we cannot use the exact same piece of tissue for all studies, we pool alternating strips within the frozen block of tissue to average out local differences. Our database is also helpful here as it enables us keep track of which tissues have been sampled, where they are stored, and whether specific molecular components have been extracted.
Identification and validation of biomarkers and drug targets for human epilepsy
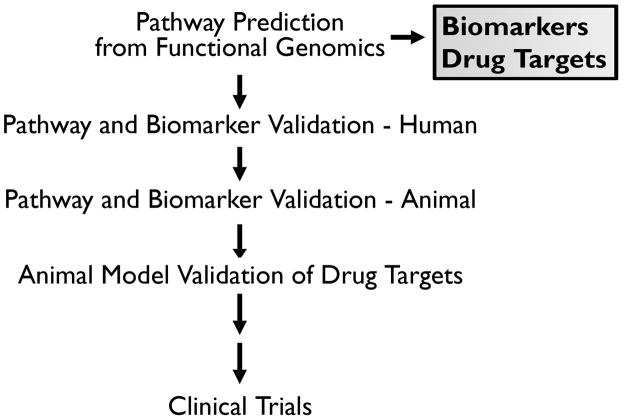
The most exciting next step is to identify biomarkers of epileptic activity as well as whether any of these biomarkers are potential drug targets (Figure 3). This is where all of the work in generating a relational database of many different systems can pay off. While each patient is linked to a variety of variables including age, sex, medications, and family history, each recording electrode location is linked to a variety of electrophysiological parameters, molecular profiles, including gene and protein expression patterns, and tissue histology. We can then generate a large number of hypotheses that can be ‘tested’ for statistical significance through a process of data mining. For example, one hypothesis we have tested is whether or not common gene expression changes exist between regions where seizures start and nearby ‘control’ areas without spontaneous seizures (Rakhade et al. 2005) (Beaumont et al. Unpublished data). The simple premise is that, while any high-throughput microarray experiment will show gene expression differences between two samples, the probably of finding the same changes across many patients by random chance is quite low, but can be realized if the right number of biological and technical replicates are performed (Wolfinger et al. 2001; Yao et al. 2004).
Figure 3. Flow chart for the validation of biomarkers and drug targets.
Once a biomarker or drug target is identified it is validated first in human tissue samples, then animal models. For potential drug targets, treatments that alter the target, such as small molecule inhibitors, are then tested in additional animal models as a prelude to clinical trials in patients.
While the expression of statistically significant individual genes may serve as epileptic biomarkers, identifying groups of differentially-expressed genes with common functions can be an even more powerful way to identify molecular pathways. Analysis of pathways based on groups of genes is often referred to as gene ontology and is a commonly used approach to make sense out of seemingly diverse groups of genes (Khatri et al. 2007). Furthermore, molecular pathway biomarkers, such as a signaling pathway, can be more readily converted from a biomarker to a drug target.
Before determining whether or not a given biomarker is a good drug target, it needs to be validated. At a minimum, validation requires reproducing the same gene expression change seen in the microarray experiment using another method such as quantitative RT-PCR. Equally important, validation of predicted pathways can be achieved by demonstrating that the implicated pathway is indeed altered (increased/decreased) in tissues displaying variable epileptic activity. Based on the tissue sampling method shown in figure 2, we use portions of subdivided human tissues from each electrode location for validation studies. For example, if a particular signaling pathway is implicated, such as the activation of the transcription factor CREB (cyclic AMP response element binding) (Rakhade et al. 2005) (Beaumont et al., unpublished data), it can be validated by showing changes in CREB phosphorylation using Western blots of protein fractions and by tissue staining experiments that have the added advantage of implicating specific cell populations that might be involved in the epileptic process.
Once a specific biomarker is validated in human tissues, the next step is to determine whether or not it is a good drug target (see figure 3). While human tissue studies can be invaluable for identifying genes and pathways, they cannot prove or disprove whether or not a particular gene or pathway is causative and hence a target for drug development. Animal studies are required for the next stage of validation to prove or disprove the value of a given biomarker as a drug target for epilepsy. The first question is how to choose an animal model from a large number of animal epileptic models many of which use combinations of genetic, chemical, electrical, or injurious processes to elicit seizures (Schwartzkroin 1993). For our purposes, we have found that a model using tetanus toxin in the neocortex replicates many of the same electrical and molecular changes we have seen in human neocortex (Barkmeier & Loeb 2009) (Beaumont et al., unpublished results). Even after a single model is chosen and found to be useful, validation with additional animal models will be needed to support a particular drug target. Finally, a means to test the drug target with a small molecule inhibitor or through genetic manipulations of the animal is truly the critical step required to validate a particular drug target. The ultimate goal is to demonstrate that disrupting a particular gene or pathway is sufficient to reduce or eliminate seizures in animals so that therapeutics can be designed for clinical trials in humans.
Acknowledgments
This work was funded by NIH/NINDS R01NS045207 and R01-NS058802 and a Wayne State University President’s Research Enhancement Program in computational biology.
Footnotes
Disclosure: The author has no conflicts of interest to disclose.
References
- Annegers JF. The Epidemiology of Epilepsy. In: Wyllie E, editor. The Treatment of Epilepsy: Principles and Practices. Lea & Febiger; Philadelphia: 1993. pp. 157–164. [Google Scholar]
- Arion D, Sabatini M, Unger T, Pastor J, Alonso-Nanclares L, Ballesteros-Yanez I, Garcia Sola R, Munoz A, Mirnics K, DeFelipe J. Correlation of transcriptome profile with electrical activity in temporal lobe epilepsy. Neurobiol Dis. 2006;22:374–87. doi: 10.1016/j.nbd.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Asano E, Chugani DC, Muzik O, Shen C, Juhasz C, Janisse J, Ager J, Canady A, Shah JR, Shah AK, Watson C, Chugani HT. Multimodality imaging for improved detection of epileptogenic foci in tuberous sclerosis complex. Neurology. 2000;54:1976–84. doi: 10.1212/wnl.54.10.1976. [DOI] [PubMed] [Google Scholar]
- Asano E, Juhasz C, Shah A, Sood S, Chugani HT. Role of subdural electrocorticography in prediction of long-term seizure outcome in epilepsy surgery. Brain. 2009;132:1038–47. doi: 10.1093/brain/awp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Muzik O, Shah A, Juhasz C, Chugani DC, Sood S, Janisse J, Ergun EL, Ahn-Ewing J, Shen C, Gotman J, Chugani HT. Quantitative interictal subdural EEG analyses in children with neocortical epilepsy. Epilepsia. 2003;44:425–34. doi: 10.1046/j.1528-1157.2003.38902.x. [DOI] [PubMed] [Google Scholar]
- Babb TL, Pretorius JK. Pathologic Substrates of Epilepsy. In: Wyllie E, editor. The Treatment of Epilepsy: Principles and Practices. Lea & Febiger; Philadelphia: 1993. pp. 55–70. [Google Scholar]
- Barkmeier DT, Loeb JA. An animal model to study the clinical significance of interictal spiking. Clin EEG Neurosci. 2009;40:234–8. doi: 10.1177/155005940904000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista RE, Cobbs MA, Spencer DD, Spencer SS. Prediction of surgical outcome by interictal epileptiform abnormalities during intracranial EEG monitoring in patients with extrahippocampal seizures. Epilepsia. 1999;40:880–90. doi: 10.1111/j.1528-1157.1999.tb00794.x. [DOI] [PubMed] [Google Scholar]
- Baybis M, Lynch D, Lee A, Patel A, McKhann G, 2nd, Chugani D, WJK, Aronica E, Crino PB. Altered expression of neurotransmitter-receptor subunit and uptake site mRNAs in hemimegalencephaly. Epilepsia. 2004;45:1517–24. doi: 10.1111/j.0013-9580.2004.16204.x. [DOI] [PubMed] [Google Scholar]
- Becker AJ, Chen J, Zien A, Sochivko D, Normann S, Schramm J, Elger CE, Wiestler OD, Blumcke I. Correlated stage- and subfield-associated hippocampal gene expression patterns in experimental and human temporal lobe epilepsy. Eur J Neurosci. 2003;18:2792–802. doi: 10.1111/j.1460-9568.2003.02993.x. [DOI] [PubMed] [Google Scholar]
- Berkovic SF, Steinlein OK. Genetics of partial epilepsies. Adv Neurol. 1999;79:375–81. [PubMed] [Google Scholar]
- Chen J, Sochivko D, Beck H, Marechal D, Wiestler OD, Becker AJ. Activity-induced expression of common reference genes in individual cns neurons. Lab Invest. 2001;81:913–6. doi: 10.1038/labinvest.3780300. [DOI] [PubMed] [Google Scholar]
- Chugani DC, Chugani HT, Muzik O, Shah JR, Shah AK, Canady A, Mangner TJ, Chakraborty PK. Imaging epileptogenic tubers in children with tuberous sclerosis complex using alpha-[11C]methyl-L-tryptophan positron emission tomography. Ann Neurol. 1998;44:858–66. doi: 10.1002/ana.410440603. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Jeon OH, Hyder K, Chenchik A, Khimani AH, Narayanan V, Hoffman EP, Kaufmann WE, Naidu S, Pevsner J. Gene expression profiling in postmortem Rett Syndrome brain: differential gene expression and patient classification. Neurobiol Dis. 2001;8:847–65. doi: 10.1006/nbdi.2001.0428. [DOI] [PubMed] [Google Scholar]
- Coulter DA. Epilepsy-associated plasticity in gamma-aminobutyric acid receptor expression, function, and inhibitory synaptic properties. Int Rev Neurobiol. 2001;45:237–52. doi: 10.1016/s0074-7742(01)45013-6. [DOI] [PubMed] [Google Scholar]
- Crino PB. Gene expression, genetics, and genomics in epilepsy: some answers, more questions. Epilepsia. 2007;48(Suppl 2):42–50. doi: 10.1111/j.1528-1167.2007.01066.x. [DOI] [PubMed] [Google Scholar]
- Doherty J, Dingledine R. The roles of metabotropic glutamate receptors in seizures and epilepsy. Curr Drug Target CNS Neurol Disord. 2002;1:251–60. doi: 10.2174/1568007023339355. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Staley KJ. Transition from interictal to ictal activity in limbic networks in vitro. J Neurosci. 2003;23:7873–80. doi: 10.1523/JNEUROSCI.23-21-07873.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French PJ, O’Connor V, Voss K, Stean T, Hunt SP, Bliss TV. Seizure-induced gene expression in area CA1 of the mouse hippocampus. Eur J Neurosci. 2001;14:2037–41. doi: 10.1046/j.0953-816x.2001.01818.x. [DOI] [PubMed] [Google Scholar]
- Gotman J. Relationships between interictal spiking and seizures: human and experimental evidence. Can J Neurol Sci. 1991;18:573–6. doi: 10.1017/s031716710003273x. [DOI] [PubMed] [Google Scholar]
- Hua J, He Y, Qin H. Multiresolution Heterogeneous Solid Modeling and Visualization Using Trivariate Simplex Splines. 9th ACM Symposium on Solid Modeling and Applications.2004. [Google Scholar]
- Kanazawa O, Blume WT, Girvin JP. Significance of spikes at temporal lobe electrocorticography. Epilepsia. 1996;37:50–5. doi: 10.1111/j.1528-1157.1996.tb00511.x. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–8. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Khatri P, Voichita C, Kattan K, Ansari N, Khatri A, Georgescu C, Tarca AL, Draghici S. Onto-Tools: new additions and improvements in 2006. Nucleic Acids Res. 2007;35:W206–11. doi: 10.1093/nar/gkm327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb JA, Beaumont TL. What goes in is what comes out: How to design and implement a successful microarray experiment. In: Krawetz S, editor. Bioinformatics for systems biology. Springer; New York: 2009. pp. 209–226. [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- McBride MC, Binnie CD, Janota I, Polkey CE. Predictive value of intraoperative electrocorticograms in resective epilepsy surgery. Ann Neurol. 1991;30:526–32. doi: 10.1002/ana.410300404. [DOI] [PubMed] [Google Scholar]
- Mody I. Ion channels in epilepsy. Int Rev Neurobiol. 1998;42:199–226. doi: 10.1016/s0074-7742(08)60611-x. [DOI] [PubMed] [Google Scholar]
- Najm I, Ying Z, Babb T, Crino PB, Macdonald R, Mathern GW, Spreafico R. Mechanisms of epileptogenicity in cortical dysplasias. Neurology. 2004;62:S9–13. doi: 10.1212/01.wnl.0000114506.49267.bb. [DOI] [PubMed] [Google Scholar]
- Noebels JL. The biology of epilepsy genes. Annu Rev Neurosci. 2003;26:599–625. doi: 10.1146/annurev.neuro.26.010302.081210. [DOI] [PubMed] [Google Scholar]
- Overweg J, Binnie CD, Oosting J, Rowan AJ. Clinical and EEG prediction of seizure recurrence following antiepileptic drug withdrawal. Epilepsy Res. 1987;1:272–83. doi: 10.1016/0920-1211(87)90002-7. [DOI] [PubMed] [Google Scholar]
- Parker A, McCaffery I, Patterson S. Examining molecular biology in humans. Biotechniques. 2009;46:358–60. doi: 10.2144/000113141. [DOI] [PubMed] [Google Scholar]
- Rakhade SN, Loeb JA. Focal reduction of neuronal glutamate transporters in human neocortical epilepsy. Epilepsia. 2008;49:226–36. doi: 10.1111/j.1528-1167.2007.01310.x. [DOI] [PubMed] [Google Scholar]
- Rakhade SN, Shah AK, Agarwal R, Yao B, Asano E, Loeb JA. Activity-dependent gene expression correlates with interictal spiking in human neocortical epilepsy. Epilepsia. 2007;48(Suppl 5):86–95. doi: 10.1111/j.1528-1167.2007.01294.x. [DOI] [PubMed] [Google Scholar]
- Rakhade SN, Yao B, Ahmed S, Asano E, Beaumont TL, Shah AK, Draghici S, Krauss R, Chugani HT, Sood S, Loeb JA. A common pattern of persistent gene activation in human neocortical epileptic foci. Ann Neurol. 2005;58:736–47. doi: 10.1002/ana.20633. [DOI] [PubMed] [Google Scholar]
- Reid CA, Berkovic SF, Petrou S. Mechanisms of human inherited epilepsies. Prog Neurobiol. 2009;87:41–57. doi: 10.1016/j.pneurobio.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA. Epilepsy: models, mechanisms, and concepts. Cambridge University Press; Cambridge; New York: 1993. [Google Scholar]
- Staley K, Hellier JL, Dudek FE. Do interictal spikes drive epileptogenesis? Neuroscientist. 2005;11:272–6. doi: 10.1177/1073858405278239. [DOI] [PubMed] [Google Scholar]
- Steinlein OK. Genetic mechanisms that underlie epilepsy. Nat Rev Neurosci. 2004;5:400–8. doi: 10.1038/nrn1388. [DOI] [PubMed] [Google Scholar]
- Tao JX, Ray A, Hawes-Ebersole S, Ebersole JS. Intracranial EEG substrates of scalp EEG interictal spikes. Epilepsia. 2005;46:669–76. doi: 10.1111/j.1528-1167.2005.11404.x. [DOI] [PubMed] [Google Scholar]
- Wolfinger RD, Gibson G, Wolfinger ED, Bennett L, Hamadeh H, Bushel P, Afshari C, Paules RS. Assessing gene significance from cDNA microarray expression data via mixed models. J Comput Biol. 2001;8:625–37. doi: 10.1089/106652701753307520. [DOI] [PubMed] [Google Scholar]
- Yao B, Rakhade SN, Li Q, Ahmed S, Krauss R, Draghici S, Loeb JA. Accuracy of cDNA microarray methods to detect small gene expression changes induced by neuregulin on breast epithelial cells. BMC Bioinformatics. 2004;5:99. doi: 10.1186/1471-2105-5-99. [DOI] [PMC free article] [PubMed] [Google Scholar]