Summary
Depression represents one of the most common comorbidities of temporal lobe epilepsy (TLE), and has profound negative impact on the quality of life of TLE patients. However, causes and mechanisms of depression in TLE remain poorly understood, and its effective therapies are lacking. We examined whether a commonly used model of TLE in rats can be used as a model of comorbidity between epilepsy and depression suitable for both mechanistic studies and for the development mechanism-based antidepressant therapies. We established that animals which had been subjected to LiCl and pilocarpine status epilepticus (SE) and developed spontaneous recurrent seizures, exhibited set of impairments congruent with depressive state: behavioral equivalents of anhedonia and despair; dysregulation of the hypothalamo-pituitary adrenocortical (HPA) axis; compromised raphe-hippocampal serotonergic transmission. Pharmacological studies suggested that depressive impairments following SE develop as a result of the enhanced interleukin-1β signaling in the hippocampus, which leads to depression via inducing perturbations in the HPA axis and subsequent deficit in the raphe-hippocampal serotonergic transmission.
Keywords: Temporal lobe epilepsy, depression, comorbidity, serotonin, hypothalamo-pituitary-adrenocortical axis, interleukin-1β
Introduction
Depression represents one of the most common comorbidities of temporal lobe epilepsy (TLE), and has profound negative impact on the quality of life of TLE patients (Kanner, 2003; Kondziella et al., 2007). However, causes and mechanisms of depression in TLE remain poorly understood, and its effective therapies are lacking. Furthermore, depression in TLE does not depend on the frequency of seizures (Gilliam et al., 2007), and a mere mitigation of seizures does not necessarily leads to the alleviation of depression (Spencer et al., 2003). Data on the effectiveness of antidepressants in epilepsy-associated depression have been scarce; in addition an empiric use of antidepressants for the treatment of depression in patients with epilepsy has been criticized as being based on “the largely untested assumption that patients with depression and epilepsy should respond to antidepressant drugs in the same manner as depressed nonepileptic patients” (Kanner, 2003). Indeed, such approach may overlook specific causes and mechanisms of depression as a comorbidity of epilepsy, as opposed to major depression.
One of challenges associated with understanding mechanisms of depression in epilepsy has been lack of validated animal models of this condition. We have been pursuing developing such a model, which could be useful both for studying mechanisms of depression in TLE, and as a screening platform for the development of therapeutic interventions. We have adopted an approach which includes examining the amenability of a commonly used model of TLE (post-LiCl and pilocarpine status epilepticus [SE] model of chronic epilepsy in rats) for concurrently reproducing depressive state.
Post-SE animals develop behavioral impairments indicative of depression
We began our inquiry with examining whether animals which develop chronic epilepsy following LiCl and pilocarpine SE exhibit behavioral equivalents of two major symptoms of depression – despair and anhedonia (i.e. the inability to experience pleasure).
In rodents, the state of despair is commonly examined using forced swim test (FST), which is based on their innate ability to adopt active strategies in the inescapable stressful situation (Pucilowski and Overstreet, 1993). Under the FST, animals exhibit two alternating behaviors - active escaping and/or exploring behavior, and relative immobility, when they move only enough to maintain their head above water and to avoid drowning. The increased immobility time in the FST has been regarded and validated as an indicator of the state of despair. In our studies, post-SE animals spent significantly longer time immobile (50% of a total 5 min test duration) as compared both with naïve rats and with themselves prior to SE (25% of a total test duration) (Table 1; Mazarati et al., 2008; Mazarati et al., 2009a), thus suggesting the state of despair. The examination of anhedonia in rodents relies on their innate preference towards sweets, and involves saccharin consumption test (Pucilowski et al., 1993). In this test, healthy subject, when given access to both tap water and saccharin solution, strongly prefer the latter, while animals with experimental depression consume equal amounts of water and saccharin. Such loss of taste preference towards saccharin has been regarded and validated as an indicator of anhedonia. Our studies established that post-SE animals exhibit loss of taste preference in the saccharin consumption test (Table 1; Mazarati et al., 2008).
Table 1.
Interictal depressive impairments in rats following LiCl and pilocarpine SE.
| Assay | Assay outcome (compared with both naïve rats and selves prior to SE) |
Corresponding hallmark of depression |
|---|---|---|
| Behavioral: Forced swim test 1,2. | Increased immobility time | Despair/hopelessness |
| Behavioral: Taste preference saccharin consumption test1. |
Loss of preference towards saccharin vs. tap water (i.e. equal consumption of each fluid). |
Anhedonia (i.e. inability to experience pleasure) |
| Endocrine: Plasma corticosterone (CORT) radioimmunoassay: basal CORT level and dexamethasone - corticotropin-releasing hormone (DEX/CRH) test2. |
Elevated CORT level; Failure of DEX to suppress CORT; Exacerbated and prolonged increase of CORT in response to CRH. |
Dysregulation of the HPA axis. |
| Biochemical: Fast cyclic voltammetry (FCV) of serotonin release from the hippocampus in response to raphe stimulation1. |
Lowered amplitude of serotonin oxidative peaks in the hippocampus. |
Compromised raphe- hippocampal serotonergic transmission. |
Thus, post-SE rats displayed at least two behavioral impairments consistent with depression – behavioral equivalents of despair and anhedonia. These impairments were not results of nonspecific motor and sensory deficits associated with SE: despite the decreased immobility time in the FST, post-SE animals’ ability to swim (as measured by swimming speed) was not impaired, and despite the loss of preference towards sweet saccharin solution, animals’ aversion to bitter quinine solution was preserved (personal unpublished data).
Dysregulation of hypothalamo-pituitary-adrenocortical (HPA) axis in post-SE rats
Dysregulation of the HPA axis represents an established hallmark of depression (Zobel et al., 2004; Kondziella et al., 2007). HPA dysregulation occurs as a result of the deficiency within a negative feedback neuroendocrine loop which controls the level of circulating glucocorticoid: the elevated level of plasma cortisol, which occurs in response to the increased release of corticotropin releasing hormone (CRH) and adrenocorticotropic hormone (ACTH) fails to inhibit further CRH release, thus resulting in the unabated and elevated level of the circulating glucocorticoid. The dysregulation of the HPA axis in depression is further revealed by the dexamethasone (DEX)/CRH test (Zobel et al., 2004). The latter is characterized by the inability of DEX to suppress plasma cortisol level and by the exacerbated and prolonged increase of plasma cortisol in response to CRH. Similar alterations are observed in animals with experimental depression. Our studies confirmed that post-SE animals exhibit interictal dysregulation of the HPA axis, which included both elevated plasma corticosterone (CORT, a major glucocorticoid in rodents) level and positive DEX/CRH test (Table 1; Mazarati et al., 2009a).
Compromised raphe-hippocampal serotonergic transmission in post-SE rats
Hippocampus receives serotonergic input from neurons located in median and dorsal raphe nuclei. Raphe-hippocampal serotonergic pathway plays an important role in regulating mood and is compromised in depression. The release of 5-HT from raphe serotonergic neurons is regulated by a number of intrinsic and external mechanisms. The most efficient mechanism consists of the short feedback autoinhibitory loop that involves somato-dendritic 5-HT1A receptors (autoreceptors). The activation of raphe 5-HT1A autoreceptors by locally released serotonin inhibits firing of serotonergic neurons and further neurotransmitter release (Riad et al., 2000). Thus, the upregulation of raphe 5-HT1A receptors would result in the compromised raphe-hippocampal serotonergic transmission. Indeed, such upregulation and subsequently enhanced autoinhibition of 5-HT release in depression have been confirmed by several clinical and experimental findings (Stockmeier et al., 1998; Boldrini et al., 2008). This mechanism may also be involved in depression associated with epilepsy: in TLE patients with concurrent depression, binding affinity of raphe 5-HT1A receptors was increased, and positively correlated with the severity of clinical symptoms of depression (Lothe et al., 2008).
We used fast cyclic voltammetry in vivo to examine the strength of the raphe hippocampal serotonergic transmission in post-SE rats. This technique involves direct electrochemical measurement of hippocampal concentration of an oxidative serotonin product (quinone) in response to electrical stimulation of raphe nucleus. Our studies revealed interictally diminished raphe-hippocampal serotonergic transmission in post-SE animals (Table 1; Mazarati et al., 2008).
We further proceeded to analyze possible involvement of raphe 5-HT1A receptors in depression observed in post-SE rats. We examined whether pharmacological blockade of raphe 5-HT1A receptors (which was achieved by the injection into the raphe nucleus of a selective 5-HT1A blocker WAY-100635) would improve symptoms of depression in post-SE animals. We found that WAY-100635 (10 and 100 nmoles) abolished abnormality in both forced swim behavior and raphe-hippocampal serotonergic deficit in post-SE rats. At 100 nmol, but not at 10 nmol, WAY-100635 also effectively shortened immobility time in the FST and increased raphe-hippocampal serotonergic transmission in naïve rats (personal unpublished data). The leftward shift in the effectiveness of WAY-100635 in post-SE rats as compared with healthy subjects suggested that epilepsy-associated depression is accompanied by the hypersensitization of raphe 5-HT1A receptors.
Role of dorsal raphe glucocorticoid receptors in epilepsy-associated depression
One of mechanisms through which the dysregulation of the HPA axis may lead to depression, is by hindering raphe hippocampal serotonergic transmission. Indeed, recent studies have suggested that glucocorticoids may positively regulate raphe 5-HT1A autoreceptors (Man et al., 2002; Bellido et al., 2004). It is thus conceivable that under conditions of the elevated plasma CORT level (as it occurs in depression and in following SE) the increased exposure of raphe glucocorticoid receptors to CORT would upregulate raphe 5-HT1A autoreceptors and thus would result in the diminished raphe-hippocampal serotonergic transmission. In such case, blocking of raphe glucocorticoid receptors would mitigate symptoms of depression. We tested this hypothesis by subjecting post-SE animals with established depression to one-week long continuous delivery of a selective glucocorticoid receptor blocker mifepristone into the dorsal raphe. In naïve rats, this treatment modified neither animals’ behavior, nor raphe hippocampal serotonergic transmission. However, in post-SE rats, blockade of raphe glucocorticoid receptors reversed both behavioral (despair) and biochemical (raphe-hippocampal serotonin deficit) hallmarks of depression (personal unpublished data). These findings suggests that raphe glucocorticoid receptors play no major role in regulating adaptive behavior in the FST and serotonergic transmission in healthy animals, but become important when the HPA axis is dysregulated and when the functional state of raphe systems that control serotonin release is impaired.
Depressive impairments in post-SE rats are resistant to fluoxetine
Selective serotonin reuptake inhibitors (SSRI) particularly fluoxetine are regarded as gold standard in the treatment of depression. However, in clinical depression at least 1/3 of patients do not respond to the SSRI therapy (Barbui et al., 2002). In our studies, post-SE depression was resistant to the two-week long treatment with fluoxetine (Mazarati et al., 2008), thus indicating that depression in the pilocarpine model belongs to a SSRI-resistant type of this mood disorder.
Depressive impairments do not depend on frequency of spontaneous recurrent seizures
In post-SE rats the severity of behavioral, endocrine and biochemical hallmarks of depression was statistically independent of the frequency of spontaneous seizures. Instead, it positively correlated with the interictal hippocampal hyperexcitability which was measured by detecting hippocampal afterdischarge properties (Mazarati et al., 2008). In addition, our earlier studies showed that animals in which chronic epileptic state had been induced by hippocampal kindling, exhibited behavioral and biochemical symptoms of depression several weeks after the completion of kindling procedure, in the absence of immediate seizures (Mazarati et al., 2007). Thus, depression in epilepsy might not be a result of recurrent seizures per se, but rather may be related to the tonic limbic dysfunction associated with epileptic state.
Activation of hippocampal interleukin-1β (IL-1β) signaling: a possible mechanism shared by TLE and concurrent depression
High incidence of depression among epilepsy patients, as well as reciprocal connection between the two conditions has led to the hypothesis that depression and epilepsy share certain mechanisms (Kanner, 2003; Kondziella et al., 2007). In the search of such mechanisms, we explored possible role of hippocampal neuroinflammation, and particularly of the enhanced interleukin-1β (IL-1β) signaling. Indeed, activation of hippocampal IL-1β and its receptor have been established hallmarks of TLE both in clinical and experimental settings (Vezzani et al., 2008). At the same time, IL-1β and may lead to depression conceivably via inducing perturbation in the HPA axis, as suggested by clinical observations, and confirmed by experimental studies (Dunn et al., 2005).
Such connections between epilepsy and IL-1β on the one hand, and IL-1β and depression on the other hand, prompted us to examine whether protracted pharmacological blockade of hippocampal IL-1 receptors exerts antidepressant effect in the post-SE animals. We found that protracted blockade of hippocampal IL-1 receptors, which was achieved by a 2 weeks-long continuous intrahippocampal infusion of human recombinant interleukin-1 receptor antagonist (hr-IL1Ra, Kalliolias and Liossis, 2008), attenuated all examined behavioral, endocrine and biochemical hallmarks of depression. This effect was not a consequence of seizure modification, since frequency of behavioral spontaneous seizures, as assessed by video monitoring, was not altered by the treatment. It should be noticed that hrIL-1Ra has been anticonvulsant in an animal model of TLE, when administered into lateral brain ventricle (Vezzani et al., 2008), or systemically (Marchi et al., 2009), which implicates different targets in its antidepressant and anticonvulsant effects. Furthermore, hrIL-1Ra improved the examined parameters of depression in epileptic rats selectively, without affecting responses in naïve animals (Mazarati et al., 2009b).
Interlekuin-1β – glucocorticoid – serotonergic hypothesis of comorbidity between TLE and depression
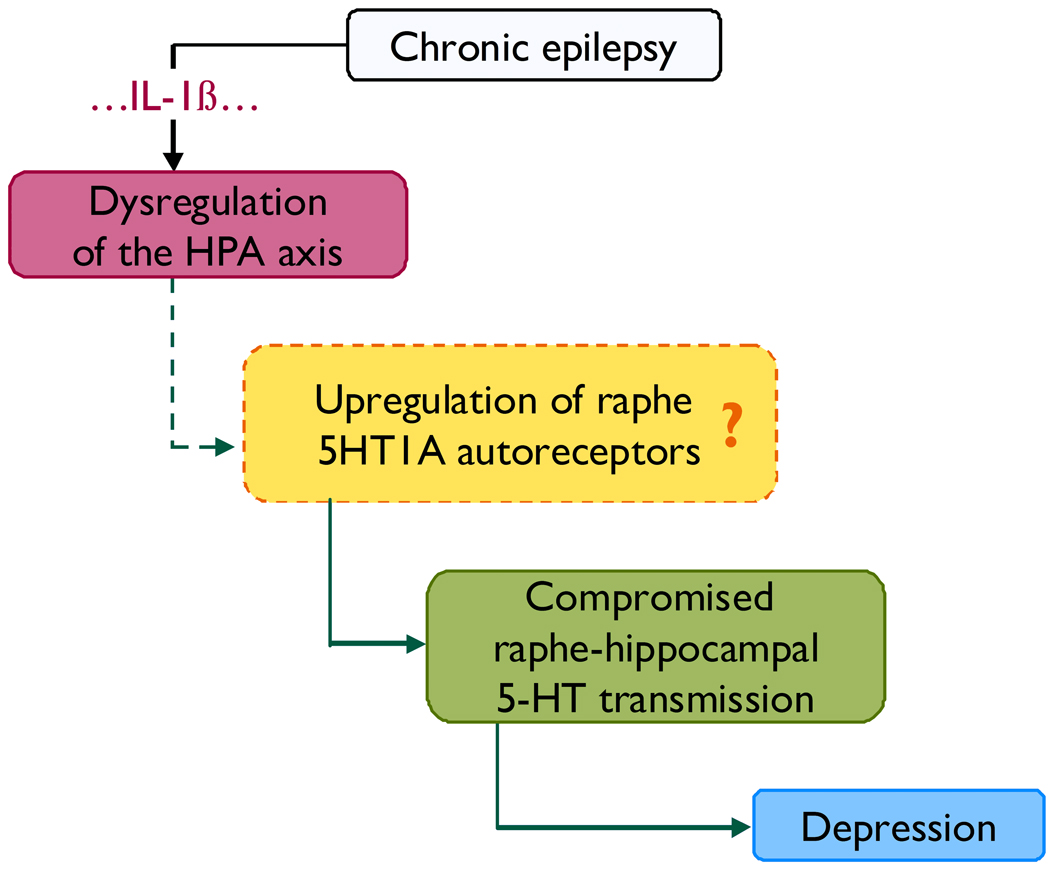
Based on studies performed up-to-date, we suggest that the following sequence of events may lead to depression associated with TLE (Fig. 1). Chronic epilepsy leads to the dysregulation of the HPA axis via several putative mechanisms, one of those being the activation of hippocampal IL-1β signaling. The enhanced level of circulating glucocorticoids and the hyperactivity of the HPA axis upregulate raphe 5-HT1A autoreceptors, and as a result compromise raphe-hippocampal serotonergic transmission. The latter serotonergic deficit leads to the development of clinical symptoms of depression.
Figure 1.
Proposed mechanisms of depression as a comorbidity of temporal lobe epilepsy. Chronic epilepsy leads to the dysregulation of the HPA axis (Mazarati et al., 2009a) via several putative mechanisms, one of those being activation of IL-1β signaling in the hippocampus (Mazarati et al., 2009b). One of possible consequences of the dysregulation of the HPA axis is the upregulation of 5-HT1A somatodendritic receptors in dorsal raphe and subsequent increased autoinhibition of serotonin release in the raphe-hippocampal pathway. The latter has not been yet confirmed directly in the pilocarpine model, but is suggested by behavioral and biochemical experiments involving the administration of glucocorticoid and 5-HT1A blockers into the raphe of post-SE rats. The resulting compromised raphe-hippocampal serotonergic transmission (Mazarati et al. 2008) leads to behavioral symptoms of depression (Mazarati et al., 2008, 2009a,b).
Conclusions
Clinical depression is a multifactorial and multisymptomatic disease, and apparently so is depression associated with epilepsy. Therefore, the validation the model of comorbidity between epilepsy and depression should continue with examining other hallmarks of depression and with the investigating other mechanisms involved. Studies undertaken by us up-to-date show that the pilocarpine model of epilepsy may be regarded as a model of comorbidity between epilepsy and depression useful both for mechanistic studies, and as a screening platform for the mechanism-driven therapeutic interventions.
Acknowledgement
The work was supported by research grants MH079933 from the National Institutes of Health and 132081 from Epilepsy Foundation of America (A. Mazarati).
Footnotes
Disclosures: None of the authors has any conflict of interest.
The authors confirm that they have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Barbui C, Hotopf M, Garattini S. Fluoxetine dose and outcome in antidepressant drug trials. Eur J Clin Pharmacol. 2002;58:379–386. doi: 10.1007/s00228-002-0497-7. [DOI] [PubMed] [Google Scholar]
- Bellido I, Hansson AC, Gomez-Luque AJ, Andbjer B, Agnati LF, Fuxe K. Corticosterone strongly increases the affinity of dorsal raphe 5-HT1A receptors. Neuroreport. 2004;15:1457–1459. doi: 10.1097/01.wnr.0000130542.06764.7f. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V. Serotonin-1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. J Psychiatr Res. 2008;42:433–442. doi: 10.1016/j.jpsychires.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Gilliam FG, Maton BM, Martin RC, Sawrie SM, Faught RE, Hugg JW, Viikinsalo M, Kuzniecky RI. Hippocampal 1H-MRSI correlates with severity of depression symptoms in temporal lobe epilepsy. Neurology. 2007;68:364–368. doi: 10.1212/01.wnl.0000252813.86812.81. [DOI] [PubMed] [Google Scholar]
- Kalliolias GD, Liossis SN. The future of the IL-1 receptor antagonist anakinra: from rheumatoid arthritis to adult-onset Still's disease and systemic-onset juvenile idiopathic arthritis. Expert Opin Investig Drugs. 2008;17:349–359. doi: 10.1517/13543784.17.3.349. [DOI] [PubMed] [Google Scholar]
- Kanner AM. Depression in epilepsy: prevalence, clinical semiology, pathogenic mechanisms, and treatment. Biol Psychiatry. 2003;54:388–398. doi: 10.1016/s0006-3223(03)00469-4. [DOI] [PubMed] [Google Scholar]
- Kondziella D, Alvestad S, Vaaler A, Sonnewald U. Which clinical and experimental data link temporal lobe epilepsy with depression? J Neurochem. 2007;103:2136–2152. doi: 10.1111/j.1471-4159.2007.04926.x. [DOI] [PubMed] [Google Scholar]
- Lothe A, Didelot A, Hammers A, Costes N, Saoud M, Gilliam F, Ryvlin P. Comorbidity between temporal lobe epilepsy and depression: a [18F]MPPF PET study. Brain. 2008;131:2765–2782. doi: 10.1093/brain/awn194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man MS, Young AH, McAllister-Williams RH. Corticosterone modulation of somatodendritic 5-HT1A receptor function in mice. J Psychopharmacol. 2002;16:245–252. doi: 10.1177/026988110201600310. [DOI] [PubMed] [Google Scholar]
- Marchi N, Fan Q, Ghosh C, Fazio V, Bertolini F, Betto G, Batra A, Carlton E, Najm I, Granata T, Janigro D. Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol Dis. 2009;33:171–181. doi: 10.1016/j.nbd.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati A, Shin D, Auvin S, Caplan R, Sankar R. Kindling epileptogenesis in immature rats leads to persistent depressive behavior. Epilepsy Behav. 2007;10:377–383. doi: 10.1016/j.yebeh.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati A, Siddarth P, Baldwin RA, Shin D, Caplan R, Sankar R. Depression after status epilepticus: behavioural and biochemical deficits and effects of fluoxetine. Brain. 2008;131:2071–2083. doi: 10.1093/brain/awn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati AM, Shin D, Kwon YS, Bragin A, Pineda E, Tio D, Taylor AN, Sankar R. Elevated plasma corticosterone level and depressive behavior in experimental temporal lobe epilepsy. Neurobiol Dis. 2009a;34:457–461. doi: 10.1016/j.nbd.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati AM, Shin D, Sankar R, Pineda E, Mazarati AM, et al. Comorbidity between epilepsy and depression: Role of hippocampal interleukin-1β. Neurobiol. Dis. 2009b doi: 10.1016/j.nbd.2009.11.001. In Press; doi:10.1016/j.nbd.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucilowski O, Overstreet DH. Effect of chronic antidepressant treatment on responses to apomorphine in selectively bred rat strains. Brain Res Bull. 1993;32:471–475. doi: 10.1016/0361-9230(93)90293-k. [DOI] [PubMed] [Google Scholar]
- Pucilowski O, Overstreet DH, Rezvani AH, Janowsky DS. Chronic mild stress-induced anhedonia: greater effect in a genetic rat model of depression. Physiol Behav. 1993;54:1215–1220. doi: 10.1016/0031-9384(93)90351-f. [DOI] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- Spencer SS, Berg AT, Vickrey BG, Sperling MR, Bazil CW, Shinnar S, Langfitt JT, Walczak TS, Pacia SV, Ebrahimi N, Frobish D. Initial outcomes in the Multicenter Study of Epilepsy Surgery. Neurology. 2003;61:1680–1685. doi: 10.1212/01.wnl.0000098937.35486.a3. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun. 2008;22:797–803. doi: 10.1016/j.bbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Zobel A, Wellmer J, Schulze-Rauschenbach S, Pfeiffer U, Schnell S, Elger C, Maier W. Impairment of inhibitory control of the hypothalamic pituitary adrenocortical system in epilepsy. Eur Arch Psychiatry Clin Neurosci. 2004;254:303–311. doi: 10.1007/s00406-004-0499-9. [DOI] [PubMed] [Google Scholar]