Abstract
Background
Dermatomyositis is an autoimmune disease of unknown etiology characterized by inflammation of the skin and muscles. Several medications have been implicated in the development of dermatomyositis1, however the disease has rarely been linked to the use of tumor necrosis factor-inhibitors (TNF-inhibitors). Herein we report four cases of dermatomyositis that developed or were exacerbated by exposure to the TNF-inhibitors etanercept and adalimumab.
Observation
Four patients with symptoms of inflammatory arthritis were treated with TNF-inhibitors, for a duration ranging from two months to two years. All patients developed symptoms consistent with dermatomyositis, including inflammatory rash and muscle weakness. Their symptoms persisted after discontinuation of the TNF-inhibitors but responded to treatment with steroids and immunosuppressive medications.
Conclusion
TNF-inhibitors have been associated with the onset of a number of autoimmune disorders, most commonly vasculitis and a lupus-like syndrome. Rarely have they been associated with dermatomyositis. The four cases presented here indicate that TNF-inhibitor use can be associated with either induction or exacerbation of dermatomyositis.
Introduction
Dermatomyositis is an autoimmune inflammatory condition of unknown etiology characterized by classic cutaneous findings and proximal muscle weakness. It can also be associated with interstitial lung disease and underlying malignancy. The primary rash is often pruritic and appears as confluent violaceous photodistributed erythema on the face, V-neck area of the chest, posterior neck and shoulders, and extensor surfaces of the arms. Other hallmark cutaneous manifestations include heliotrope periocular erythema, malar rash involving the nasolabial folds, Gottron’s papules, periungual telangectasias, mechanic’s hands, poikiloderma, and flagellate erythema2. The etiology is unknown, however there have been reports of cases of dermatomyositis that appear to be drug-induced1. Nineteen different medications have been implicated, the most common being hydroxyurea (36 cases), penicillamine (10 cases), and HMG-CoA reductase inhibitors (6 cases). Only two cases have been described in association with tumor necrosis factor (TNF) inhibitors, namely lenercept and etanercept3-5. We herein report four additional cases of dermatomyositis associated with TNF-inhibitors.
Report of Cases
Case 1
A 33-year-old woman with arthralgias and low titer rheumatoid factor (RF) positivity was diagnosed with rheumatoid arthritis (RA) and treated sequentially with etanercept followed by adalimumab for five months. When her symptoms did not improve, she saw a different rheumatologist who diagnosed her with fibromyalgia and stopped the adalimumab. Over the course of the next year, her arthralgias persisted and she developed mild proximal muscle weakness and pain as well as faint periocular erythema and swelling.
She developed an exacerbation of symptoms following sun exposure, consisting of arthralgias and mild malar and heliotrope erythema. Her original rheumatologist treated her with a single in-office injection of etanercept. Within days she developed very severe myalgias, arthralgias, exacerbation of her rash, shortness of breath, and fevers to 104.5 °F. She was admitted to the intensive care unit of an outside hospital and treated with antibiotics for possible sepsis, although her infectious workup was negative. Soon thereafter, she developed a generalized pruritic morbilliform rash and was placed on oral prednisone for a possible drug reaction.
She then presented to our institution with continued fevers, weakness and generalized rash. She underwent an extensive autoimmune work-up which revealed the following negative labs: ANA, double-stranded DNA (dsDNA), Scl-70, Smith, SSA, SSB, RNP, histone, anticardiolipin antibodies, RF, ANCA, HLA-B27, cryoglobulins, Mi-2, Jo-1, PM-Scl, PL-7, PL-12, EJ, OJ, KU, and SRP. C3 and C4 were normal. Creatinine kinase (CK) and anti-mitochondrial antibody were normal, however aldolase was elevated (18 U/L; reference range 1.2-7.6 U/L). Ferritin levels were persistently markedly elevated (16,282 ng/mL, reference 9-120 ng/mL). An infectious workup, including blood and urine cultures and serologies for Rocky Mountain spotted fever, lyme, ehrlichia, and parvovirus B19, was negative. A punch biopsy from a sun exposed area showed an interface dermatitis with a mixed inflammatory infiltrate.
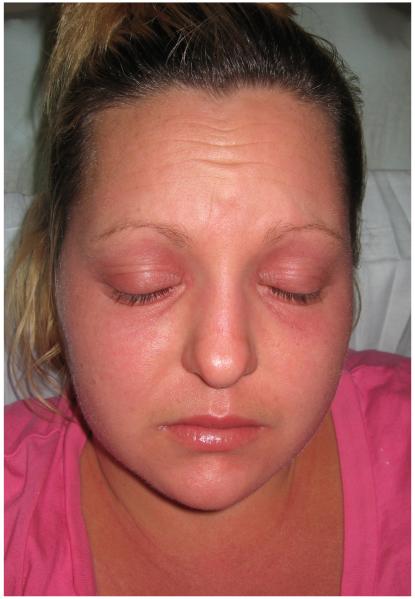
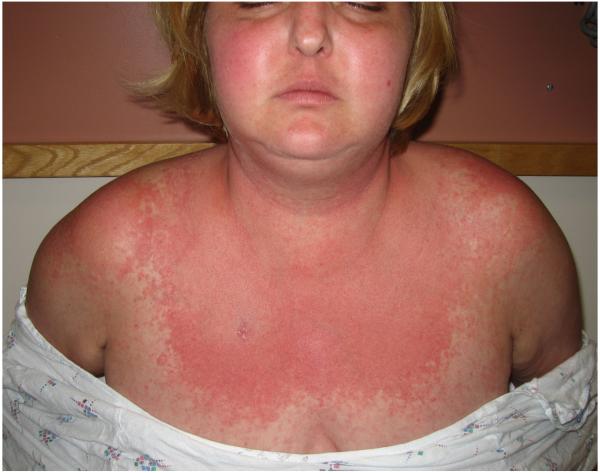
Based on the results of the skin biopsy, the elevated aldolase and ferritin, the morbilliform rash, and the fevers, underlying dermatomyositis, drug reaction, or Still’s disease were suspected. The patient was started on IV followed by oral methylprednisolone, resulting in prompt resolution of both the fevers and rash. As her steroids were tapered, however, she developed new skin findings consistent with dermatomyositis, including a heliotrope rash, Gottron’s papules on the elbows and interphalangeal joints, malar erythema involving the nasolabial folds, and mechanic’s hands. She also had fixed, violaceous patches on the V-neck of her chest, extensor surfaces of the arms and legs, back, and abdomen (Fig. 1).
Figure 1.
Clinical photographs of patient 1 show heliotrope erythema of the eyelids (A) and a violaceous patch in the v-neck area (B).
An MRI of the thigh and electromyography (EMG) while the patient was on steroids did not show evidence of active myositis or myopathy. Pulmonary function tests (PFTs) showed mild restrictive lung disease with decreased carbon monoxide diffusing capacity (DLCO), but a high-resolution CT scan of the chest was normal. Given the results of the biopsy, the elevated aldolase, the new rash, and the abnormal PFTs, a diagnosis of dermatomyositis was made.
A malignancy screening including a colonoscopy, pap smear, mammogram, positron emission tomography (PET) scan, bone scan, peripheral blood flow cytometry, CT scans of chest, abdomen, and pelvis, and transvaginal and retroperitoneal ultrasounds was unremarkable.
As an outpatient, she was treated with mycophenolate mofetil and later hydroxychloroquine, which resulted in a marked clinical improvement. Six months later, she has stable proximal muscle weakness and shortness of breath with unchanged DLCO. Her rash remains in remission on low-dose methylprednisolone, mycophenolate mofetil, hydroxychloroquine, medium potency topical steroids, and broad spectrum sun protection.
Case 2
A 40-year-old woman with symptoms of inflammatory arthritis and a low titer RF positivity was diagnosed with RA. She was started on etanercept therapy and two years later developed pruritus and erythema in a photodistributed pattern exacerbated by sun exposure. The etanercept was discontinued, and she was treated with topical steroids, systemic corticosteroids and hydroxychloroquine. Eight months later, the rash continued to progress and she developed symmetric proximal lower extremity muscle weakness. She had no pulmonary symptoms. On physical exam, she had Gottron’s papules on the proximal interphalangeal joints, periungual erythema with cuticular hypertrophy and telangiectatic vessels, facial erythema, and poikilodermatous patches her on neck, chest, upper back, trunk and thighs.
An autoimmune workup showed an elevated ANA (255 AU/mL, reference range 0-99 AU/mL) and elevated RF (14.8 IU/mL, reference range 0-13.9). Mitochondrial antibody, histone antibody, Jo-1, SSA, SSB, dsDNA, C3, C4, Smith, RNP, ESR, were normal. CRP was mildly elevated (6.9 mg/L, reference range 0.0-4.9). A urinalysis was normal. CK (1245 U/L, reference range 24-173 U/L) and aldolase were elevated (20.4, reference range 1.2-7.6). A punch biopsy from a poikilodermatous patch on her abdomen showed a focal interface dermatitis, focal vacuolar alteration and a superficial perivascular infiltrate. PFTs and chest x-ray were normal.
A malignancy screen including a mammogram, pap smear, CBC, and CT scans of the chest, abdomen, and pelvis is in progress.
She was treated with high dose corticosteroids and rituximab, and her rash and muscle weakness improved.
Case 3
A 29-year-old woman with a history of a seronegative inflammatory arthritis and a family history of psoriasis was treated with adalimumab and methotrexate. She previously had malar erythema but no additional skin findings, muscle weakness, or shortness of breath. Three months after starting the adalimumab, she developed photosensitivity and proximal muscle weakness in the lower extremities bilaterally. On physical exam, she had diffuse V-neck erythema, Gottron’s papules over the interphalangeal joints, periungual erythema, and pitting of the nails.
An autoimmune workup demonstrated a positive ANA (1:640) and normal dsDNA, Jo-1, SSA, SSB, Smith, RNP, C3, C4, CRP, and ESR. CK (95 U/L, reference range 20-150 U/L) and aldolase (3.7 U/L, reference range 1.5-8.1) were within normal limits. Lyme antibody titers were negative. A biopsy showed an interface dermatitis with increased mucin deposition and a lymphocytic perivascular infiltrate. An MRI of the thigh exhibited muscle edema. EMGs showed evidence of myopathy in the proximal muscles of the upper extremities. PFTs were normal.
A malignancy workup, including a pap smear, transvaginal pelvic ultrasound, chest x-ray, and CTs of the chest, abdomen, and pelvis, was normal.
The adalimumab was discontinued and she was treated with successive additions of prednisone, methotrexate, hydroxychloroquine, azathioprine and quinacrine. Her muscle weakness improved with therapy. She had a slight improvement of her rash initially, but it ultimately remained active despite treatment.
Case 4
A 51-year-old man with a history of a dermatomyositis / RA overlap syndrome (RF positive and anti-cyclic citrullinated peptide antibody positive) refractory to methotrexate and azathioprine presented with worsening pulmonary symptoms after treatment with adalimumab. When he was initially diagnosed with dermatomyositis overlap, he had myalgias in the proximal muscles, mechanic’s hands, a heliotrope rash, Gottron’s sign on the interphalangeal joints, and palmar erythema. A malignancy workup, including an endoscopy, colonoscopy, prostate-specific antigen, and CTs of the chest, abdomen, and pelvis was unremarkable. A high resolution CT scan showed mild interstitial fibrosis at the bases, but PFTs were normal. Two months after starting adalimumab, he noted increased dry cough and his DLCO decreased from 98% to 58% predicted. The adalimumab was stopped, and he was treated with prednisone, hydroxychloroquine, and cyclophosphamide. One year later, his DLCO has increased to 68% predicted and his exercise tolerance has improved.
Comment
TNF-inhibitors have been associated with the development of a wide array of autoimmune diseases, including lupus-like syndromes, vasculitis, interstitial lung disease, sarcoidosis, autoimmune hepatitis and uveitis, and antiphospholipid syndrome5, 6. Of these, lupus and vasculitis are the most common, together comprising 60% of documented cases of TNF-induced autoimmune disease5. Moreover, the incidence of ANA-positivity increases three-fold with anti-TNF therapy, even in the absence of a lupus-like syndrome7. TNF-induced dermatomyositis, however, is rare, constituting less than one percent of reported cases of TNF-induced autoimmunity. Previous case series and open label studies of TNF-inhibitors used to treat dermatomyositis have suggested these agents may exacerbate disease; in a case series of five patients treated with etanercept for dermatomyositis, all experienced worsening of their muscle weakness and did not have any improvement of their rash8.
In our case series, three out of four patients did not carry a diagnosis of dermatomyositis prior to exposure to the TNF-inhibitors, although all had a history of inflammatory arthritis. For several years, their complaints of joint pain were attributed to other disease processes, such as RA. Because patients with dermatomyositis can develop arthritis, it is difficult to ascertain whether the patients truly had another disease or if the arthritis was an early symptom of dermatomyositis. If the latter were true, it would seem that the anti-TNF medications either uncovered or accelerated the progression of underlying dermatomyositis in patients with otherwise limited symptoms.
Regardless, each patient in our series worsened after treatment with TNF-inhibitors, and in one instance (case 1) the effects seemed rapid and dramatic when the medication was reintroduced after a prolonged hiatus. Further, all patients in our series showed some improvement in their symptoms when both the TNF-inhibitor was stopped and appropriate therapy for dermatomyositis was started. Thus, while we cannot say with certainty that TNF-inhibitors induced dermatomyositis in these cases, exposure seemed to coincide with worsening and/or progression of symptoms that may not be explained by the natural history of dermatomyositis alone.
At first glance, the notion that TNF-inhibition can exacerbate dermatomyositis seems counterintuitive; TNF-α and its receptor are overexpressed in dermatomyositis, suggesting a contribution to the pathogenesis of the disease9-11. However, attempts at pharmacologically blocking TNF-α have led to disease flares in patients with inflammatory myopathies8, 12. It is not yet fully understood why this occurs, however several mechanisms have been proposed13. The cytokine-shift hypothesis suggests that the inhibition of TNF-α promotes the expression of type I interferon (IFN) by altering the balance between Th1 and Th2 cytokine production12, 14, 15. This increase in type I IFN, which has been shown to be important in the pathogenesis of dermatomyositis, may contribute to the exacerbation of symptoms16, 17. Another possibility is that TNF-α blockade interferes with apoptosis, allowing for the increased formation of autoantibodies18. This hypothesis is supported by the observation that when patients with RA are treated with TNF-inhibitors, they develop antibodies against ANA and ds DNA7.
Our cases differed significantly with respect to the temporal relationship between the initiation of anti-TNF therapy and the worsening of dermatomyositis, ranging from two months to approximately two years. This is consistent with other reports in the literature of general drug induced dermatomyositis, which has been shown to occur after a wide range of duration of therapy1. Similarly, exacerbation of inflammatory myopathies specifically following treatment with a TNF-inhibitor has also occurred at varied times, ranging from one week to ten months8, 12. The reasons for this heterogeneity are not clear but may be related to unidentified host factors.
A recent review on drug-induced dermatomyositis indicates that over 90% of patients noted improvement in their rash and myositis simply after discontinuing the medication1, however the patients reported here required additional therapy with corticosteroids and immunosuppressive medications to control their symptoms. This observation may reflect the previously described notion that TNF-inhibition may cause sustained alterations in the immune system which are not easily overcome by simply removing the offending agent.
In conclusion, TNF-inhibitors are a valuable tool in treating a number of inflammatory and autoimmune disorders. While they can be an important asset to patient care, their use has rarely been associated with serious adverse events, including infections, certain malignancies, demyelinating disorders, congestive heart failure, and autoimmune disease19-23. As their use continues to become more widespread, it is important for providers to be aware of, and monitor for, these complications. In addition, patients with inflammatory arthritis being treated with TNF-inhibitors who develop weakness, skin rash, or pulmonary symptoms should be promptly evaluated for dermatomyositis. Physicians should also be cautious in using these medications in patients with known dermatomyositis.
Acknowledgment
We are indebted to Dr. Joan Von Feldt for her critical review of the manuscript.
Footnotes
Financial Disclosure: None reported. The authors report no conflicts of interest.
References
- 1.Seidler AM, Gottlieb AB. Dermatomyositis induced by drug therapy: a review of case reports. J Am Acad Dermatol. 2008 Nov;59(5):872–880. doi: 10.1016/j.jaad.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 2.Sontheimer RD. Skin manifestations of systemic autoimmune connective tissue disease: diagnostics and therapeutics. Best Pract Res Clin Rheumatol. 2004 Jun;18(3):429–462. doi: 10.1016/j.berh.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Flendrie M, Vissers WH, Creemers MC, de Jong EM, van de Kerkhof PC, van Riel PL. Dermatological conditions during TNF-alpha-blocking therapy in patients with rheumatoid arthritis: a prospective study. Arthritis Res Ther. 2005;7(3):R666–676. doi: 10.1186/ar1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall HA, Zimmermann B. Evolution of dermatomyositis during therapy with a tumor necrosis factor alpha inhibitor. Arthritis Rheum. 2006 Dec 15;55(6):982–984. doi: 10.1002/art.22358. [DOI] [PubMed] [Google Scholar]
- 5.Ramos-Casals M, Brito-Zeron P, Soto MJ, Cuadrado MJ, Khamashta MA. Autoimmune diseases induced by TNF-targeted therapies. Best Pract Res Clin Rheumatol. 2008 Oct;22(5):847–861. doi: 10.1016/j.berh.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Ramos-Casals M, Brito-Zeron P, Munoz S, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore) 2007 Jul;86(4):242–251. doi: 10.1097/MD.0b013e3181441a68. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson C, Engstrand S, Sundqvist KG, Rantapaa-Dahlqvist S. Autoantibody formation in patients with rheumatoid arthritis treated with anti-TNF alpha. Ann Rheum Dis. 2005 Mar;64(3):403–407. doi: 10.1136/ard.2004.024182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iannone F, Scioscia C, Falappone PC, Covelli M, Lapadula G. Use of etanercept in the treatment of dermatomyositis: a case series. J Rheumatol. 2006 Sep;33(9):1802–1804. [PubMed] [Google Scholar]
- 9.De Bleecker JL, Meire VI, Declercq W, Van Aken EH. Immunolocalization of tumor necrosis factor-alpha and its receptors in inflammatory myopathies. Neuromuscul Disord. 1999 Jun;9(4):239–246. doi: 10.1016/s0960-8966(98)00126-6. [DOI] [PubMed] [Google Scholar]
- 10.Lundberg IE, Dastmalchi M. Possible pathogenic mechanisms in inflammatory myopathies. Rheum Dis Clin North Am. 2002 Nov;28(4):799–822. doi: 10.1016/s0889-857x(02)00025-x. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu T, Tomita Y, Son K, Nishinarita S, Sawada S, Horie T. Elevation of serum soluble tumour necrosis factor receptors in patients with polymyositis and dermatomyositis. Clin Rheumatol. 2000;19(5):352–359. doi: 10.1007/s100670070027. [DOI] [PubMed] [Google Scholar]
- 12.Dastmalchi M, Grundtman C, Alexanderson H, et al. A high incidence of disease flares in an open pilot study of infliximab in patients with refractory inflammatory myopathies. Ann Rheum Dis. 2008 Dec;67(12):1670–1677. doi: 10.1136/ard.2007.077974. [DOI] [PubMed] [Google Scholar]
- 13.Williams EL, Gadola S, Edwards CJ. Anti-TNF-induced lupus. Rheumatology (Oxford) 2009 May 4; doi: 10.1093/rheumatology/kep080. [DOI] [PubMed] [Google Scholar]
- 14.Romagnani S. Th1/Th2 cells. Inflamm Bowel Dis. 1999 Nov;5(4):285–294. doi: 10.1097/00054725-199911000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Singh VK, Mehrotra S, Agarwal SS. The paradigm of Th1 and Th2 cytokines: its relevance to autoimmunity and allergy. Immunol Res. 1999;20(2):147–161. doi: 10.1007/BF02786470. [DOI] [PubMed] [Google Scholar]
- 16.Eloranta ML, Barbasso Helmers S, Ulfgren AK, Ronnblom L, Alm GV, Lundberg IE. A possible mechanism for endogenous activation of the type I interferon system in myositis patients with anti-Jo-1 or anti-Ro 52/anti-Ro 60 autoantibodies. Arthritis Rheum. 2007 Sep;56(9):3112–3124. doi: 10.1002/art.22860. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg SA, Pinkus JL, Pinkus GS, et al. Interferon-alpha/beta-mediated innate immune mechanisms in dermatomyositis. Ann Neurol. 2005 May;57(5):664–678. doi: 10.1002/ana.20464. [DOI] [PubMed] [Google Scholar]
- 18.Lorenz HM, Herrmann M, Winkler T, Gaipl U, Kalden JR. Role of apoptosis in autoimmunity. Apoptosis. 2000 Nov;5(5):443–449. doi: 10.1023/a:1009692902805. [DOI] [PubMed] [Google Scholar]
- 19.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006 May 17;295(19):2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 20.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003 Jul 1;107(25):3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 21.Genovese MC, Bathon JM, Fleischmann RM, et al. Longterm safety, efficacy, and radiographic outcome with etanercept treatment in patients with early rheumatoid arthritis. J Rheumatol. 2005 Jul;32(7):1232–1242. [PubMed] [Google Scholar]
- 22.Mohan N, Edwards ET, Cupps TR, et al. Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthritides. Arthritis Rheum. 2001 Dec;44(12):2862–2869. doi: 10.1002/1529-0131(200112)44:12<2862::aid-art474>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 23.van Oosten BW, Barkhof F, Truyen L, et al. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology. 1996 Dec;47(6):1531–1534. doi: 10.1212/wnl.47.6.1531. [DOI] [PubMed] [Google Scholar]