Abstract
p66Shc, a 66 kDa proto-oncogene Src homologous-collagen homologue (Shc) adaptor protein, is classically known in mediating receptor tyrosine kinase signaling and recently identified as a sensor to oxidative stress-induced apoptosis and as a longevity protein in mammals. The expression of p66Shc is decreased in mice and increased in human fibroblasts upon aging and in aging-related diseases, including prostate cancer. p66Shc protein level correlates with the proliferation of several carcinoma cells and can be regulated by steroid hormones. Recent advances point that p66Shc protein plays a role in mediating cross-talk between steroid hormones and redox signals by serving as a common convergence point in signaling pathways on cell proliferation and apoptosis. This article first reviews the unique function of p66Shc protein in regulating oxidative stress-induced apoptosis. Subsequently, we discuss its novel role in androgen-regulated prostate cancer cell proliferation and metastasis and the mechanism by which it mediates androgen action via the redox signaling pathway. The data together indicate that p66Shc might be a useful biomarker for the prognosis of prostate cancer and serve as an effective target for its cancer treatment.
Keywords: p66Shc, Apoptosis, Redox, Cell proliferation, Metastasis, Prostate cancer
1 Introduction
Cell proliferation and apoptosis are the two indispensable physiologically balanced, associated, and regulated cellular processes [1]. Disruption in this homeostasis forms the basis for several life-threatening diseases, for example, cancer, neurodegenerative, and autoimmune diseases [2–4]. The foremost and fundamental mechanism that governs these two processes is the signal transduction machinery that is mediating this process [1, 5, 6].
Adaptor proteins are in general signaling molecules lacking enzymatic activity while they have the ability to interact with other proteins mediating cell signaling. The prototype Src homologue and collagen homologue (Shc) adaptor proteins were cloned by using an SH2-coding sequence as a probe, and this family of proteins includes three members with molecular masses of 46, 52, and 66 kDa, which are encoded by the same gene at chromosome 1q21 [7]. The Shc adaptor protein has been considered as one of the most studied adaptor proteins for exploring the role of adaptor proteins in cellular signaling; therefore the role of Shc proteins in mediating diverse signaling pathways has received much attention [7–11]. The expression of these adaptor proteins, especially p66Shc, might be regulated by steroid hormones [10] that play a distinct role in the regulation of tumor development, cancer cell proliferation, progression, and metastatic processes of major types of cancers [12]. Moreover, cross-talks between tyrosine phosphorylation signaling and steroid hormones have been well established [13–17]. It is now evident that either in the early stage of carcinogenesis or in the advanced metastatic phenotype, steroid hormone action goes far beyond the classical receptor-mediated gene regulation. These steroid hormone-related cancers share some common mechanisms of carcinogenesis, such as DNA damage/mutation and aberrant growth regulation due to the elevated levels of various growth factors induced by the excess of steroids [18–20].
Growth factors and their receptors are known to be involved in regulating various steps of carcinogenesis, including cell proliferation, motility, invasion and/or migration of various cell types [21–29]. The activated receptors triggered by growth factors, cytokines or adhesion molecules facilitate the docking of Shc adaptor molecules, containing Src homology 2 (SH2) and phosphotyrosine-binding (PTB) domains, which transduce signals to downstream intracellular cascades. Thus, Shc proteins play a conceivable role in various stages of carcinogenesis.
Moreover, several lines of evidence indicate that Shc proteins mediate diverse biological activities other than merely serve as adaptors in tyrosine phosphorylation signaling. One such functional role of Shc proteins, specifically p66Shc, is in mediating redox signaling and thus is deserving further studies ever since it is reported to play a prominent role in oxidative stress-induced apoptosis and the life span of mammals. [30]. Other Shc members have also been implicated in the longevity and apoptosis of C. elegans and Drosophila [31, 32]. Indeed, p66Shc expression is decreased in mice with advanced age and is reduced in mouse lung cells upon treatment with aurintricarboxylic acid that promotes cell survival, suggesting p66Shc as one of the lifespan determinants in mammals [33].
Aberrant expression of p66Shc could also be involved in various stages of carcinogenesis [9, 10, 34–38]. Despite the fact that Shc proteins could be differentially phosphorylated in different types of primary tumors and tumor cell lines [28, 39, 40], a direct correlation between the protein level of p66Shc and prostate cancer cell proliferation is observed, insisting the importance of p66Shc adaptor protein in the tumorigenicity of human prostate cancer [10, 41, 42]. Thus, in the light of above facts, the present review will emphasis both the extremities of p66Shc adaptor protein, including its apoptotic potential in the control of lifespan in mammals and its novel role in androgen-regulated prostate cancer cell proliferation and metastasis and the mechanism by which it mediates the androgen action via redox signaling pathway.
2 p66Shc —a unique isoform of ShcA adaptor protein
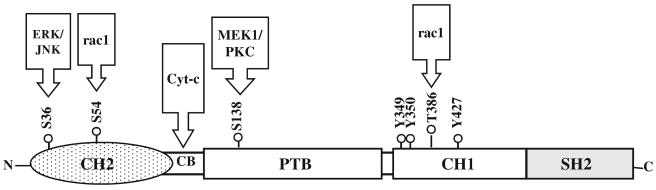
p66Shc is an isoform of ShcA family of adaptor proteins, transcribed from a promoter in the first intron of Shc locus [43]. It contains four functional domains, a SH2 domain (∼100 amino acids) at the COOH-terminal that mediates the formation of multiprotein complexes during signaling [44, 45] and a PTB binding domain, which is separated by a collagen homology (CH1) domain, enriched in proline and glycine residues and contains the essential tyrosine phosphorylation sites (Fig. 1) [7, 9]. Structurally, p66Shc differs from p52Shc and p46Shc by virtue of its unique NH2-terminal, a 110-amino acid CH2 region, which is also rich in proline and glycine residues and contains the inimitable serine phosphorylation (S36 & S54) sites [8]. In addition to these domains, p66Shc also carries a cytochrome c-binding (CB) region within the CH2-PTB domains that is primarily implicated in the mitochondrial regulation of oxidative stress [46].
Fig. 1.
A schematic organization of p66Shc. The p66Shc consists of a SH2 domain at the COOH-terminal, three major tyrosine phosphorylation sites and a threonine phosphorylation site within the central CH1 domain and a serine phosphorylation site (S138) in the PTB domain. It also contains a unique CH2 domain [110 amino acids] comprising the serine phosphorylation sites (S36 and S54) at the NH2-terminal. p66Shc also possesses a cytochrome c-binding region (CB) within the CH2-PTB domains
2.1 Ambiguous presence of p66Shc
Unlike the ubiquitous expression of p52Shc and p46Shc isoforms, p66Shc expression is vastly constrained that may be due to the utilization of two alternative promoters and the selective regulation of the transcriptional inclusion of the N-terminal CH2 domain, which is specific for p66Shc [47]. p66Shc is expressed primarily in epithelial cells [8] whereas its expression is missing or very stumpy in neurons, hematopoietic cell lines and peripheral blood lymphocytes, and further its expression varies in breast and prostate cancer cell lines [7, 34, 40, 41, 48]. Intriguingly, peripheral blood lymphocytes, mouse thymocytes and splenic T-cells attain the ability to express p66Shc in response to oxidative stress stimuli [49], which might be attributed to the transcriptional regulation of p66Shc gene promoter.
2.2 Tweaking of p66Shc expression
The p66Shc protein level can be regulated at the levels of post-translation and transcription. The post-translational modifications of p66Shc protein include protein phosphorylation and prolyl-isomerization. For example, serine 36 (S36) phosphorylation of p66Shc upon oxidative stress results in shift in the mobility of p66Shc protein in SDS-gels in various cell types [30, 50–53] and also rac1-mediated phosphorylation of S54 in the CH2 domain and T386 in the CH1 domain of p66Shc (Fig. 1) causes decreased p66Shc protein turnover by disguising their proteolytic PEST signal sequence that can be recognized by the ubiquitin-26S proteasome degradative pathway [54]. The later event of post-translational modification also implicates the substantial role of rac1 in the stability of p66Shc. Cis-trans isomerization of phosphorylated p66Shc at S36 by Prolyl isomerase can result in a conformational change that facilitates the transfer of p66Shc from cytosol to mitochondria in mouse embryo fibroblasts (MEFs) [55]. A S36-phosphorylation independent pathway has also been established for the translocation of p66Shc from cytosol to mitochondria induced by androgens in prostate cancer cells [42], which will be discussed later.
Regulation of p66Shc expression at the transcriptional level includes the silencing of p66Shc by epigenetic modifications of its promoter such as DNA methylation and histone deacetylation. Evidently, the expression of p66Shc is restored in primary, immortal and transformed cells upon treatments of histone deacetylase inhibitors and demethylation agents, and also an inverse correlation is observed between p66Shc promoter methylation and its expression in the cell lines expressing various amounts of p66Shc [43, 56]. This methylation disparity in the p66Shc promoter is consecutively involved in the process of both aging and tumorigenesis, as age-related methylation possibly serves as a basic marker in cancer subjects [57–61]. Strikingly, it has been found that the promoter methylation does not have any influence on the p66Shc expression level in the centenarians; while the p66Shc expression in these subjects is affected by p53 codon 72 polymorphism following oxidative stress [62]. Studies by Mooijaart et al. [63] exemplify the correlation of the Met410Val polymorphism in p66Shc gene with the regulation of lifespan in humans. However, the contradictions in the relationship of p66Shc expression in cellular senescence need further investigations.
2.3 Tyrosine phosphorylation of p66Shc
ShcA proteins are the targets of receptor tyrosine kinases (RTKs) and are concerned in the transmission of mitogenic signals to Ras [7]. In this context, like other isoforms of p52Shc/p46Shc, p66Shc is also tyrosine phosphorylated by RTKs upon growth factor stimulation and binds effectively to Grb2, while it is unable to activate the Ras-MAPK-Fos pathways and also unable to transform NIH3T3 mouse fibroblasts [8, 30]. Indeed, over expression of p66Shc result in the inhibition of Ras signaling pathway in response to EGF or to cytokines [8, 49, 53]. p66Shc also inhibits the IGF-1 stimulation of MEK/ERK pathway that is required for actin cytoskeleton phosphorylation in skeleton muscle myoblasts [64]. The possible explanation for the inhibitory effect of p66Shc on mitogenic signaling are (1) upon growth factor (e.g., EGF) stimulation, p66Shc competes with p52Shc/p46Shc isoforms for binding to the limited cellular pool of Grb2 and seizes the Grb2/SOS complex, but is unable to recruit SOS, thus results in the dominant negative inhibition of p52Shc/p46Shc–mediated MAPK pathway (Fig. 2). (2) The reason could also be that increased expression of p66Shc has resulted in an elevated basal activity of ERK/MAPK in the absence of stimulus, which thus minimizes the extent of further activation by growth factors such as EGF [41]. (3) Serine/threonine phosphorylation of p66Shc that takes place upon EGF stimulation and following tyrosine phosphorylation is proposed to be crucial for the termination of Ras/ERK/MEK pathways as this event may uncouple the association of p66Shc·Grb2 complex from EGFR via forming the nonproductive complex with Grb2 (Fig. 2) [49, 53]. Further, the inhibitory effect on the c-fos promoter activation by p66Shc is primarily mediated by the N-terminal CH2 domain rather than by the critical CH1 domain; the later is in common with the p52Shc/p46Shc mediated Ras-signaling [8]. These facts further exploit the functional importance of the unique N-terminal CH2 domain of p66Shc and it could be proposed that p66Shc plays a role independent of Ras pathway.
Fig. 2.
A possible mechanism for the inhibitory effect of p66Shc on mitogenic signaling. Upon growth factor, e.g., EGF, stimulation, p66Shc competes with p52Shc/p46Shc isoforms for binding to the limited cellular pool of Grb2 and seizes the Grb2/SOS complex, but is unable to recruit SOS. Subsequently, serine 36 (S36) phosphorylation of p66Shc following tyrosine phosphorylation uncouples the association of p66Shc·Grb2 complex from EGFR via forming the nonproductive complex with Grb2, and thus may result in the dominant negative inhibition of p52Shc/p46Shc-mediated Ras-MAPK pathway
2.4 Serine phosphorylation of p66Shc
The major serine phosphorylation sites of p66Shc are S36 and S54 in its CH2 domain and S138 in the PTB domains (Fig. 1). Several stimuli that are engaged in tyrosine phosphorylation of p66Shc also trigger the phosphorylation of S138 in the PTB domain [65]. The S54 site in the CH2 domain of p66Shc is found to be critical for the stability of p66Shc [54]. The S36 site is unique to p66Shc isoform and is responsible for oxidative stress response whereas the S138 site is homologous to S29 in p52Shc that is regulated by PKC and MEK1 (Fig. 1) [65, 66]. Further, S36 phosphorylation of p66Shc has also been shown to exert a pro-apoptotic effect in different cell types. The variation in the responses of S36 phosphorylation both in vivo and in vitro depend on the kinase(s) (MAPK, stress-activated JNK and p38) that mediate this process that may vary depending on the cell type, cellular milieu and the distinctiveness of the inducement [50, 52, 53, 67].
Rapid phosphorylation on S36 is observed upon EGF and H2O2 treatments in fibroblasts. Significantly, p66Shc −/− cells are unable to restore the pro-apoptotic response to H2O2 upon expression of p66S36A mutant into these cells, thus substantiating the role of S36 phosphorylation in inducing apoptosis [30]. Severe oxidative stress induces ERK/JNK-dependent S36 phosphorylation of p66Shc in renal proximal tubule cells that causes cell death via negative feedback inhibition of Ras/MEK/ERK survival pathway by the early activation of ERK and JNK [68]. In Chinese hamster ovary (CHO) cells, p66Shc negatively regulates the EGF-induced Ras/Raf/MEK/ERK pathway via MEK-dependent S36 phosphorylation of p66Shc, which is followed by tyrosine phosphorylation of p66Shc [53]. Serine phosphorylation of p66Shc also takes place in response to other factors including insulin, TPA, FGF-2, taxol and endothelin-1 [52, 53, 66, 69]. Taxol-induced serine phosphorylation of p66Shc in both macrophage-like cell line (RAW 264.7) and in non-macrophage-like human cell line (human lung carcinoma cells-A549) stimulates the signaling cascade that lead to cell death in a MEK-dependent and MEK-independent manner, respectively, via inducing TNF-α expression in RAW 264.7 cells and inducing PARP cleavage in A549 cells [52, 67]. In CHO cells, insulin also induces MEK-dependent S36 phosphorylation of p66Shc and results in the inactivation of Ras [51] in a manner similar to the insulin-stimulated MEK-dependent SOS phosphorylation [70]. Nevertheless, the biological significance of insulin-induced S36 phosphorylation of p66Shc in these cells is unclear.
It has been reported that serine phosphorylation of p66Shc promotes the binding of p66Shc to the sequestering protein 14-3-3 and protein-tyrosine phosphatase (PTP) PEST [71]. Endothelin-1-induced serine phosphorylation in glomerular mesangial cells promotes the binding of p66Shc to the sequestering protein 14-3-3 in a MEK/ERK dependent pathway and exerts an anti-apoptotic effect [65]. It is thus proposed that this may occur via seizing the apoptosis-inducing protein BAD, i.e., Bcl-xL/Bcl-2 associated death promoter, by the sequestering protein 14-3-3 from mitochondria to cytosol [72]. Moreover, serine phosphorylation of p66Shc by endothelin-1 through ERK has been reported to inhibit apoptosis in vascular smooth muscle cells [73]. Nevertheless, it is unclear whether S36 or S138 or both is the requisite for binding of p66Shc to the sequestering protein. On the contrary, in NIH3T3 fibroblasts, TPA-induced S138 phosphorylation in the PTB domain of p66Shc leads to p66Shc·Grb2 association and the activation of ERK [69] that is indirectly promoted by the binding of p66Shc to PTP-PEST in a PKC-dependent manner [66]. However, the functional role of TPA-induced S138 phosphorylation of p66Shc has not been clearly shown and further incompatibility persists with the binding of p66Shc to PTP-PEST [74, 75]. These facts therefore imply the inevitable role of p66Shc serine phosphorylation in regulating both apoptosis and cell survival.
3 p66Shc and oxidative stress
Several lines of evidence for p66Shc and its pivotal play in the oxidative stress response result from the information on p66Shc-null mice showing enhanced resistance to oxidative stress in vivo upon treatment with paraquat, and amazingly, the p66Shc−/− mice have a 30% increase in their life span when compared with their normal animals [30]. Consistently, p66Shc−/− mice show reduced levels of systemic (isoprostane) and tissue (nitrotyrosines, 8-oxo-dG) oxidative stress markers [76–79] and the enhanced resistance to oxidative stress induced by hypercholesterolemia (atherogenesis) [78], acute ischemia [80], angiotensin II [81], carbon tetrachloride [46] and ethanol [82]. Migliaccio et al. [30] have established the critical role of p66Shc in the regulation of oxidative stress. p66Shc inhibits the c-fos promoter activity triggered upon UV irradiation and by H2O2 [83], while the effect of p66Shc on stress response is found to be in a dissimilar manner. Upon exposure of mouse embryo fibroblasts (MEFs) to UV, H2O2 and EGF, EGF stimulated the tyrosine phosphorylation whereas UV and H2O2 induced serine phosphorylation of p66Shc, thus suggesting its role in redox signaling. This notion was supported by inducing apoptosis in p66Shc-ablated and -over expressing MEFs via UV and H2O2 treatments. It is observed that p66Shc−/− cells have enhanced resistance to apoptosis, whereas over expression of p66Shc increases the sensitivity to apoptosis [30, 77]. In fact, several p66Shc−/− cells in general show increased resistance to apoptosis upon other different signals, such as, growth factor deprivation, taxol, calcium ionophore and CD3-CD4 cross-linking [49], staurosporine [46, 84] and amyloid-β peptide [85]. The oxidative damage induced by p66Shc is attributed to serine phosphorylation (S36) in its CH2 domain as oxidative damage causes the activation of serine/threonine kinases [86]. Therefore, taken together these facts further exploit the apoptotic potential of p66Shc that could act as a sensor of intracellular ROS concentrations.
3.1 p66Shc and p53—interplay
It was initially proposed that the oxidative stress response of p66Shc is mediated by the tumor suppressor p53, as p53 is upregulated upon UV and H2O2 treatments and further like p66Shc−/− cells, p53−/− MEFs showed resistance to UV- and H2O2-induced apoptosis [30]. This was confirmed by the studies of Trinei et al. [77] demonstrating that p66Shc serves as a downstream effector of p53. A significant and incessant increase of p66Shc was observed upon augmented expression of p53 in both WT MEFs and in the DLD-1 colon cancer cells, the later possesses inactive p53, thus insisting the requisite of p53 for the upregulation of p66Shc and consecutively is accomplished by increasing the stability of p66Shc[77]. Further, the apoptotic function of p53 is mediated through a p66Shc dependent manner, to be precise, p66Shc−/− MEFs showed resistance to apoptosis induced by increased expression of p53, and a simultaneous increase in apoptosis was observed in the p53-activated DLD-1 cells upon mounting the expression of p66Shc in these cells. In addition, p66Shc regulates the p53-dependent apoptosis but does not involve in other functions of p53 such as cell cycle arrest [77]. Activation of p53 induces redox-related genes, release of cytochrome c from mitochondria and promotes apoptosome assembly, which ultimately lead to a persistent increase in the ROS levels [87–89]. p66Shc−/− MEFs were unable to trigger cytochrome c release, while p53 is activated upon H2O2 treatment, whereas the deficit was restored upon re-expression of p66Shc in these cells, suggesting the involvement of p66Shc in the regulation of p53-induced cytochrome c release. Further, p66Shc−/− cells failed to increase the ROS levels upon increasing the expression of p53; while there was a marked increase in the ROS levels after expression of p66Shc in p66Shc−/− MEFs and p53-null DLD-1 cells, supporting the pivotal role of p66Shc and p53 in the regulation of intracellular ROS levels yet in the absence of apoptogenic stimuli [77]. Fascinatingly, Tiberi and coworkers [90] have demonstrated that p53-independent S36 phosphorylation of p66Shc and a marked increase of oxidative stress-induced apoptosis upon expression of p66Shc in p53−/− human cell lines, such as, Saos-2 and Hela cells. Furthermore, several studies on the role of p66Shc in embryo arrest validate that p66Shc and oxidative stress are associated with a p53-independent early embryonic arrest in vitro [91–95].
3.2 p66Shc and forkhead proteins—interplay
The regulation of intracellular levels of ROS by S36-phosphorylated p66Shc is further mediated by modulating the transcriptional activity of Forkhead (FKHD) transcriptional factor FKHRL1 that is similar to the FKHD family member DAF-16, which regulates lifespan in C. elegans [76]. FKHRL1 serves as a downstream target of activated p66Shc and is vital for initiating tolerance to stress and cell survival [76]. Further, though it has been contemplated that the cell survival and apoptotic effects of FKHD proteins depends on cell-specific effect and/or gene-dosing effect, too high or too low FKHD protein may both lead to cell death [96, 97]. Several studies have demonstrated the association of FKHRL1 activation and apoptosis by serum and growth factor stimulation in lymphocytes [98], whereas in fibroblasts and neuronal cells, p66Shc plays a pivotal role in the regulation of FKHRL1 activity upon exposure to oxidative stress [76]. Further, FKHRL1 has been reported to stimulate the antioxidant enzymes including superoxide dismutase and catalase and also in the release of pro-apoptotic protein such as Bim [76, 99, 100]. Accordingly, in dormant cells, FKHRL1 is localized in the nucleus and is engaged in the control of the expression of the antioxidant enzymes, such as catalase. Upon phosphorylation of FKHRL1 by oxidative stress, it is translocated to the cytoplasm and becomes inactive. Considering the role of p66Shc in this task, p66Shc−/− MEFs articulated an augmented resistance to H2O2 induced apoptosis via increasing the FKHRL1-transcriptional activity and consequently, inducing the expression of antioxidant genes [76]. Thus substantiating the crucial act of p66Shc in the oxidative stress-dependent inhibition of FKHRL1 transcriptional activity and, subsequently, in the regulation of intracellular ROS levels and in the ageing process. Nevertheless, the transcriptional control of FKHRL1 by p66Shc needs further analysis.
3.3 p66Shc in mitochondrial control of apoptosis
The increased intracellular ROS alters the mitochondrial membrane potential, leading to the activation of mitochondrial permeability transition pore (MPTP) and the release of cytochrome c [46, 77, 84]. Cyclosporin A, an inhibitor of MPTP and apoptosis, retarded the oxidative stress-induced apoptosis in p66Shc-expressing wild type MEFs and also averted the capability of re-expressed p66Shc to regain the H2O2-induced apoptosis in p66Shc−/− cells, suggesting the requirement of p66Shc in the regulation of MPTP and it also revealed that MPTP serves as a downstream target of p66Shc [77]. Therefore, it is apparent that p66Shc regulates oxidative stress in part through H2O2 generation that is involved in mitochondrial control of apoptosis. Due to the importance of apoptosis involving in carcinogenesis, the functional role of p66Shc in this mode of regulation has received much attention and thus will be discussed in the following sub-sections. In light of current findings and understandings, the possible role of p66Shc in oxidative stress-induced apoptosis is summarized in Fig. 3.
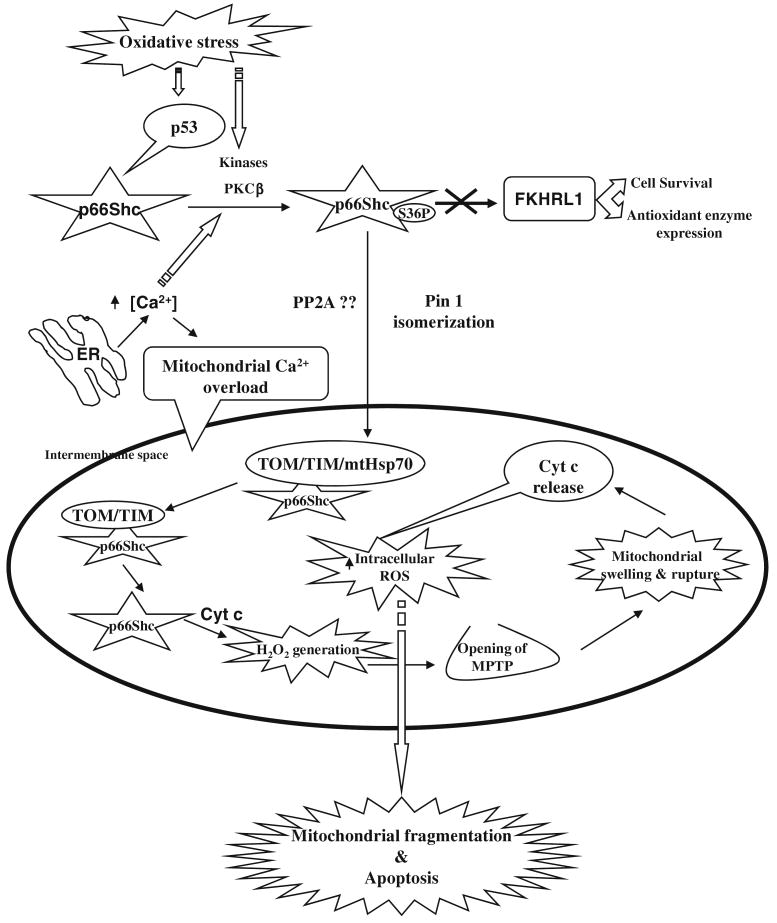
Fig. 3.
A schematic representation for the possible role of p66Shc in oxidative stress-induced apoptosis. In the cytosol, p66Shc is phosphorylated at S36 upon oxidative stress and is mediated by the activation of p53, kinases and also due to modulation in the calcium ion concentration. The phosphorylated p66Shc is then translocated to the mitochondria by binding to a peptidyl-prolyl isomerase Pin1. In the mitochondrial intermembrane space, p66Shc associates to the mitochondrial super complex import machinery (TIM44, TIM20, TIM23 & mtHsp70) at the basal state, whereas upon oxidative stress signals mtHsp70 dissociates from TIM44 and facilitates the discharge of p66Shc (active) that has been associated with TOM-TIM complex. The monomeric p66Shc then interacts and oxidizes cytochrome c (cyt c) resulting in the generation of H2O2 via transferring electrons from reduced cytochrome c to oxygen and that increases the intracellular ROS levels. The increased intracellular ROS alters the mitochondrial membrane potential, leading to the opening of MPTP, swelling of mitochondria, release of cytochrome c and finally result in the mitochondrial fragmentation and apoptosis. The regulation of intracellular levels of ROS by S36-phosphorylated p66Shc is further mediated by inhibiting the transcriptional activity of the transcriptional factor FKHRL1
3.3.1 S36-phosphorylation—a critical key for p66Shc translocation to mitochondria
Most of the p66Shc protein is distributed throughout the cytosol and a fraction of p66Shc localizes within the inner membrane and intermembrane space of mitochondria and, oxidative stress stimulates an increase in the mitochondrial p66Shc [46, 84, 101, 102]. However, the mechanism by which p66Shc translocates into mitochondria remains unclear. It is possible that p66Shc translocates into the mitochondria by the influence of ER mitochondrial network such as inositol 1,4,5-triphosphate-dependent Ca2+ release channel [103]. Further, mitochondrial Ca2+ uptake is altered by protein kinase C (β) (PKC) [104], which is sensitive to oxidative stress [105]. Although S36 phosphorylation is critical for apoptotic potential of p66Shc, mitochondrial p66Shc is not phosphorylated, suggesting the nonmitochondrial role of S36 phosphorylation of p66Shc in manipulating the pro-apoptotic response. Pinton and coworkers [55] have proposed a marked interdependence between the PKCβ-dependent phosphorylation of p66Shc and the early mitochondrial responses to oxidative stimulus in MEFs, such responses include mitochondrial fragmentation and repression of Ca2+ signal transmission to the mitochondria, pursued by apoptosis. Accordingly, p66ShcS36A mutant was deficient in triggering the early mitochondrial response to oxidative stress induced by PKCβ activation, thus implicating the requisite of S36 phosphorylation of p66Shc in the control of mitochondrial oxidative response. In addition, Pellegrini et al., [106] have reported that Ca2+-dependent S36 phosphorylation of p66Shc is essential for mitochondrial dysfunction and impairment of Ca2+ homeostasis, which encourages T cell apoptosis. Nevertheless, the mitochondrial apoptotic responses mediated by p66Shc, in part, but not firmly depend on increase in [Ca2+]c [cytosolic free Ca2+ concentration] [106]. The phosphorylated p66Shc is then translocated to the mitochondria, which is mediated by binding of phosphorylated p66Shc to a peptidyl-prolyl isomerase Pin1 [55]. Pin1 stimulates cis-trans isomerization of phosphorylated Ser-Pro bonds (pSer-Pro); granting phosphorylation-dependent conformational change that is pertinent for the translocation of p66Shc to mitochondria. Further, it has been reported that phosphatase PP2A recognizes and dephosphorylates pSer-Pro, once isomerized by Pin1 [55, 107]. However, the subcellular location of dephosphorylation by phosphatase PP2A remains further investigation.
3.3.2 Mitochondrial super complex import machinery—a driving source for p66Shc translocation
In the intermembrane space, mitochondrial p66Shc is associated with the high-molecular-weight complex involving import machinery, namely, translocase of the outer membrane/translocase of the inner membrane (TOM/TIM) super complex (TIM44, TIM20, TIM23, and mtHsp70) [84, 108]. In the basal condition, the mitochondrial p66Shc associates with the mitochondrial heat-shock protein (mtHsp70), which is a stress response protein that protects cells from the oxidative stress-induced mitochondrial damage [84]. The mtHsp70 is in complex with the mitochondrial import machinery TIM44 subunit and regulates the import of proteins into mitochondrial vesicles. Upon oxidative stress signals, including UVC and H2O2, mtHsp70 dissociates from TIM44, which may facilitate the discharge of the monomeric p66Shc (active) that has been associated with TOM–TIM complex, while the later process is affected by the variations in mitochondrial energetic status [46, 84, 108]. Thus the data together signify the importance of mtHsp70 in retarding the apoptotic potential of p66Shc [84], which is delicately tuned by both cytosolic stimulus and intrinsic mitochondrial machinery.
3.3.3 Redox-active sequence of p66Shc and electron transfer in mitochondria
Within the mitochondrial inter-membrane space, p66Shc oxidizes cytochrome c resulting in the generation of H2O2 via transferring electrons from reduced cytochrome c to oxygen and that increases the intracellular ROS levels. The N-terminal CH2-PTB regions of p66Shc possessing a redox-active sequence (Fig. 1) have been implicated in the binding of cytochrome c and in the transfer of electrons, and the mutation of amino acid residue(s) within the redox region impairs this pro-apoptotic function of p66Shc, leaving unaltered the other cellular effects of p66Shc such as binding to the activated receptors [46]. In fact, recent report demonstrated the presence of two redox centers in CH2-PTB regions of p66Shc including a copper-sensitive catalytic site for ROS formation and a regulatory disulphide/thiol site arbitrating a reversible dimmer-tetramer switch [109].
3.3.4 p66Shc—induced oxidative damage
Increase in the level of ROS increases the damage of cellular proteins, lipids, and DNA and the accumulation of this oxidative cellular damage caused by ROS is apparently the primary origin of ageing, supportively, mutations and deletions of both nuclear and mitochondrial DNA are common phenomena in aged mammals [110–112]. In consistence with this notion, it has been illustrated that cells derived from p66Shc−/− mice showed reduction in both the oxidation of the C8 of guanine (8-oxo-dG) (a most profuse form of oxidation-damaged nuclear DNA [113]) and in the mutation of mitochondrial DNA. Moreover, the expression of p66Shc correlates with the intensity of oxidative damage, which is greater in the lung, spleen, liver and skin, while there is no marked oxidative damage observed in brain where p66Shc expression is absent [77]. Thus collectively, it is apparent that decreased intracellular ROS is accountable for the prolonged life span of p66Shc −/− mice.
4 p66Shc and human longevity
Interestingly, the studies on p66Shc expression and human longevity illustrate that p66Shc expression in dermal fibroblasts increases with age [62], which is converse to the role of p66Shc in the elongation of life span in mice [30, 33]. The greatest level of p66Shc is found in fibroblasts from centenarians upon oxidative stress when compared to fibroblasts from old and young individuals. It is thus speculated to be an adaptive response exerted by the centenarians to oxidative cellular damage accumulated with age. Further, several reports reveal the association between the increased expression (mRNA and protein) of p66Shc and cellular senescence and oxidative stress up-regulation of ROS [114–116]. Nevertheless, the molecular means by which p66Shc mediates aging responses in humans are obscure.
Overall the above facts emphasize the central role of p66Shc in ageing process via regulating oxidative stress-induced apoptosis in mammals. On the other side, in vivo and in vitro studies demonstrated that p66Shc regulates the mtDNA replication, following Ras-induced cell proliferation, which is triggered by but not as such depending on Ras signaling [117]. Indeed, p66Shc mutants that lack the ability to get phosphorylated at S36 or to induce apoptosis that upon transferred into p66Shc−/− MEFs were able to enhance mtDNA content, suggesting the pioneering mechanism of p66Shc apart from its role in ROS generation [117]. Moreover p66Shc−/− old mice show marked reduction in the level of tissue (liver, skin and muscle) mtDNA that has been found to be increased in the tissues of the corresponding wild type (wt) old mice, enumerating the relation between ageing, p66Shc and mtDNA replication [117, 118]. Thus, the data taken together is insisting the role of mtDNA replication in the regulation of cell proliferation involving Ras and p66Shc.
5 Role of p66Shc in prostate cancer cell proliferation and metastasis
In general, cell proliferation, migration and adhesion to the target tissues are the critical steps that allow tumor cells to obtain the metastatic phenotype. Cell proliferation and migration depend on intracellular signals transmitted by growth factors and adhesion proteins within the extracellular matrix [119]. The common intracellular signaling molecules involved in these processes include Rho family proteins and ERK cascades [119–122]. It is now evident that p66Shc plays a crucial role in cell migration and adhesion in addition to its role in mediating cell proliferation induced by growth factor receptor signaling [123, 124]. These processes require the rearrangement of actin cytoskeleton, the formation of new integrin substratum contacts, cell contraction and release of pre-existing cell-matrix contacts [125].
Noteworthily, these data are clinically significant because in prostate cancer archival specimens, p66Shc protein level is significantly higher in prostate adenocarcinomatous cells than in adjacent benign glandular cells [10]. Similarly, the expression level of p66Shc is elevated in estrogen-regulated tumors, including metastatic breast and ovarian and estrogen-treated breast cancer cells, and also other tumors including thyroid tumors and may serve as a useful prognostic marker for stage IIA colon cancer [10, 34, 38, 126, 127]. It should be noted that some data showed that p66Shc protein is down-regulated in primary tumors of breast cancer [36]. We will thus discuss the role of p66Shc in these processes and emphasize on androgen regulation in prostate cancer cell proliferation and metastasis, as the growth and progression of prostate cancer including advanced cancers are dependent on androgens and the receptors.
5.1 p66Shc in prostate cancer cell proliferation
In tyrosine phosphorylation signal transduction pathway, p66Shc is conventionally known as an adaptor protein and exhibits the distinct biological function compared to other Shc isoforms. As aforementioned, p66Shc is unable to transform NIH3T3 mouse fibroblasts in culture cells despite that it is phosphorylated at its tyrosine residues upon EGF stimulation and forms complexes with Grb2 [30]. It also could not augment the activation of EGF-induced extracellular signal-regulated kinases/mitogen-activated protein kinases (ERK/MAPK) in cell cultures such as HeLa, CHO, and COS-1 cells [8, 53]. As discussed above, one reason could be that increased expression of p66Shc has resulted in an elevated level of the basal activity of ERK/MAPK even in the absence of stimulus, which thus minimizes the extent of further activation by growth factors such as EGF [41].
p66Shc plays a critical role in mediating proliferation of epithelial cells. Strikingly, the protein level of p66Shc closely correlates with the growth rate of prostate cancer cells. In rapidly growing prostate cancer cells such as PC-3 and DU145 cells, p66Shc protein level is approximately 4–13-folds of that in slow-growing LNCaP C-33 cells and is over 10-fold of even slower-growing MDA PCa2b cells [41]. In the presence of androgens or EGF, in slow-growing human prostate cancer LNCaP C-33 and MDA PCa2b cells, both the p66Shc protein level and cell proliferation rate are increased, higher than the corresponding cells cultured in the absence of stimulus [10]. In parallel, in estrogen-treated breast cancer MCF-7 cells, increased p66Shc protein concurs with accelerated cell growth [10]. Thus, p66Shc protein level is associated with the epithelial cell growth.
The direct role of p66Shc protein in regulating the growth of prostate cancer cells is clearly supported by both cDNA and siRNA approaches [41]. Elevated expression of p66Shc by cDNA transfection correlates with increased cell proliferation. On the contrary, a decreased cell growth rate is observed when p66Shc protein is knocked down by its siRNA. It should also be noted that phosphorylation of S36 at p66Shc serves as a sensor of apoptotic pathway, androgen-stimulated cell proliferation correlates with decreased S36 phosphorylation [42] (Fig. 4). The data clearly establish the causal relationship of p66Shc protein with prostate cell growth. Furthermore, p66Shc mediates growth stimulation by androgens and elevated expression of p66Shc might lead to androgen-independent proliferation [42]. Collectively, the data show that the elevated level of p66Shc protein in androgen-regulated prostate cancer cells plays a critical role in up-regulating those cancer cell proliferation and thus contributes to the tumorigenicity of those cancer cells. The role of p66Shc in this mode of regulation requires further investigation.
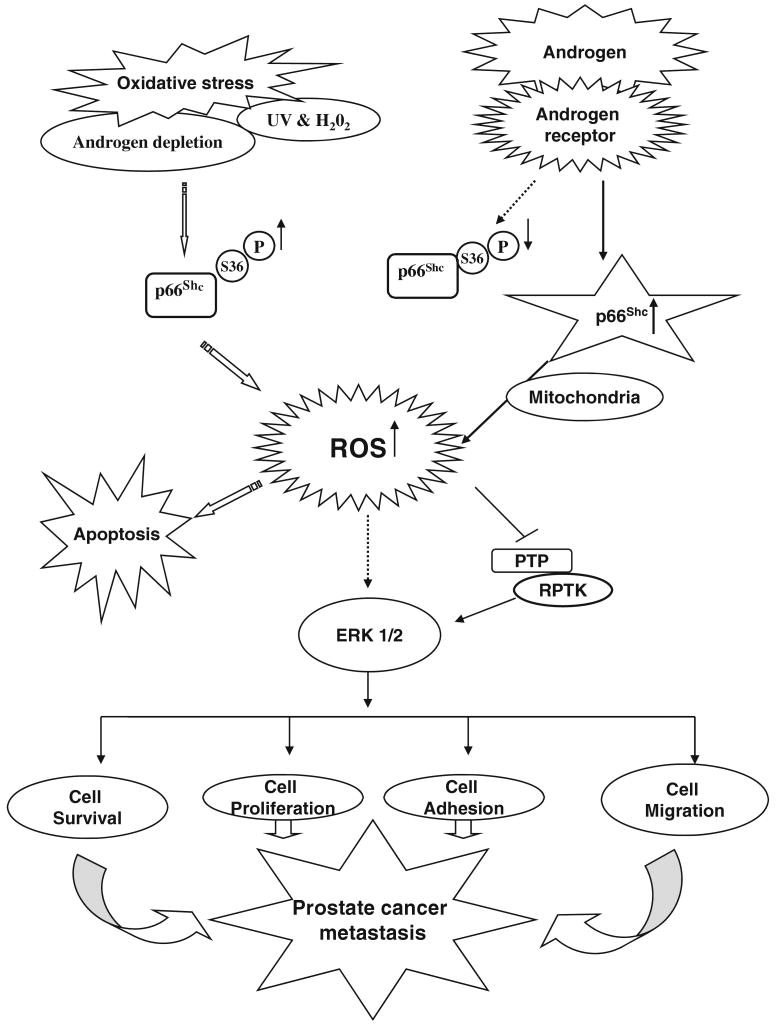
Fig. 4.
p66Shc signaling and its novel mechanism regulated by androgens in prostate cancer cells. Oxidative stress including androgen depletion, UV and H2O2 induces the phosphorylation at S36 of p66Shc protein that leads to the increased production of ROS. The later mediates diverse biological functions, including cell proliferation, adhesion, migration and apoptosis. In prostate cancer cells, androgen (DHT) treatment also increases the ROS level via increasing the p66Shc protein level and decreasing the S36-phosphorylation of p66Shc, which lead to prostate cancer cell proliferation. p66Shc protein mediates androgen action on tumor progression. Upon stimulation by androgens, p66Shc is translocated to mitochondria via Ser-36 phosphorylation-independent manner resulting in the generation of ROS. ROS may then inhibit PTP, leading to corresponding RPTK activation and ERK/MAPK activation. ROS may also activate ERK/MAPK directly. Together, this activated signal can promote cell proliferation, survival as well as migration that collectively lead to tumor metastasis
5.2 p66Shc and integrin-associated signaling in angiogenesis
Integrins play vital roles in cancer progression via regulating various intracellular signaling molecules those are essential for cell motility, cell survival and proliferation [128, 129]. Among them, αvβ3 integrin is thought to play a crucial role in the metastasis of cancer cells to bone marrow [130]. In prostate cancer cells, αvβ3 is expressed in bone foci-derived human PC-3 cells, but not in lymph node foci-derived LNCaP cells [131]. αvβ3 integrin is also expressed in breast and lung cancer cells that were originally derived from the bone marrow aspirates, supporting a role of integrin signaling in bone metastasis
Recent studies revealed that αvβ3 integrin-associated signaling by influencing VEGF expression regulates the growth of both prostate and breast tumors. The up-regulation of VEGF expression depends on αvβ3 clustering where it promotes the recruitment of p66Shc and subsequently the phosphorylation of β3-associated p66Shc. Thus, phosphorylation of p66Shc is a critical step for αvβ3-mediated potentiation of VEGF expression and tumor vascularization [37]. Supportively, down-regulation of p66Shc inhibits VEGF expression as well as the tumor growth and angiogenesis in vivo [37]. These findings provide insights into the role of αvβ3 and p66Shc interaction as a regulator of angiogenesis.
5.3 Role of p66Shc in metastasis
p66Shc is apparently involved in tumor metastasis including cellular invasion, motility and migration in different types of cancers. An association between the expression of p66Shc and prostate cancer cell motility is observed, i.e., an elevated expression of p66Shc is associated with increased motility of cDNA transfected slow growing LNCaP C-33 cells [Yuan TC, Lin FF and Lin MF, unpublished data]. Furthermore, LNCaP C-81 and PC-3 prostate cancer cells express higher levels of p66Shc and exhibit higher metastatic potential than LNCaP C-33 cells in xenograft animals [41, Sebeger J and Lin MF, unpublished data]. Thus, p66Shc is apparently involved in prostate cell motility, an early step of metastasis [34] and may play a role in androgen-regulated prostate cancer metastatic process.
In parallel, xenograft bone metastasis of breast cancer MDA-MB-231cell line expresses p66Shc and its metastatic variant F-11 cells have a 3-fold higher level of p66Shc [34]. Increased expression of p66Shc in lymph-node positive breast cancers also correlates with lymph node metastasis [34]. Supportively, higher levels of p66Shc are observed in breast cancer specimens with higher metastatic potential [34]. Conversely, decrease in the Shc A levels or the expression of a dominant-negative Shc A mutant blocked TGFβ-induced motility and the invasion of Neu/ErbB-2-expressing breast cancer cells [132]. Together, the data indicate that Shc A is involved in regulating tumor cell metastasis. Recent studies with transgenic mouse models have clearly validated the role of Shc A signaling in regulating the carcinogenesis of breast epithelia [133]. The observations on the increased expression of p66Shc in different cancer cell lines with higher metastatic ability emphasize the importance of p66Shc as a potential therapeutic target in combating different types of cancers including the prostate.
6 A novel mechanism: androgen action via p66Shc-dependent redox signaling in prostate cancer cells
p66Shc mediates androgen-stimulated prostate cancer cell proliferation in part via a non-genomic redox signaling pathway. In rapidly growing cells, including androgen-stimulated PCa cells, increased oxidative stress might contribute to the elevated level of p66Shc protein (Fig. 4). As discussed earlier, p66Shc protein can function as a stress sensor and is involved in regulating intracellular level of reactive oxygen species (ROS) [77]. ROS mediates diverse biological functions, including cell proliferation, adhesion, migration and apoptosis (Fig. 4).
Steroid hormones, such as androgens, and growth factors, such as EGF, can all up-regulate ROS production in cells and thereby promote cell proliferation [134, 135]. The functional role of ROS as a positive regulator of prostate cancer cells is at least in part by inhibiting the PTPase activity and thus the corresponding RPTK can be activated [135–137] (Fig. 4). This is in consistent with our findings that androgenic treatment of androgen-sensitive LNCaP C-33 and MDA PCa 2b cells promotes cell proliferation in part via increasing p66Shc protein level [10] and ROS production, leading to decreased cellular prostatic acid phosphatase (cPAcP) activity, a PTPase exhibiting the growth inhibitory activity [138–140], and increased ErbB-2 tyrosine phosphorylation [13, 42].
Supportively, in prostate cancer archival specimens, the higher level of ROS correlates with the higher proliferation index in cancerous cells than in non-cancerous cells [141]. Furthermore, androgens promote the translocalization of p66Shc via a S36-phosphorylation-independent mechanism from cytosol into mitochondria where it interacts with cytochrome c for ROS production because androgen treatment results in decreased S36 phosphorylation and elevated p66Shc mitochondrial level [42] (Fig. 4). Supportively, the redox-negative mutant p66Shc W134F could not increase ROS production nor growth promotion [42]. Additionally, p66Shc may also increase ROS production through Rac1-SOS signal pathway [142], possibly leading to androgen-independent prostate cancer cell proliferation [143]. Since ROS may play a critical role in the various stages of prostate carcinogenesis including the initiation and the progression of prostate cancer, anti-oxidants may be useful for this cancer prevention [144] despite the initial insignificant results of Selenium and Vitamin E Cancer Prevention Trial, which might be in part due to the usage of different forms of selenium compound [145]. The data collectively support the notion that p66Shc can mediate the non-genomic steroid action on prostate cancer cell proliferation and carcinogenesis including metastasis; while the molecular mechanisms require further investigations.
7 Potential clinical applications of p66Shc in prostate cancer and its perspectives
To date, numerous factors such as cytokines, growth factors and receptors have been demonstrated for their vital roles in the various stages of prostate carcinogenesis. Further understanding the molecular mechanisms should pave ways to identify new prognostic markers that might also serve as therapeutic targets for the prevention and treatment of prostate cancer. In the journey of hunting surrogate markers for determining the prostate origin of metastatic cancers, cPAcP prior to PSA was used as a biomarker, due to its cell-specific expression [146, 147]. Nevertheless, its cellular level negatively correlates with prostatic carcinogenesis [139, 147–150]. Recently, several studies have established the marked effects of the adaptor protein p66Shc, a molecule that serves as a common convergence point in signaling pathways on cancer cell proliferation, migration, metastasis, and cell death, since the expression level of p66Shc increases with age and in age-related diseases including cancer, especially prostate cancer [15, 34].
In this review, we have outlined the striking role of p66Shc in mediating the oxidative stress-induced apoptotic responses in general and discussed its functional role in cell growth, survival, migration and ultimate metastasis of prostate cancer. The inflammation has been proposed to play a critical role in prostate carcinogenesis. Our preliminary data reveal a possible association of p66Shc protein expression with the atrophy of prostate epithelia (Lin FF, Johansson SL and Lin MF, un-published observations) while the biological significance remains further investigation. We also present a novel mechanism by which androgen signaling via p66Shc, an authentic oxidase, promotes prostate cancer cell proliferation. Interestingly, in prostate cancer cells, the level of p66Shc is inversely correlated with cPAcP expression [139] and has a positive correlation with the activation of ErbB-2 as well as ERK/MAPK [41]. This inverse correlation of cPAcP to p66Shc expression level and ErbB2 as well as ERK/MAPK activation is also clinically relevant [10, 138, 139, 150–156]. Hence, despite the fact that cPAcP alone may serve as a good prognostic marker for metastatic prostate cancer [153]; because of decreased cPAcP level upon tumor progression as well as increased p66Shc protein level in PCa tumors, we contemplate that the p66Shc/cPAcP ratio may serve as a competitive surrogate biomarker for predicting the prognosis of prostate carcinomas. Furthermore, this positive correlation to prostate cancer makes p66Shc a soaring precedence therapeutic target for combating against the progression of prostate cancer. Indeed, knockdown expression of p66Shc reduces the tumorigenicity of prostate cancer cells [41, 42].
In summary, we propose that the participation of p66Shc in cell proliferation and/or apoptosis depends on the type of cell origin, the signals and kinases[s] that are involved in the signal transduction network and as well the cellular milieu. In prostate cancer, p66Shc plays a critical role in tumor progression, while the molecular mechanism of inverse relationship between cPAcP and p66Shc protein and the upstream regulators and downstream effectors engaged in p66Shc signaling pathway relating to androgens remain further investigations. The results may identify p66Shc as a surrogate marker for the prognosis of prostate cancer and lead to the development of novel therapy against prostate cancer.
Acknowledgments
This study was supported in part by National Cancer Institute, National Institutes of Health [R01 CA88184], Department of Defense [W81XWH-06-1-0070 and W81XWH-08-1-0459], Nebraska Research Initiative for Cancer Metastasis, and Nebraska Cancer and Smoking Disease Research Program LB 506 [2008-20 and 2010-18]. We thank Dr. Shouqiang Ouyang for reading, and Ms. Fen-Fen Lin for her tremendous contributions toward our studies on the functional role of p66Shc in carcinogenesis.
Contributor Information
Mythilypriya Rajendran, Department of Biochemistry and Molecular Biology, College of Medicine, University of Nebraska Medical Center, 985870 Nebraska Medical Center, Omaha, NE 68198-5870, USA.
Paul Thomes, Department of Biochemistry and Molecular Biology, College of Medicine, University of Nebraska Medical Center, 985870 Nebraska Medical Center, Omaha, NE 68198-5870, USA.
Li Zhang, Department of Biochemistry and Molecular Biology, College of Medicine, University of Nebraska Medical Center, 985870 Nebraska Medical Center, Omaha, NE 68198-5870, USA.
Suresh Veeramani, Department of Biochemistry and Molecular Biology, College of Medicine, University of Nebraska Medical Center, 985870 Nebraska Medical Center, Omaha, NE 68198-5870, USA.
Ming-Fong Lin, Email: mlin@unmc.edu, Department of Biochemistry and Molecular Biology, College of Medicine, University of Nebraska Medical Center, 985870 Nebraska Medical Center, Omaha, NE 68198-5870, USA, Eppley Institute for Cancer Research, University of Nebraska Medical Center, Omaha, NE 68198, USA.
References
- 1.Guo M, Hay BA. Cell proliferation and apoptosis. Current Opinion in Cell Biology. 1999;11:745–752. doi: 10.1016/s0955-0674(99)00046-0. [DOI] [PubMed] [Google Scholar]
- 2.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 3.Rathmell JC, Thompson CB. The central effectors of cell death in the immune system. Annual Review of Immunology. 1999;17:781–828. doi: 10.1146/annurev.immunol.17.1.781. [DOI] [PubMed] [Google Scholar]
- 4.Ginaldi L, De Martinis M, D'Ostilio A, Marini L, Loreto MF, Corsi MP, et al. Cell proliferation and apoptosis in the immune system in the elderly. Immunological Research. 2000;21:31–38. doi: 10.1385/IR:21:1:31. [DOI] [PubMed] [Google Scholar]
- 5.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes and Development. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 6.Budd RC. Death receptors couple to both cell proliferation and apoptosis. Journal of Clinical Investigation. 2002;109:437–441. doi: 10.1172/JCI15077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pelicci G, Lanfrancone L, Grignani F, McGlade J, Cavallo F, Forni G, et al. A novel transforming protein [SHC] with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992;70:93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- 8.Migliaccio E, Mele S, Salcini AE, Pelicci G, Lai KM, Superti-Furga G, et al. Opposite effects of the p52Shc/p46Shc and p66Shc splicing isoforms on the EGF receptor-MAP kinase-fos signaling pathway. The EMBO Journal. 1997;16:706–716. doi: 10.1093/emboj/16.4.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ravichandran KS. Signaling via Shc family adapter proteins. Oncogene. 2001;20:6322–6330. doi: 10.1038/sj.onc.1204776. [DOI] [PubMed] [Google Scholar]
- 10.Lee MS, Igawa T, Chen SJ, Van Bemmel D, Lin JS, Lin FF, et al. p66Shc protein is upregulated by steroid hormones in hormone-sensitive cancer cells and in primary prostate carcinomas. International Journal of Cancer. 2004;108:672–678. doi: 10.1002/ijc.11621. [DOI] [PubMed] [Google Scholar]
- 11.Migliaccio E, Giorgio M, Pelicci PG. Apoptosis and aging: role of p66Shc redox protein. Antioxidant and Redox Signaling. 2006;8:600–608. doi: 10.1089/ars.2006.8.600. [DOI] [PubMed] [Google Scholar]
- 12.Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–433. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 13.Meng TC, Lee MS, Lin MF. Interaction between protein tyrosine phosphatase and protein tyrosine kinase is involved in androgen-promoted growth of human prostate cancer cells. Oncogene. 2000;19:2664–2677. doi: 10.1038/sj.onc.1203576. [DOI] [PubMed] [Google Scholar]
- 14.Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. Journal of the National Cancer Institute. 2001;93:1687–1697. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- 15.Kraus S, Gioeli D, Vomastek T, Gordon V, Weber MJ. Receptor for activated C kinase 1 [RACK1] and Src regulate the tyrosine phosphorylation and function of the androgen receptor. Cancer Research. 2006;66:11047–11054. doi: 10.1158/0008-5472.CAN-06-0596. [DOI] [PubMed] [Google Scholar]
- 16.Guo Z, Dai B, Jiang T, Xu K, Xie Y, Kim O. Regulation of androgen receptor activity by tyrosine Phosphorylation. Cancer Cell. 2006;10:309–319. doi: 10.1016/j.ccr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Weigel NL, Moore NL. Kinases and protein phosphorylation as regulators of steroid hormone action. Nuclear Receptor Signaling. 2007;5:e005. doi: 10.1621/nrs.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickson RB, Lippman ME. Estrogenic regulation of growth and polypeptide growth factor secretion in human breast carcinoma. Endocrine Reviews. 1987;8:29–43. doi: 10.1210/edrv-8-1-29. [DOI] [PubMed] [Google Scholar]
- 19.Dabrosin C, Ollinger K, Ungerstedt U, Hammar M. Variability of glutathione levels in normal breast tissue and subcutaneous fat during the menstrual cycle: an in vivo study with microdialysis technique. Journal of Clinical Endocrinology and Metabolism. 1997;82:1382–1384. doi: 10.1210/jcem.82.5.3957. [DOI] [PubMed] [Google Scholar]
- 20.Devanesan P, Santen RJ, Bocchinfuso WP, Korach KS, Rogan EG, Cavalieri E. Catechol estrogen metabolites and conjugates in mammary tumors and hyperplastic tissue from estrogen receptor-alpha knock-out [ERKO]/Wnt-1 mice: Implications for initiation of mammary tumors. Carcinogenesis. 2001;22:1573–1576. doi: 10.1093/carcin/22.9.1573. [DOI] [PubMed] [Google Scholar]
- 21.Engebraaten O, Bjerkvig R, Pedersen PH, Laerum OD. Effects of EGF, bFGF, NGF, and PDGF [bb] on cell proliferative, migratory, and invasive capacities of human braintumour biopsies in vitro. International Journal of Cancer. 1993;53:209–214. doi: 10.1002/ijc.2910530206. [DOI] [PubMed] [Google Scholar]
- 22.Hamada J, Nagayasu H, Takayama M, Kawano T, Hosokawa M, Takeichi N. Enhanced effect of epidermal growth factor on pulmonary metastasis and in vitro invasion of rat mammary carcinoma cells. Cancer Letters. 1995;89:161–167. doi: 10.1016/0304-3835(95)03686-q. [DOI] [PubMed] [Google Scholar]
- 23.Mignatti P, Morimoto T, Rifkin DB. Basic fibroblast growth factor released by single, isolated cells stimulates their migration in an autocrine manner. Proceedings of National Academy of Sciences. 1991;88:11007–11011. doi: 10.1073/pnas.88.24.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor WR, Greenberg AH, Turley EA, Wright JA. Cell motility, invasion, and malignancy induced by overexpression of K-FGF or bFGF. Experimental cell research. 1993;204:295–301. doi: 10.1006/excr.1993.1036. [DOI] [PubMed] [Google Scholar]
- 25.Choudhury GG, Karamitsos C, Hernandez J, Gentilini A, Bardgette J, Abboud HE. PI-3-kinase and MAPK regulate mesangial cell proliferation and migration in response to PDGF. Amarican Journal of Physiology. 1997;273:F931–F938. doi: 10.1152/ajprenal.1997.273.6.F931. [DOI] [PubMed] [Google Scholar]
- 26.Stracke ML, Engel JD, Wilson LW, Rechler MM, Liotta LA, Schiffmann E. The type I insulin-like growth factor receptor is a motility receptor in human melanoma cells. Journal of Biological Chemistry. 1989;264:21544–21549. [PubMed] [Google Scholar]
- 27.Sachs M, Weidner KM, Brinkmann V, Walther I, Obermeier A, Ullrich A, et al. Motogenic and morphogenic activity of epithelial receptor tyrosine kinases. The Journal of Cell Biology. 1996;133:1095–1107. doi: 10.1083/jcb.133.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelicci G, Giordano S, Zhen Z, Salcini AE, Lanfrancone L, Bardelli A, et al. The motogenic and mitogenic responses to HGF are amplified by the Shc adaptor protein. Oncogene. 1995;10:1631–1638. [PubMed] [Google Scholar]
- 29.Ratner S, Patrick P, Bora G. Lymphocyte development of adherence and motility in extracellular matrix during IL-2 stimulation. The Journal of Immunology. 1992;149:681–688. [PubMed] [Google Scholar]
- 30.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 31.Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 32.Taub J, Lau JF, Ma C, Hahn JH, Hoque R, Rothblatt J, et al. A cytosolic catalase is needed to extend adult lifespan in C. elegans daf-C and clk-1 mutants. Nature. 1999;399:162–166. doi: 10.1038/20208. [DOI] [PubMed] [Google Scholar]
- 33.Sagi O, Wolfson M, Utko N, Muradian K, Fraifeld V. p66ShcA and ageing: modulation by longevity-promoting agent aurintricarboxylic acid. Mechanisms of Ageing and Development. 2005;126:249–254. doi: 10.1016/j.mad.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Jackson JG, Yoneda T, Clark GM, Yee D. Elevated levels of p66 Shc are found in breast cancer cell lines and primary tumors with high metastatic potential. Clinical Cancer Research. 2000;6:1135–1139. [PubMed] [Google Scholar]
- 35.Luzi L, Confalonieri S, Di Fiore PP, Pelicci PG. Evolution of Shc functions from nematode to human. Current Opinion in Genetics and Development. 2000;10:668–674. doi: 10.1016/s0959-437x(00)00146-5. [DOI] [PubMed] [Google Scholar]
- 36.Davol PA, Bagdasaryan R, Elfenbein GJ, Maizel AL, Frackelton AR., Jr Shc proteins are strong, independent prognostic markers for both node negative and node positive primary breast cancer. Cancer Research. 2003;63:6772–6783. [PubMed] [Google Scholar]
- 37.De S, Razorenova O, McCabe NP, O'Toole T, Qin J, Byzova TV. VEGF-integrin interplay controls tumor growth and vascularization. Proceedings of the National Academy of Sciences. 2005;102:7589–7594. doi: 10.1073/pnas.0502935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grossman SR, Lyle S, Resnick MB, Sabo E, Lis RT, Rosinha E, et al. p66 Shc tumor levels show a strong prognostic correlation with disease outcome in stage IIA colon cancer. Clinical Cancer Research. 2007;13:5798–5804. doi: 10.1158/1078-0432.CCR-07-0073. [DOI] [PubMed] [Google Scholar]
- 39.Biscardi JS, Belsches AP, Parsons SJ. Characterization of human epidermal growth factor receptor and c-Src interactions in human breast tumor cells. Molecular Carcinoenesis. 1998;21:261–272. doi: 10.1002/(sici)1098-2744(199804)21:4<261::aid-mc5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 40.Stevenson LE, Frackelton AR., Jr Constitutively tyrosine phosphorylated p52 Shc in breast cancer cells: correlation with ErbB2 and p66 Shc expression. Breast Cancer Research and Treatment. 1998;49:119–128. doi: 10.1023/a:1006007227747. [DOI] [PubMed] [Google Scholar]
- 41.Veeramani S, Igawa T, Yuan TC, Lin FF, Lee MS, Lin JS, et al. Expression of p66 [Shc] protein correlates with proliferation of human prostate cancer cells. Oncogene. 2005;24:7203–7212. doi: 10.1038/sj.onc.1208852. [DOI] [PubMed] [Google Scholar]
- 42.Veeramani S, Yuan TC, Lin FF, Lin MF. Mitochondrial redox signaling by p66 Shc is involved in regulating androgenic growth stimulation of human prostate cancer cells. Oncogene. 2008;27:5057–5068. doi: 10.1038/onc.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ventura A, Luzi L, Pacini S, Baldari CT, Pelicci PG. The p66 Shc longevity gene is silenced through epigenetic modifications of an alternative promoter. Journal of Biological Chemistry. 2002;277:22370–22376. doi: 10.1074/jbc.M200280200. [DOI] [PubMed] [Google Scholar]
- 44.Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- 45.Yoshida S, Masaki T, Feng H, Yuji J, Miyauchi Y, Funaki T, et al. Enhanced expression of adaptor molecule p46 Shc in nuclei of hepatocellular carcinoma cells: study of LEC rats. International Journal of Oncology. 2004;25:1089–1096. [PubMed] [Google Scholar]
- 46.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, et al. Electron transfer between cytochrome c and p66 Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 47.Pellegrini M, Pacini S, Baldari CT. p66SHC: the apoptotic side of Shc proteins. Apoptosis. 2005;10:13–18. doi: 10.1007/s10495-005-6057-8. [DOI] [PubMed] [Google Scholar]
- 48.Xie Y, Hung MC. p66Shc isoform down-regulated and not required for HER-2/neu signaling pathway in human breast cancer cell lines with HER-2/neu overexpression. Biochemical and Biophysical Research Communications. 1996;221:140–145. doi: 10.1006/bbrc.1996.0559. [DOI] [PubMed] [Google Scholar]
- 49.Pacini S, Pellegrini M, Migliaccio E, Patrussi L, Ulivieri C, Ventura A, et al. p66SHC promotes apoptosis and antagonizes mitogenic signaling in T cells. Molecular Cell Biology. 2004;24:1747–1757. doi: 10.1128/MCB.24.4.1747-1757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le S, Connors TJ, Maroney AC. c-Jun N-terminal kinase specifically phosphorylates p66ShcA at serine 36 in response to ultraviolet irradiation. Journal of Biological Chemistry. 2001;276:48332–48336. doi: 10.1074/jbc.M106612200. [DOI] [PubMed] [Google Scholar]
- 51.Kao AW, Waters SB, Okada S, Pessin JE. Insulin stimulates the phosphorylation of the 66- and 52-kilodalton Shc isoforms by distinct pathways. Endocrinology. 1997;138:2474–2480. doi: 10.1210/endo.138.6.5203. [DOI] [PubMed] [Google Scholar]
- 52.Yang CP, Horwitz SB. Taxol mediates serine phosphorylation of the 66-kDa Shc isoform. Cancer Research. 2000;60:5171–5178. [PubMed] [Google Scholar]
- 53.Okada N, Wada K, Goldsmith BA, Koizumi S. The 66-kDa Shc isoform is a negative regulator of the epidermal growth factor-stimulated mitogen- activated protein kinase pathway. Journal of Biological Chemistry. 1997;272:28042–28049. doi: 10.1074/jbc.272.44.28042. [DOI] [PubMed] [Google Scholar]
- 54.Khanday FA, Yamamori T, Mattagajasingh I, Zhang Z, Bugayenko A, Naqvi A, et al. Rac1 leads to phosphorylation-dependent increase in stability of the p66shc adaptor protein: role in Rac1-induced oxidative stress. Molecular Biology of the Cell. 2006;17:122–129. doi: 10.1091/mbc.E05-06-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, et al. Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science. 2007;315:659–663. doi: 10.1126/science.1135380. [DOI] [PubMed] [Google Scholar]
- 56.Geisel J, Schorr H, Heine GH, Bodis M, Hübner U, Knapp JP, et al. Decreased p66Shc promoter methylation in patients with end-stage renal disease. Clinical Chemistry Laboratory Medicine. 2007;45:1764–1770. doi: 10.1515/CCLM.2007.357. [DOI] [PubMed] [Google Scholar]
- 57.Richardson BC. Role of DNA methylation in the regulation of cell function: autoimmunity, aging and cancer. The Journal of Nutrition. 2002;132:2401S–2405S. doi: 10.1093/jn/132.8.2401S. [DOI] [PubMed] [Google Scholar]
- 58.Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Advances in Cancer Research. 1998;72:141–196. [PubMed] [Google Scholar]
- 59.Ahuja N, Issa JP. Aging, methylation and cancer. Histology and Histopathology. 2000;15:835–842. doi: 10.14670/HH-15.835. [DOI] [PubMed] [Google Scholar]
- 60.Ahuja N, Li Q, Mohan AL, Baylin SB, Issa JP. Aging and DNA methylation in colorectal mucosa and cancer. Cancer Research. 1998;58:5489–5494. [PubMed] [Google Scholar]
- 61.Issa JP, Ottaviano YL, Celano P, Hamilton SR, Davidson NE, Baylin SB. Methylation of the oestrogen receptor CpG island links ageing and neoplasia in human colon. Nature Genetics. 1994;7:536–540. doi: 10.1038/ng0894-536. [DOI] [PubMed] [Google Scholar]
- 62.Pandolfi S, Bonafè M, Di Tella L, Tiberi L, Salvioli S, Monti D, et al. p66[shc] is highly expressed in fibroblasts from centenarians. Mechanism of Ageing and Development. 2005;126:839–844. doi: 10.1016/j.mad.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Mooijaart SP, van Heemst D, Schreuder J, van Gerwen S, Beekman M, Brandt BW, et al. Variation in the SHC1 gene and longevity in humans. Experimental Gerontology. 2004;39:263–268. doi: 10.1016/j.exger.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 64.Natalicchio A, Laviola L, De Tullio C, Renna LA, Montrone C, Perrini S, et al. Role of the p66Shc isoform in insulin-like growth factor I receptor signaling through MEK/Erk and regulation of actin cytoskeleton in rat myoblasts. Journal of Biological Chemistry. 2004;279:43900–43909. doi: 10.1074/jbc.M403936200. [DOI] [PubMed] [Google Scholar]
- 65.Foschi M, Franchi F, Han J, La Villa G, Sorokin A. Endothelin-1 induces serine phosphorylation of the adaptor protein p66Shc and its association with 14-3-3 protein in glomerular mesangial cells. Journal of Biological Chemistry. 2001;276:26640–26647. doi: 10.1074/jbc.M102008200. [DOI] [PubMed] [Google Scholar]
- 66.Faisal A, el-Shemerly M, Hess D, Nagamine Y. Serine/threonine phosphorylation of ShcA. Regulation of proteintyrosine phosphatase-pest binding and involvement in insulin signaling. Journal of Biological Chemistry. 2002;277:30144–30152. doi: 10.1074/jbc.M203229200. [DOI] [PubMed] [Google Scholar]
- 67.Yang CP, Horwitz SB. Distinct mechanisms of taxol-induced serine phosphorylation of the 66-kDa Shc isoform in A549 and RAW 264.7 cells. Biochimica et Biophysica Acta. 2002;1590:76–83. doi: 10.1016/s0167-4889(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 68.Arany I, Faisal A, Nagamine Y, Safirstein RL. p66shc inhibits pro-survival epidermal growth factor receptor/ERK signaling during severe oxidative stress in mouse renal proximal tubule cells. Journal of Biological Chemistry. 2008;283:6110–6117. doi: 10.1074/jbc.M708799200. [DOI] [PubMed] [Google Scholar]
- 69.El-Shemerly MY, Besser D, Nagasawa M, Nagamine Y. 12-O- Tetradecanoylphorbol-13-acetate activates the Ras/extracellular signal-regulated kinase [ERK] signaling pathway upstream of SOS involving serine phosphorylation of Shc in NIH3T3 cells. Journal of Biological Chemistry. 1997;272:30599–30602. doi: 10.1074/jbc.272.49.30599. [DOI] [PubMed] [Google Scholar]
- 70.Holt KH, Kasson BG, Pessin JE. Insulin stimulation of a MEK- dependent but ERK-independent SOS protein kinase. Molecular and Cellular Biology. 1996;16:577–583. doi: 10.1128/mcb.16.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Purdom S, Chen QM. p66[Shc]: at the crossroad of oxidative stress and the genetics of aging. Trends in Molecular Medicine. 2003;9:206–210. doi: 10.1016/s1471-4914(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 72.Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annual Review of Immunology. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 73.Shichiri M, Yokokura M, Marumo F, Hirata Y. Endothelin-1 inhibits apoptosis of vascular smooth muscle cells induced by nitric oxide and serum deprivation via MAP kinase pathway. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:989–997. doi: 10.1161/01.atv.20.4.989. [DOI] [PubMed] [Google Scholar]
- 74.Habib T, Herrera R, Decker SJ. Activators of protein kinase C stimulate association of Shc and the PEST tyrosine phosphatase. Journal of Biological Chemistry. 1994;269:25243–25246. [PubMed] [Google Scholar]
- 75.Charest A, Wagner J, Jacob S, McGlade CJ, Tremblay ML. Phosphotyrosine-independent binding of SHC to the NPLH sequence of murine protein-tyrosine phosphatase-PEST. Evidence for extended phosphotyrosine binding/phosphotyrosine interaction domain recognition specificity. Journal of Biological Chemistry. 1996;271:8424–8429. doi: 10.1074/jbc.271.14.8424. [DOI] [PubMed] [Google Scholar]
- 76.Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-depedent signaling pathway. Science. 2002;295:2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 77.Trinei M, Giorgio M, Cicalese A, Barozzi S, Ventura A, Migliaccio E, et al. A p53-p66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis. Oncogene. 2002;21:3872–3878. doi: 10.1038/sj.onc.1205513. [DOI] [PubMed] [Google Scholar]
- 78.Napoli C, Martin-Padura I, de Nigris F, Giorgio M, Mansueto G, Somma P, et al. Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2112–2116. doi: 10.1073/pnas.0336359100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Francia P, delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, et al. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation. 2004;110:2889–2895. doi: 10.1161/01.CIR.0000147731.24444.4D. [DOI] [PubMed] [Google Scholar]
- 80.Zaccagnini G, Martelli F, Fasanaro P, Magenta A, Gaetano C, Di Carlo A, et al. p66ShcA modulates tissue response to hindlimb ischemia. Circulation. 2004;109:2917–2923. doi: 10.1161/01.CIR.0000129309.58874.0F. [DOI] [PubMed] [Google Scholar]
- 81.Graiani G, Lagrasta C, Migliaccio E, Spillmann F, Meloni M, Madeddu P, et al. Genetic deletion of the p66Shc adaptor protein protects from angiotensin II-induced myocardial damage. Hypertension. 2005;46:433–440. doi: 10.1161/01.HYP.0000174986.73346.ba. [DOI] [PubMed] [Google Scholar]
- 82.Koch OR, Fusco S, Ranieri SC, Maulucci G, Palozza P, Larocca LM, et al. Role of the life span determinant P66[shcA] in ethanol-induced liver damage. Laboratory Investigations. 2008;88:750–760. doi: 10.1038/labinvest.2008.44. [DOI] [PubMed] [Google Scholar]
- 83.Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. The FASEB Journal. 1996;10:709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- 84.Orsini F, Migliaccio E, Moroni M, Contursi C, Raker VA, Piccini D, et al. The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. Journal of Biological Chemistry. 2004;279:25689–25695. doi: 10.1074/jbc.M401844200. [DOI] [PubMed] [Google Scholar]
- 85.Smith WW, Norton DD, Gorospe M, Jiang H, Nemoto S, Holbrook NJ, et al. Phosphorylation of p66Shc and forkhead proteins mediates Aβ toxicity. The Journal of Cell Biology. 2005;169:331–339. doi: 10.1083/jcb.200410041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stevenson MA, Pollock SS, Coleman CN, Calderwood SK. X- irradiation, phorbol esters, and H2O2 stimulate mitogen-activated protein kinase activity in NIH-3T3 cells through the formation of reactive oxygen intermediates. Cancer Research. 1994;54:12–15. [PubMed] [Google Scholar]
- 87.Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 88.Li PF, Dietz R, von Harsdorf R. p53 regulates mitochondrial membrane potential through reactive oxygen species and induces cytochrome c-independent apoptosis blocked by Bcl-2. The EMBO Journal. 1999;18:6027–6036. doi: 10.1093/emboj/18.21.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schuler M, Bossy-Wetzel E, Goldstein JC, Fitzgerald P, Green DR. p53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. Journal of Biological Chemistry. 2000;275:7337–7342. doi: 10.1074/jbc.275.10.7337. [DOI] [PubMed] [Google Scholar]
- 90.Tiberi L, Faisal A, Rossi M, Di Tella L, Franceschi C, Salvioli S. p66[Shc] gene has a pro-apoptotic role in human cell lines and it is activated by a p53-independent pathway. Biochemical and Biophysical Research Communications. 2006;342:503–508. doi: 10.1016/j.bbrc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 91.Favetta LA, St John EJ, King WA, Betts DH. High levels of p66shc and intracellular ROS in permanently arrested early embryos. Free Radical Biology and Medicine. 2007;42:1201–1210. doi: 10.1016/j.freeradbiomed.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 92.Favetta LA, Robert C, St John EJ, Betts DH, King WA. p66shc, but not p53, is involved in early arrest of in vitro-produced bovine embryos. Molecular Human Reproduction. 2004;10:383–392. doi: 10.1093/molehr/gah057. [DOI] [PubMed] [Google Scholar]
- 93.Matwee C, Betts DH, King WA. Apoptosis in the early bovine embryo. Zygote. 2000;8:57–68. doi: 10.1017/s0967199400000836. [DOI] [PubMed] [Google Scholar]
- 94.Velez-Pardo C, Morales AT, Del Rio MJ, Olivera-Angel M. Endogenously generated hydrogen peroxide induces apoptosis via mitochondrial damage independent of NF-kappaB and p53 activation in bovine embryos. Theriogenology. 2007;67:1285–1296. doi: 10.1016/j.theriogenology.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 95.Betts DH, Madan P. Permanent embryo arrest: molecular and cellular concepts. Molecular Human Reproduction. 2008;14:445–453. doi: 10.1093/molehr/gan035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tang ED, Nuñez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. Journal of Biological Chemistry. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 97.Biggs WH, 3rd, Cavenee WK, Arden KC. Identification and characterization of members of the FKHR [FOX O] subclass of winged-helix transcription factors in the mouse. Mammalian Genome. 2001;12:416–425. doi: 10.1007/s003350020002. [DOI] [PubMed] [Google Scholar]
- 98.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 99.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 100.Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Current Biology. 2000;10:1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 101.Ventura A, Maccarana M, Raker VA, Pelicci PG. A cryptic targeting signal induces isoform-specific localization of p46Shc to mitochondria. Journal of Biological Chemistry. 2004;279:2299–2306. doi: 10.1074/jbc.M307655200. [DOI] [PubMed] [Google Scholar]
- 102.Nemoto S, Combs CA, French S, Ahn BH, Fergusson MM, Balaban RS, et al. The mammalian longevity-associated gene product p66shc regulates mitochondrial metabolism. Journal of Biological Chemistry. 2006;281:10555–10560. doi: 10.1074/jbc.M511626200. [DOI] [PubMed] [Google Scholar]
- 103.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 104.Pinton P, Leo S, Wieckowski MR, Di Benedetto G, Rizzuto R. Long-term modulation of mitochondrial Ca2 + signals by protein kinase C isozymes. The Journal of Cell Biology. 2004;165:223–232. doi: 10.1083/jcb.200311061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radical Biology of Medicine. 2000;28:1349–1361. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 106.Pellegrini M, Finetti F, Petronilli V, Ulivieri C, Giusti F, Lupetti P, et al. p66SHC promotes T cell apoptosis by inducing mitochondrial dysfunction and impaired Ca2+ homeostasis. Cell Death and Differentiation. 2007;14:338–347. doi: 10.1038/sj.cdd.4401997. [DOI] [PubMed] [Google Scholar]
- 107.Wulf G, Finn G, Suizu F, Lu KP. Phosphorylation-specific prolyl isomerization: is there an underlying theme? Nature Cell Biology. 2005;7:435–441. doi: 10.1038/ncb0505-435. [DOI] [PubMed] [Google Scholar]
- 108.Orsini F, Moroni M, Contursi C, Yano M, Pelicci P, Giorgio M, et al. Regulatory effects of the mitochondrial energetic status on mitochondrial p66Shc. Biological Chemistry. 2006;387:1405–1410. doi: 10.1515/BC.2006.176. [DOI] [PubMed] [Google Scholar]
- 109.Gertz M, Fischer F, Wolters D, Steegborn C. Activation of the lifespan regulator p66Shc through reversible disulfide bond formation. Proceedings of the National Academic of Sciences of the United States of America. 2008;105:5705–5709. doi: 10.1073/pnas.0800691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sastre J, Pallardó FV, Viña J. The role of mitochondrial oxidative stress in aging. Free Radical Biology of Medicine. 2003;35:1–8. doi: 10.1016/s0891-5849(03)00184-9. [DOI] [PubMed] [Google Scholar]
- 111.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lithgow GJ, Kirkwood TB. Mechanisms and evolution of aging. Science. 1996;273:80. doi: 10.1126/science.273.5271.80. [DOI] [PubMed] [Google Scholar]
- 113.Helbock HJ, Beckman KB, Ames BN. 8-Hydroxydeoxyguanosine and 8-hydroxyguanine as biomarkers of oxidative DNA damage. Methods in Enzymology. 1999;300:156–166. doi: 10.1016/s0076-6879(99)00123-8. [DOI] [PubMed] [Google Scholar]
- 114.Favetta LA, Robert C, King WA, Betts DH. Expression profiles of p53 and p66shc during oxidative stress-induced senescence in fetal bovine fibroblasts. Experimental Cell Research. 2004;299:36–48. doi: 10.1016/j.yexcr.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 115.Jiang X, Edstrom E, Altun M, Ulfhake B. Differential regulation of Shc adaptor proteins in skeletal muscle, spinal cord and forebrain of aged rats with sensorimotor impairment. Aging Cell. 2003;2:47–57. doi: 10.1046/j.1474-9728.2003.00030.x. [DOI] [PubMed] [Google Scholar]
- 116.Klein LE, Freeze BS, Smith AB, 3rd, Horwitz SB. The microtubule stabilizing agent discodermolide is a potent inducer of accelerated cell senescence. Cell Cycle. 2005;4:501–507. doi: 10.4161/cc.4.3.1550. [DOI] [PubMed] [Google Scholar]
- 117.Trinei M, Berniakovich I, Pelicci PG, Giorgio M. Mitochondrial DNA copy number is regulated by cellular proliferation: a role for Ras and p66[Shc] Biochim Biophysics Acta. 2006;1757:624–630. doi: 10.1016/j.bbabio.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 118.Masuyama M, Iida R, Takatsuka H, Yasuda T, Matsuki T. The pregnane X receptor regulates gene expression in a ligand- and promoter-selective fashion. Molecular Endocrinology. 2005;19:1170–1180. doi: 10.1210/me.2004-0434. [DOI] [PubMed] [Google Scholar]
- 119.Pages G, Lenormand P, L'Allemain G, Chambard JC, Meloche S, Pouyssegur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proceedings of National Academy of Sciences. 1993;90:8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 121.Anand-Apte B, Zetter BR, Viswanathan A, Qiu RG, Chen J, Ruggieri R, et al. Platelet-derived growth factor and fibronectin-stimulated migration are differentially regulated by the Rac and extracellular signal-regulated kinase pathways. Journal of Biological Chemistry. 1997;272:30688–30692. doi: 10.1074/jbc.272.49.30688. [DOI] [PubMed] [Google Scholar]
- 122.Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. The Journal of Cell Biology. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Klemke RL, Yebra M, Bayna EM, Cheresh DA. Receptor tyrosine kinase signaling required for integrin alpha v beta 5-directed cell motility but not adhesion on vitronectin. The Journal of Cell Biology. 1994;127:859–866. doi: 10.1083/jcb.127.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huttenlocher A, Sandborg RR, Horwitz AF. Adhesion in cell migration. Current Opinion in Cell Biology. 1995;7:697–706. doi: 10.1016/0955-0674(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 125.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 126.Abdollahi A, Gruver BN, Patriotis C, Hamilton TC. Identification of epidermal growth factor-responsive genes in normal rat ovarian surface epithelial cells. Biochemical and Biophysical Research Communications. 2003;18:188–197. doi: 10.1016/s0006-291x(03)01140-9. [DOI] [PubMed] [Google Scholar]
- 127.Park YJ, Kim TY, Lee SH, Kim H, Kim SW, Shong M, et al. p66shc expression in proliferating thyroid cells is regulated by thyrotropin receptor signaling. Endocrinology. 2005;146:2473–2480. doi: 10.1210/en.2004-1588. [DOI] [PubMed] [Google Scholar]
- 128.Ruoslahti E, Reed JC. Anchorage dependence, integrins, and apoptosis. Cell. 1994;77:477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 129.Hynes RO, Bader BL, Hodivala-Dilke K. Integrins in vascular development. Brazil Journal of Medicinal Biological Research. 1999;32:501–510. doi: 10.1590/s0100-879x1999000500002. [DOI] [PubMed] [Google Scholar]
- 130.Cooper CR, Chay CH, Pienta KJ. The role of alpha[v]beta[3] in prostate cancer progression. Neoplasia. 2002;4:191–194. doi: 10.1038/sj.neo.7900224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR. Prostatic carcinoma cell migration via alpha[v]beta3 integrin is modulated by a focal adhesion kinase pathway. Cancer Research. 1999;59:1655–1664. [PubMed] [Google Scholar]
- 132.Northey JJ, Chmielecki J, Ngan E, Russo C, Annis MG, Muller WJ, et al. Signaling through ShcA is required for transforming growth factor beta- and Neu/ErbB-2-induced breast cancer cell motility and invasion. Molecular and Cellular Biology. 2008;28:3162–3176. doi: 10.1128/MCB.01734-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ursini-Siegel J, Muller WJ. The ShcA adaptor protein is a critical regulator of breast cancer progression. Cell Cycle. 2008;7:1936–1943. doi: 10.4161/cc.7.13.6205. [DOI] [PubMed] [Google Scholar]
- 134.Sundaresan M, Yu ZX, Ferrans VJ, Sulciner DJ, Gutkind JS, Irani K, et al. Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochemical Journal. 1996;318:379–382. doi: 10.1042/bj3180379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu SL, Lin X, Shi DY, Cheng J, Wu CQ, Zhang YD. Reactive oxygen species stimulated human hepatoma cell proliferation via cross-talk between PI3-K/PKB and JNK signaling pathways. Archives of Biochemistry and Biophysics. 2002;406:173–182. doi: 10.1016/s0003-9861(02)00430-7. [DOI] [PubMed] [Google Scholar]
- 136.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;9:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 137.Lou YW, Chen YY, Hsu SF, Chen RK, Lee CL, Khoo KH, et al. Redox regulation of the protein tyrosine phosphatase PTP1B in cancer cells. FEBS Journal. 2008;275:69–88. doi: 10.1111/j.1742-4658.2007.06173.x. [DOI] [PubMed] [Google Scholar]
- 138.Lin MF, Meng TC. Tyrosine phosphorylation of a 185 kDa phosphoprotein [pp 185] inversely correlates with the cellular activity of human prostatic acid phosphatase. Biochemical and Biophysical Research Communications. 1996;226:206–213. doi: 10.1006/bbrc.1996.1334. [DOI] [PubMed] [Google Scholar]
- 139.Lin MF, Lee MS, Zhou XW, Andressen JC, Meng TC, Johansson SL. Decreased expression of cellular prostatic acid phosphatase increases umorigenicity of human prostate cancer cells. The Journal of Urology. 2001;166:1943–1950. [PubMed] [Google Scholar]
- 140.Veeramani S, Yuan TC, Chen SJ, Lin FF, Petersen JE, Shaheduzzaman S, et al. Cellular prostatic acid phosphatase: a protein tyrosine phosphatase involved in androgen-independent proliferation of prostate cancer. Endocr-Related Cancer. 2005;12:805–822. doi: 10.1677/erc.1.00950. [DOI] [PubMed] [Google Scholar]
- 141.Lim SD, Sun C, Lambeth JD, Marshall F, Amin M, Chung L, et al. Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate. 2005;62:200–207. doi: 10.1002/pros.20137. [DOI] [PubMed] [Google Scholar]
- 142.Khanday FA, Santhanam L, Kasuno K, Yamamori T, Naqvi A, Dericco J, et al. Sos-mediated activation of rac1 by p66shc. The Journal of Cell Biology. 2006;172:817–822. doi: 10.1083/jcb.200506001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Knight-Krajewski S, Welsh CF, Liu Y, Lyons LS, Faysal JM, Yang ES, et al. Deregulation of the Rho GTPase, Rac1, suppresses cyclin-dependent kinase inhibitor p21 [CIP1] levels in androgen-independent human prostate cancer cells. Oncogene. 2004;23:5513–5522. doi: 10.1038/sj.onc.1207708. [DOI] [PubMed] [Google Scholar]
- 144.Veeramani S, Lin MF. Role of reactive oxygen species in carcinogenesis. In: Banarjee R, editor. Redox biochemistry. New Jersey: Wiley; 2007. pp. 212–218. [Google Scholar]
- 145.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) The Journal of the American Medical Association. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sakai H, Igawa T, Saha PK, Nomata K, Yushita Y, Kanetake H, et al. A case of prostatic carcinoma presenting as a metastatic orbital tumor. Hinyokika Kiyo. Acta Urologica Japonica [Kyoto] 1992;38:77–80. [PubMed] [Google Scholar]
- 147.Chu TM, Lin MF. PSA and acid phosphatase in the diagnosis of prostate cancer. Journal of Clinical Ligand Assay. 1998;21:24–34. [Google Scholar]
- 148.Reif AE, Schlesinger RM, Fish CA, Robinson CM. Acid phosphatase isozymes in cancer of the prostate. Cancer. 1973;31:689–699. doi: 10.1002/1097-0142(197303)31:3<689::aid-cncr2820310331>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 149.Foti AG, Cooper JF, Herschman H, Malvaez RR. Detection of prostatic cancer by solid-phase radioimmunoassay of serum prostatic acid phosphatase. New England Journal of Medicine. 1977;297:1357–1361. doi: 10.1056/NEJM197712222972501. [DOI] [PubMed] [Google Scholar]
- 150.Loor R, Wang MC, Valenzuela L, Chu TM. Expression of prostatic acid phosphatase in human prostate cancer. Cancer Letters. 1981;14:63–69. doi: 10.1016/0304-3835(81)90010-0. [DOI] [PubMed] [Google Scholar]
- 151.Pontes JE, Rose NR, Ercole C, Pierce JM., Jr Immunofluorescence for prostatic acid phosphatase: clinical applications. The Journal of Urology. 1981;126:187–189. doi: 10.1016/s0022-5347(17)54440-7. [DOI] [PubMed] [Google Scholar]
- 152.Solin T, Kontturi M, Pohlmann R, Vihko P. Gene expression and prostate specificity of human prostatic acid phosphatase [PAP]: evaluation by RNA blot analyses. Biochimica et Biophysica Acta. 1990;1048:72–77. doi: 10.1016/0167-4781(90)90024-v. [DOI] [PubMed] [Google Scholar]
- 153.Sakai H, Yogi Y, Minami Y, Yushita Y, Kanetake H, Saito Y. Prostate specific antigen and prostatic acid phosphatase immunoreactivity as prognostic indicators of advanced prostatic carcinoma. The Journal of Urology. 1993;149:1020–1023. doi: 10.1016/s0022-5347(17)36285-7. [DOI] [PubMed] [Google Scholar]
- 154.Gioeli D, Mandell JW, Petroni GR, Frierson HF, Jr, Weber MJ. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Research. 1999;59:279–284. [PubMed] [Google Scholar]
- 155.Price DT, Della Rocca G, Guo C, Ballo MS, Schwinn DA, Luttrell LM. Activation of extracellular signal regulated kinase in human prostate cancer. The Journal of Urology. 1999;162:1537–1542. [PubMed] [Google Scholar]
- 156.Lee MS, Igawa T, Yuan TC, Zhang XQ, Lin FF, Lin MF. ErbB2 signaling is involved in regulating PSA secretion in androgen-independent human prostate cancer LNCaP C-81 cells. Oncogene. 2003;22:781–796. doi: 10.1038/sj.onc.1206066. [DOI] [PubMed] [Google Scholar]