Abstract
Background
The Ukrainian American Cohort Study was established to evaluate the risk of thyroid disorders in a group exposed as children and adolescents to 131I by the Chornobyl accident (arithmetic mean thyroid dose=0.79 Gray). Subjects are screened by palpation and ultrasound and referred to surgery according to fine needle aspiration biopsy (FNA). However, the accuracy of FNA cytology for detecting histopathologically confirmed malignancy following this level of internal exposure to radioiodines is unknown.
Methods
As a result of the first screening cycle (1998-2000), 13,243 individuals were examined, 356 with thyroid nodules were referred for FNA, 288 completed the procedure, 85 were referred to surgery, 82 were operated upon, and pre-operative cytology was available for review in 78. Cytological interpretation for the nodule that resulted in surgical referral was correlated with final pathomorphology; discrepancies were retrospectively reviewed; and sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of FNA cytology were calculated.
Results
All 24 cytological interpretations definite for papillary thyroid cancer (PTC) were histopathologically confirmed (PPV=100%) and of 11 suspect for PTC, 10 were confirmed (PPV=90.9%). Ten of 41 FNAs interpreted as either definite or suspect for follicular neoplasm (FN) were confirmed as malignant (PPV=24.4%): 2 follicular thyroid cancers (FTC) and 8 PTCs (all but one of the follicular or mixed subtypes). Depending on whether a cytological interpretation of FN was considered a “positive” or “negative”, sensitivity was 100% or 77.3%, respectively; similarly, specificity was 17.6% or 97.1 %, PPV 61.1% or 97.1% and NPV 100% or 76.7%.
Conclusions
Among children and adolescents exposed to 131I following Chornobyl and evaluated 12 to 14 years later, thyroid cytology has a sensitivity and predictive value similar to that reported in unexposed populations.
INTRODUCTION
In April 1986 the Chornobyl (Chernobyl in Russian) accident exposed the residents of northern Ukraine to massive amounts of radioactive isotopes of iodine, mainly 131I that was acquired by drinking contaminated milk (1). The most important health consequence of the catastrophe has been an enormous increase in the number of thyroid cancers, largely of the papillary type, among those exposed as children and adolescents (2,3). Although many of the early papillary cancers demonstrated a prominent or exclusively solid growth pattern and aggressive clinical behavior (4-8), longer latency has been associated with more typical papillary carcinomas that are less invasive (9,10).
The Ukrainian-American Cohort Study of Thyroid Cancer and Other Thyroid Diseases (UkrAm) was established to evaluate risk of thyroid cancer and other thyroid disorders in a group of individuals under age 18 years when exposed to Chornobyl fallout, and who had direct thyroid radioactivity measurements taken within several weeks after the accident. Subjects are screened for thyroid nodules by palpation and ultrasound (US) and referral is made to surgery if fine needle aspiration biopsy (FNA) is definite or suspect papillary cancer or follicular neoplasm.
FNA cytology of thyroid nodules is widely recognized to be accurate and, except for follicular neoplasms (11,12), to have a high positive predictive value for thyroid malignancy among unexposed adults (13-15) and children (16). However, there have been only a few reports concerning cytological findings in irradiated individuals and what is known comes largely from those who were externally exposed to x-irradiation during childhood (17-20). High-dose x-ray exposure (17) and internal irradiation with therapeutic doses of 131I (21,22) result in marked nuclear and cellular atypia that potentially lowers the accuracy of FNA for detecting malignancy, whereas following lower external doses the reported predictive value of thyroid cytology is similar to that in unexposed populations (18,19). The accuracy of FNA following lower thyroid 131I doses from environmental exposure has seldom been examined. There have been only three previous reports of thyroid cytology in Chornobyl-exposed children (23-25) and no previous cyto-histopathologic correlations in this population.
In this paper we describe our experience with pre-operative FNA cytology in those UkrAm cohort members who underwent surgery for thyroid nodules detected during the first cycle of screening (1998-2000). We correlate the cytological and histopathologic features in 78 cases, discuss discordant conclusions, and examine the accuracy of FNA cytology in this unique population, which has been evaluated according to a strict protocol.
MATERIALS AND METHODS
Study cohort
Details of the organization and methods of UkrAm and a parallel program in Belarus have been published (26,27). The Ukrainian cohort consists of subjects less than 18 years of age on 26 April 1986 who were residents of the heavily contaminated Kyiv, Chernihiv, and Zhytomyr Oblasts and who had direct thyroid radioactivity measurements taken in May or June 1986. An oblast is an administrative region similar to a state or province.
Screening, fine needle aspiration, and referral to surgery
During the first screening cycle (1998-2000), 13,243 subjects were examined and FNA was carried out according to protocol-specified indications. The screenings were conducted mainly by mobile teams working in regional hospitals and patients were referred to the Institute of Endocrinology and Metabolism (IEM) in Kyiv for FNA, which was performed on focal thyroid nodules detected either on palpation or US that measured ≥ 10 mm in greatest dimension and on all sonographically suspicious lesions (based on hypoechogenicity, irregular shape/contour, microcalcifications, extension through thyroid capsule, interval growth, abnormal adenopathy) that measured from 5 to 10 mm in greatest dimension. Patients were referred for surgery if cytology was diagnostic or suspicious for malignancy in either a nodule or a lymph node or for a follicular neoplasm in a nodule, or at the discretion of the attending physician. In the case of an inadequate specimen, the FNA was repeated either at the same or a subsequent visit until sufficient cytological material was obtained. Up to three attempts were carried out within any given 12-month period.
Out of 13,243 cohort members screened during the first cycle, 356 (2.7%) with thyroid nodules were referred for FNA and of these 62 (17.4 %) were deemed not to require the procedure after re-evaluation at the IEM. Of the 294 patients for whom FNA was indicated, 288 (98.0%) completed the procedure at least once. After the first FNA, an adequate cytological specimen was obtained in 218 (75.7%); after the second in 52 (18.1%); after the third in 14 (4.9%); and after the fourth in 2 (0.7%). Since subjects lacking a satisfactory FNA specimen were not referred to surgery, two patients (0.7%) with uninformative aspirates who refused additional sampling are not included in the analysis.
Of the 286 subjects with adequate specimens, almost 60% of cytological interpretations were nodular goiter; autoimmune thyroiditis and benign cyst were much less common conclusions. Eighty-five cohort members with suspicious biopsies were referred to surgery and 82 (96.5%) were operated on between 1998 and 2007, 78 at the IEM and the remaining four at regional hospitals, two in Chernihiv and one each in Kyiv and Zhytomyr. All 82 histopathogical specimens were evaluated (re-diagnosed in four cases) at the IEM. Eighty cases had surgery performed between 1998 and 2004 and have been described in detail (10, 27). Of the 82 operated patients, 78 (95%) had preoperative FNA performed at the IEM and are the subjects of this report. Four cases were excluded: one FNA performed at a regional hospital in Zhytomyr and not reviewed at the IEM and three that did not have a preoperative FNA (one each with toxic diffuse goiter, simple diffuse goiter, and bilateral multinodular goiter).
Cytological procedures
FNA of the thyroid was performed under US guidance using a 21-G needle, usually without a syringe or suction, a technique that produced a high cellular yield and no excessive bleeding. In order to maximize efficiency and minimize patient waiting time, the presence of an adequate number of epithelial cells was determined on-site by a cytologist’s examination of native (unstained) smears with the light microscope’s condenser lowered to one half of the lens’ aperture. In the laboratory, the smears were fixed for five minutes in methanol followed by 30 minutes of staining in Giemsa dye diluted in 0.067M phosphate buffer. Adequacy of the stained smears was defined by the presence of at least two smears with at least six to eight groups of well-preserved follicular epithelial cells. Cytological findings were classified according to conventional criteria (28) as:
Negative for malignant cells (nonneoplastic: nodular goiter [NG] or autoimmune thyroiditis);
Definite or suspect papillary thyroid carcinoma (PTC);
Definite or suspect follicular neoplasm (FN) or Hurthle cell tumor;
Non-diagnostic due to insufficient or distorted epithelial cells.
Cases were given a unique laboratory identification number and the slides were also labeled with a bar code number corresponding to their identification number in the Chornobyl Cohort Screening Project. Cytological interpretations were made by two of the authors (YB, AZ) and reviewed by a third (EG). The consensus cytological findings and conclusions for each FNA were entered on a paper form and submitted to the UkrAm Data Coordinating Center in the IEM for entry into the project database. In addition to choosing specific diagnostic categories with a manual check mark on the form, the cytologist entered the diagnosis as free text as a back up for the data entry staff.
Histopathological procedures
Postoperative study of paraffin sections stained with hematoxylin-eosin was performed at the IEM Pathology Laboratory in all cases. The diagnosis was made by two of the authors (TIB, LYZ) according to the World Health Organization histological classification system (29), and another author (EG) reviewed all cases. The International Pathology Panel, established in the framework of the Chernobyl Tissue Bank (available at URL www.Chernobyltissuebank.com [accessed April 2008]), confirmed all histopathologic diagnoses. PTCs were subdivided into several subtypes, depending on the dominant structural component (10). Final pathomorphological analysis revealed 45 thyroid carcinomas, including 43 PTCs (95.6%) and 2 follicular thyroid carcinomas (FTCs) (4.4%) (10). Among the PTCs, 8 exhibited the classical papillary histologic pattern (18.6%), 14 exhibited a follicular histologic pattern (32.6%), 5 exhibited a solid histologic pattern (11.6%), and 16 exhibited a mixed histologic pattern (37.2%). Both FTCs had a microfollicular-solid structure. In two patients who underwent surgery for multinodular goiter, occult PTCs that measured 1 and 9 mm were detected only at final pathomorphological examination and are not considered in the current analysis. Retrospective review of the FNA cytology was performed by three of the authors (YB, TIB, EG) on all cases in which there was a discrepancy between the cytological interpretation and the final histopathologic diagnosis.
Statistical analysis
In order to estimate the accuracy of FNA in our cohort, we analyzed the data in several ways. Initially, we calculated positive predictive values (PPV) for the specific cytological interpretations in Table 1. However, we could not accurately estimate the PPV of a benign aspirate as individuals with non-neoplastic FNAs were not referred to surgery, with the exception of two cases operated upon for clinical reasons.
Table 1.
Correlation of pre-operative FNA cytological conclusions and final histopathological diagnoses in 78 patients. * Ukrainian-American Cohort Study of Thyroid Cancer and Other Thyroid Diseases (1998-2000).
| Histopathological diagnosis | |||||
|---|---|---|---|---|---|
| Cytological interpretation |
PTC | FTC | FA | NG | Total |
| PTC | 24 | 24 | |||
| Suspect PTC | 10 | 1 | 11 | ||
| FN | 8 | 2 | 18 | 9† | 37 |
| Suspect FN | 2 | 2 | 4 | ||
| NG | 1 | 1 | 2 | ||
| Total | 42 | 2 | 22 | 12 | 78 |
Abbreviations:
PTC papillary thyroid cancer
FTC follicular thyroid cancer
FA follicular adenoma
NG nodular goiter (uninodular and multinodular goiter)
FN follicular neoplasm
Correlation is for the nodule that resulted in referral to surgery.
One nodule classified on FNA as FN was diagnosed histopathologically as a non-neoplastic nodule with fibrosclerotic and cystic changes.
Because a cytological conclusion of FN does not differentiate between benign and malignant nodules, some authors have considered FN a “positive” (malignant or neoplastic) finding, while others have considered it a ”negative” (11,12). To compare our findings with the majority of studies and to evaluate how FNA accuracy is influenced by how FN is categorized, we analyzed the data using both approaches and determined overall sensitivity, specificity, positive predictive value, and negative predictive value according to the definitions and formulas given in Table 2. We first considered a cytological interpretation of definite FN as a “positive” finding and if the corresponding histopathology was FA or NG, the case was considered a false positive. The single case where the cytology was suspect PTC and the final diagnosis was FA was also considered a false positive. For the purposes of analysis, the two cases of NG that underwent operation at the discretion of the attending physician and the four cases of suspect FN were considered “negative”. We then performed the calculations by treating a cytological finding of definite FN as a “negative” result (11) and if the final histopathology was PTC or FTC, the case was considered a false negative.
Table 2.
Overall sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of FNA for a histopathological diagnosis of thyroid cancer according to two treatments for a cytological interpretation of follicular neoplasm*. Ukrainian-American Cohort Study of Thyroid Cancer and Other Thyroid Diseases (1998-2000).
| Cytological interpretation |
||
|---|---|---|
| PTC+Suspect PTC+FN¶ |
PTC+Suspect PTC† | |
| Sensitivity | 100% (44/44)** | 77.3% (34/44) |
| Specificity | 17.6% (6/34) | 97.1% (33/34) |
| PPV | 61.1% (44/72) | 97.1% (34/35) |
| NPV | 100% (6/6) | 76.7% (33/43) |
|
| ||
| Sensitivity | TP/ (TP + FN) | |
| Specificity | TN/ (TN+ FP) | |
| PPV | TP/ (TP+FP) | |
| NPV | TN/ (TN+FN) | |
Histopathology is considered positive if diagnosis is PTC or FTC, otherwise it is considered negative.
Cytology is considered positive if FNA interpretation is PTC, suspect PTC, or FN, otherwise it is considered negative
Cytology is considered positive if FNA interpretation is PTC or suspect PTC, otherwise it is considered negative.
From Table 1
The study was reviewed and approved by Institutional Review Boards in Ukraine and the United States and all participants signed an informed consent form.
RESULTS
The study population
The female to male ratio among the 78 study subjects was 50:28 (1.8:1). At operation, 2 (2.6%) were children 14 years old, 13 (16.7%) were adolescents aged 15 to 18 years, and 63 (80.8%) were young adults aged 19 to 35 years (mean age at operation 23.5 years). At the time of the accident, the great majority (72 of 78; 92.3%) were children under 14 years of age and of these, 50 (64.1% of the total) were under 10 years of age; 6 patients (7.7%) were adolescents 15 to 18 years old (mean age at exposure 8.5 years). The mean latency (time elapsed between exposure and surgery) was 15.1 years (range 12.5-21.6 years).
Correlation of cytology and histopathology
The correlation of pre-operative cytological interpretations and final histopathological diagnoses in 78 UkrAm patients is shown in Table 1. Although three patients had two nodules biopsied, the correlation presented is for the nodule that resulted in referral to surgery. The highest PPV was for an FNA finding of definite PTC, since all 24 cases were confirmed on histopathology (PPV=100%). Of the 11 suspect PTC, 10 were confirmed as PTC (PPV= 90.9 %) and a single case (9.1 %) was found to be a follicular adenoma (FA). Ten of 41 FNAs interpreted as either definite or suspect follicular neoplasm (FN) were confirmed as malignant (PPV=24.4%): two FTCs and eight PTCs. Of the rest, 20 (48.8 %) were diagnosed as FAs and 11 (26.8%) as nodular goiter (NG). One of two cases interpreted as NG on FNA was histopathologically confirmed and the other was revealed to be a FA.
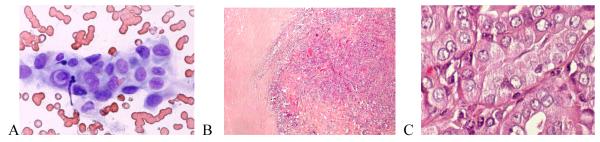
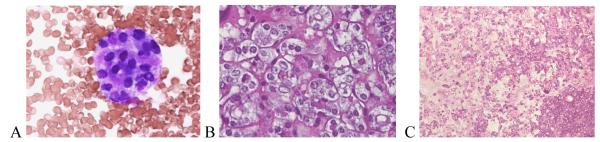
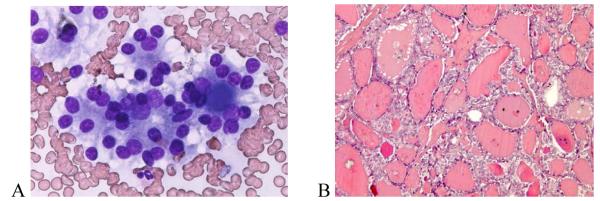
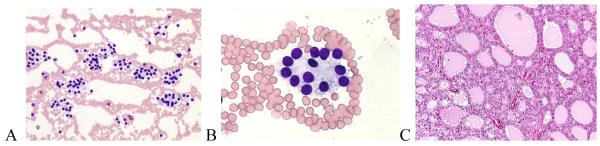
The only false positive FNA interpretation of PTC was a FA that exhibited fibrosclerotic and cystic histopathological changes (Figure 1). On cytology, the follicular cells showed nuclear enlargement, angulation, and rare small intranuclear cytoplasmic inclusions (INCIs) (Figure 1 A). Along with nuclear clearing, these changes, although typical of a PTC, can also be seen in a FA and were likely caused by fibrous-sclerosing changes and degeneration (Figure 1 B, C). None of the eight FNA cases interpreted as FN that proved to be PTC on histopathology were of the classical papillary subtype. Five (62.5%) were of the follicular and one each was of the solid, solid-follicular, and papillary-follicular-solid variants. For the follicular and mixed subtypes, the most common reason for discrepancy was the prominence in the FNA of a micro-follicular pattern of cellular proliferation usually without oval nuclear shapes, powdery chromatin, INCIs, prominent nuclear grooves, or psammoma bodies typical of PTC. Upon retrospective review, two of these cytological specimens were found to have had rare foci in which the nuclei had powdery chromatin and a third case had rare equivocal INCIs (Figure 2). In one FNA, the nuclear chromatin was indistinct, making the type of tumor difficult to determine (Figure 2 A). Upon histopathologic evaluation, the tumor was largely necrotic (Figure 2 B), but after thorough histopathologic evaluation of the peripheral viable areas it became possible to diagnose the nodule as PTC- follicular variant (Figure 2 B, C). Histopathologically, three papillary cancers, all of follicular subtype, had focal nuclear features typical of PTC (Figure 3) and in one case the histology showed that some areas of the tumor had coarse, compact nuclear chromatin. The FNA cytology of the single case of PTC-solid variant exhibited large, round or oval nuclei, a few of which demonstrated powdery chromatin and others denser chromatin, but none showed INCIs. A similar mixture of nuclear features was present in the histopathology, which could explain the discordance between the cytological and histological diagnoses.
Figure 1.
FNA of a thyroid nodule classified as “suspect PTC” based on nuclear enlargement, angulation, and grooves, powdery chromatin, and intranuclear cytoplasmic inclusions (INCI) (A). Histopathologically it was a follicular adenoma with fibrous-sclerosing changes (B) and focal nuclear clearing (C). (A – Giemsa × 40; B – H&E × 10; C – H&E × 40 ).
Figure 2.
Indistinct nuclear chromatin in FNA (A) and tissue (B) from a necrotic PTC- follicular variant (C).(A–Giemsa × 40; B - H&E × 40; C - H&E × 10 ).
Figure 3.
Microfollicles in a case of PTC classified on FNA as FN (A). The clear nuclei were seen only focally at histopathology (B). (A – Giemsa × 40; B - H&E × 10).
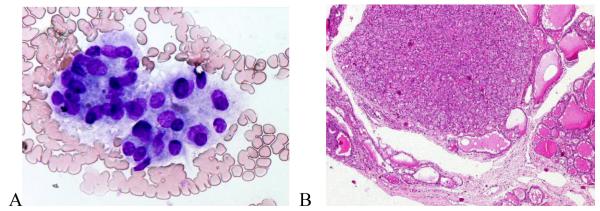
Nine cases cytologically interpreted as FN were histopathologically non-neoplastic and included 8 NGs and 1 fibrotic-degenerative nodule. In three cases, the FNA cytology was predominantly microfollicular in appearance (Figure 4), with one demonstrating prominent Hurthle cell features; all corresponding histopathologic diagnoses were adenomatous multinodular goiter (MNG) with microfollicular-solid structure and one also had prominent Hurthle cell hyperplasia. In five others, the FNA cytology also exhibited a predominantly microfollicular architecture, but pathologic analysis showed that such patterns were only focal in the resected nodules (Figure 5), and final pathology diagnosis was NG in all (one MNG). One case of FN by cytology was diagnosed pathologically as a non-neoplastic fibrotic-degenerative nodule. Despite the discordant classification, we believe that the degenerative cytomorphological changes in the tissue may have obscured the features of a FN.
Figure 4.
FNA with microfollicular structures interpreted cytologically as “follicular neoplasm” (A) and histopathologically as multinodular goiter with adenomatous hyperplasia, mixed microfollicular-solid structure (B). (A – Giemsa × 40; B – H&E × 4 ).
Figure 5.
Microfollicular structures interpreted as “follicular neoplasm” by cytology (A, B) and as nodular goiter on histopathology due to focal distribution of microfollicles (C). (A – Giemsa × 10; B- Giemsa × 40; C – H&E × 10).
Two of four cases that were suspect for FN on cytology were confirmed as FA by histopathology, while the other two proved to be NG. The discrepancy is not unexpected, largely due to overlapping cytological features between FN and NG such as microfollicular and focal Hurthle cell proliferation.
Accuracy of FNA for diagnosing thyroid cancer
The overall sensitivity, specificity, PPV and NPV of FNA cytology for a histopathological diagnosis of thyroid cancer are presented in Table 2. Depending on whether a cytological interpretation of FN was considered a “positive” or “negative” finding, the sensitivity was 100% or 77.3%, respectively; similarly, the specificity was 17.6% or 97.1 %, PPV 61.1% or 97.1% and NPV 100% or 76.7%.
DISCUSSION
In this cohort exposed to 131I as a result of the Chornobyl accident, we have found a very high PPV (97.1%) for malignancy when the cytological interpretation was definite or suspect PTC and a lower, but substantial, one (24.4%) when cytology was definite or suspect FN. The major strengths of our study are screening of the entire cohort according to a standardized protocol irrespective of 131I dose; referral to FNA and surgery according to strictly defined criteria; a high rate of compliance with FNA (98.0%) and surgery (96.5%); a low rate of inadequate cytological specimens (0.7%); and independent review of histopathological specimens by an international panel of experts. All these characteristics make our series unique in terms of interpreting FNA accuracy relative to histopathology. The major limitation of our study is the relatively small number of cases as the present analysis is restricted to patients operated upon as a result of only the first cycle of screening. It should also be noted that we could determine if cellular atypia was a consequence of 131I exposure, since cytological interpretations were performed without regard to dose.
Other studies of radiation-exposed individuals have recorded a high frequency of inadequate specimens (18) and a large number of small cancers that have escaped cytological diagnosis (19, 20). Therefore, our protocol specified biopsy of up to three sonographically suspicious nodules as small as 5 mm, immediate assessment of cytological material, and recall visits for those with inadequate specimens (26). In our previously published series of 45 cancers, 10 (23.3%) were less than or equal to10 mm in greatest dimension and only two (5%) were first detected at final pathologic analysis (10). By way of comparison, among a group of externally exposed children, 50% of the cancers were less than 10 mm in size and in more than half of the cases the malignant nodule was not the one that was biopsied (19).
Our results are similar to those obtained from a general population (13-16, 30, 31), where FNA cytology has a predictive value approaching 99% for malignancy (13-14) and from 15 to 30% for FN (11, 12, 32-34). However, they contrast with those from patients treated externally with between 25 and 40 Gy (17) or internally with therapeutic doses of 131I (21, 22), which typically delivers up to 100 Gy to the thyroid (35). In these cases, severe nuclear and cytoplasmic changes can result in an erroneous cytological conclusion of malignancy and lower the predictive value of FNA. We did not observe these cytological alterations in our cohort, which was exposed to an arithmetic mean dose of only 0.79 Gy (36).
Very little has been published concerning thyroid cytology in those exposed to Chornobyl fallout as children (23-25). Thyroid cancer, all of the papillary type was found in 2.3% and FN in 6.4% of successful aspirates from a population screened by ultrasound, and the largest cause of nodularity was non-neoplastic, mainly chronic thyroiditis and cysts (23). Another study of the cytological features in 20 cases of pathologically confirmed PTC concluded that the main diagnostic FNA findings were similar to those in unexposed adult cases, with the exception of more prominent nuclear atypia, which correlated with solid proliferation on histology, and a high prevalence of psammoma bodies on FNAs from the exposed children (24). Although we are unable to confirm a high prevalence of nuclear atypia and psammoma bodies in our PTCs, it should be noted that our subjects were older at surgery (mean 23.5 years compared to 12 years in the earlier study) and had cancers of longer latency (15.1 years compared to 7.9 years). Since long-latency cancers are potentially more differentiated than those of short latency (9,10), a direct comparison of our results with those of the previous study may not be justified.
In our cohort, the PPV for a cytological interpretation of PTC or suspect PTC was 97.1% and for one of definite or suspect FN was 24.4%, similar to what has been reported in unexposed populations (11-14). It is also worth noting that sensitivity, specificity, PPV, and NPV varied depending on how a cytological conclusion FN was classified. When we considered FN as a “positive” finding, the overall sensitivity was high (100%) and specificity low (17.6 %), largely due to the absence of false negative results. When FN was regarded as “negative” finding, the sensitivity decreased to 77.3% and specificity increased to 97.1 %. These differences demonstrate that specificity is more affected than sensitivity by how FN is treated and emphasize that in our cohort histopathologically confirmed cancer cases were found less often than non-cancer cases when the cytological conclusion was FN.
Among those exposed to the Chornobyl accident as children and adolescents and evaluated between 1998 and 2000, the sensitivity, specificity, and predictive value of FNA for diagnosing malignancy are similar to that seen in unexposed subjects. Although a cytological conclusion of FN is often incorrect, it is also comparable to that found in a general population. We conclude that exposure to 131I at doses received by our study group has not lowered the accuracy of thyroid cytology for either malignancy or FN in nodules detected 12 to 14 years later.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge diagnostic confirmation provided by the International Pathology Panel of the Chornobyl Tissue Bank: Drs. A. Abrosimov, T.I. Bogdanova, M. Ito, V. LiVolsi, J. Rosai and E.D. Williams. We also thank T. Odnolko, V. Shpak, and A. Zvinchuk for database management; the late Professor O. Epshtein for organization of the FNA and cytology examinations; Dr. Y. Naida for performing ultrasound-guided FNA biopsies; and Dr. Arthur B. Schneider and the late Dr. Jacob Robbins for their helpful suggestions during the preparation and writing of the manuscript.
This study was funded under the Contract #NO1-CP-21178 by the National Cancer Institute, DHHS. The Department of Energy provided funding at the initial stages of the study, and the Nuclear Regulatory Commission provided the initial funds for purchase of equipment.
REFERENCES
- 1.Robbins J. Lessons from Chernobyl: the event, the aftermath fallout: radioactive, political, social. Thyroid. 1997;7:189–192. doi: 10.1089/thy.1997.7.189. [DOI] [PubMed] [Google Scholar]
- 2.Williams D. Health consequences of the Chornobyl accident. Science. 2001;292:2010–2011. doi: 10.1126/science.292.5524.2010. [DOI] [PubMed] [Google Scholar]
- 3.Hatch M, Ron E, Bouville A, Zablotska L, Howe G. The Chernobyl disaster: cancer following the accident at the Chernobyl nuclear power plant. Epidemiol Rev. 2005;27:56–66. doi: 10.1093/epirev/mxi012. [DOI] [PubMed] [Google Scholar]
- 4.Likhtarev IA, Sobolev BG, KairoI A, Tronko ND, Bogdanova TI, Oleinic VA, Epshtein EV, Beral V. Thyroid cancer in the Ukraine. Nature. 1995;375:365. doi: 10.1038/375365a0. [DOI] [PubMed] [Google Scholar]
- 5.Tronko MD, Bogdanova TI, Komissarenko IV, Epstein OV, Oliynik VA, Kovalenko A.Ye., Likhtarev IA, Kairo IA, Peters SB, LiVolsi VA. Thyroid carcinoma in children and adolescents in Ukraine after the Chernobyl accident: statistical data and clinicomorphologic characteristics. Cancer. 1999;86:149–156. doi: 10.1002/(sici)1097-0142(19990701)86:1<149::aid-cncr21>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 6.Tronko ND, Bogdanova TI. Thyroid cancer in children and adolescents. In: Vozianov A, Bebeshko V, Bazyka D, Chumak A, editors. Health effects of Chornobyl accident. DIALtd; Kyiv: 2003. pp. 60–68. [Google Scholar]
- 7.Tronko M, Bogdanova T, Likhtarev I, Komisarenko I, Kovalenko A, Epshtein O, Tereshchenko V, Shpak V, Gulak L. Thyroid gland and radiation (fundamental and applied aspects): 20-years after the Chernobyl accident. In: Shibata Y, Namba H, editors. Radiation Risk Perspectives. Elsevier; Amsterdam: 2007. pp. 46–53. [Google Scholar]
- 8.Bogdanova T, Zurnadzhy L, Tronko M, Namba H, Yamashita Sh., Thomas G. Pathology of thyroid cancer in children and adolescents of Ukraine having been exposed as a result of the Chernobyl accident. In: Shibata Y, Namba H, editors. Radiation Risk Perspectives. Elsevier; Amsterdam: 2007. pp. 256–270. [Google Scholar]
- 9.Williams ED, Abrosimov A, Bogdanova TI. Thyroid cancer after Chernobyl. Latent period, morphology and aggressiveness. Br J Cancer. 2004;90:2219–2224. doi: 10.1038/sj.bjc.6601860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogdanova TI, Zurnadzhy LY, Greenebaum E, McConnell RJ, Robbins J, Epstein OV, Olijnyk VA, Hatch M, Zablotska LB, Tronko MD. A cohort study of thyroid cancer and other thyroid disease following the Chornobyl accident: pathology analysis of thyroid cancer cases in Ukraine detected during first screening (1998-2000) Cancer. 2006;107:2559–2566. doi: 10.1002/cncr.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greaves TS, Olvera M, Florentine BD, Raza AS, Cobb CJ, Tsao-Wei DD, Groshen S, Singer P, Lopresti J, Martin SE. Follicular lesions of thyroid; a 5-year fine-needle aspiration experience. Cancer (Cancer Cytopathol) 2000;90:3335–341. [PubMed] [Google Scholar]
- 12.Baloch ZW, Fleisher S, LiVolsi VA, Gupta PK. Diagnosis of “follicular neoplasm”: a gray zone in thyroid fine-needle aspiration cytology. Diagnostic Cytopathol. 2002;26:41–44. doi: 10.1002/dc.10043. [DOI] [PubMed] [Google Scholar]
- 13.Sangalli G, Serio G, Zampatti C, Bellotti M, Lomuscio G. Fine needle aspiration cytology of the thyroid: a comparison of 5469 cytological and final histological diagnoses. Cytopathology. 2006;17:245–250. doi: 10.1111/j.1365-2303.2006.00335.x. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Schnadig V, Logrono R, Wasserman PG. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer. 2007;111:306–315. doi: 10.1002/cncr.22955. [DOI] [PubMed] [Google Scholar]
- 15.Kim DL, Song KH, Kim SK. High prevalence of carcinoma in ultrasonography-guided fine needle aspiration cytology of thyroid nodules. Endocr J. 2008 doi: 10.1507/endocrj.k07-120. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Corrias A, Einaudi S, Chiorboli E, Weber G, Crino A, Andreo M, Cesaretti G, De Sanctis L, Messina MF, Segni M, Cicchetti M, Vigone M, Pasquino AM, Spera S, De Luca F, Mussa GC, Bona G. Accuracy of fine needle aspiration biopsy of thyroid nodules in detecting malignancy in childhood: comparison with conventional clinical, laboratory, and imaging approaches. J Clin Endocrinol Metab. 2001;86:4644–4648. doi: 10.1210/jcem.86.10.7950. [DOI] [PubMed] [Google Scholar]
- 17.Pretorius HT, Katikineni M, Kinsella TJ, Barsky SH, Brennan MF, Chu EW, Robbins J. Thyroid nodules after high-dose external radiotherapy. Fine-needle aspiration cytology in diagnosis and management. JAMA. 1982;247:3217–3220. [PubMed] [Google Scholar]
- 18.Rosen IB, Palmer JA, Bain J, Strawbridge H, Walfish PG. Efficacy of needle biopsy in postradiation thyroid disease. Surgery. 1983;94:1002–1007. [PubMed] [Google Scholar]
- 19.Hatipoglu BA, Gierlowski T, Shore-Freedman E, Recant W, Schneider AB. Fine-needle aspiration of thyroid nodules in radiation-exposed patients. Thyroid. 2000;10:63–69. doi: 10.1089/thy.2000.10.63. [DOI] [PubMed] [Google Scholar]
- 20.Kikuchi S, Perrier ND, Ituarte PHG, Treseler PA, Siperstein AE, Duh Q-Y, Greenspan FS, Clark OH. Accuracy of fine-needle aspiration cytology in patients with radiation-induced thyroid neoplasms. Brit J Surg. 2003;90:755–758. doi: 10.1002/bjs.4198. [DOI] [PubMed] [Google Scholar]
- 21.Centeno BA, Szyfelbein WM, Daniels GH, Vickery AL. Fine needle aspiration biopsy of the thyroid gland with prior Graves’ disease treated with radioactive iodine. Morphologic findings and potential pitfalls. Acta Cytol. 1996;40:1189–1197. doi: 10.1159/000333979. [DOI] [PubMed] [Google Scholar]
- 22.Verma RN, Dhananjayan G, Saini JS, Banerjee AK. Fine needle aspiration biopsy-a critical investigation in thyrotoxicosis. Indian J. Pathol Microbiol. 1992;35:209–218. [PubMed] [Google Scholar]
- 23.Ito M, Yamashita S, Ashizawa K, Namba H, Hoshi M, Shibata Y, Sekine I, Nagataki S, Shigematsu I. Childhood thyroid diseases around Chernobyl evaluated by ultrasound examination and fine needle aspiration cytology. Thyroid. 1995;5:365–368. doi: 10.1089/thy.1995.5.365. [DOI] [PubMed] [Google Scholar]
- 24.Ito M, Sekine I, Ashizawa K, Nishikawa T, Nagataki S, Yamashita S, Kotova L, Panasyuk GD. Cytologic characteristics of pediatric thyroid carcinoma around Chernobyl, Republic of Belarus. Acta Cytol. 1997;41:1642–1644. [PubMed] [Google Scholar]
- 25.Ito M, Yamashita S. Summary of the cytological diagnosis of childhood thyroid diseases around Chernobyl. Chernobyl: Message for the 21st century International Congress Series; May 2002.pp. 185–192. [Google Scholar]
- 26.Stezhko VA, Buglova EE, Danilova LI, Drozd VM, Krysenko NA, Lesnikova NR, Minenko VF, Ostapenko VA, Petrenko SV, Polyanskaya ON, Rzheutski VA, Tronko MD, Bobylyova OO, Bogdanova TI, Epshtein OV, Kairo IA, Kostin OV, Likhtarev IA, Markov VV, Oliynik VA, Shpak VM, Tereshchenko VP, Zamotayeva GA, Beebe GW, Bouville AC, Brill AB, Burch JD, Fink DJ, Greenebaum E, Howe GR, Luckyanov NK, Masnyk IJ, McConnell RJ, Robbins J, Thomas TL, Voilleque PG, Zablotska LB. A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: objectives, design, and methods. Radiation Research. 2004;161:481–492. doi: 10.1667/3148. [DOI] [PubMed] [Google Scholar]
- 27.Tronko M, Howe G, Bogdanova T, Bouville A, Epstein O, Brill A, Likhtarev I, Fink D, Markov V, Greenebaum E, Olijnyk V, Masnyk I, Shpak V, McConnell R, Tereshchenko V, Robbins J, Zvinchuk O, Zablotska L, Hatch M, Luckyanov N, Ron E, Thomas T, Voillequé P, Beebe G. A cohort study of thyroid cancer and other thyroid disease following the Chornobyl accident: thyroid cancer cases in Ukraine detected during first screening. J.Nat.Cancer Inst. 2006;98:896–903. doi: 10.1093/jnci/djj244. [DOI] [PubMed] [Google Scholar]
- 28.Kini SR. Thyroid. In: Kline TS, editor. Guides to clinical aspiration biopsy. Igaku-Shoin; N.Y.: 1987. p. 368. [Google Scholar]
- 29.DeLelis R, Lloyd R, Heitz Ph., Eng Ch., editors. WHO classification of tumours. IARC Press; Lyon: 2004. Pathology and genetics of tumours of endocrine organs; p. 320. [Google Scholar]
- 30.Ylagan LR, Farkas T, Dehner LP. Fine needle aspiration of the thyroid: a cytologic correlation and study of discrepant cases. Thyroid. 2004;14:35–41. doi: 10.1089/105072504322783821. [DOI] [PubMed] [Google Scholar]
- 31.Sidawy MK, Del Vecchio DM, Knoll SM. Fine-needle aspiration of thyroid nodules: correlation between cytology and histology and evaluation of discrepant cases. Cancer. 1997;81:253–259. doi: 10.1002/(sici)1097-0142(19970825)81:4<253::aid-cncr7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 32.Sahin M, Gursoy A, Tutuncu NB, Guvener DN. Prevalence and prediction of malignancy in cytologically indeterminate thyroid nodules. Clin Endocrinol. 2006;65:514–518. doi: 10.1111/j.1365-2265.2006.02625.x. [DOI] [PubMed] [Google Scholar]
- 33.Deveci MS, Deveci G, LiVolsi VA, Baloch ZW. Fine-needle aspiration of follicular lesions of the thyroid. Diagnosis and follow-up. 2006 doi: 10.1186/1742-6413-3-9. http://www.cytojournal.com/content/3/1/9. [DOI] [PMC free article] [PubMed]
- 34.Kapur U, Wojcik EM. Follicular neoplasm of the thyroid-vanishing cytologic diagnosis? Diagn. Cytopathol. 2007;35:525–528. doi: 10.1002/dc.20676. [DOI] [PubMed] [Google Scholar]
- 35.Bajnok L, Mezosi E, Nagy E, Szabo J, Sztojka I, Varga J, Galuska L, Leovey A. Calculation of the radioiodine dose for the treatment of Graves’ hyperthyroidism: is more than seven-thousand rad target dose necessary? Thyroid. 1999;9:865–869. doi: 10.1089/thy.1999.9.865. [DOI] [PubMed] [Google Scholar]
- 36.Likhtarev I, Bouville A, Kovgan L, Luckyanov NK, Voilleque PG. Questionnaire- and measurement-based individual thyroid doses in Ukraine resulting from the Chornobyl nuclear reactor accident. Radiat.Res. 2006;166:271–286. doi: 10.1667/RR3545.1. [DOI] [PubMed] [Google Scholar]