Abstract
Elevated methylmalonic acid in five asymptomatic newborns whose fibroblasts showed decreased uptake of transcobalamin-bound cobalamin (holo-TC), suggested a defect in the cellular uptake of cobalamin. Analysis of TCblR/CD320, the gene for the receptor for cellular uptake of holo-TC, identified a homozygous single codon deletion, c.262_264GAG (p.E88del), resulting in the loss of a glutamic acid residue in the low-density lipoprotein receptor type A-like domain. Inserting the codon by site-directed mutagenesis fully restored TCblR function.
Keywords: methylmalonic acid, homocysteine, transcobalamin-receptor, cobalamin, vitamin B12, TCblR, CD320
Introduction
Although genetic defects affecting the absorption, transport and metabolism of vitamin B12 (cobalamin, Cbl) are now known [Fowler et al., 2008], no defects in the receptor TCblR (CD320; MIM# 606475) for cellular uptake of Cbl have been identified to date. TCblR expressed on the plasma membrane binds transcobalamin (TC) saturated with Cbl (holo-TC) and mediates cellular uptake of Cbl. TCblR expression is coupled to the cell cycle with highest expression in actively proliferating cells and down-regulation in quiescent cells [Amagasaki et al., 1990]. The receptor has a 29-fold higher affinity for holo-TC than for apo TC [Quadros et al., 2005]. The only route for physiologic transport of TC-bound Cbl into cells is via TCblR. The existence of a receptor for holo-TC was inferred from Ca++-dependent binding and uptake of holo-TC by cells [DiGirolamo and Huennekens 1975], but the identity of the protein and the gene encoding this receptor had eluded researchers for decades. Definitive purification of the receptor protein from human placenta provided the protein sequence for the identification of the CD320 gene on chromosome 19.p13.2 as the gene that encodes the receptor [Quadros et al., 2009]. This 282aa glycoprotein with a single transmembrane domain and a cytoplasmic tail is ubiquitously expressed. This report describes the first gene defect of a single amino acid deletion in the receptor leading to elevated methylmalonic acid (MMA) in newborns.
Case Report
The index case was born at term after a pregnancy (gravida1, para1) complicated by mild anemia and gestational diabetes, controlled by diet. The parents are of mixed European background and are nonconsanguineous. Blood for a newborn screen was collected on the second day of life and revealed an elevated C3-acylcarnitine of 6.22 µmol/l (cutoff 5.71). The C3/C2 ratio was 0.21 (cutoff 0.2). Urine organic acid analysis showed moderately elevated MMA and trace methylcitric acid without other propionate metabolites. The plasma vitamin B12 level was 269 ng/l (reference range, RR; 180–914), and total plasma homocysteine was 8.6 µmol/l (RR 0–11). The hematocrit was 44% (RR 42–70), and the mean corpuscular volume (MCV) was 96.9 FL (RR 90–115). A repeat newborn screen, done on day 14 of life was normal; the C3-acylcarnitine was below cutoff at 3.2 µmol/l, the C3/C2 ratio was 0.17. Lactate, ammonia, electrolytes, liver function tests, coagulation studies, blood gas, CBC, urinalysis and a carnitine panel were normal. Repeat MMA levels were obtained during this period and ranged from 5.3 to 7.7 µmol/l. One milligram of hydroxocobalamin was administered intramuscularly (i.m.) at 21 days of age; the MMA level 24 hr later was normal at 0.82 µmol/l and remained within the reference range for the patient’s age. When last tested at 9 months of age, MMA was 0.7 µmol/l and the vitamin B12 level, without additional supplementation, was elevated at > 1,400 ng/l. Maternal vitamin B12 level 2 weeks after delivery was 312 ng/l (RR 180–914); homocysteine was 7.8 µmol/l (RR 5.1–13.9) and MMA was 0.58 µmol/l (RR <0.89). All hematological parameters were within the normal range with no indication of vitamin B12 deficiency. Following the positive newborn screen, a skin biopsy was obtained and a fibroblast culture was established.
Identification of the TCblR gene defect in the index case prompted the review of previous undiagnosed cases that were referred for genetic testing due to elevated levels of MMA in newborn screens.
The index case and the additional cases reported here were all referred to the medical genetics laboratory at McGill University as a result of an abnormal newborn screen in the form of an elevated C3-acylcarnitine and methylmalonic aciduria. This cell line and four additional lines with the identical deletion are available from the Repository for Mutant Human Cell Strains, Montreal Children’s Hospital, Montreal, Canada (http://www.cellbank.mcgill.ca/). The protocol was approved by the Royal Victoria Hospital, Research Ethics Board.
Methods
Propionate Incorporation in Cells
Fibroblasts were plated in 35-mm tissue culture dishes at a density of 400,000 cells per dish. After cells had attached, medium was removed and replaced with Puck’s F medium supplemented with 15% (v/v) fetal bovine serum and 1–[14C]propionate (GE Health Sciences, Piscataway, NY) diluted with cold propionate to a final specific activity of 10 µCi/µmol. Cultures were incubated with labeled medium for 18 hr. At the end of this period, medium was removed and cellular macromolecules were precipitated by incubation in 5% trichloroacetic acid. The precipitate was dissolved in 0.2N NaOH and radioactivity was determined by liquid scintillation counting. The assay was done in the presence and absence of 3.75 µM OHCbl and provides a measure of methylmalonylCoA mutase function in intact cells [Willard et al., 1978].
Methyltetrahydrofolate Incorporation in Cells
This procedure is identical to the propionate incorporation test except for the use of methionine-free minimal essential medium supplemented with 100 µM homocysteine thiolactone, 50 µg/ml sodium ascorbate, and 10% (v/v) dialyzed fetal bovine serum containing 0.5 µCi/ml (60 mCi/mmol) 5-[14C]methyltetrahydrofolate (GE Health Sciences). This assay provides a measure of methionine synthase function in intact cells [Rosenblatt et al., 1984].
Synthesis of Cobalamin Coenzymes
Confluent cultures of fibroblasts in 175 cm2 culture flasks were incubated for 96 hr in MEM supplemented with 25 pg/ml [57Co]cyanocobalamin bound to TC in human serum. Cells were harvested by trypsinization and cobalamins were extracted in hot ethanol and separated by high-performance liquid chromatography (HPLC) using a modification of the method of Jacobsen et al. [1982].
Holo-TC Uptake Studies
Skin fibroblasts were cultured in Dulbecco’s modified Eagle’s minimal essential medium (DMEM) with 10% fetal bovine serum. Binding and uptake of Cbl was determined by incubating cells in fresh medium containing recombinant TC saturated with [57Co]cyanocobalamin.
Homocysteine and Methylmalonic Acid in Fibroblast Cultures
Cells were cultured with varying amounts of holo-TC. Medium was analyzed for Hcy by an HPLC-based method with fluorescence detection [Jacobsen et al., 1994] and MMA by gas chromatography-mass spectrometry [Rasmussen, 1989].
Analysis of the TCblR Gene
Total RNA and genomic DNA were prepared from cultured fibroblasts using Trizol reagent (Invitrogen, Carlsbad, CA). The mRNA level was measured by SYBR GreenER (ABI PRISM)-based real-time PCR (qPCR) (Primer set: Forward [F]-AAGTTCCAGTGCCGCACCAGT/Reverse (R)-AGTCACTGACGCCGGTGCAGG; 95°C, 10 min, followed by 40 cycles of 95°C, 15 sec, and 60°C, 1min). The size and sequence of the transcript was determined by reverse transcription using random primers followed by PCR amplification of the cDNA (Primer set: F-ACAGCATGAGCGGCGGTTGGA/RGCTACGCCCAGGGCTGAGTGA; 94°C, 2min, followed by 30 cycles of 94°C, 40 sec, 60°C, 40 sec, 72°C, 40 sec, and one cycle at 72°C, 10 min). The nucleotide sequence encoding the mRNA for TCblR was determined by sequencing the PCR amplified cDNA fragment and confirmed by amplification (Primer set: F-TATATCCCGGGACTTCGCCTGTCTCC/ R-ATACCACAGCCGAGCTCGTCGCT; 94°C, 2 min, followed by 30 cycles of 94°C, 40 sec, 58°C, 40 sec, 72°C, 40 sec, and one cycle at 72°C, 10 min), and sequencing the region of the gene corresponding to the mutation in the genomic DNA. The missing codon was reinserted into the patient’s cDNA using the QuikChange Lightning site-directed mutagenesis kit (Stratagene, La Jolla, CA). The patient’s cDNA in pCDNA3.1 vector was amplified by PCR using overlapping mutagenic primers (Primer set: F-GGCGATGAGGAGGAGTGCAGGATTGAGCC/R-GGCTCAATCCTGCACTCCTCCTCATCGCTGC). The PCR amplification, cDNA isolation and cloning was carried out as per instructions provided with the kit and the insertion of the missing codon confirmed by sequencing. Nucleotide numbering reflects cDNA numbering with +1 corresponding to the A of the ATG translation initiation codon in the reference sequence (GenBank NM_016579.3). The initiation codon is codon 1.
Results
Cellular Vitamin B12 Metabolism
Studies in the index case with fibroblasts cultured for 18 hr with and without 3.7 µM hydroxocobalamin showed normal [14C]propionate (9.5 and 12.2 nmol/mg protein) and [14C]methyltetrahydrofolate (79 and 960 nmol/mg protein) incorporation. Initial testing in cultured fibroblasts revealed low uptake of [57Co]cyanocobalamin (1.6 pg/106 cells) bound to TC in human serum but conversion to adenosylcobalamin (11%) and methylcobalamin (52%) was normal. The decreased Cbl uptake suggested a potential defect in TC-receptor mediated cellular uptake of holo-TC and a detailed study to identify the genetic abnormality was initiated.
Binding and Uptake of TC-Cbl
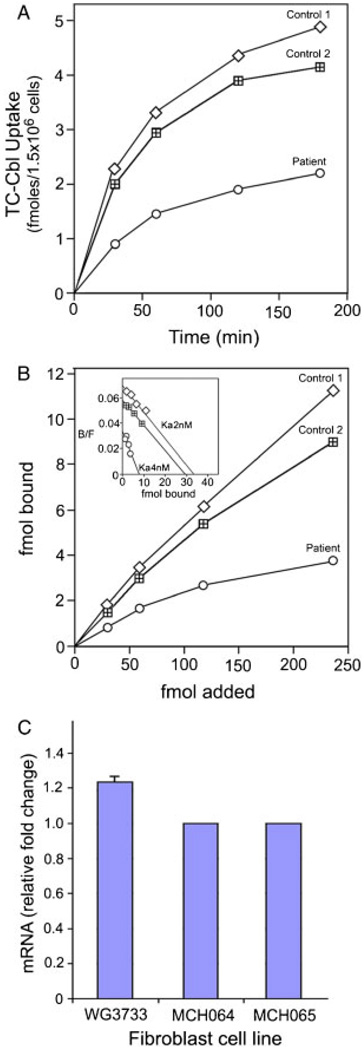
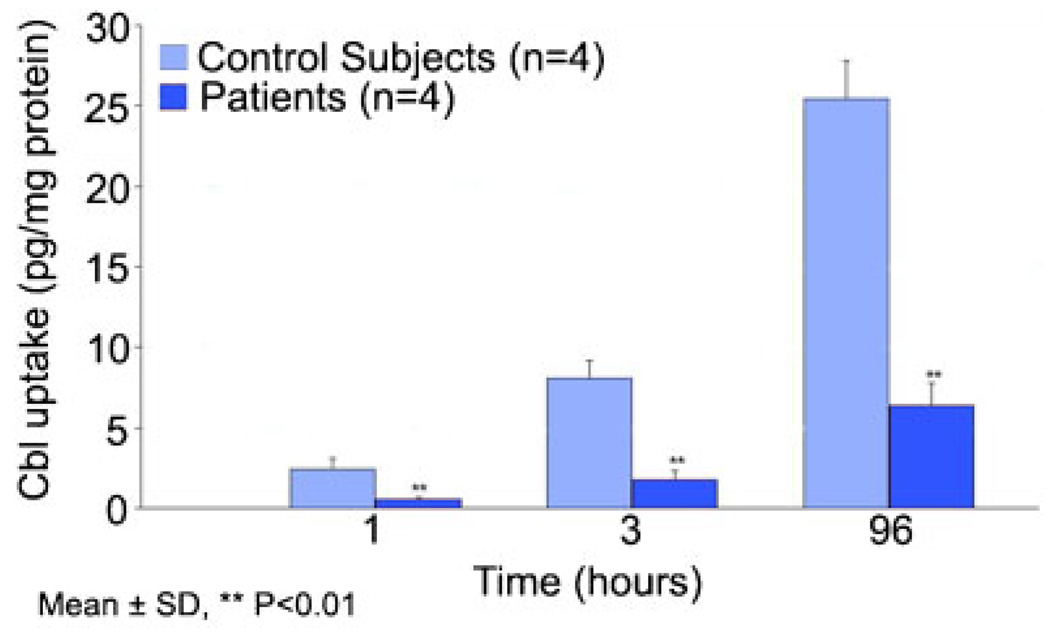
Uptake of [57Co]cyanocobalamin bound to human recombinant TC in fibroblast cultures showed consistently lower binding and uptake throughout the 3-hr time period tested (Fig. 1A). On average, the uptake in the patient’s cells was about half that observed in two control cell lines. This difference in uptake appeared to be due to a twofold decrease in the affinity of the receptor for holo-TC (Ka = 4 nM−1) compared to the control fibroblasts (Ka = 2 nM−1), and a fourfold decrease in maximum binding (Bmax) (Fig. 1B). The fibroblast cultures from the four additional cases also showed decreased uptake of TC-Cbl (Fig. 2).
Figure 1.
A: Holo-TC uptake in skin fibroblasts in culture. Cells were seeded at 0.2 × 106 cells/2 ml in six-well plates overnight. For uptake of holo-TC, [57Co]cyanocobalamin-TC (0.03 pmol) was added in 1ml DMEM and incubated at 37°C. Following removal of the medium and washing, the cells were detached by incubating with 0.5 ml 0.05% trypsin/EDTA. The radioactivity in the trypsin/EDTA solution and the cells was determined as a measure of TCblR-mediated binding and uptake of holo-TC. B: The kinetics of holo-TC binding to TCblR was determined by incubating 1.5 × 106 cells with 0.03 to 0.24 pmol of holo-TC for 1 hr at room temperature. Inset shows the Scatchard analysis of the binding data. C: TCblR mRNA in fibroblasts from the patient (WG3733) and two control cell lines (MCH 064 and MCH 065) as determined by qPCR.
Figure 2.
Uptake of TC-Cbl in fibroblast cultures from four additional cases with the single amino acid deletion in the TCblR protein.
Homocysteine and Methylmalonate in Fibroblast Cultures
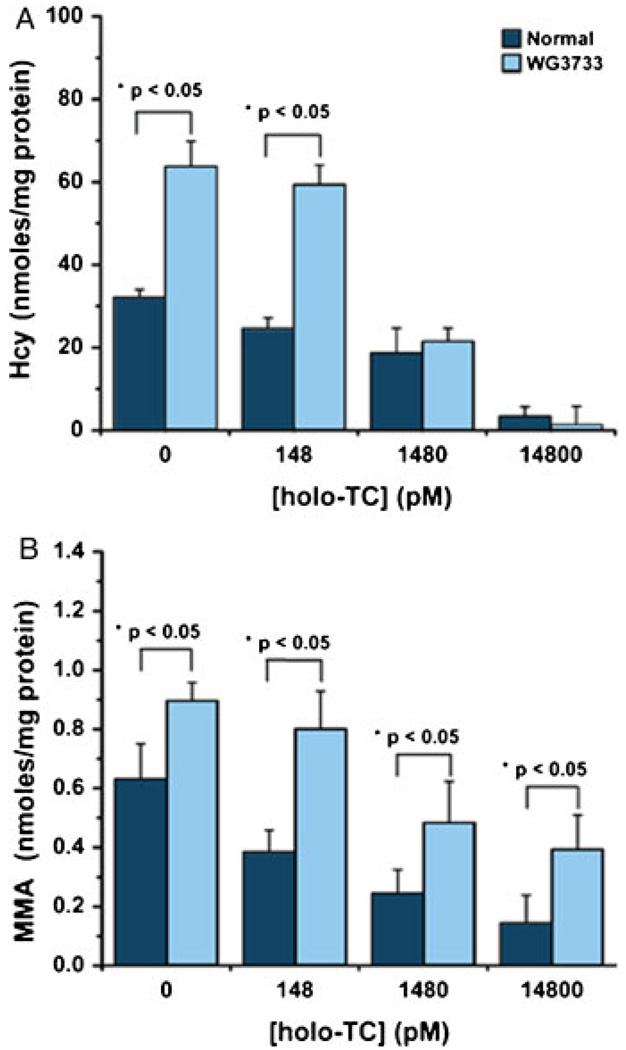
The fibroblasts from the index case accumulated significantly higher Hcy and MMA in the medium compared to controls during 8 days in culture (Fig. 3). Both the normal and the TCblR mutant cell line showed less Hcy and MMA with increasing concentrations of holo-TC. Production of Hcy in the presence of 0 or 148 pmol/l holo-TC was significantly higher for the TCblR mutant than for the control cell line (Fig. 3A). The concentration of Hcy produced by the TCblR mutant cells decreased to normal levels when the concentration of holo-TC was increased to > 1.4 nmol/l. In contrast, although a substantial decrease in the production of MMA was observed with increasing concentrations of holo-TC, the TCblR mutant cell line produced significantly higher concentration of MMA at all concentrations tested (Fig. 3B).
Figure 3.
Accumulation of homocysteine (Hcy) (A) and methylma-lonic acid (MMA) (B) in culture medium of fibroblasts grown in the presence of increasing concentrations of holo-TC. Bovine recombinant holo-TC was added to 2ml culture medium containing 0.2 × 106 cells. The medium collected after 8 days of culture was used for the determination of Hcy and MMA. Significant increases in Hcy at 0 and 140 pmol/l and MMA at 0–14,800 pmol/l were observed in the medium from the patient’s fibroblasts (WG3733) compared to a control cell line under identical culture conditions. The statistical analysis was performed using the Microcal Origin v7.0 software. The independent Student t-test with a confidence interval of 95% was used to establish significant differences.
Analysis of the TCblR Gene
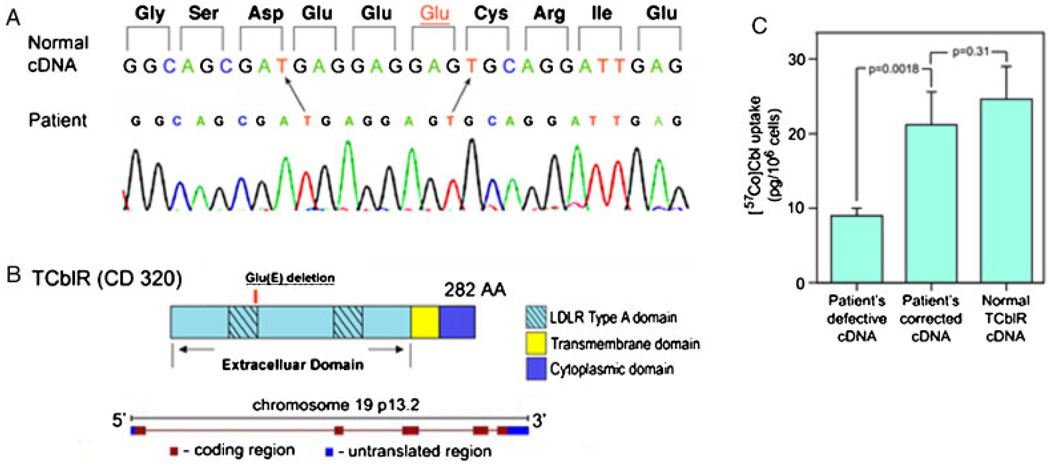
Genomic DNA and mRNA were extracted from cultured skin fibroblasts. The size of the transcript determined by PCR amplification of the mRNA and agarose gel electrophoresis was consistent with the full-length cDNA. In addition, the amount of the transcript as determined by qPCR was similar to that in control fibroblasts (Fig. 1C). However, the nucleotide sequence showed deletion of three base pairs (c.262_264delGAG) (Fig. 4A), resulting in loss of codon 88 (p.E88del) and deletion of a single glutamic acid residue in the 3′ end of the first LDLR type A domain (Fig. 4B). The patient was presumed to be homozygous for this deletion, because direct sequencing of the PCR amplified cDNA as well as the genomic DNA failed to show the presence of a normal allele. DNA from parents was not available. Other changes in the nucleotide sequence noted were c.489A>G (p.S142G), which is not a known polymorphism, c.723G>A (rs2336573; p.G220R), and the silent polymorphisms c.512G>T (rs2232783) and c.548C>T (rs2232784). The phenotype was confirmed by transfecting HEK 293 cells with the patient’s cDNA containing the deletion. Reinserting the missing codon by site directed mutagenesis corrected the cell line’s decreased Cbl uptake (Fig. 4C).
Figure 4.
A: The nucleotide sequence of the region corresponding to the codon deletion in the first LDLR type A domain in the patient. B: The organization of the protein and the gene encoding TCblR. C: Normalization of holo-TC uptake in cells transfected with patient’s cDNA that has the missing codon (GAG) inserted by site directed mutagenesis. The cDNA in pcDNA3.1 plasmid (4 µg) was transfected into HEK 293 cells seeded at a density of 0.6 × 106/well in six-well plates. Binding and uptake of holo-TC was determined 48 hr after transfection for 1 hr at 37°C as described for Figure 1A
Discussion
Following an unremarkable pregnancy, a newborn screen positive for elevated C3-acylcarnitine led to the identification of methylmalonic acidemia, prompting additional studies to determine its cause. Somatic cell studies failed to identify any abnormalities in the synthesis of Cbl coenzymes or Cbl-dependent enzymes. However, a substantial decrease in the accumulation of Cbl in fibroblast cultures prompted additional studies to identify the underlying cause.
Both acquired and inherited causes of functional Cbl deficiency have been described. Juvenile and adult onset pernicious anemia due to intrinsic factor deficiency is primarily an autoimmune disease and mutations in the genes encoding intrinsic factor, cubilin, and amnionless result in disorders of Cbl absorption [Fyfe et al., 2004; Tanner et al., 2005; Yassin et al., 2004]. Inherited TC deficiency leads to Cbl insufficiency after birth, typically manifesting as decreased absorption and cellular uptake of the vitamin [Burman et al., 1979] due to the dual role of TC in the translocation of the absorbed Cbl in the gut as well as its delivery to tissue cells [Quadros et al., 1999]. Mutations in the genes for the two enzymes requiring Cbl or in the enzymes involved in the pathways leading to the synthesis of adenosyl- and methyl-Cbl affect intracellular Cbl metabolism [Coelho et al., 2008; Hannibal et al., 2009; Rutsch et al., 2009]. Depending on the location of the defect, these disorders can present with elevated Hcy and/or MMA, with disorders of Cbl transport typically presenting with lower levels of these metabolites when compared to disorders affecting intracellular metabolism. Early detection and treatment with pharmacologic doses of vitamin B12 can prevent or reverse neuropathologic changes [Hall, 1990]. Therefore, newborn screening for Hcy and MMA, two sensitive indicators of intracellular Cbl deficiency, is recommended for early detection. Although testing for Hcy is not routine, testing for MMA through elevated C3-acylcarnitine is becoming widespread.
In the index case reported, elevated MMA was identified after an abnormal newborn screen. Failure to associate the high MMA with known genetic defects prompted additional studies, among them measurement of cellular TC-Cbl uptake to detect a possible defect in cellular uptake and processing of TC-Cbl. Preliminary studies indicated a potential defect in the binding of TC-Cbl to the cell surface receptor. The receptor belongs to a class of membrane receptors that share structural features such as the LDL receptor type A domains found in the LDL receptor family of proteins, separated by a CUB-like domain found in a number of multiligand binding receptors such as cubilin, megalin, and epidermal growth factor receptor; these domains are involved in ligand binding [Kozyraki and Gofflot, 2007]. In the case of TCblR, the 2 LDLR- type A domains with consensus sequence for Ca++ binding are involved in holo-TC/TCblR interaction. Epitope mapping studies [Fedosov et al., 2005] and the crystal structure of holo-TC [Wuerges et al., 2006] have identified the positively charged heparin-binding region of TC as the likely site of interaction with the receptor. This is further supported by the observation that a monoclonal antibody either to the second LDLR-A domain of TCblR (unpublished data) or to sequences near the heparin binding domain of TC [Fedosov et al., 2005], blocks holo-TC/TCblR interaction. The identification of a single amino acid deletion in the first LDLR-A domain of TCblR, which is involved in Ca++ and TC-Cbl binding is consistent with the observation of decreased uptake contributing to intracellular Cbl deficiency and elevated MMA. This conclusion is further supported by the observation of decreased Hcy and MMA in fibroblast cultures when 10–100-fold excess holo-TC is available for Cbl uptake. Compensating for the decreased affinity by providing adequate TC-Cbl facilitates additional binding and uptake to lower the intracellular production of Hcy and MMA.
The discovery of four additional cases with the identical gene defect [Anastasio et al., 2009] suggests that this defect may not be rare, and that an abnormal newborn screen with elevated MMA, with or without elevated Hcy, should be investigated for this genetic defect. In all of these cases the elevation in MMA can be considered as moderate, which is consistent with the moderate decrease in binding and cellular uptake of Cbl. The Hcy level appears to vary from normal to moderately elevated. Early clinical studies have shown that hyperhomocysteinemia does not always accompany methylmalonic academia in Cbl deficiency [Stabler et al., 1990]. This disparity in MMA and Hcy levels has been previously reported in cases of Cbl deficiency in infants [Campbell et al., 2005; Monsen et al., 2003]. Thus, the MMA level appears to be a more sensitive indicator of subclinical Cbl deficiency. From the cases reported here, it is also evident that hematological changes are not apparent at this deficiency level in newborns and infants, and therefore, screening for acylcarnitine and MMA would be required. Earlier studies have also reported a decrease in Cbl level during the first weeks of life with corresponding benign and transient increase in MMA and Hcy that either remain elevated without clinical symptoms or revert to normal levels with time [Ledley et al., 1984; Shih et al., 1976]. The elevated Hcy observed in the in vitro studies done with fibroblast cultures from the index case contrasts with the in vivo data of normal Hcy. Although unconfirmed, it is possible that the metabolism of Hcy in the liver by the alternate betaine–homocysteine methyltransferase (BHMT) pathway could account for the normal plasma Hcy. Skin fibroblasts do not express BHMT [Wang et al., 1991], and therefore, reflect the effect of cellular Cbl deficiency on both the MMA and Hcy pathways. It should be noted, however, that serum Hcy levels were elevated in two of the additional cases of TCblR deficiency. In all cases, indirect measures of propionate catabolism and conversion of homocysteine to methionine, with and without added Cbl, as well as synthesis of Cbl cofactors from exogenous cyanocobalamin was normal. The only defect identified in all five cases with the single codon deletion was decreased uptake of TC-Cbl.
Many inborn errors of Cbl metabolism cause severe functional blocks and present early in life. Patients with genetic abnormalities of TC, the ligand for TCblR, develop severe cellular Cbl deficiency, pancytopenia, megaloblastic anemia, and neurological sequelae within the first 2 months of life due to complete loss of TC function [Prasad et al., 2008; Ratschmann et al., 2009]. The moderate MMA and the absence of an early clinical phenotype in these cases is likely due to the defect producing a mild but sustained intracellular Cbl deficiency. The elevated plasma vitamin B12 level 9 months after a single injection of vitamin B12 in the index case further attests to the defect in cellular uptake. The long-term effects of this condition are yet to be unraveled, but TCblR defects should be considered in individuals with elevated blood and urine MMA who do not have other defects in Cbl metabolism.
Acknowledgments
We thank Lydia Vezina, Lara Reichman, and Laura Dempsey-Nunez for excellent technical support and discussions as part of their undergraduate research project. This study was supported by NIH Grant DK 064732 (E.V.Q.), HL71907 and HL52234 (D.W.J.), and CIHR Grant 160439 (D.S.R.).
References
- Amagasaki T, Green R, Jacobsen DW. Expression of transcobalamin II receptors by human leukemia K562 and HL-60 cells. Blood. 1990;76:1380–1386. [PubMed] [Google Scholar]
- Anastasio N, Watkins D, Vezina L, Dempsey-Nunez L, Reichman L, Quadros EV, Rosenblatt DS. Mutations in TCblR, the gene for the transcobalamin receptor, result in decreased cellular uptake of vitamin B12 and methylmalonic aciduria. 11th International Congress of Inborn Errors of Metabolism. Mol Genet Metab. 2009;98:122. Abstract 615. [Google Scholar]
- Burman JF, Mollin DL, Sourial NA, Sladden RA. Inherited lack of transcobalamin II in serum and megaloblastic anaemia: a further patient. Br J Haematol. 1979;43:27–38. doi: 10.1111/j.1365-2141.1979.tb03716.x. [DOI] [PubMed] [Google Scholar]
- Campbell CD, Ganesh J, Ficicioglu C. Two newborns with nutritional vitamin B12 deficiency: challenges in newborn screening for vitamin B12 deficiency. Haematologica. 2005;90:e119–e121. [PubMed] [Google Scholar]
- Coelho D, Suormala T, Stucki M, Lerner-Ellis JP, Rosenblatt DS, Newbold RF, Baumgartner MR, Fowler B. Gene identification for the cblD defect of vitamin B12 metabolism. N Engl J Med. 2008;358:1454–1464. doi: 10.1056/NEJMoa072200. [DOI] [PubMed] [Google Scholar]
- DiGirolamo PM, Huennekens FM. Transport of vitamin B12 into mouse leukemia cells. Arch Biochem Biophys. 1975;168:386–393. doi: 10.1016/0003-9861(75)90267-2. [DOI] [PubMed] [Google Scholar]
- Fedosov SN, Orning L, Lovli T, Quadros EV, Thompson K, Berglund L, Petersen TE. Mapping the functional domains of human transcobalamin using monoclonal antibodies. FEBS J. 2005;272:3887–3898. doi: 10.1111/j.1742-4658.2005.04805.x. [DOI] [PubMed] [Google Scholar]
- Fowler B, Leonard JV, Baumgartner MR. Causes of and diagnostic approach to methylmalonic acidurias. J Inherit Metab Dis. 2008;31:350–360. doi: 10.1007/s10545-008-0839-4. [DOI] [PubMed] [Google Scholar]
- Fyfe JC, Madsen M, Hojrup P, Christensen EI, Tanner SM, de la Chapelle A, He Q, Moestrup SK. The functional cobalamin (vitamin B12)-intrinsic factor receptor is a novel complex of cubilin and amnionless. Blood. 2004;103:1573–1579. doi: 10.1182/blood-2003-08-2852. [DOI] [PubMed] [Google Scholar]
- Hall CA. Function of vitamin B12 in the central nervous system as revealed by congenital defects. Am J Hematol. 1990;34:121–127. doi: 10.1002/ajh.2830340208. [DOI] [PubMed] [Google Scholar]
- Hannibal L, Kim J, Brasch NE, Wang S, Rosenblatt DS, Banerjee R, Jacobsen DW. Processing of alkylcobalamins in mammalian cells: a role for the MMACHC (cblC) gene product. Mol Genet Metab. 2009;97:260–266. doi: 10.1016/j.ymgme.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen DW, Gatautis VJ, Green R, Robinson K, Savon SR, Secic M, Ji J, Otto JM, Taylor LM., Jr Rapid HPLC determination of total homocysteine and other thiols in serum and plasma: sex differences and correlation with cobalamin and folate concentrations in healthy subjects. Clin Chem. 1994;40:873–881. [PubMed] [Google Scholar]
- Jacobsen DW, Green R, Quadros EV, Montejano YD. Rapid analysis of cobalamin coenzymes and related corrinoid analogs by high-performance liquid chromatography. Anal Biochem. 1982;120:394–403. doi: 10.1016/0003-2697(82)90363-3. [DOI] [PubMed] [Google Scholar]
- Kozyraki R, Gofflot F. Multiligand endocytosis and congenital defects: roles of cubilin, megalin and amnionless. Curr Pharm Des. 2007;13:3038–3046. doi: 10.2174/138161207782110507. [DOI] [PubMed] [Google Scholar]
- Ledley FD, Levy HL, Shih VE, Benjamin R, Mahoney MJ. Benign methylmalonic aciduria. N Engl J Med. 1984;311:1015–1018. doi: 10.1056/NEJM198410183111604. [DOI] [PubMed] [Google Scholar]
- Monsen AL, Refsum H, Markestad T, Ueland PM. Cobalamin status and its biochemical markers methylmalonic acid and homocysteine in different age groups from 4 days to 19 years. Clin Chem. 2003;49:2067–2075. doi: 10.1373/clinchem.2003.019869. [DOI] [PubMed] [Google Scholar]
- Prasad C, Rosenblatt DS, Corley K, Cairney AE, Rupar CA. Transcobalamin (TC) deficiency—potential cause of bone marrow failure in childhood. J Inherit Metab Dis. 2008 doi: 10.1007/s10545-008-0864-3. DOI: 10.1007/s10545-008-0864-3. [DOI] [PubMed] [Google Scholar]
- Quadros EV, Nakayama Y, Sequeira JM. The binding properties of the human receptor for the cellular uptake of vitamin B12. Biochem Biophys Res Commun. 2005;327:1006–1010. doi: 10.1016/j.bbrc.2004.12.103. [DOI] [PubMed] [Google Scholar]
- Quadros EV, Nakayama Y, Sequeira JM. The protein and the gene encoding the receptor for the cellular uptake of transcobalamin-bound cobalamin. Blood. 2009;113:186–192. doi: 10.1182/blood-2008-05-158949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadros EV, Regec AL, Khan KM, Quadros E, Rothenberg SP. Transcobalamin II synthesized in the intestinal villi facilitates transfer of cobalamin to the portal blood. Am J Physiol. 1999;277:G161–G166. doi: 10.1152/ajpgi.1999.277.1.G161. [DOI] [PubMed] [Google Scholar]
- Rasmussen K. Solid-phase extraction for rapid determination of methylmalonic acid in serum and urine by stable-isotope-dilution method. Clin Chem. 1989;35:260–264. [PubMed] [Google Scholar]
- Ratschmann R, Minkov M, Kis A, Hung C, Rupar T, Mühl A, Fowler B, Nexo E, Bodamer OA. Transcobalamin II deficiency at birth. Mol Genet Metab. 2009;98:265–288. doi: 10.1016/j.ymgme.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Rosenblatt DS, Cooper BA, Pottier A, Lue-Shing H, Matiaszuk N, Grauer K. Altered vitamin B12 metabolism in fibroblasts from a patient with megaloblastic anemia and homocystinuria due to a new defect in methionine biosynthesis. J Clin Invest. 1984;74:2149–2156. doi: 10.1172/JCI111641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutsch F, Gailus S, Miousse IR, Suormala T, Sagne C, Toliat MR, Nurnberg G, Wittkampf T, Buers I, Sharifi A, Stucki M, Becker C, Baumgartner M, Robenek H, Marquardt T, Höhne W, Gasnier B, Rosenblatt DS, Fowler B, Nürnberg P. Identification of a putative lysosomal cobalamin exporter altered in the cblF defect of vitamin B12 metabolism. Nat Genet. 2009;41:234–239. doi: 10.1038/ng.294. [DOI] [PubMed] [Google Scholar]
- Shih VE, Coulombe JT, Maties M, Levy HL. Methylmalonic aciduria in the newborn. N Engl J Med. 1976;295:1320–1321. doi: 10.1056/nejm197612022952319. [DOI] [PubMed] [Google Scholar]
- Stabler SP, Allen RH, Savage DG, Lindenbaum J. Clinical spectrum and diagnosis of cobalamin deficiency. Blood. 1990;76:871–881. [PubMed] [Google Scholar]
- Tanner SM, Li Z, Perko JD, Oner C, Cetin M, Altay C, Yurtsever Z, David KL, Faivre L, Ismail EA, Gräsbeck R, de la Chapelle A. Hereditary juvenile cobalamin deficiency caused by mutations in the intrinsic factor gene. Proc Natl Acad Sci USA. 2005;102:4130–4133. doi: 10.1073/pnas.0500517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JA, Dudman NP, Lynch J, Wilcken DE. Betaine:homocysteine methyltransferase—a new assay for the liver enzyme and its absence from human skin fibroblasts and peripheral blood lymphocytes. Clin Chim Acta. 1991;204:239–249. doi: 10.1016/0009-8981(91)90235-5. [DOI] [PubMed] [Google Scholar]
- Willard HF, Mellman IS, Rosenberg LE. Genetic complementation among inherited deficiencies in methylmalonylCoA mutase activity: evidence for a new class of human cobalamin mutant. Am J Hum Genet. 1978;30:1–13. [PMC free article] [PubMed] [Google Scholar]
- Wuerges J, Garau G, Geremia S, Fedosov SN, Petersen TE, Randaccio L. Structural basis for mammalian vitamin B12 transport by transcobalamin. Proc Natl Acad Sci USA. 2006;103:4386–4391. doi: 10.1073/pnas.0509099103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassin F, Rothenberg SP, Rao S, Gordon MM, Alpers DH, Quadros EV. Identification of a 4-base deletion in the gene in inherited intrinsic factor deficiency. Blood. 2004;103:1515–1557. doi: 10.1182/blood-2003-07-2239. [DOI] [PubMed] [Google Scholar]