Abstract
DNA vaccines have emerged as a potential alternative to current strategies to control cancer for their safety, stability and ease of preparation. We have previously demonstrated that a DNA vaccine encoding calreticulin (CRT) linked to human papillomavirus type 16 (HPV-16) E7 antigen (CRT/E7) can generate significant E7-specific immune responses and antitumor effects in vaccinated mice, thus enhancing DNA vaccine potency. Another strategy to improve DNA vaccine potency is by enhancing the level of expression of the antigen encoded in the vaccine. DNA methylation has been shown to lead to silencing of the genes that would affect the expression of the encoded antigen of the DNA vaccines. In the current study, we reasoned that CRT/E7 DNA vaccination combined with demethylating agent, 5-aza-2’-deoxycytidine (DAC) would lead to upregulation of CRT/E7 expression, resulting in improved DNA vaccine potency. We found that pretreatment with DAC led to increased CRT/E7 DNA expression, leading to enhanced E7-specific CD8+ T cell immune responses as well as the antitumor effects generated by the CRT/E7 DNA vaccine. Thus, our data suggest that combination of CRT/E7 DNA vaccination with DAC treatment may represent a potentially promising approach to control HPV-associated malignancies. The clinical implications of this study are discussed.
Keywords: DNA vaccine, HPV-16 E7, calreticulin (CRT), 5-aza-2’-deoxycytidine (DAC)
2.0 Introduction
Cancer immunotherapy using DNA vaccines have emerged as a potentially promising approach in addition to current therapies such as chemotherapy and radiotherapy for the control of tumors based on their safety, stability and ease of preparation (for review, see [1, 2]). However, naked DNA vaccines suffer from limited immunogenicity and therefore require additional strategies to improve their ability to generate strong immune responses. We have previously employed gene gun administration system to directly deliver DNA vaccines to the professional antigen presenting cells (APCs) in the skin, the Langerhans cells. The tumor antigen-expressing Langerhans cells eventually become mature dendritic cells (DCs), which are most important for activating cytotoxic T lymphocytes. Using the gene gun administration system, we have developed a number of innovative strategies to enhance DNA vaccine potency by modifying the properties of DCs (for review, see [3, 4]).
One approach to enhance DNA vaccine potency is the employment of intracellular targeting strategies to improve the MHC class I presentation of the antigen encoded by DNA vaccines in APCs. In previous studies, we explored the DNA vaccine encoding antigen linked to a chaperone protein, calreticulin (CRT), a Ca2+-binding protein normally located in the endoplasmic reticulum (ER) (for review, see [5]). We used a model tumor antigen, HPV-16 E7 because HPV type 16 is accountable for the pathogenesis of more than 50 % of all cervical cancer cases [6]. In addition, E7 oncogenic protein is constantly present in cervical cancer cells and responsible for the malignant transformation of HPV-associated cancer cells. Thus, HPV-16 E7 represents an ideal target for the development of therapeutic HPV DNA vaccines. We have previously shown that vaccination of mice with the CRT/E7 DNA generated significantly higher E7-specific CD8+ T cell immune responses and impressive antitumor effects against E7-positive tumors compared to vaccination with DNA encoding wild type E7 [7]. Furthermore, in a head-to-head comparison study, DNA vaccine encoding CRT/E7 was shown to be one of the best strategies to induce E7-specific CD8+ T cell immune responses and antitumor effects among various DNA vaccines that employed different intracellular targeting strategies [8, 9]. Currently, clinical grade CRT/E7 DNA vaccine has been prepared for clinical trials in stage IBI cervical cancer patients.
Another strategy to improve DNA vaccine potency is by enhancing the level of expression of the antigen encoded in the vaccine [10]. Methylation of CpG islands in the cytomegalovirus (CMV) promoter regions has been shown to lead to silencing of the gene expression [11, 12]. This silencing would significantly affect the expression of the encoded antigen of the DNA vaccines, since most DNA vaccines use expression vectors containing CMV promoters. One strategy to overcome the limitation of gene silencing by DNA methylation is the employment of demethylating agents such as 5-aza-2’-deoxycytidine (DAC). The nucleotide analogue 5-aza-2'-deoxycytidine has been shown to inhibit DNA methyltransferase, resulting in the reactivation of several methylation-silenced genes [13–15]. DAC is currently being employed in combination with other anti-cancer drugs in clinical trials in patients with a variety of solid tumors [16]. Furthermore, DAC is currently an FDA approved antineoplastic drug for the treatment of myelodysplastic syndrome [17]. Thus, the employment of DAC represents a potentially promising chemotherapeutic strategy that may be used in combination with DNA vaccines.
In the current study, we have reasoned that CRT/E7 DNA vaccine combined with demethylating agent, 5-aza-2’-deoxycytidine (DAC) would lead to upregulation of CRT/E7 expression, resulting in improved DNA vaccine potency. We found that DAC treatment led to the upregulation of the transgene expression in DNA transfected cells in vitro as well as in DNA-vaccinated mice in vivo. Furthermore, we found that treatment with DAC enhanced the E7-specific CD8+ T cell immune responses as well as the antitumor effects generated by the CRT/E7 DNA vaccine. Thus, our data suggest that combination of immunotherapy utilizing CRT/E7 DNA vaccine with chemotherapy utilizing DAC may represent a potentially feasible approach to controlling HPV-associated malignancies. The clinical implications of this study are discussed.
3.0 Materials and Methods
Mice
6–8 week-old female C57BL/6 mice purchased from the National Cancer Institute (Frederick, MD) were used for experiments unless stated elsewhere. The mice were housed under specific pathogen-free conditions. All procedures were done according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals.
Cell culture and reagents
TC-1 cells were generated as described previously [18]. DC-1 cells were derived from the dendritic cell line provided by Dr. Kenneth Rock (University of Massachusetts, Boston, MA) through continuous culture. BHK21 cells were purchased from ATCC. All the cells were maintained in a humidified 5% CO2 atmosphere at 37 °C and in RPMI medium supplemented with 10% FBS, 2 mM glutamine, 0.1 mM non-essential amino acid, 1 mM sodium pyruvate and 55 µM 2-mercaptoethanol. Transfection of BHK21 and DC-1 cells was performed using LipofectAMINE 2000 (Invitrogen) according to the manufacture’s instruction. 5-aza-2′-deoxycytidine (DAC, 5-aza-CdR, decitabine, dacogen, DAX), (Sigma, St. Louis, MO) was used as indicated in the figure legends.
Reverse Transcription (RT)-PCR
Total RNAs were isolated using TRIzol method (Invitrogen). 10 ng of the total RNA was subjected to RT-PCR analysis using SuperScript One-Step RT-PCR with Platinum Taq system (Invitrogen) with the following primers: mIFN-γ upstream, 5′-CTGAGACAATGAACGCTACAC-3′, mIFN-γ downstream: 5′-TTGCTGTTGCTGAAGAAGGT-3′; GAPDH upstream, 5′-CCGGATCCTGGGAAGCTTGTCATCAACGG-3′, downstream, 5′-GGCTCGAGGCAGTGATGGCATGGACTG-3′.
DNA vaccination
DNA-coated particles were prepared as described previously [19]. The mice received the particles at the shaved abdominal region via a helium-driven gene gun (Bio-Rad, Hercules, CA) at a discharge pressure of 400 psi. For pcDNA3-CRT/E7 vaccination, the mice were immunized with 2 µg/mouse of DNA and boosted once with the same dose at one-week interval.
In vivo tumor treatment experiments
C57BL/6 mice (five per group) were inoculated with TC-1 cells (5×104 per mouse) subcutaneously (s.c.) at day 0. At days 4, 7 and 11, the mice were treated with or without 0.25mg/kg body weight of DAC intraperitoneally. At days 11 and 18, the mice were treated with or without 2µg per mouse of CRT/E7 DNA via gene gun. Tumors were measured with a pair of calipers twice a week from day 14. Tumor growth was monitored twice a week by palpation and measuring the length (L) and width (W) of the tumor with Vernier calipers, and the tumor size was calculated as (L×W2)/2.
Flow cytometry
Detection of cellular surface CD8a and intracellular IFNγ was performed using flow cytometry as described previously [19]. In brief, the cells were incubated overnight with 1 µg/ml of GolgiPlug (BD Pharmingen) in the presence (for E7-specific CD8+ T cells) or absence of 1 µg/ml of E7 peptide (49–57). After washed twice with FACScan buffer, the cells were stained with phycoerythrin-conjugated anti-mouse CD8a antibody. The cells were then incubated with BD cytofix/cytoperm solution (BD Pharmingen) followed by staining with FITC-conjugated anti-mouse IFNγ antibody. The flow cytometry analysis was performed on a Becton-Dickinson FACSCalibur with CELLQuest software (BD Biosciences, Mountain View, CA).
Analysis of tumor-infiltrating lymphocytes
C57BL/6 mice (3 per group) were inoculated with TC-1 cells (5×104 per mouse) subcutaneously (s.c.) at day 0. At days 4, 7 and 11, the mice were treated with or without 0.25mg/kg body weight of DAC intraperitoneally. At days 11 and 18, the mice were treated with or without 2µg per mouse of CRT/E7 DNA via gene gun. One week after the second vaccination, the tumors were dissected and single cell suspension was prepared as follows. The tumors were cut into small pieces and digested with 0.05% (w/v) of trypsin and 0.1% (w/v) of collagenase D in HBSS for one hour at 37 °C with vortex every 15 minutes. The suspension was then passed through cell strainers before washing with growth medium. 5×106 cells were incubated with GolgiPlug in the presence or absence of E7 peptide (49–57) overnight in duplicate wells using 24-well plate. The tumor-infiltrating E7-specific CD8+ T cells were analyzed by flow cytometry.
In vitro stimulation of splenocytes
48-well plates were coated with or without 5 µg/ml of anti-CD3ε antibody (145-2C11, BD Pharmingen) in PBS for 90 minutes at 37°C and washed three times with PBS before use. The splenocytes (1×106/well) isolated from mice treated with or without DAC were stimulated with the coated plates and 1 µg/ml of anti-CD28 antibody (37.51, BD Pharmingen) in the presence of GolgiPlug overnight at 37°C. And then the cells were harvested and analyzed for the CD8+/IFNγ+ population using flow cytometry.
Western blot
Cells were lysed with protein extraction reagent (Pierce, Rockford, IL). Equal amounts of whole cell lysates (30 µg) were resolved by SDS-PAGE, and the fractionated proteins were transferred onto nitrocellulose membrane (0.45 µM, Bio-Rad). After being blocked with 5% (w/v) non-fat dry milk in TBST (Tris-buffered saline/Tween; 50 mM Tris/HCl, pH 7.5, 50 mM NaCl and 0.1% Tween 20), the membranes were incubated with the E7-specific monoclonal antibody (Invitrogen, Carlsbad, CA) diluted in the blocking buffer overnight at 4°C using the methods similar to what we have described previously [20]. The bound primary antibodies were detected using the appropriate horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences, UK) and Amersham ECL Western Blotting Detection Reagents (GE Health) and visualized using ChemiDoc XRS chemiluminescent detection system (Bio-Rad).
Bioluminescence imaging
To analyze the luciferase activity in cells, the cells were incubated with 20 µg/ml of luciferin (Promega, Madison, WI) for 5 minutes before the luminescence intensity was measured using the IVIS Imaging System Series 200 (Xenogen, Cranbury, NJ). To analyze the luciferase activity in mice, the mice were intraperitoneally injected with 0.8 mg/mouse of luciferin. 10 minutes after the injection, the anesthesized mice were imaged using the IVIS Imaging System Series 200.
Statistical Analysis
All quantitative data were expressed as mean±SE. Comparison between two groups was made by Student’s t test, and comparison among multiple groups was by one-way analysis of variance (ANOVA). Linear trend of dose effect was analyzed by linear regression. The survival analysis was made by the Kaplan-Meier method and one-sided log-rank statistic. p<0.05 was considered statistically significant.
4.0 Results
Combination treatment of TC-1 tumor-bearing mice with 5-aza-2′-deoxycytidine (DAC) and CRT/E7 DNA generated the best therapeutic antitumor effects
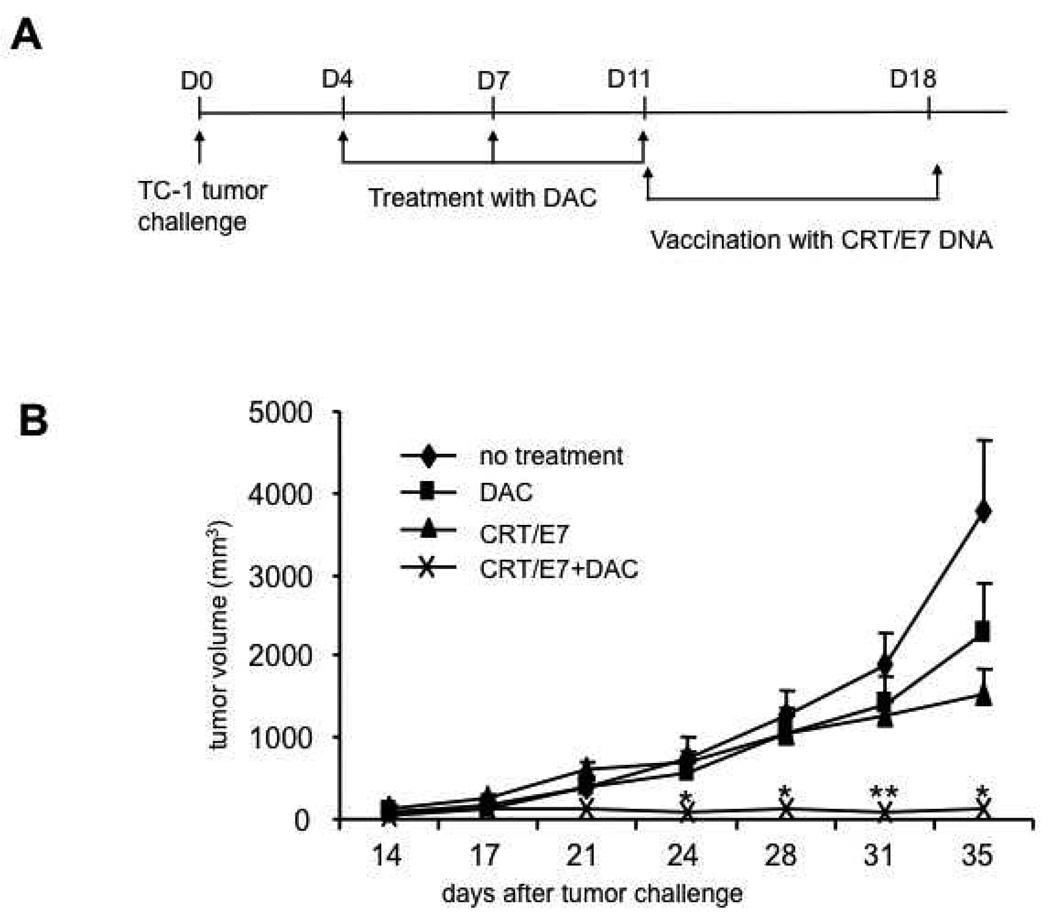
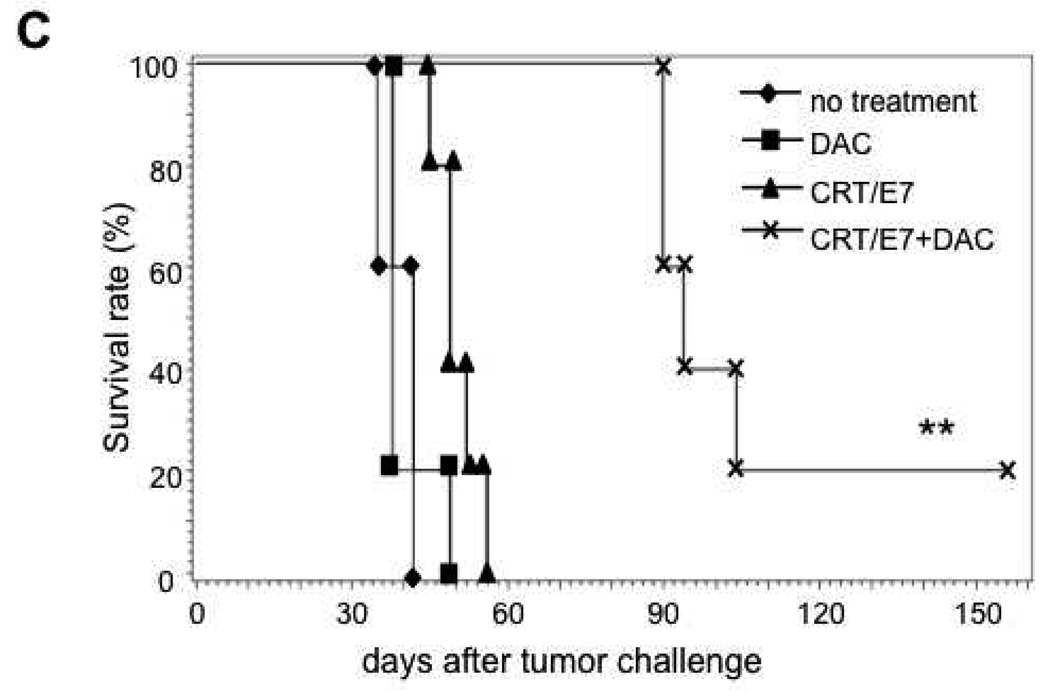
To determine if the enhanced E7-specific CD8+ T cell immune responses observed in CRT/E7 DNA-vaccinated mice pre-treated with DAC could translate into enhanced antitumor effects, we performed in vivo tumor treatment experiments. We first challenged C57BL/6 mice (3 per group) subcutaneously with 5×104 /mouse of TC-1 tumor cells on day 0. On day 4, 7 and 11 after tumor challenge, we treated the mice with or without 0.25 mg/kg body weight of DAC and from day 11, we treated the mice with CRT/E7 DNA intradermally via gene gun twice with a 1 week interval. The treatment regimens are outlined in Figure 1A. As shown in Figure 1B, the TC-1 tumor-bearing mice pre-treated with DAC followed by CRT/E7 DNA vaccination exhibited significantly decreased tumor growth compared to tumor-bearing mice treated with DAC alone or CRT/E7 DNA vaccine alone. We further characterized the survival of the different treatment groups using Kaplan-Meier survival analysis. As shown in Figure 1C, tumor-bearing mice pre-treated with DAC followed by CRT/E7 DNA vaccination exhibited improved survival compared to tumor-bearing mice treated with DAC alone or CRT/E7 DNA vaccine alone. Taken together, our results indicate that pre-treatment with DAC significantly enhances the therapeutic antitumor effects generated by treatment with CRT/E7 DNA vaccine.
Figure 1. In vivo tumor treatment experiments.
(A) Schematic diagram of the in vivo treatment schedule. C57BL/6 mice (5 per group) were inoculated with TC-1 cells (5×104 per mouse) subcutaneously (s.c.) on day 0. On days 4, 7 and 11, the mice were treated with or without 0.25mg/kg body weight of DAC intraperitoneally. On days 11 and 18, the mice were treated with or without 2µg per mouse of CRT/E7 DNA intradermally via gene gun. Tumor size and volume was measured as described in the Materials and Methods. (B) Line graph depicting the tumor volumes over time in the treated groups of tumor-bearing mice. (C) Kaplan-Meier survival analysis of the treated groups of tumor-bearing mice. Representative data from one of three independent experiments are shown. ** indicates p<0.01* indicates p<0.05.
Treatment with 5-aza-2′-deoxycytidine (DAC) led to upregulation of the proteins encoded by DNA in transfected cells
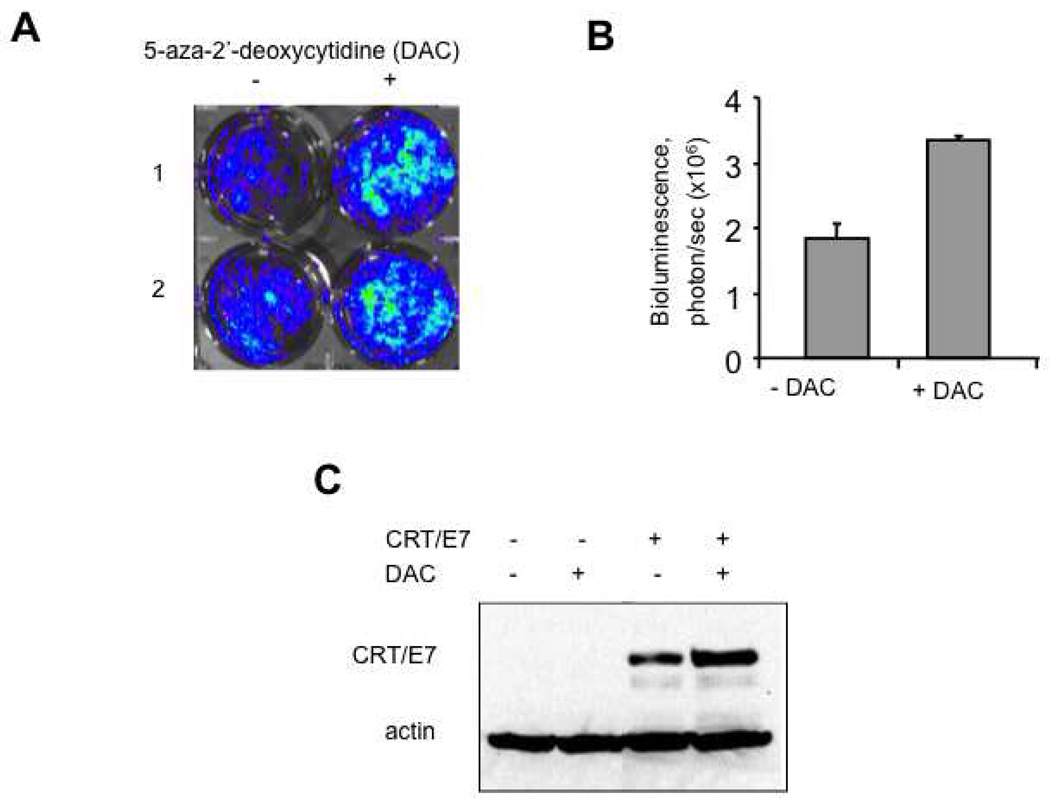
To examine if the expression of the protein encoded by the DNA in transfected cells can be upregulated by treatment with 5-aza-2′-deoxycytidine (DAC), we first transfected BHK21 cells with pcDNA3-luciferase. 16 hours later, the transfected cells were treated with or without DAC. The treated cells were characterized for luciferase expression using luminescence imaging 24 hours after treatment with DAC. As shown in Figure 3A and B, DNA transfected cells treated with DAC demonstrated significantly higher luciferase expression compared to the transfected cells without DAC treatment. We further characterized the effect of DAC on the expression of CRT/E7 using a dendritic cell line, DC-1 transfected with pcDNA3-CRT/E7. The transfected DC-1 cells were then treated with or without DAC and analyzed for the expression of CRT/E7 using Western blot analysis. As shown in Figure 2C, the transfected cells treated with DAC demonstrated significantly higher expression of CRT/E7 compared to the transfected cells without DAC treatment. These results are consistent with previous studies using different antigenic systems [21, 22]. Thus, our data indicate that treatment with DAC enhanced the expression of the protein encoded by the DNA in transfected cells.
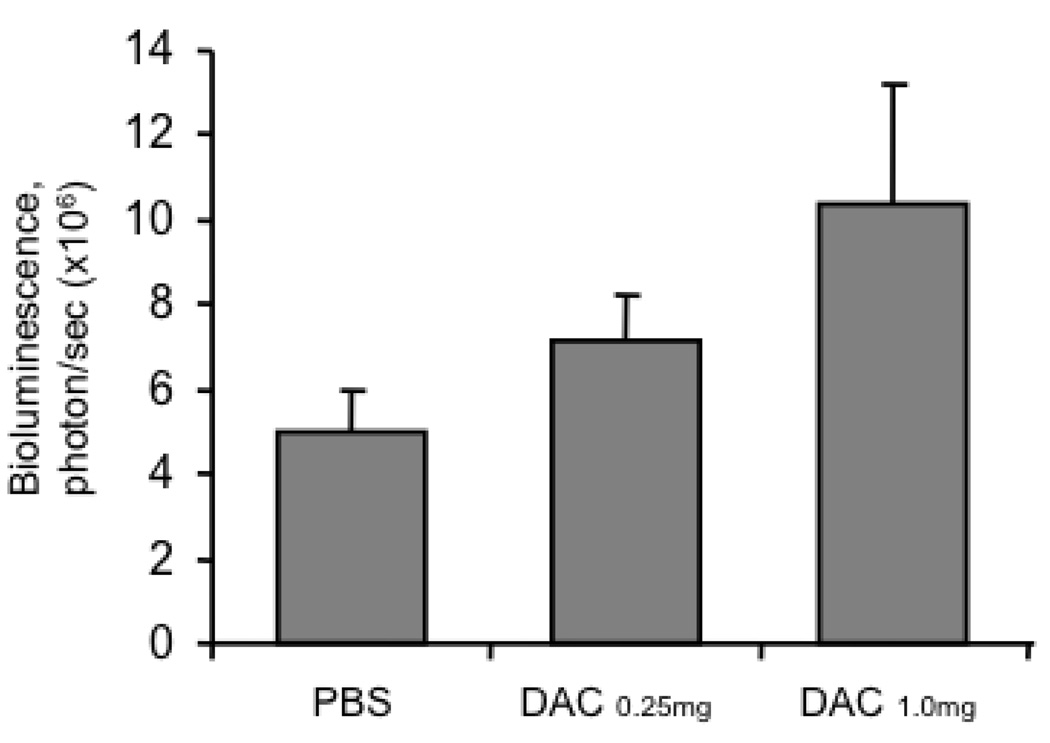
Figure 3. Characterization of the in vivo luciferase expression in pcDNA3-luciferase vaccinated mice pre-treated with or without DAC.
C57BL/6 mice (5 per group) were administered 0, 0.25 or 1.0 mg/kg body weight of DAC on days 0, 3 and 7. On the last day of administration of DAC, the mice were administered pcDNA3-luciferase intradermally via gene gun. 24 hours later, the mice were analyzed for luciferase activity using the IVIS Imaging System. Bar graphs depicting the levels of luciferase activity in the different groups of mice are shown. Note: we found a linear trend in the dose effect with a p-value of 0.0397.
Figure 2. Characterization of the expression of luciferase or chimeric protein CRT/E7 in transfected cells treated with or without DAC.
A and B. BHK21 cells were transfected with plasmid pcDNA3-luciferase and incubated for 16 hours followed by treatment with or without 5µM of 5-aza-2′-deoxycytidine (DAC). 24 hours after DAC treatment, the cells were analyzed for luciferase activity using the IVIS Imaging System Series 200. (A) Representative luminescence images of the luciferase-expressing BHK21 cells treated with or without DAC. (B) Bar graphs depicting the levels of luciferase activity in transfected cells with or without DAC treatment. (C) Western blot analysis to determine the expression of CRT/E7 in DC-1 cells transfected with plasmid pcDNA3-CRT/E7 (CRT/E7) followed by treatment with or without 5µM of DAC using HPV-16 E7-specific monoclonal Ab. Representative data from one of two independent experiments are shown.
Treatment with 5-aza-2′-deoxycytidine (DAC) enhanced the luciferase expression in mice vaccinated with pcDNA3-luciferase
In order to determine if treatment with DAC would influence the expression of the protein encoded by the DNA vaccine administered in vivo, we first challenged C57BL/6 mice (5 per group) with increasing doses of DAC by intraperitoneal injection on days 0, 3 and 7 and then vaccinated the mice with pcDNA3-luciferase intradermally via gene gun on last day of DAC administration. 24 hours later, the mice were analyzed for luciferase activity using bioluminescence imaging. As shown in Figure 3, vaccinated mice treated with DAC demonstrated a significant increase in bioluminescence intensity in a dose dependent manner compared to vaccinated mice without DAC treatment. Thus, our data indicate that treatment of mice with DAC leads to the upregulation of proteins encoded by DNA vaccines in vivo.
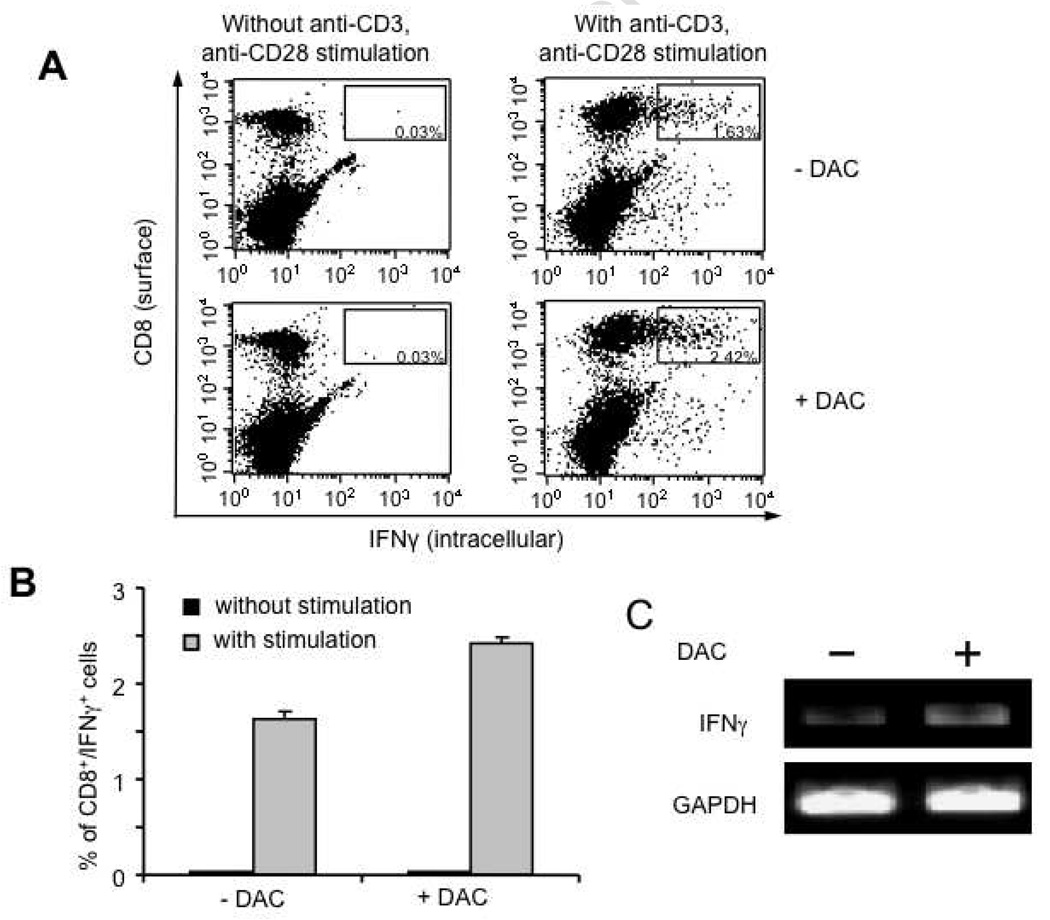
Pre-treatment of mice with 5-aza-2′-deoxycytidine (DAC) led to the enhanced activation of CD8+ T cells during in vitro stimulation
To determine if treatment of mice with 5-aza-2′-deoxycytidine (DAC) would influence the activation ability of splenocytes, C57BL/6 mice (3 per group) were treated with or without 0.25 mg/kg body weight of DAC on days 0, 3, and 7. One day later, the splenocytes from each group were harvested and stimulated with plate-bound anti-CD3 and soluble anti-CD28 overnight. The splenocytes were then analyzed for CD8 and IFNγ expression using intracellular cytokine staining followed by flow cytometry analysis. As shown in Figure 4A and B, in vitro stimulation of splenocytes from DAC-treated mice with anti-CD3 and anti-CD28 antibodies led to significantly increased numbers of CD8+/IFNγ+ T cells compared to in vitro stimulation of splenocytes from mice without DAC treatment. We also measured mRNA levels of IFNγ in the stimulated splenocytes from each mouse group using RT-PCR followed by gel electrophoresis. The mRNA level of GAPDH was used as loading control. As shown in Figure 4C, we found that the mRNA level of IFNγ was higher in the stimulated splenocytes from the DAC-treated mice as compared to the stimulated splenocytes from the mice without DAC treatment. Thus, our data suggest that treatment of mice with DAC sensitizes the splenocytes to further activation with anti-CD3 and anti-CD28 monoclonal antibodies.
Figure 4. Characterization of the activation ability of the splenocytes derived from mice treated with or without DAC.
C57BL/6 mice (3 per group) were injected with or without 0.25 mg/kg body weight of DAC on days 0, 3 and 7. 24 hours after the last treatment, the splenocytes from each group were pooled together and stimulated with plate-bound anti-CD3 (5µg/ml) and soluble anti-CD28 (1µg/ml) monoclonal antibodies. The stimulated splenocytes were then analyzed for CD8+/IFN-γ+ cells by intracellular cytokine staining followed flow cytometry analysis or analyzed for IFN-γ mRNA by RT-PCR with primers specific for murine IFN-γ or GAPDH. (A) Representative flow cytometry data indicating the percentages of the CD8+/IFNγ+ splenocytes from each of the treated groups. (B) Bar graph depicting the percentages of CD8+/IFNγ+ splenocytes from each of the treated groups. Representative data from one of two independent experiments are shown. (C) Representative gel electrophoresis data illustrating the mRNA levels of IFN-γ by RT-PCR in the stimulated splenocytes from mice treated with or without DAC.
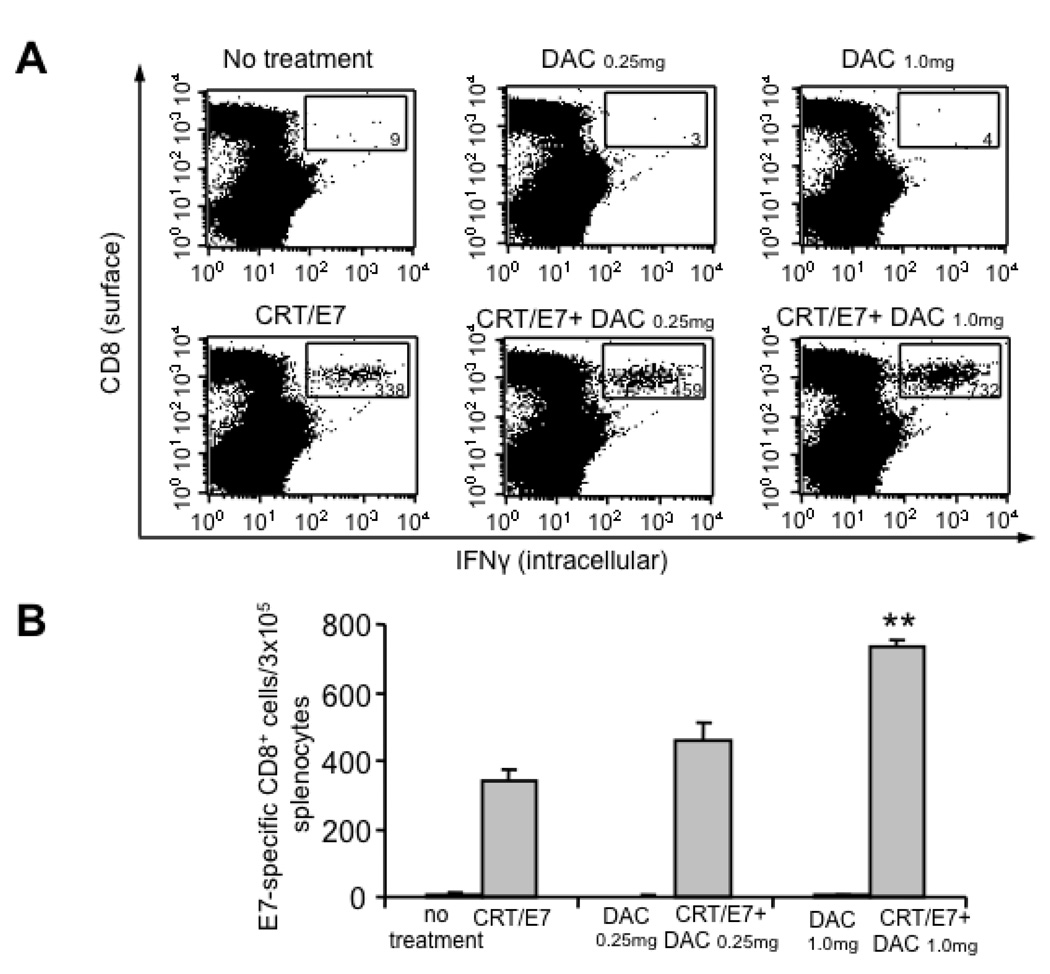
Pre-treatment with 5-aza-2′-deoxycytidine (DAC) led to enhanced E7-specific CD8+ T cell immune responses in CRT/E7 DNA vaccinated mice
In order to determine if treatment of mice with DAC would lead to enhanced E7-specific CD8+ T cell immune responses generated by vaccination with CRT/E7 DNA, we first treated C57BL/6 mice (3 per group) with or without increasing doses of DAC on days 0, 3 and 7. We then vaccinated the mice with CRT/E7 DNA on the last day of DAC treatment. The mice received a booster vaccination with the same dose 1 week later. Unvaccinated mice treated with or without DAC were used as controls. One week after the second vaccination, splenocytes were harvested from the different groups of mice and analyzed for E7-specific CD8+ T cell immune responses using intracellular cytokine staining followed by flow cytometry analysis. As shown in Figure 5A and B, the CRT/E7 DNA-vaccinated mice pre-treated with DAC generated significantly greater numbers of E7-specific CD8+ T cells in a dose-dependent manner compared to CRT/E7 DNA-vaccinated mice without pre-treatment with DAC. We also observed that mice treated with DAC alone without DNA vaccination did not generate detectable E7-specific CD8+ T cell immune responses. Therefore, our results indicate that pre-treatment of mice with DAC enhances the E7-specific CD8+ T cell immune responses generated by CRT/E7 DNA vaccine.
Figure 5. Characterization of the E7-specific CD8+ T cell immune responses in CRT/E7-vaccinated mice pre-treated with or without DAC.
C57BL/6 mice (3 per group) were pre-treated with increasing does of DAC (0.25 and 1.0 mg/kg body weight) on day 0, 3 and 7. On day 7, the mice were immunized with or without CRT/E7 DNA intradermally via gene gun. The mice received a booster with the same dose and regimen 1 week later. One week after the last vaccination, the splenocytes from each group were harvested and characterized for E7-specific CD8+ T cells by intracellular IFNγ staining followed by flow cytometry analysis. (A) Representative flow cytometry data indicating the number of E7-specific CD8+ T cells per 3×105 splenocytes from each mouse group. (B) Bar graph depicting the numbers of E7-specific CD8+ cells per 3×105 splenocytes among each group. Data shown are representative of three independent experiments performed (mean±SE). ** indicates p<0.01. Note: we found a linear trend in the dose effect with a p-value to be less than 0.0001.
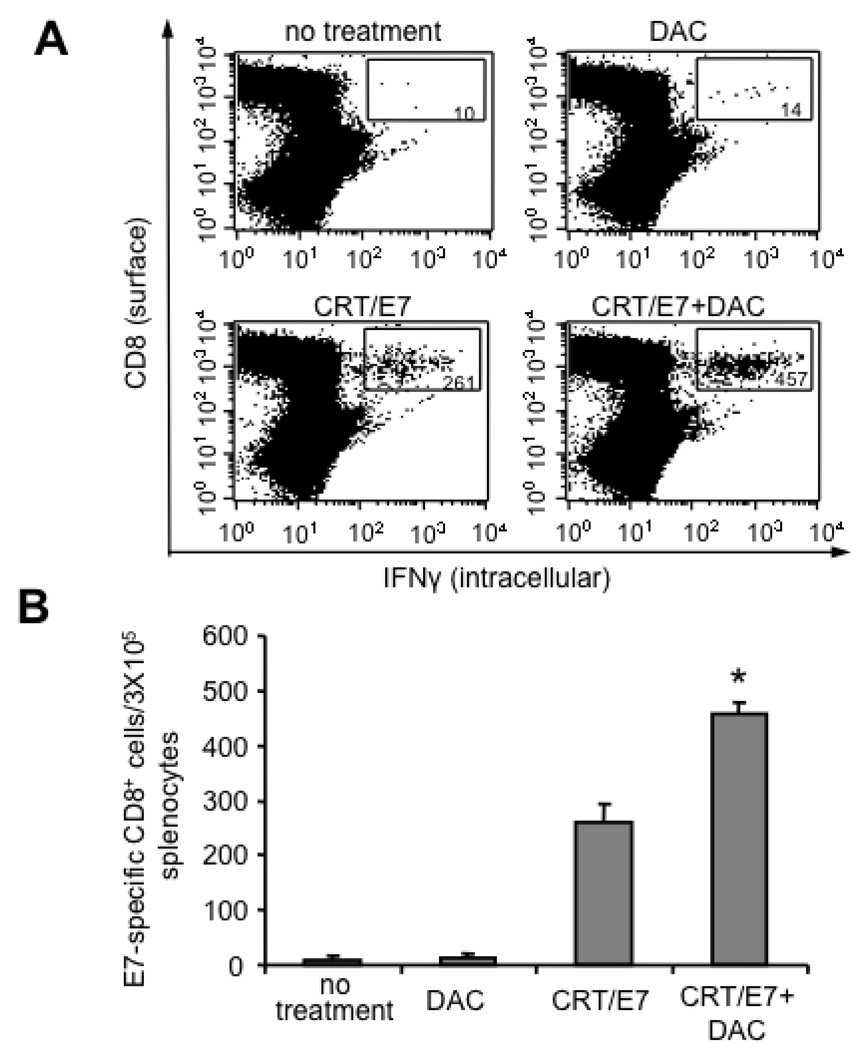
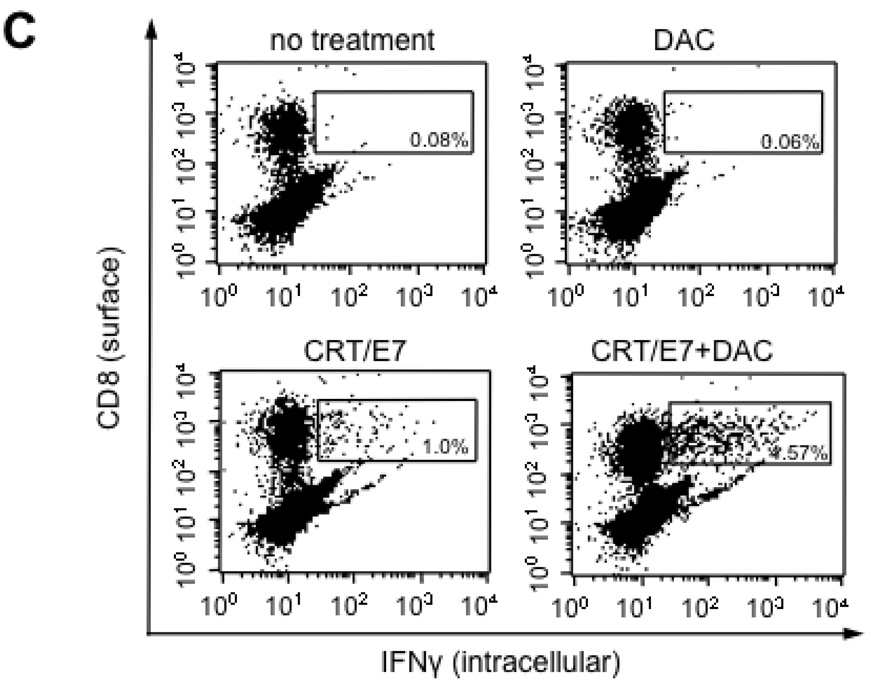
Treatment with 5-aza-2′-deoxycytidine (DAC) enhanced E7-specific CD8+ T cell immune responses generated in tumor-bearing mice treated with CRT/E7 DNA
To determine if DAC enhanced the E7-specific CD8+ T cell immune responses generated by CRT/E7 DNA vaccination in the spleens and tumors of tumor-bearing mice, tumor-bearing C57BL/6 mice (5 per group) were treated with DAC and/or CRT/E7 DNA using the regimen described in Figure 1A. One week after the last treatment, we characterized the E7-specific CD8+ T cell immune responses in the spleens and tumors of tumor-bearing mice. Spleens and tumors were harvested from the different groups of mice and the isolated cells were then analyzed for E7-specific CD8+ T cells using intracellular cytokine staining followed by flow cytometry analysis. As shown in Figure 6A and B, tumor-bearing mice treated with DAC followed by CRT/E7 DNA vaccination generated significantly higher numbers of E7-specific CD8+ T cells in the spleens compared to tumor-bearing mice treated with DAC alone or CRT/E7 DNA vaccine alone. Furthermore, as shown in Figure 6C, tumor-bearing mice treated with DAC followed by CRT/E7 DNA vaccination generated significantly higher numbers of E7-specific CD8+ tumor-infiltrating lymphocytes compared to tumor-bearing mice treated with DAC alone or CRT/E7 DNA vaccine alone. Taken together, our data indicate that pre-treatment with DAC significantly enhances the numbers of E7-specific CD8+ T cells in the spleens and tumors of tumor-bearing mice generated by treatment with CRT/E7 DNA vaccine.
Figure 6. Characterization of the E7-specific CD8+ T cell immune responses in the spleens and tumors of tumor-bearing mice treated with DAC and/or CRT/E7 DNA.
C57BL/6 mice (three per group) were inoculated with TC-1 cells and treated as depicted in Figure 5A. On day 25 after tumor inoculation, the spleens and tumors were excised from the mice. The splenocytes and tumor-infiltrating lymphocytes (TILs) from each group were harvested and characterized for E7-specific CD8+ T cells by intracellular cytokine staining followed by flow cytometry analysis. (A) Representative flow cytometry data depicting the number of E7-specific CD8+ cells in the spleens of treated tumor-bearing mice. (B) Bar graph depicting the numbers of E7-specific CD8+ cells per 3×105 splenocytes among each group of treated mice. The mean±SE of two independent experiments is shown. The groups with statistical significance are indicated by asterisks, *p<0.05 (CRT/E7 vs CRT/E7+DAC). (C) Representative flow cytometry data depicting the percentage of E7-specific CD8+ cells in the tumors of treated tumor-bearing mice.
5.0 Discussion
In the current study we examined a strategy to enhance DNA vaccine potency by improving antigen expression using a demethylating agent, 5-aza-2’-deoxycytidine (DAC). We showed that treatment of DNA-transduced cells with DAC led to the upregulation of the encoded gene in vitro and in vivo. We further found that DAC enhances the E7-specific CD8+ T cell immune responses and antitumor effects generated by CRT/E7 DNA vaccine in tumor-bearing mice. Thus, our data suggest that treatment with a demethylating agent followed by CRT/E7 DNA vaccination may represent a potentially promising approach to improve the therapeutic effects of CRT/E7 DNA vaccine for the control of HPV-associated malignancies.
Our results are consistent previous observations that the DAC could activate the expression of the protein encoded by the DNA constructs. Previously, Grassi et al. showed that DAC treatment could increase the expression of a reporter gene controlled by cytomegalovirus (CMV) promoter in a stably transfected neuroblastoma cell line [22]. Another study using human pancreatic cancer and Chinese Hamster Ovary cells stably transfected with CMV promoter driven glycogenes showed that the expression of the glycogenes was upregulated in cells treated with DAC [21]. Thus, our data are consistent with previous findings that DAC treatment leads to increased expression of transgenes.
In our study, we showed that treatment of mice with DAC sensitizes the splenocytes to further activation with anti-CD3 and anti-CD28 monoclonal antibodies (Figure 3). Based on our data, treatment with DAC alone does not lead to activation of CD8+ T cells by releasing IFN-γ. One possibility is that treatment with DAC may potentially lead to the upregulation of the genes that are involved in T cell activation. Alternatively, DAC treatment may also increase the expression of key molecules in the other immune cells that may influence the activation of CD8+ T cells. Thus, it will be of interest to characterize the expression of the molecules expressed by different immune cells, which are involved in T cell activation with or without DAC treatment in the future.
We observed that treatment of TC-1 tumor-bearing mice with DAC and CRT/E7 DNA generated the best therapeutic antitumor effects (Figure 5). Our data suggest that the observed antitumor effects may be contributed by the enhancement of antigen expression and DNA vaccine immunogenicity by DAC. However, we cannot exclude the possibility that the observed effects could be a direct consequence of the anti tumor activity of DAC, or due to a non specific activation of immune cells by DAC. Further exploration of these potential mechanisms may facilitate a better understanding of the role of DAC in enhancing the antitumor effects induced by the DNA vaccine.
For clinical translation, it is essential to employ a DNA vector that could potentially used for clinical trials without safety concerns. The pcDNA3 vector, used in the current study may not be feasible for clinical translation since it contains an ampicillin resistance gene. We have previously used pNGVL4a vector obtained from the NIH National Gene Vector Laboratory for DNA vaccine development for our clinical trials [23]. This vector is a second-generation plasmid derived from pNGVL-3, which has been previously used for human clinical trials [24]. The pNGVL4a vector lacks the ampicillin resistance gene and thus would be suitable for clinical translation of the current DNA vaccines.
In summary, this study demonstrates a novel strategy utilizing demethylating agents to improve the antigen expression for the enhancement of DNA vaccine potency. Several studies have demonstrated that the combination of chemotherapeutic agents with immunotherapy can further enhance therapeutic vaccine potency [25–28] For clinical translation, it will be important to further characterize the optimal dose and regimen of DAC in order to generate the best therapeutic effects in preclinical models [29]. Furthermore, the successful example of the current study warrants the exploration of other demethylation agents that has been used in clinical trials, such as 5-azacytidine. Future endeavors should also focus on exploring novel demethylating agents that are capable of enhancing the potency of DNA vaccines for clinical translation.
Acknowledgements
This work was supported by the American Cancer Society, NCDDG (1U19 CCA113341) and National Cancer Institute SPORE in Cervical Cancer P50 CA098252 and the 1 RO1 CA114425-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 2.Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization*. Annu Rev Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 3.Hung CF, Wu TC. Improving DNA vaccine potency via modification of professional antigen presenting cells. Curr Opin Mol Ther. 2003;5(1):20–24. [PubMed] [Google Scholar]
- 4.Tsen SW, Paik AH, Hung CF, Wu TC. Enhancing DNA vaccine potency by modifying the properties of antigen-presenting cells. Expert Rev Vaccines. 2007;6(2):227–239. doi: 10.1586/14760584.6.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelebart P, Opas M, Michalak M. Calreticulin, a Ca2+-binding chaperone of the endoplasmic reticulum. Int J Biochem Cell Biol. 2005;37(2):260–266. doi: 10.1016/j.biocel.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 6.Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55(4):244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng WF, Hung CF, Chai CY, Hsu KF, He L, Ling M, et al. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J Clin Invest. 2001;108(5):669–678. doi: 10.1172/JCI12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JW, Hung CF, Juang J, He L, Kim TW, Armstrong DK, et al. Comparison of HPV DNA vaccines employing intracellular targeting strategies. Gene Ther. 2004;11(12):1011–1018. doi: 10.1038/sj.gt.3302252. [DOI] [PubMed] [Google Scholar]
- 9.Peng S, Tomson TT, Trimble C, He L, Hung CF, Wu TC. A combination of DNA vaccines targeting human papillomavirus type 16 E6 and E7 generates potent antitumor effects. Gene Ther. 2006;13(3):257–265. doi: 10.1038/sj.gt.3302646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pamer E, Cresswell P. Mechanisms of MHC class I--restricted antigen processing. Annu Rev Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 11.Ochiai H, Harashima H, Kamiya H. Intranuclear disposition of exogenous DNA in vivo: silencing, methylation and fragmentation. FEBS Lett. 2006;580(3):918–922. doi: 10.1016/j.febslet.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Brooks AR, Harkins RN, Wang P, Qian HS, Liu P, Rubanyi GM. Transcriptional silencing is associated with extensive methylation of the CMV promoter following adenoviral gene delivery to muscle. J Gene Med. 2004;6(4):395–404. doi: 10.1002/jgm.516. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki H, Gabrielson E, Chen W, Anbazhagan R, van Engeland M, Weijenberg MP, et al. A genomic screen for genes upregulated by demethylation and histone deacetylase inhibition in human colorectal cancer. Nature genetics. 2002;31(2):141–149. doi: 10.1038/ng892. [DOI] [PubMed] [Google Scholar]
- 14.Yu N, Wang M. Anticancer drug discovery targeting DNA hypermethylation. Current medicinal chemistry. 2008;15(14):1350–1375. doi: 10.2174/092986708784567653. [DOI] [PubMed] [Google Scholar]
- 15.Arai M, Imazeki F, Sakai Y, Mikata R, Tada M, Seki N, et al. Analysis of the methylation status of genes up-regulated by the demethylating agent, 5-aza-2'-deoxycytidine, in esophageal squamous cell carcinoma. Oncology reports. 2008;20(2):405–412. [PubMed] [Google Scholar]
- 16.Appleton K, Mackay HJ, Judson I, Plumb JA, McCormick C, Strathdee G, et al. Phase I and pharmacodynamic trial of the DNA methyltransferase inhibitor decitabine and carboplatin in solid tumors. J Clin Oncol. 2007;25(29):4603–4609. doi: 10.1200/JCO.2007.10.8688. [DOI] [PubMed] [Google Scholar]
- 17.Yoo CB, Jeong S, Egger G, Liang G, Phiasivongsa P, Tang C, et al. Delivery of 5-aza-2'-deoxycytidine to cells using oligodeoxynucleotides. Cancer research. 2007;67(13):6400–6408. doi: 10.1158/0008-5472.CAN-07-0251. [DOI] [PubMed] [Google Scholar]
- 18.Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer research. 1996;56(1):21–26. [PubMed] [Google Scholar]
- 19.Chen CH, Wang TL, Hung CF, Yang Y, Young RA, Pardoll DM, et al. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer research. 2000;60(4):1035–1042. [PubMed] [Google Scholar]
- 20.Kim D, Gambhira R, Karanam B, Monie A, Hung CF, Roden R, et al. Generation and characterization of a preventive and therapeutic HPV DNA vaccine. Vaccine. 2008;26(3):351–360. doi: 10.1016/j.vaccine.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi KH, Basma H, Singh J, Cheng PW. Activation of CMV promoter-controlled glycosyltransferase and beta -galactosidase glycogenes by butyrate, tricostatin A, and 5-aza-2'-deoxycytidine. Glycoconj J. 2005;22(1–2):63–69. doi: 10.1007/s10719-005-0326-1. [DOI] [PubMed] [Google Scholar]
- 22.Grassi G, Maccaroni P, Meyer R, Kaiser H, D'Ambrosio E, Pascale E, et al. Inhibitors of DNA methylation and histone deacetylation activate cytomegalovirus promoter-controlled reporter gene expression in human glioblastoma cell line U87. Carcinogenesis. 2003;24(10):1625–1635. doi: 10.1093/carcin/bgg118. [DOI] [PubMed] [Google Scholar]
- 23.Trimble CL, Peng S, Kos F, Gravitt P, Viscidi R, Sugar E, et al. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clin Cancer Res. 2009;15(1):361–367. doi: 10.1158/1078-0432.CCR-08-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trimble C, Lin CT, Hung CF, Pai S, Juang J, He L, et al. Comparison of the CD8+ T cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe. Vaccine. 2003;21(25–26):4036–4042. doi: 10.1016/s0264-410x(03)00275-5. [DOI] [PubMed] [Google Scholar]
- 25.Tseng CW, Monie A, Trimble C, Alvarez RD, Huh WK, Buchsbaum DJ, et al. Combination of treatment with death receptor 5-specific antibody with therapeutic HPV DNA vaccination generates enhanced therapeutic anti-tumor effects. Vaccine. 2008;26(34):4314–4319. doi: 10.1016/j.vaccine.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tseng CW, Monie A, Wu CY, Huang B, Wang MC, Hung CF, et al. Journal of molecular medicine. 8. Vol. 86. Berlin, Germany: 2008. Treatment with proteasome inhibitor bortezomib enhances antigen-specific CD8+ T-cell-mediated antitumor immunity induced by DNA vaccination; pp. 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bae SH, Park YJ, Park JB, Choi YS, Kim MS, Sin JI. Therapeutic synergy of human papillomavirus E7 subunit vaccines plus cisplatin in an animal tumor model: causal involvement of increased sensitivity of cisplatin-treated tumors to CTL-mediated killing in therapeutic synergy. Clin Cancer Res. 2007;13(1):341–349. doi: 10.1158/1078-0432.CCR-06-1838. [DOI] [PubMed] [Google Scholar]
- 28.Tseng CW, Hung CF, Alvarez RD, Trimble C, Huh WK, Kim D, et al. Pretreatment with cisplatin enhances E7-specific CD8+ T-Cell-mediated antitumor immunity induced by DNA vaccination. Clin Cancer Res. 2008;14(10):3185–3192. doi: 10.1158/1078-0432.CCR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemaire M, Chabot GG, Raynal NJ, Momparler LF, Hurtubise A, Bernstein ML, et al. Importance of dose-schedule of 5-aza-2'-deoxycytidine for epigenetic therapy of cancer. BMC Cancer. 2008;8(1):128. doi: 10.1186/1471-2407-8-128. [DOI] [PMC free article] [PubMed] [Google Scholar]